Effect of Pulsed Light Irradiation on Patterning of Reduction Graphene Oxide-Graphene Oxide Interconnects for Power Devices
Abstract
:1. Introduction
2. Material and Methods
2.1. Synthesis of GO
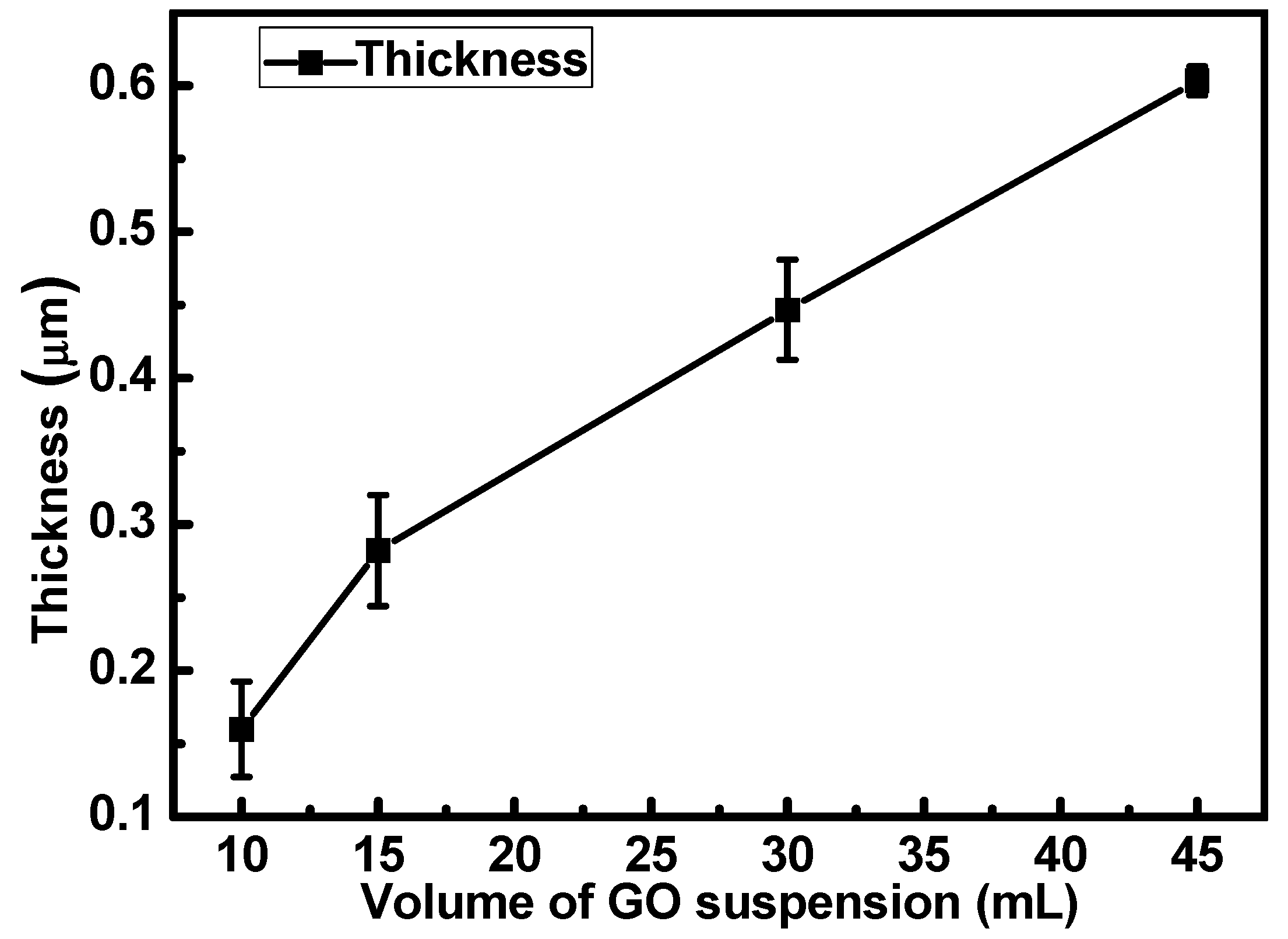
2.2. Deposition of GO Thin Films and TiO2-GO Thin Films
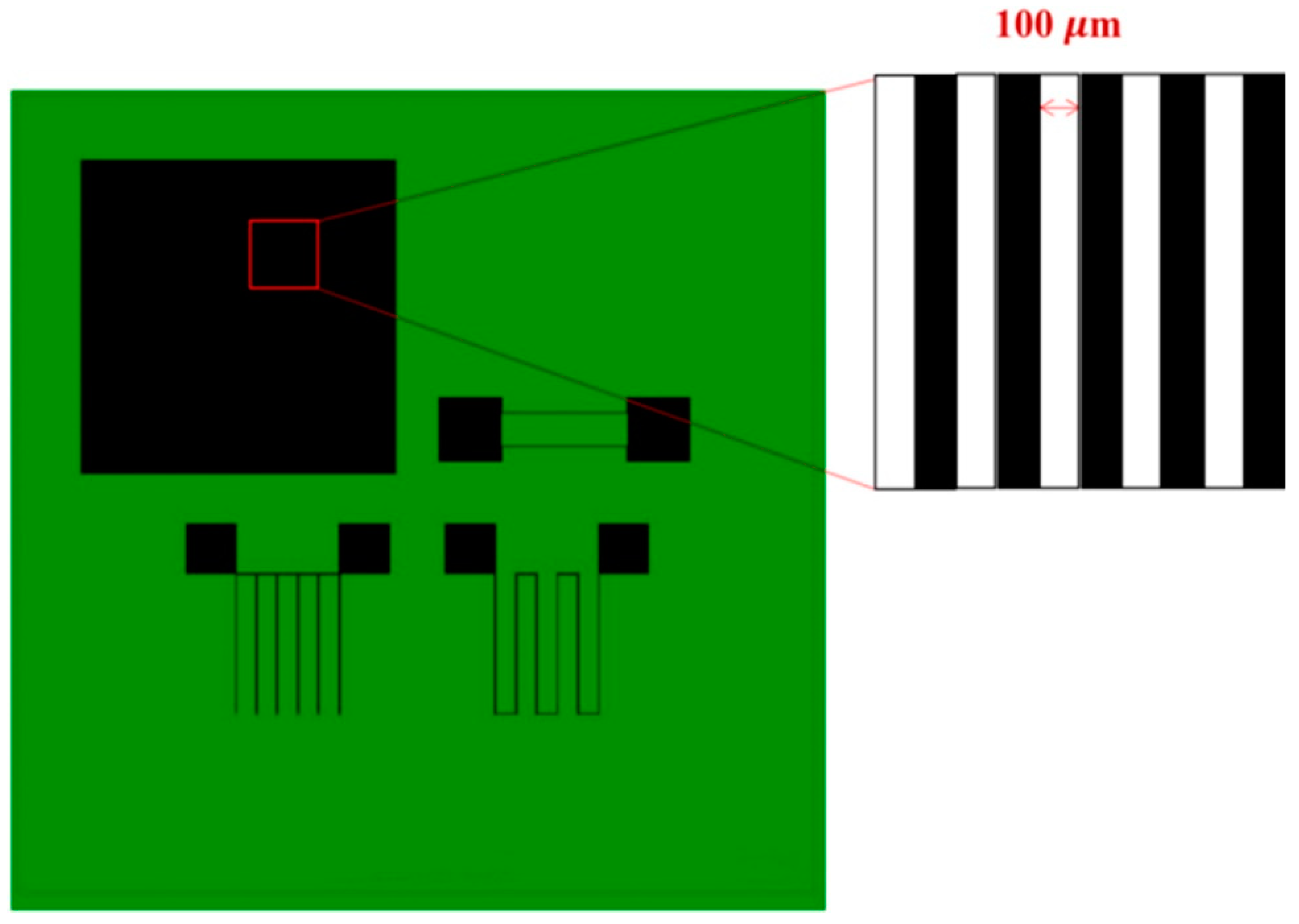
2.3. Graphene Patterning
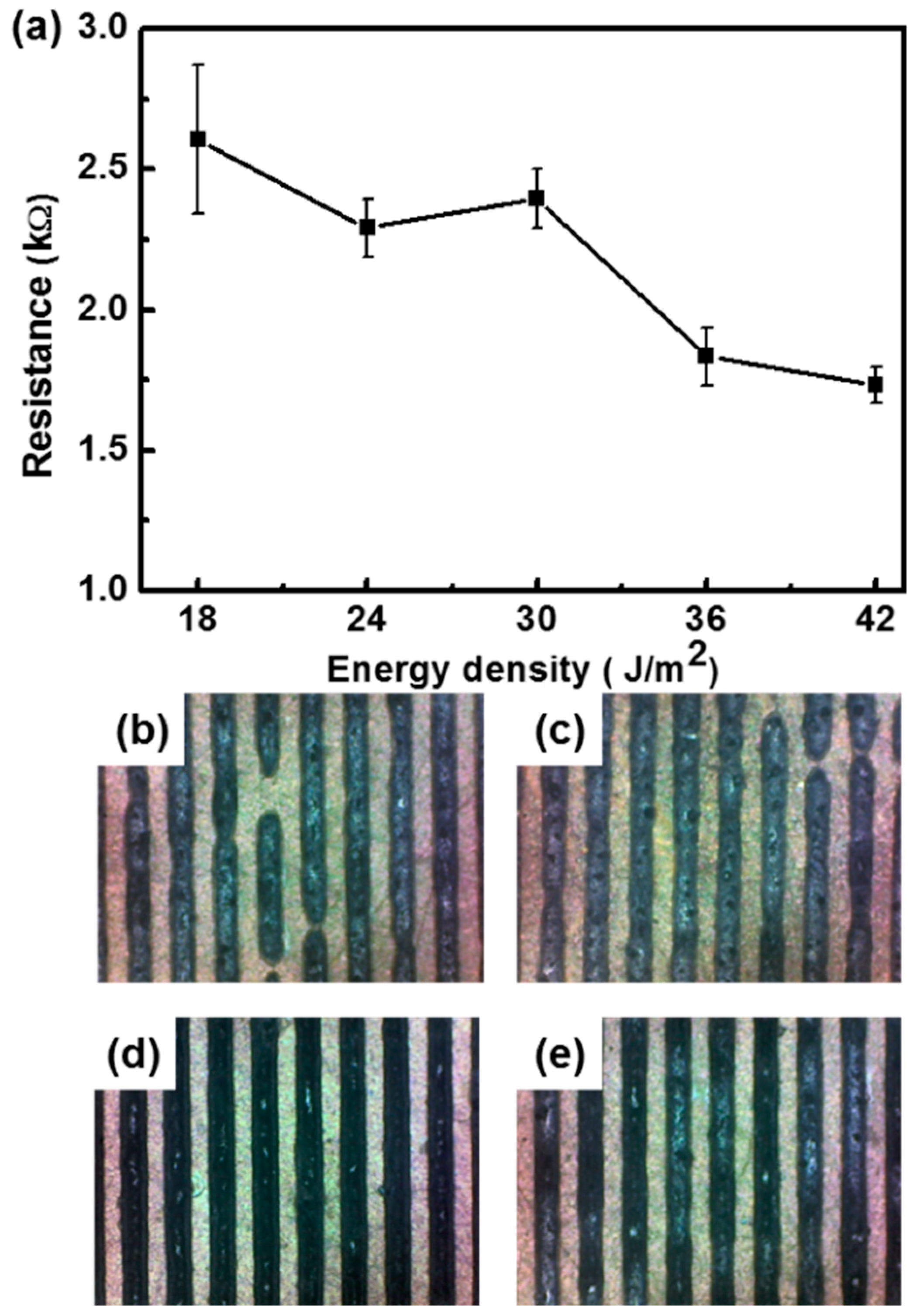
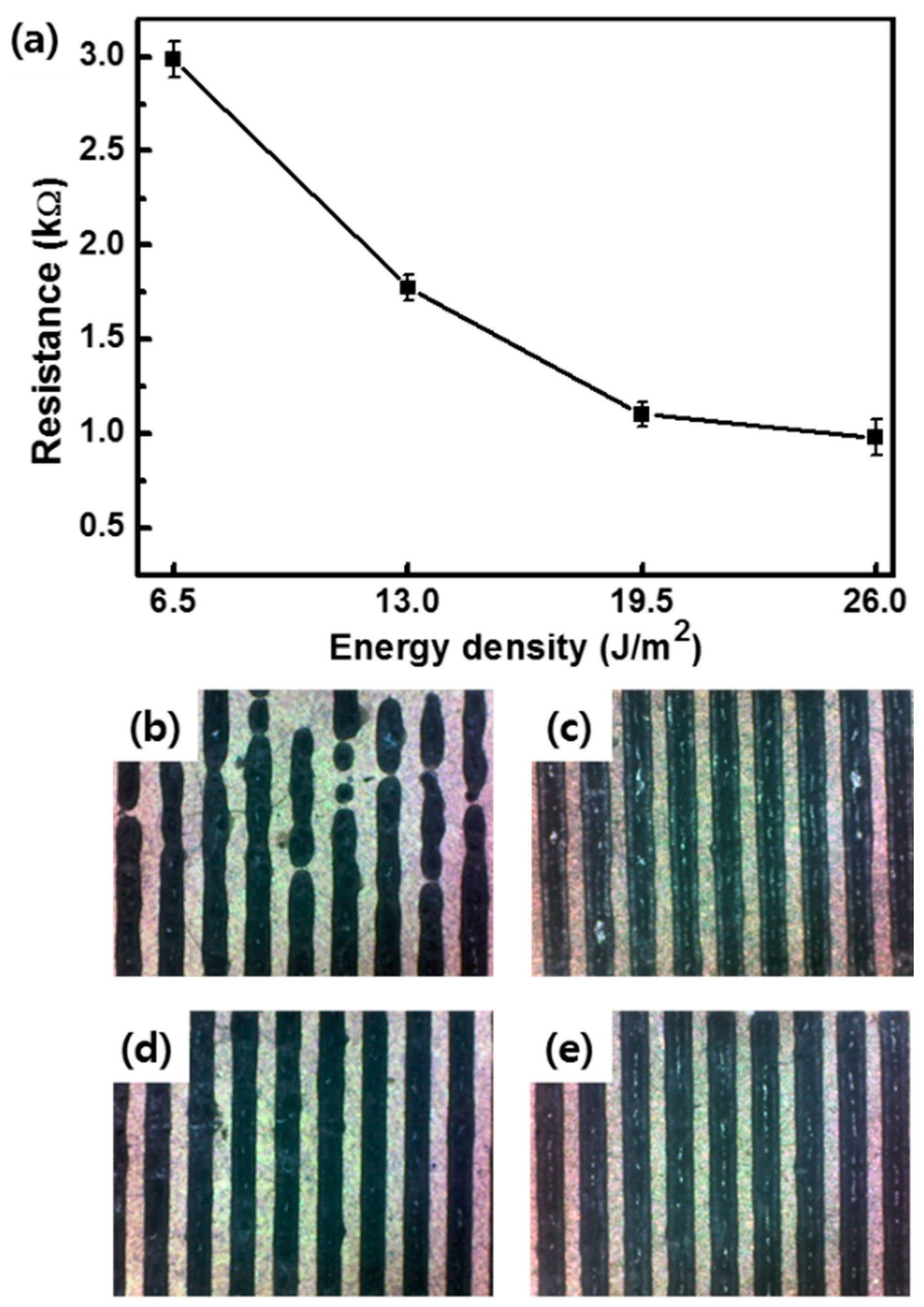
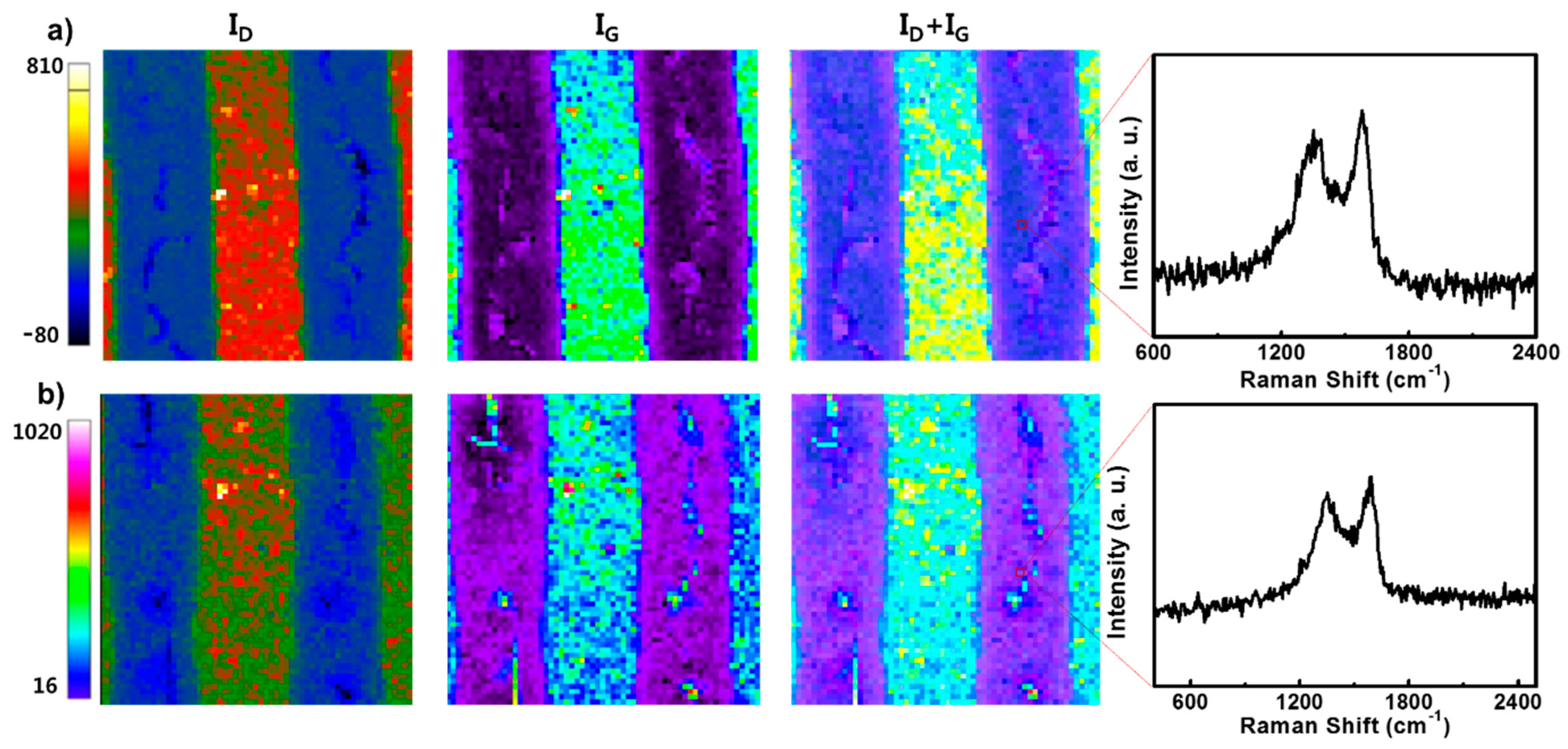
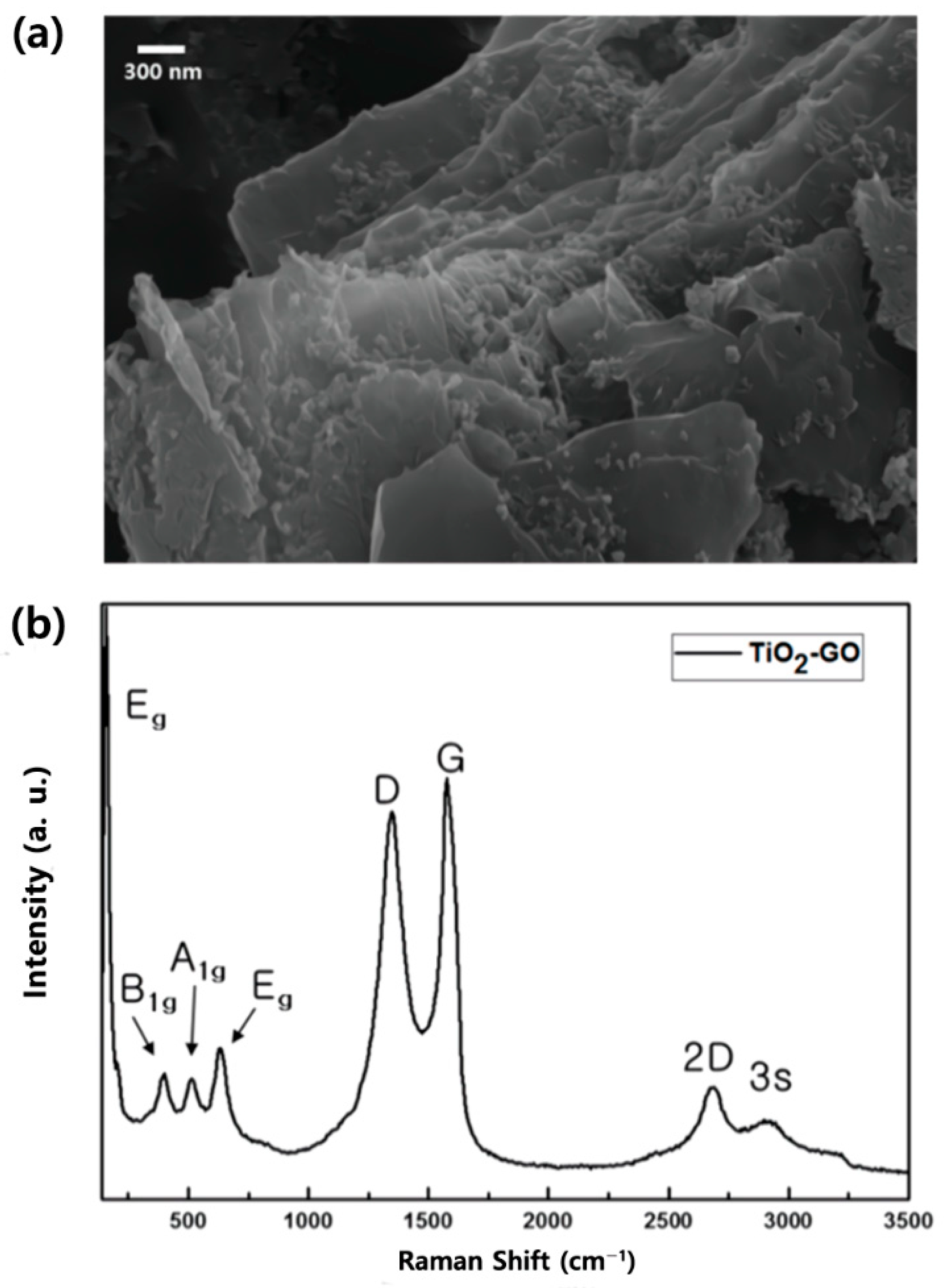
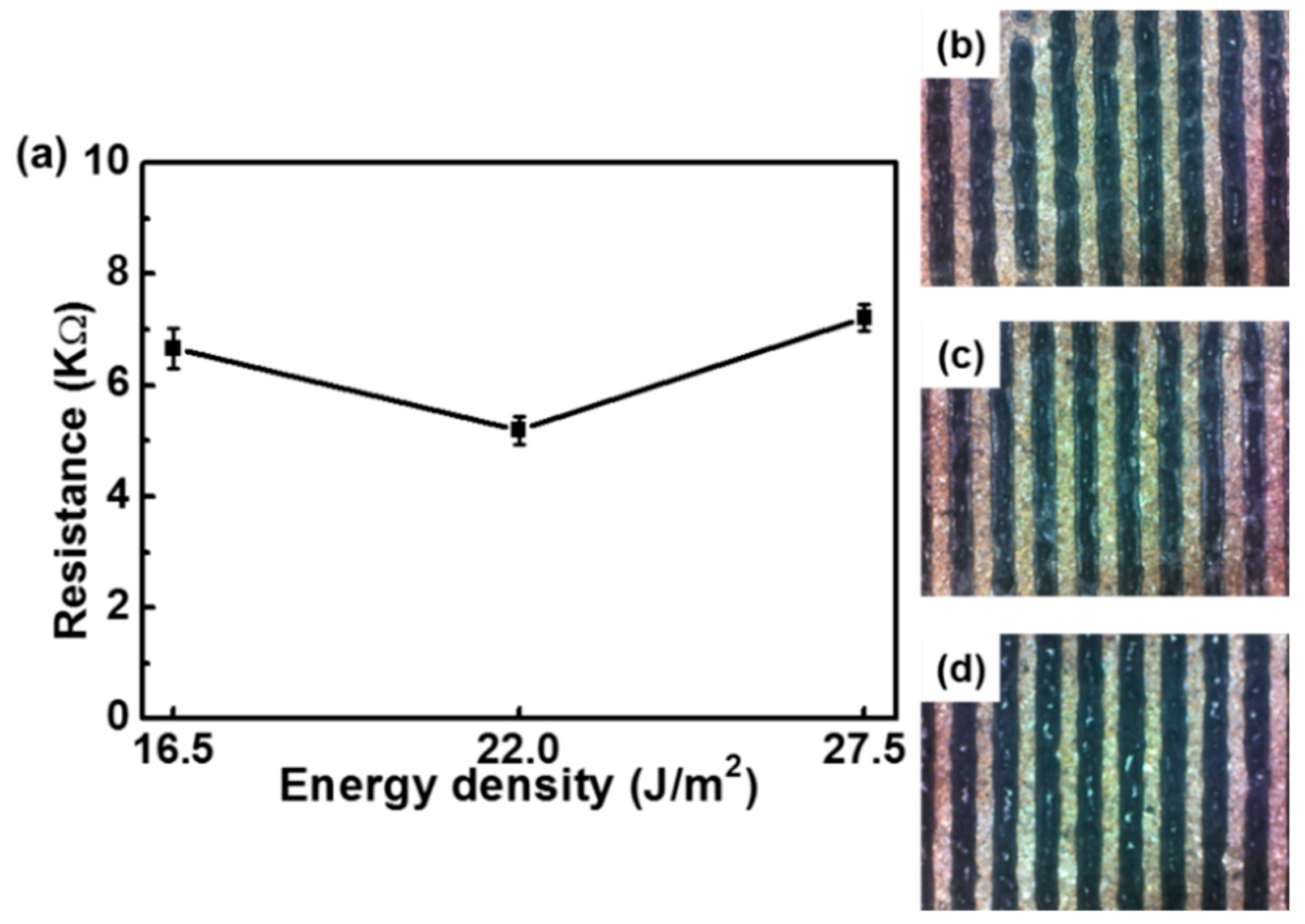
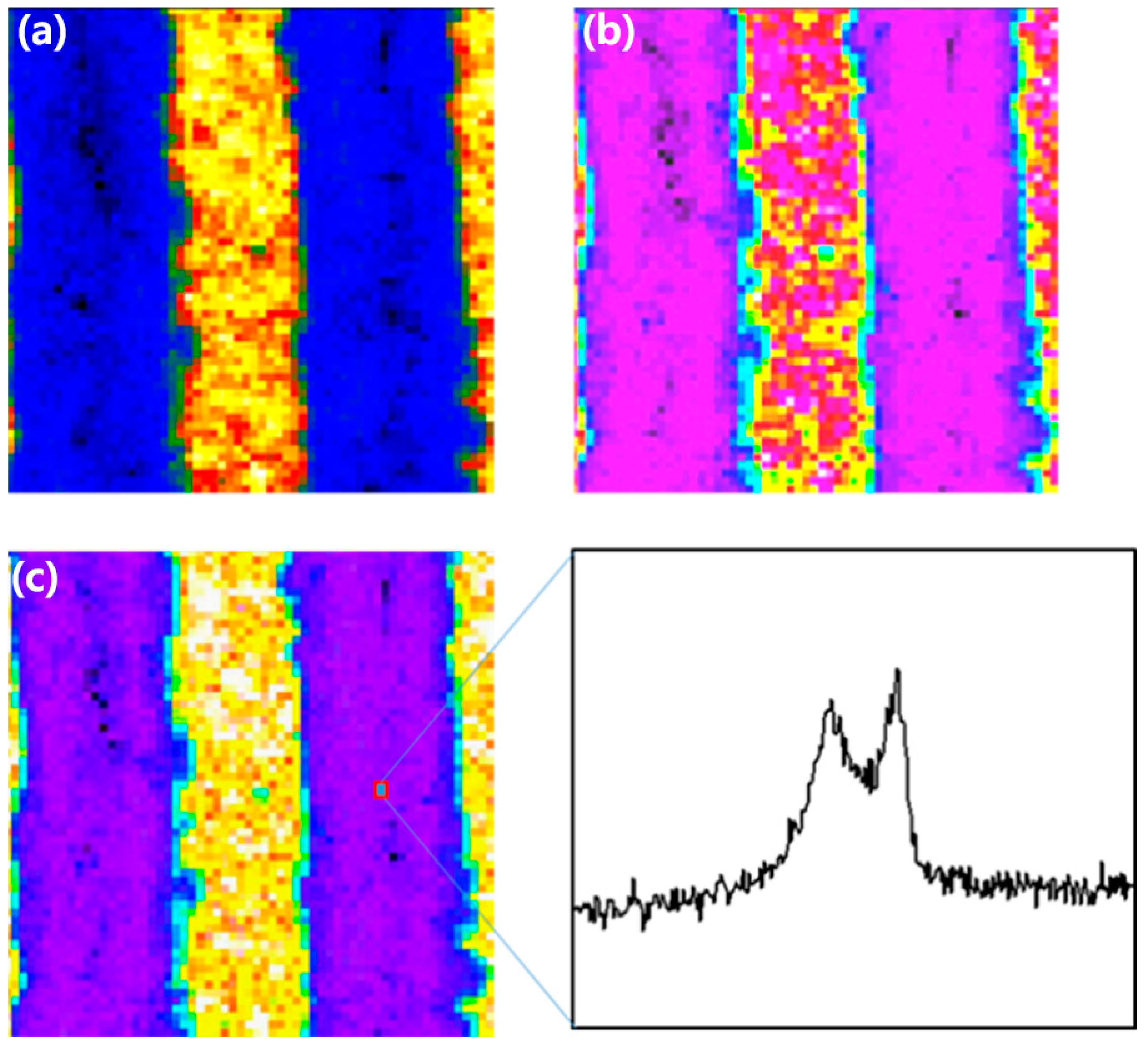
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Zhao, Z.; Nie, N.; Wang, F.; Zhu, W. Dynamic through-silicon-via filling process using copper electrochemical deposition at different current densities. Sci. Rep. 2017, 7, 46639. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.-W.; Chen, K.-N. Three-dimensional integrated circuit (3D IC) key technology: Through-silicon via (TSV). Nanoscale Res. Lett. 2017, 12, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, W.; Watanabe, N.; Shimamoto, H.; Kikuchi, K.; Aoyagi, M. Methods to Reduce Thermal Stress for TSV Scaling~TSV with Novel Structure: Annular-Trench-Isolated TSV. In Proceedings of the 2015 IEEE 65th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 26–29 May 2015; pp. 1057–1062. [Google Scholar]
- Kim, D.H.; Lim, S.K. Impact of Through-Silicon-via Scaling on the Wirelength Distribution of Current and Future 3D ICs. In Proceedings of the 2011 IEEE International Interconnect Technology Conference and 2011 Materials for Advanced Metallization (IITC/MAM), Dresden, Germany, 8–12 May 2011; pp. 1–3. [Google Scholar]
- Lu, K.H.; Zhang, X.; Ryu, S.K.; Huang, R.; Ho, P.S. Thermal Stresses Analysis of 3-D Interconnect. In Proceedings of the AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2009; pp. 224–230. [Google Scholar]
- Park, H.; Seo, H.; Kim, S.E. Characteristics of copper nitride nanolayer used in 3d cu bonding interconnects. Electron. Mater. Lett. 2021. [Google Scholar] [CrossRef]
- Mortazavi, B.; Shahrokhi, M.; Rabczuk, T.; Pereira, L.F.C. Electronic, optical and thermal properties of highly stretchable 2D carbon Ene-yne graphyne. Carbon 2017, 123, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Lei, T.; Chen, X.; Pitner, G.; Wong, H.-S.P.; Bao, Z. Removable and recyclable conjugated polymers for highly selective and high-yield dispersion and release of low-cost carbon nanotubes. J. Am. Chem. Soc. 2016, 138, 802–805. [Google Scholar] [CrossRef]
- Park, O.-K.; Tiwary, C.S.; Yang, Y.; Bhowmick, S.; Vinod, S.; Zhang, Q.; Colvin, V.L.; Asif, S.S.; Vajtai, R.; Penev, E.S. Magnetic field controlled graphene oxide-based origami with enhanced surface area and mechanical properties. Nanoscale 2017, 9, 6991–6997. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Zou, X.; Wu, S.; Wan, D.; Cao, A.; Liao, L.; Yuan, Q.; Duan, X. Ultrafine graphene nanomesh with large on/off ratio for high-performance flexible biosensors. Adv. Funct. Mater. 2017, 27, 1604096. [Google Scholar] [CrossRef]
- Yang, M.K.; Lee, J.K. CNT/AgNW Multilayer electrodes on flexible organic solar cells. Electron. Mater. Lett. 2020, 16, 573–578. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.-H.; Lee, J.-Y.; Kim, K.-K.; Choa, Y.-H.; Lim, J.-H. Single-walled carbon nanotube-based chemi-capacitive sensor for hexane and ammonia. Electron. Mater. Lett. 2019, 15, 712–719. [Google Scholar] [CrossRef]
- Choi, E.; Kim, D.; Lee, I.; Chae, S.J.; Kim, A.; Pyo, S.G.; Yoon, S. SnO2-graphene nanocomposite free-standing film as anode in lithium-ion batteries. Electron. Mater. Lett. 2015, 11, 836–840. [Google Scholar] [CrossRef]
- Chen, X.Y.; Akinwande, D.; Lee, K.J.; Close, G.F.; Yasuda, S.; Paul, B.C.; Fujita, S.; Kong, J.; Wong, H.S.P. Fully integrated graphene and carbon nanotube interconnects for gigahertz high-speed cmos electronics. IEEE Trans. Electron. Dev. 2010, 57, 3137–3143. [Google Scholar] [CrossRef]
- Ghosh, K.; Ranjan, N.; Verma, Y.K.; Tan, C.S. Graphene-CNT hetero-structure for next generation interconnects. RSC Adv. 2016, 6, 53054–53061. [Google Scholar] [CrossRef]
- Maffucci, A.; Miano, G. Number of conducting channels for armchair and zig-zag graphene nanoribbon interconnects. IEEE Trans. Nanotechnol. 2013, 12, 817–823. [Google Scholar] [CrossRef]
- Hwang, U.G.; Kim, K.; Kim, W.; Shin, W.H.; Seo, W.-S.; Lim, Y.S. Thermoelectric transport properties of interface-controlled p-type bismuth antimony telluride composites by reduced graphene oxide. Electron. Mater. Lett. 2019, 15, 605–612. [Google Scholar] [CrossRef]
- Kim, J.; Choi, E.; Lee, I.; Kim, D.; Han, S.; Pyo, S.G.; Yoon, S. Investigation of the charge-storage behavior of electrochemically activated graphene oxide on supercapacitor electrodes in acidic electrolyte. Electron. Mater. Lett. 2017, 13, 434–441. [Google Scholar] [CrossRef]
- Choi, E.; Kim, J.; Cui, Y.; Choi, K.; Gao, Y.; Han, S.; Pyo, S.G.; Yoon, S. Effect of the graphene oxide reduction temperature on supercapacitor performance. Electron. Mater. Lett. 2017, 13, 324–329. [Google Scholar] [CrossRef]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutiérrez, M.C.; del Monte, F. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Qi, Z.J.; Rodríguez-Manzo, J.A.; Hong, S.J.; Park, Y.W.; Stach, E.A.; Drndić, M.; Johnson, A.C. Direct electron beam patterning of sub-5nm monolayer graphene interconnects. In Proceedings of the SPIE Advanced Lithography; International Society for Optics and Photonics: Bellingham, WA, USA, 2013; p. 86802. [Google Scholar]
- Wei, Z.J.; Hou, Y.X.; Jiang, C.; Liu, H.Y.; Chen, X.R.; Zhang, A.Y.; Liu, Y.X. Graphene enhanced electrical properties of polyethylene blends for high-voltage insulation. Electron. Mater. Lett. 2019, 15, 582–594. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2011, 134, 15–18. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Ji, L.-C.; Xie, Y.-Q.; Wang, T.; Shi, W.-Z. Pulsed laser assisted reduction of graphene oxide. Carbon 2011, 49, 2431–2436. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite-and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Prezioso, S.; Perrozzi, F.; Donarelli, M.; Stagnini, E.; Treossi, E.; Palermo, V.; Santucci, S.; Nardone, M.; Moras, P.; Ottaviano, L. Dose and wavelength dependent study of graphene oxide photoreduction with VUV Synchrotron radiation. Carbon 2014, 79, 478–485. [Google Scholar] [CrossRef]
- Prezioso, S.; Perrozzi, F.; Donarelli, M.; Bisti, F.; Santucci, S.; Palladino, L.; Nardone, M.; Treossi, E.; Palermo, V.; Ottaviano, L. Large area extreme-UV lithography of graphene oxide via spatially resolved photoreduction. Langmuir 2012, 28, 5489–5495. [Google Scholar] [CrossRef]
- Cullis, A.G.; Webber, H.C.; Chew, N.G.; Poate, J.M.; Baeri, P. Transitions to defective crystal and the amorphous state induced in elemental si by laser quenching. Phys. Rev. Lett. 1982, 49, 219–222. [Google Scholar] [CrossRef]
- Pei, L.M.; Li, Y.F. Rapid and efficient intense pulsed light reduction of graphene oxide inks for flexible printed electronics. RSC Adv. 2017, 7, 51711–51720. [Google Scholar] [CrossRef] [Green Version]
- Al-Hamry, A.; Kang, H.; Sowade, E.; Dzhagan, V.; Rodriguez, R.D.; Müller, C.; Zahn, D.R.T.; Baumann, R.R.; Kanoun, O. Tuning the reduction and conductivity of solution-processed graphene oxide by intense pulsed light. Carbon 2016, 102, 236–244. [Google Scholar] [CrossRef]
- de Lima, B.S.; Bernardi, M.I.B.; Mastelaro, V.R. Wavelength effect of ns-pulsed radiation on the reduction of graphene oxide. Appl. Surf. Sci. 2020, 506, 144808. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Spanò, S.F.; Compagnini, G.; Sinatra, M.; D’Urso, L.; Fazio, E.; Privitera, V.; Scalese, S. Modification of graphene oxide and graphene oxide–TiO2 solutions by pulsed laser irradiation for dye removal from water. Mater. Sci. Semicond. Process. 2016, 42, 50–53. [Google Scholar] [CrossRef]
- Menazea, A.; Ahmed, M.K. Silver and copper oxide nanoparticles-decorated graphene oxide via pulsed laser ablation technique: Preparation, characterization, and photoactivated antibacterial activity. Nano-Struct. Nano-Objects 2020, 22, 100464. [Google Scholar] [CrossRef]
- Sachan, R.; Gupta, S.; Narayan, J. Nonequilibrium structural evolution of Q-carbon and interfaces. ACS Appl. Mater. Interfaces 2020, 12, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Trusovas, R.; Ratautas, K.; Račiukaitis, G.; Barkauskas, J.; Stankevičienė, I.; Niaura, G.; Mažeikienė, R. Reduction of graphite oxide to graphene with laser irradiation. Carbon 2013, 52, 574–582. [Google Scholar] [CrossRef]
- Sokolov, D.A.; Shepperd, K.R.; Orlando, T.M. Formation of graphene features from direct laser-induced reduction of graphite oxide. J. Phys. Chem. Lett. 2010, 1, 2633–2636. [Google Scholar] [CrossRef]
- Gupta, S.; Narayan, J. Non-equilibrium processing of ferromagnetic heavily reduced graphene oxide. Carbon 2019, 153, 663–673. [Google Scholar] [CrossRef]
- Gupta, S.; Narayan, J. Reduced graphene oxide/amorphous carbon P–N junctions: Nanosecond laser patterning. ACS Appl. Mater. Interfaces 2019, 11, 24318–24330. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, D.A.; Rouleau, C.M.; Geohegan, D.B.; Orlando, T.M. Excimer laser reduction and patterning of graphite oxide. Carbon 2013, 53, 81–89. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, A.; Anand, V.K.; Sood, S.C. Synthesis of graphene oxide using modified hummer’s method and its reduction using hydrazine hydrate. Int. J. Eng. Trends Technol. 2016, 40, 67–71. [Google Scholar] [CrossRef]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and reduced graphene oxide as efficient photocatalysts for hydrogen evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- Moraes, F.C.; Freitas, R.G.; Pereira, R.; Gorup, L.F.; Cuesta, A.; Pereira, E.C. Coupled electronic and morphologic changes in graphene oxide upon electrochemical reduction. Carbon 2015, 91, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, T.; Konishi, Y.; Tada, H.; Tohge, N.; Nishii, J.; Ito, S. A patterned TiO2 (anatase)/TiO2 (rutile) bilayer-type photocatalyst: Effect of the anatase/rutile junction on the photocatalytic activity. Angew. Chem. 2002, 114, 2935–2937. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Wang, P.; Wang, J.; Ming, T.; Wang, X.; Yu, H.; Yu, J.; Wang, Y.; Lei, M. Dye-sensitization-induced visible-light reduction of graphene oxide for the enhanced TiO2 photocatalytic performance. ACS Appl. Mater. Interfaces 2013, 5, 2924–2929. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25. [Google Scholar] [CrossRef]
- Feng, F.; Guo, H.; Li, D.; Wu, C.; Wu, J.; Zhang, W.; Fan, S.; Yang, Y.; Wu, X.; Yang, J. Highly efficient photothermal effect by atomic-thickness confinement in two-dimensional ZrNCl nanosheets. ACS Nano 2015, 9, 1683–1691. [Google Scholar] [CrossRef]
- Park, J.; Xiong, W.; Gao, Y.; Qian, M.; Xie, Z.; Mitchell, M.; Zhou, Y.; Han, G.; Jiang, L.; Lu, Y. Fast growth of graphene patterns by laser direct writing. Appl. Phys. Lett. 2011, 98, 123109. [Google Scholar] [CrossRef]
- Park, M.; Choi, H.; Park, Y.; Lee, W.; Lee, J.; Jeon, M. Fabrication and characterization of graphene-based electrochemical sensors for glucose measurement. J. Nanosci. Nanotechnol. 2015, 15, 7891–7894. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.; Pyo, S. Effect of Pulsed Light Irradiation on Patterning of Reduction Graphene Oxide-Graphene Oxide Interconnects for Power Devices. Coatings 2021, 11, 1042. https://doi.org/10.3390/coatings11091042
Choi E, Pyo S. Effect of Pulsed Light Irradiation on Patterning of Reduction Graphene Oxide-Graphene Oxide Interconnects for Power Devices. Coatings. 2021; 11(9):1042. https://doi.org/10.3390/coatings11091042
Chicago/Turabian StyleChoi, Eunmi, and Sunggyu Pyo. 2021. "Effect of Pulsed Light Irradiation on Patterning of Reduction Graphene Oxide-Graphene Oxide Interconnects for Power Devices" Coatings 11, no. 9: 1042. https://doi.org/10.3390/coatings11091042
APA StyleChoi, E., & Pyo, S. (2021). Effect of Pulsed Light Irradiation on Patterning of Reduction Graphene Oxide-Graphene Oxide Interconnects for Power Devices. Coatings, 11(9), 1042. https://doi.org/10.3390/coatings11091042





