3.2. Effects of the Heated Showerhead on Source Density Distributions
Kim and Lee stated that the application of the appropriate inlet boundary conditions can be critical to precisely determining the plasma and radical distributions in a CCP deposition reactor [
21]. Similarly, Sansonnens et al. attempted to determine how film uniformity can be achieved in a showerhead-type CCP reactor using a simple one-dimensional model [
6]. They proposed the following equations:
where
x is the spatial coordinate;
kd is the electron impact dissociation rate;
ni (
i =
A,
B, or
C) is the density of species
i;
Di is the diffusivity of species
i;
S(
x) represents the spatial source term of ingredient mixture gas (
A);
v(
x) is defined as the gas velocity averaged over the electrode spacing;
A is dissociated by the electron impact reaction (with
kd) to generate
B and
C, which are reactive radicals; and
B is consumed on the surface by film deposition with
ks.
For their analytical study, they concluded that a uniform showerhead gas injection (i.e., S(x) = constant) can achieve uniform reactant transport in an isothermal and isobaric system, assuming spatially uniform electron density and energy. Based on this finding, it is inferred that the modification of S(x) can induce the modification of the product distribution. This means that the inlet boundary conditions should be carefully considered in CCP process simulations: in certain cases, it would be necessary to obtain information about the inlet gas distributions separately, as in the work of Kim and Lee. As the showerhead temperature (Tsh) was controlled by the Ohmic heating module in this study, as stated above, the enforced heat flux ensured that the radial uniformities of the gas temperature and density were sufficient along the showerhead radius in the three-dimensional gas flow simulations. In this study, their non-uniformities were less than 3% with Tsh = 373 K and less than 6% with Tsh = 473 K.
Figure 3 depicts the spatial variations in the gas temperature distributions for Cases 2, 3, 4 and 5. In
Figure 3a–c, axial variations in the gas temperature distributions, owing to the temperature conditions of the wall, are clearly observed. These variations are attributed to the depletion of the source gas and thus the changing properties of the mixture such as the changes in
ρmix [
27]. However, in
Figure 3d, because the showerhead temperature and the heater temperature were set to the same value (i.e.,
Tsh =
Th = 673 K), the temperature distribution is vertically symmetric.
Figure 4 and
Figure 5 depict the spatial variations in the density distributions of SiH
4 and Si
2H
6, respectively. We selected Si
2H
6 for the analysis because SiH
4 is rapidly consumed at gas pressures higher than 100 Pa; thus, Si
2H
6 is produced via a fast gas phase reaction between SiH
4 and SiH
2, as shown below.
Here,
kr is the reaction rate coefficient. In addition, Si
2H
6 acts as an important seed on which to grow to the high-order silanes, and finally the particles (such as a killer defect).
Figure 3.
Spatial variations in the gas temperature (Tg) distributions (K) for (a) Case 2 (Tsh = 373 K), (b) Case 3 (Tsh = 423 K), (c) Case 4 (Tsh = 473 K), and (d) Case 5 (Tsh = 673 K) for r ≥ 120 mm.
Figure 3.
Spatial variations in the gas temperature (Tg) distributions (K) for (a) Case 2 (Tsh = 373 K), (b) Case 3 (Tsh = 423 K), (c) Case 4 (Tsh = 473 K), and (d) Case 5 (Tsh = 673 K) for r ≥ 120 mm.
Figure 4.
Effects of the showerhead temperature (Tsh) on the spatial distribution of the reactive gas. Spatial variations in the SiH4 density distributions (m−3) for (a) Case 2 (Tsh = 373 K), (b) Case 3 (Tsh = 423 K), (c) Case 4 (Tsh = 473 K), and (d) Case 5 (Tsh = 673 K) for r ≥ 120 mm. The profiles are radially uniform for r < 120 mm. (e) SiH4 densities are plotted on the vertical line at rh (r = 154.3 mm) for Cases 2, 3, 4, and 5.
Figure 4.
Effects of the showerhead temperature (Tsh) on the spatial distribution of the reactive gas. Spatial variations in the SiH4 density distributions (m−3) for (a) Case 2 (Tsh = 373 K), (b) Case 3 (Tsh = 423 K), (c) Case 4 (Tsh = 473 K), and (d) Case 5 (Tsh = 673 K) for r ≥ 120 mm. The profiles are radially uniform for r < 120 mm. (e) SiH4 densities are plotted on the vertical line at rh (r = 154.3 mm) for Cases 2, 3, 4, and 5.
Figure 5.
Effects of the showerhead temperature (Tsh) on the spatial distribution of the source gas. Spatial variations in the Si2H6 density distributions (m−3) for (a) Case 2 (Tsh = 373 K), (b) Case 3 (Tsh = 423 K), (c) Case 4 (Tsh = 473 K), and (d) Case 5 (Tsh = 673 K) for r ≥ 120 mm. The profiles are radially uniform for r < 120 mm.
Figure 5.
Effects of the showerhead temperature (Tsh) on the spatial distribution of the source gas. Spatial variations in the Si2H6 density distributions (m−3) for (a) Case 2 (Tsh = 373 K), (b) Case 3 (Tsh = 423 K), (c) Case 4 (Tsh = 473 K), and (d) Case 5 (Tsh = 673 K) for r ≥ 120 mm. The profiles are radially uniform for r < 120 mm.
Notably, in various PECVD examples, neutral species can diffuse to the reactor surface rapidly. This efficient transport is expected by the fact that gas kinetic theory states that the diffusion coefficients of neutral species increase under lower pressure conditions. Howling et al. used an SiH
4/H
2 mixture to calculate the Peclet number (Pe =
dū/
D, where
d is the characteristic length,
ū is the characteristic velocity, and
D is the diffusion coefficient) in their CCP process conditions. Taking the binary diffusion coefficient for SiH
4 and H
2 at 100 Pa and 473 K to be 0.14 m
2 s
−1, the Pe was calculated as only 0.03 [
8]. As our process conditions are similar to those of Howling et al., we confidently assumed that our gas transport phenomena are also observed under conditions of rapid diffusion. We verified that the diffusion coefficients for silicon compounds are sufficiently high under the given process conditions; for example, the diffusion coefficient of SiH
4 is 0.057 m
2 s
−1 at 673 K. For a characteristic length of
d = 0.015 m, a characteristic velocity of
ū = 1.35 m s
−1, and a diffusion coefficient of
D = 0.057 m
2 s
−1, the spatially averaged value of Pe (Pe =
d ū/
D) is calculated to be only 0.36. Although our Pe is sufficiently low (i.e., less than one), convection effects are still observed in the density distribution contours of SiH
4 and Si
2H
6.
In
Figure 4, for all four cases, in the discharge volume, their SiH
4 density distributions have higher values at
r ≤
rh than at
r >
rh. These variations have their origins in both the source gas consumption routine (based on gas convection) of our showerhead reactor and the transport mechanism resulting from the fast consumption of SiH
4. Here, it should be noted that the transport phenomena of neutral species are mainly determined by gas flow streams, which can be described in terms of streamlines. Owing to the common features of the spatial variations in the gas temperature distributions, the spatial variations in the SiH
4 density distributions are similar to each other for Cases 2, 3 and 4. In these three cases, the SiH
4 density distributions vary axially in the inter-electrode region, and in addition, radial variations are clearly observed near the electrode edge. The expansion of an ideal gas and the efficient depletion of SiH
4 are both responsible for the distribution features. Conversely, in Case 5, the SiH
4 density distribution is noticeably changed by the increase in the showerhead temperature.
Figure 4e depicts that the axial variations become negligible in Case 5. Therefore, one can state that in Case 5, an increase in the showerhead temperature significantly modifies the SiH
4 density distributions.
Figure 5 shows that for all four cases, their Si
2H
6 densities indicate low values at the inlet (the initial position). However, because of the relatively slow consumption (and fast production) of Si
2H
6 and the gas convection effect, its density increases in the axial direction until it reaches the midplane of the discharge volume (i.e., the middle point between the showerhead and the heater). In addition, its density increases in the radial direction until it reaches the sidewall: the depletion of Si
2H
6 is not as fast as that of radicals such as H, SiH, SiH
2, and SiH
3 in this study. Note that both the lowest density distribution and the thickest boundary layer occur near the showerhead surface for Case 5.
3.3. Effects of the Heated Showerhead on Plasma Density Distributions
Figure 6 depicts the spatial distributions of the ion production rates. To allow for easier comparison, Cases 2 (
Tsh = 373 K) and 5 (
Tsh = 673 K) were selected and analyzed more carefully. The production of SiH
2+ (via direct impact ionization: e
− + SiH
4 → SiH
2+ + H + H + e
− + e
−) and that of Si
+ (via dissociative excitation: He
* + SiH
4 → Si
+ + 2H + H
2 + He + e
−) were chosen because of their significance under our conditions. As depicted in
Figure 6a,b, the SiH
2+ production rates are enhanced near the heater edge. Although the SiH
4 density is lower near the showerhead, the SiH
2+ production rate of Case 5 is higher than that of Case 2. In addition, in Case 5, the axial variation in the SiH
2+ production rate is bell-shaped, whereas the variation has two maxima in the inter-electrode region in Case 2. The difference in the profiles arises because the higher showerhead temperature lowers the gas density in the immediate surroundings of the showerhead to a sufficient degree, and thus the relative contribution of the mid-plane to the ionization is increased. In
Figure 6c,d, the rates at which Si
+ is produced are also enhanced near the heater edge in Cases 2 and 5. As similarly observed in
Figure 6a,b, the axial profile has two maxima in Case 2, whereas the profile is bell-shaped in Case 5. However, the decrease in the SiH
4 density as a result of the higher showerhead temperature lowers the Si
+ production rate in Case 5 relative to that in Case 2 near the showerhead surface. Because He dilution is used in this study, the peak value of the Si
+ production rate is higher than that of the SiH
2+ production rate. The rate coefficient for the Si
+ production used in this study was set as [
28]:
As depicted in
Figure 7a,b, the ion production rates in
Figure 6 are responsible for the distributions of the time-averaged electron density reaching a maximum near the heater edge: an off-axis maximum of the electron density,
Neo, is detected at locations similar to the peak ion production rates. As a result of the lower SiH
4 density, the peak value of Case 5 is lower than that of Case 2:
Neo is 5.90 × 10
15 m
−3 in Case 2, whereas
Neo is 3.93 × 10
15 m
−3 in Case 5. As shown in
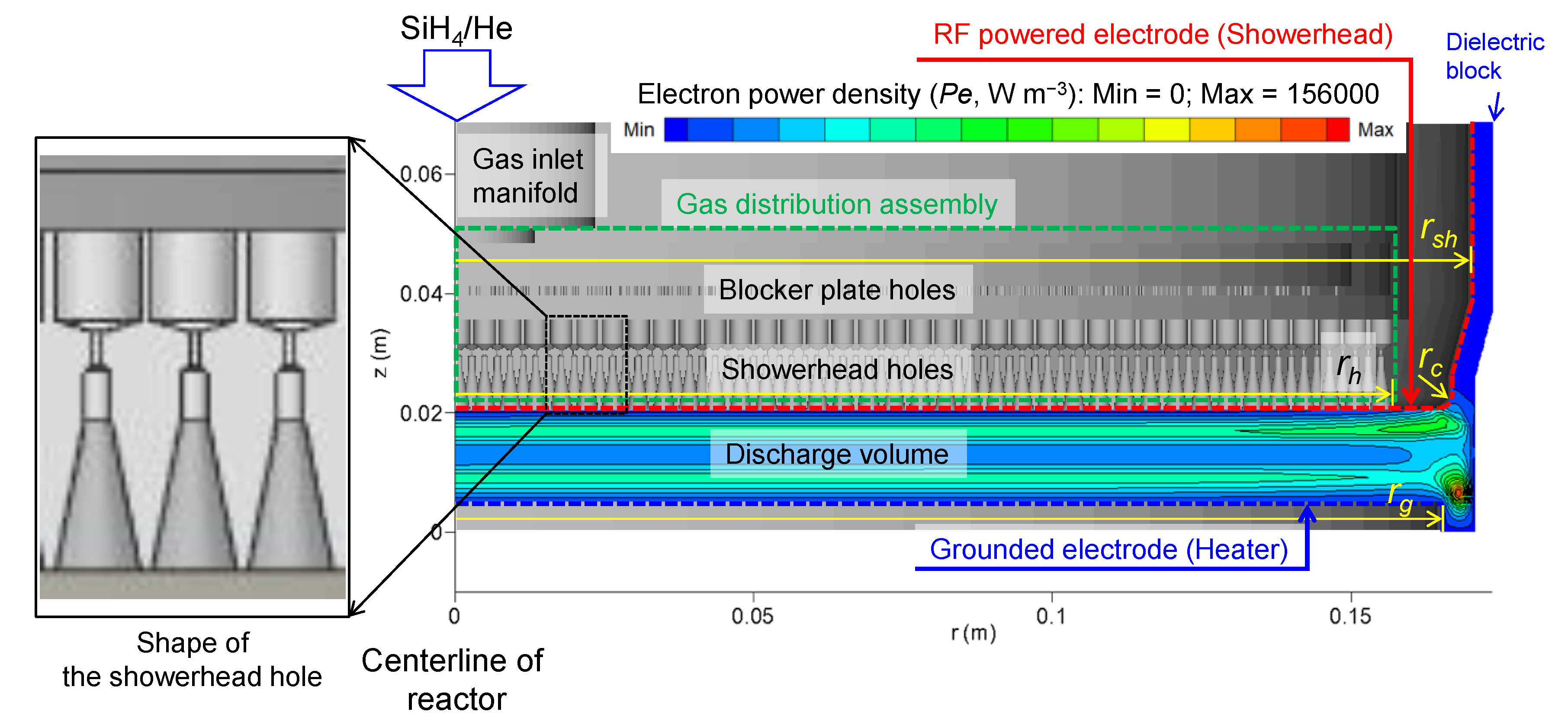
Figure 1, the electron power density (
Pe = −
e Je·
E, W m
−3) is also localized near the heater edge. This localization enhances the mean electron energy near the heater edge. Therefore, the locally enhanced production rates mentioned above are strongly correlated with the localization of the electron power density for Cases 2 and 5, as depicted in
Figure 7c,d, respectively. The contours in
Figure 7c,d also demonstrate that their peak values are affected by the change in the showerhead temperature.
3.4. Effects of the Heated Showerhead on Radical Density Distributions
Figure 8 provides the contours of the spatial variations in the SiH
3 densities (m
−3) for Cases 2, 3, 4, and 5. SiH
3 was selected for analysis from among various other radicals and stable molecules because of its intrinsic importance in the deposition of an a-Si:H film [
29]. A common feature between each of the different distributions is that the peak values are detected near the heater edge.
In the meantime, although the SiH
3 density distributions exhibit higher maximum values with lower showerhead temperature, the values detected near the heater decrease against the expectation, as shown in
Figure 8. This behavior can be understood by the SiH
3 being quickly depleted in the bulk by the enhancement of the disproportionation process as a result of the higher SiH
3 density:
In addition, SiH
4 depletion is suppressed with higher showerhead temperature, as observed in
Figure 4 and
Figure 5: the maximum SiH
4 density of Case 2 (7.64 × 10
20 m
−3) becomes higher than that of Case 5 (7.07 × 10
20 m
−3), whereas the minimum SiH
4 density of Case 2 becomes lower than that of Case 5. As stated above, the same trend was observed for the SiH
3 density distribution. The increase in the minimum densities of both SiH
4 and SiH
3 originates from the higher showerhead temperature suppressing their depletion not only by decreasing the reactant densities in the bulk, but also by decreasing the resistance time. Consequently, the Si
2H
6 density decreases as a consequence of the suppressed depletion of SiH
4 (SiH
4 + SiH
2 → Si
2H
6) in Case 5, as shown in
Figure 4 and
Figure 5. As a result, as depicted in
Figure 8, the axial variations in the SiH
3 density distributions decrease with the higher showerhead temperature.
3.5. Effects of the Showerhead Temperature on the Deposition Rate Profile
Figure 9 shows that for Cases 2 (
Tsh = 373 K), 3 (
Tsh = 423 K), 4 (
Tsh = 473 K), 5 (
Tsh = 673 K), and 6, both their deposition rate profiles and averaged rates are affected by the showerhead temperature. For convenience, we compare the spatial features by normalizing the profiles with their corresponding averaged values.
For Case 6, we averaged the 3D gas simulation results of the case with
Tsh = 473 K (i.e., similarly observed in Case 1) over the azimuthal angle and used the resulting 2D inlet density profile as the input (or the inlet boundary condition) to the 2D plasma simulation. In
Figure 9a, the deposition rate profiles are plotted for Cases 2, 3, 4, and 5. For Cases 2 and 4, their experimental data are superimposed on the profiles in
Figure 9b,c, respectively. The experimental data are denoted with larger circle symbols. The simulated deposition rate profiles are in good correspondence with the corresponding experimental data.
Note that, because of
the modified inlet boundary condition, the simulated deposition rate profile of Case 6 more closely agrees with the corresponding experimental data than that of Case 4, as depicted in
Figure 9c. Although the uniformities are different, all the profiles are convex. As depicted in
Figure 8, the boundary layers of the SiH
3 density thicken, approaching
r = 150 mm; the thicknesses of the boundary layers tend to decrease slightly, further approaching each other near the heater edge (at
r = 165 mm). The lower SiH
3 surface fluxes result in the boundary layers of the SiH
3 density field becoming thicker; thus, the deposition rates decrease in the vicinity of
r = 150 mm. At the heater edge, the origin of the higher SiH
3 surface flux can be understood by the electron-impact dissociation being enhanced by the
Neo there, and thus excess SiH
3 is produced and transported to the heater surface.
In
Figure 9d, for Cases 2, 3, 4, and 5, the averaged values of the normalized deposition rates are plotted against the showerhead temperature. The values are normalized with the average value of Case 2 for the clear comparison. Notably, the average rates are largely affected by the showerhead temperature: the average deposition rate of Case 5 is approximately 30% higher than that of Case 2, although the maximum value of the SiH
3 density of Case 5 is lower than that of Case 2. The increase in the deposition rate can be understood by observing the increase in the SiH
3 density near the heater surface in
Figure 8.
3.6. Effects of the Showerhead Temperature on the Particle Deposition
To analyze the effects of increasing the temperature of the showerhead on lowering the number of particles transported onto the heater surface, the number of particles that were deposited was experimentally measured by counting the total number of particles. The input parameters were identical to those of Cases 2, 3, 4, and 5. In
Figure 10, the number of particles of which the size is characteristically larger than 45 nm is plotted against the showerhead temperature: in the inset, simulation results of Si
2H
6 density distributions are depicted for Cases 2 and 5. The higher showerhead temperature decreases the number of deposited particles. Note that the number of particles of which the size exceeds 45 nm is decreased by approximately 93% by increasing the showerhead temperature from 373 to 553 K.
Understanding the tendency of the number of particles to decrease, as depicted in
Figure 10, would require us to analyze the effects of the heated showerhead on the spatial distributions of the high-order silanes such as Si
nH
2n+2 (
n ≥ 5).
Figure 11 and
Figure 12 demonstrate that for Cases 2, 3, 4, and 5, Si
5H
12 and Si
12H
26 reach the heater surface less easily when the showerhead temperature is higher, respectively. The thickness of the diffusion layer formed near the showerhead inlet becomes slightly larger with higher showerhead temperature, regardless of the value of
n in the high-order silane. As a result of the slower growth, the compositions of these species are lowered at the heater surface.
As similarly observed in
Figure 5 (Si
2H
6), in all four cases, the density distribution of Si
5H
12 is initially low at the showerhead inlet and increases as it approaches the bulk plasma region in
Figure 11. Case 5 has the lowest density distribution of Si
5H
12 near the heater surface. This similar growth trend originates from Si
5H
12 being grown from Si
2H
6 by cluster formation as a result of SiH
2 insertion, as shown below:
where
n is larger than one [
9]. As Si
12H
26 is also generated from the above SiH
2 insertion reaction, the difference in the Si
12H
26 density distribution between Cases 2 and 5 increases, as shown in
Figure 12 (note that the observed difference is two orders of magnitude).