The Low-Temperature Ring during Droplet Impact on a Superhydrophilic Surface
Abstract
:1. Introduction
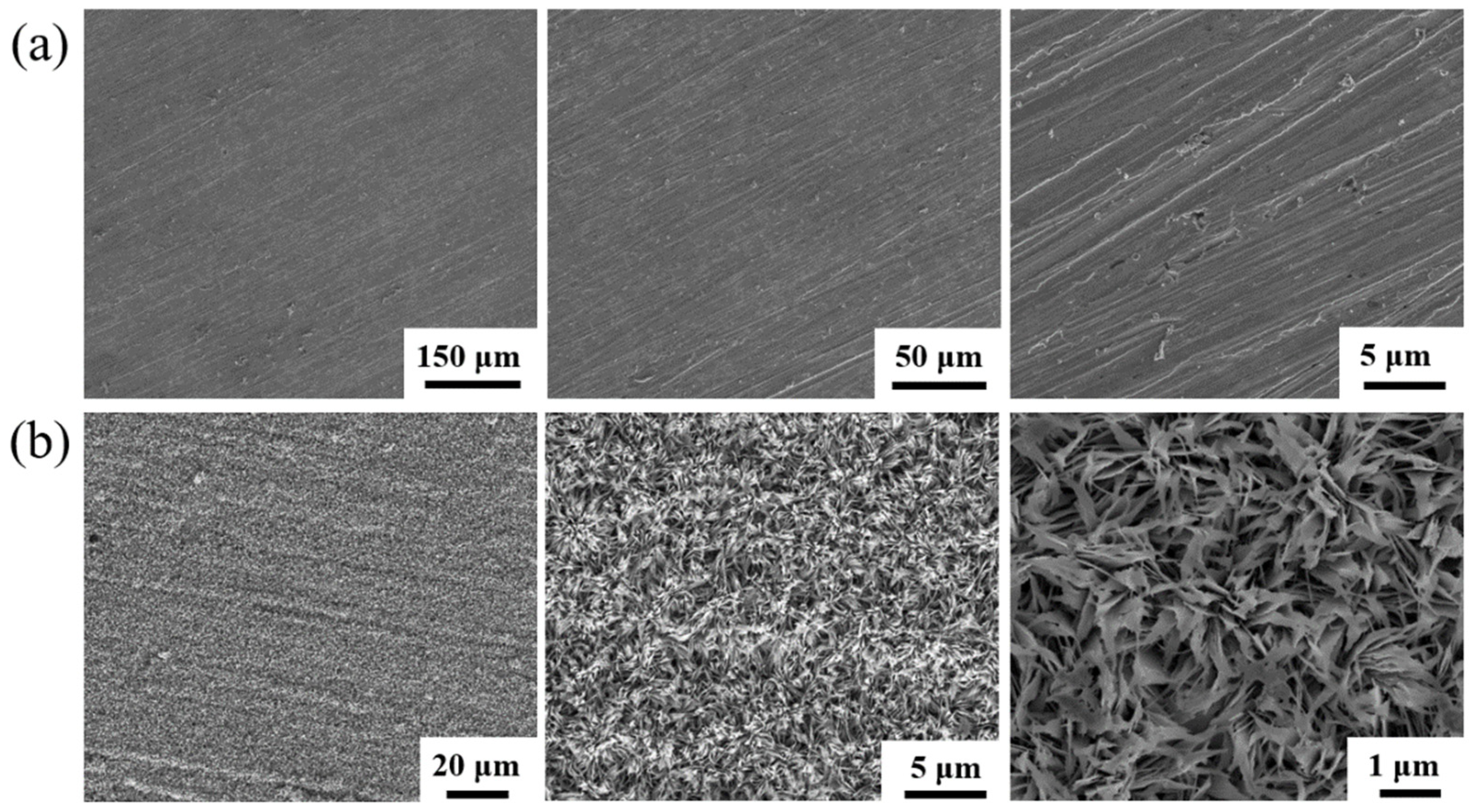
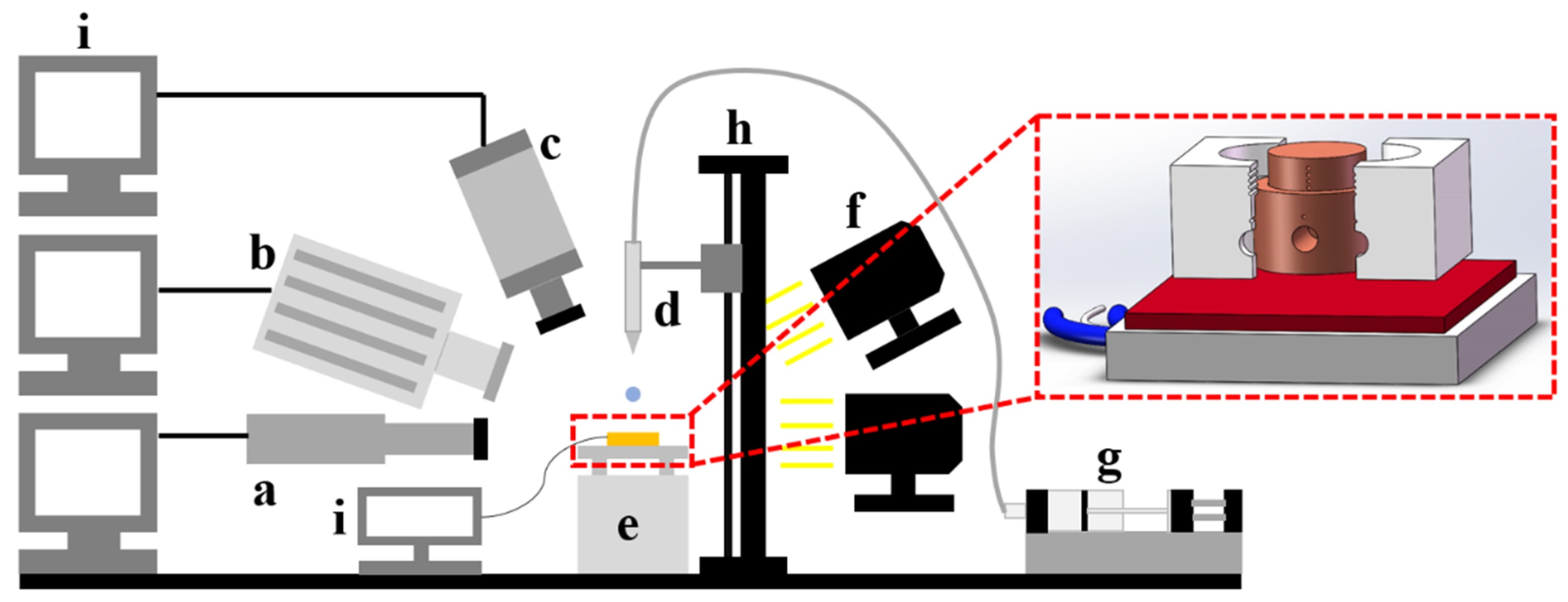
2. Experimental Method
3. Results
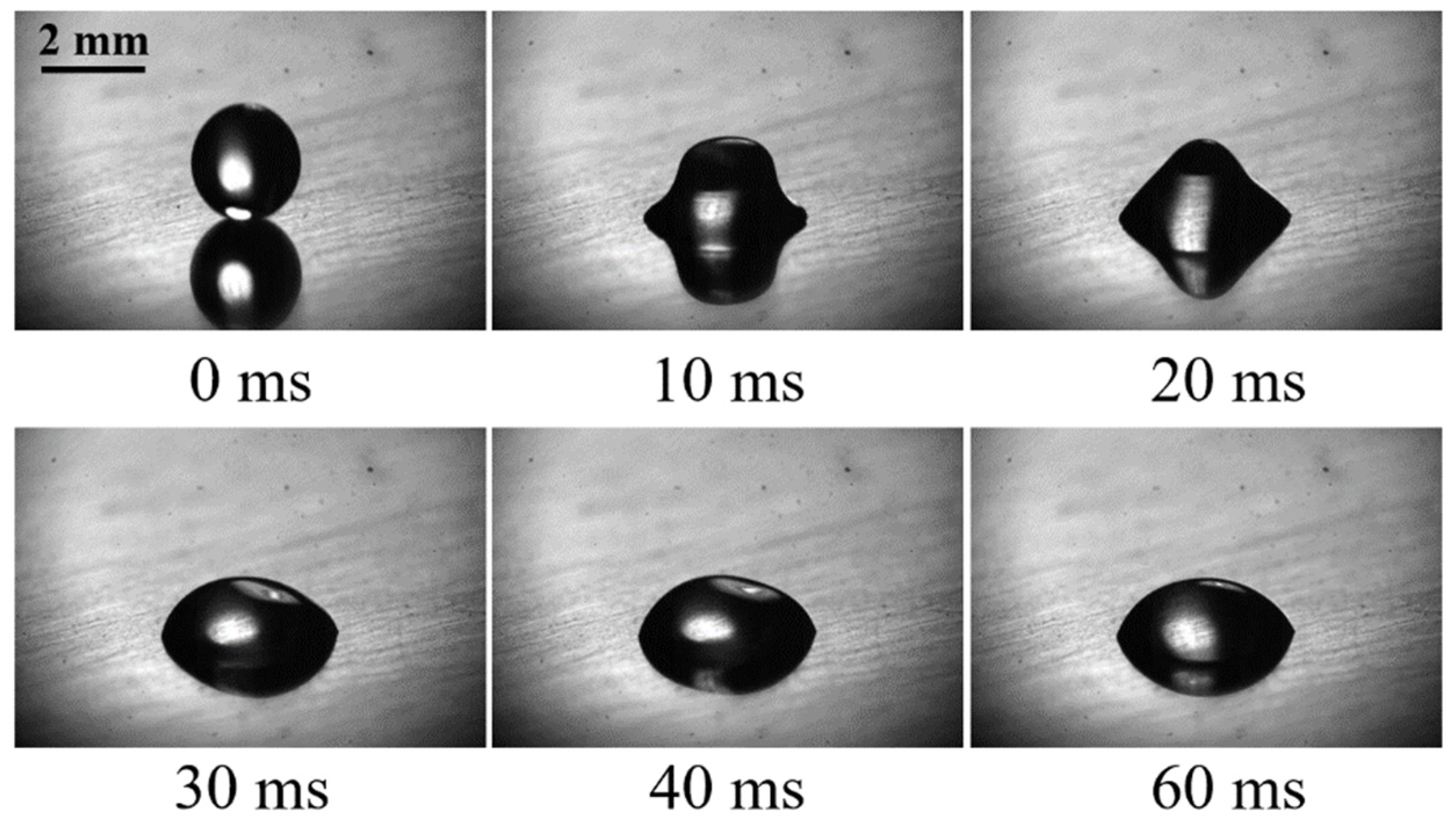
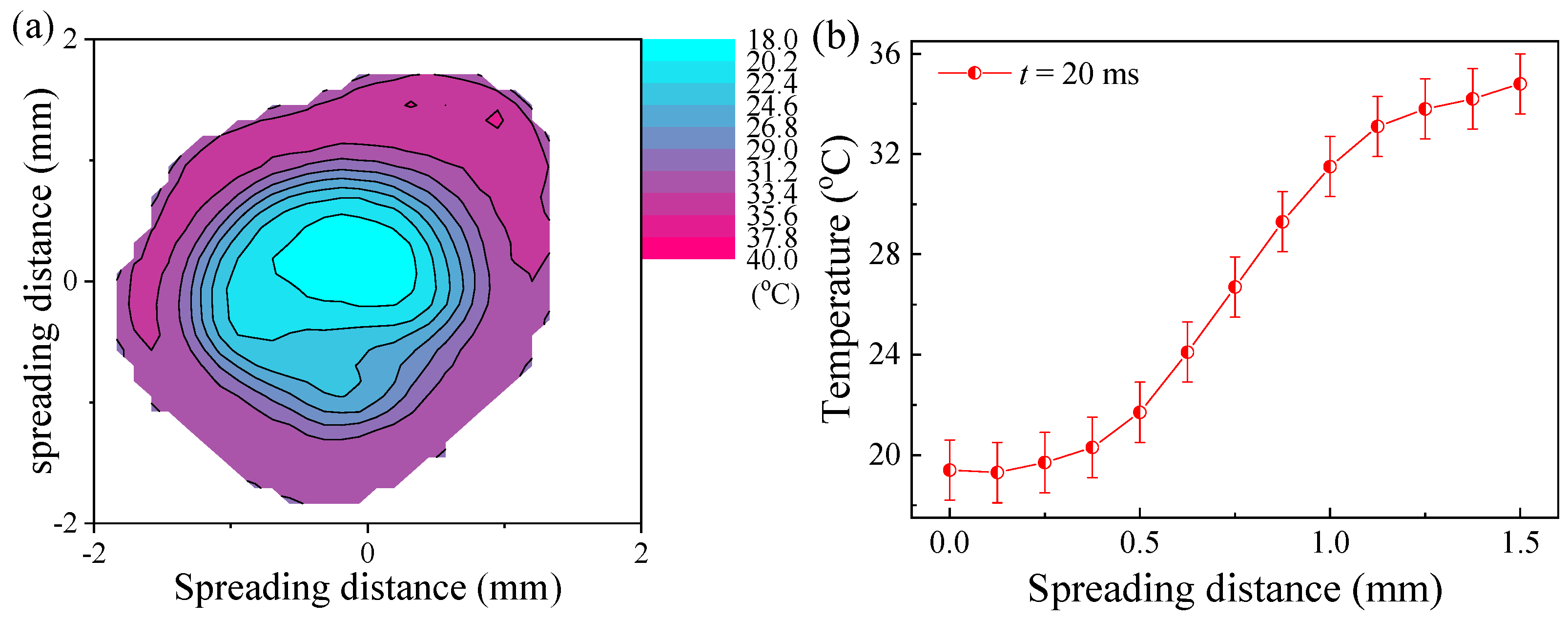
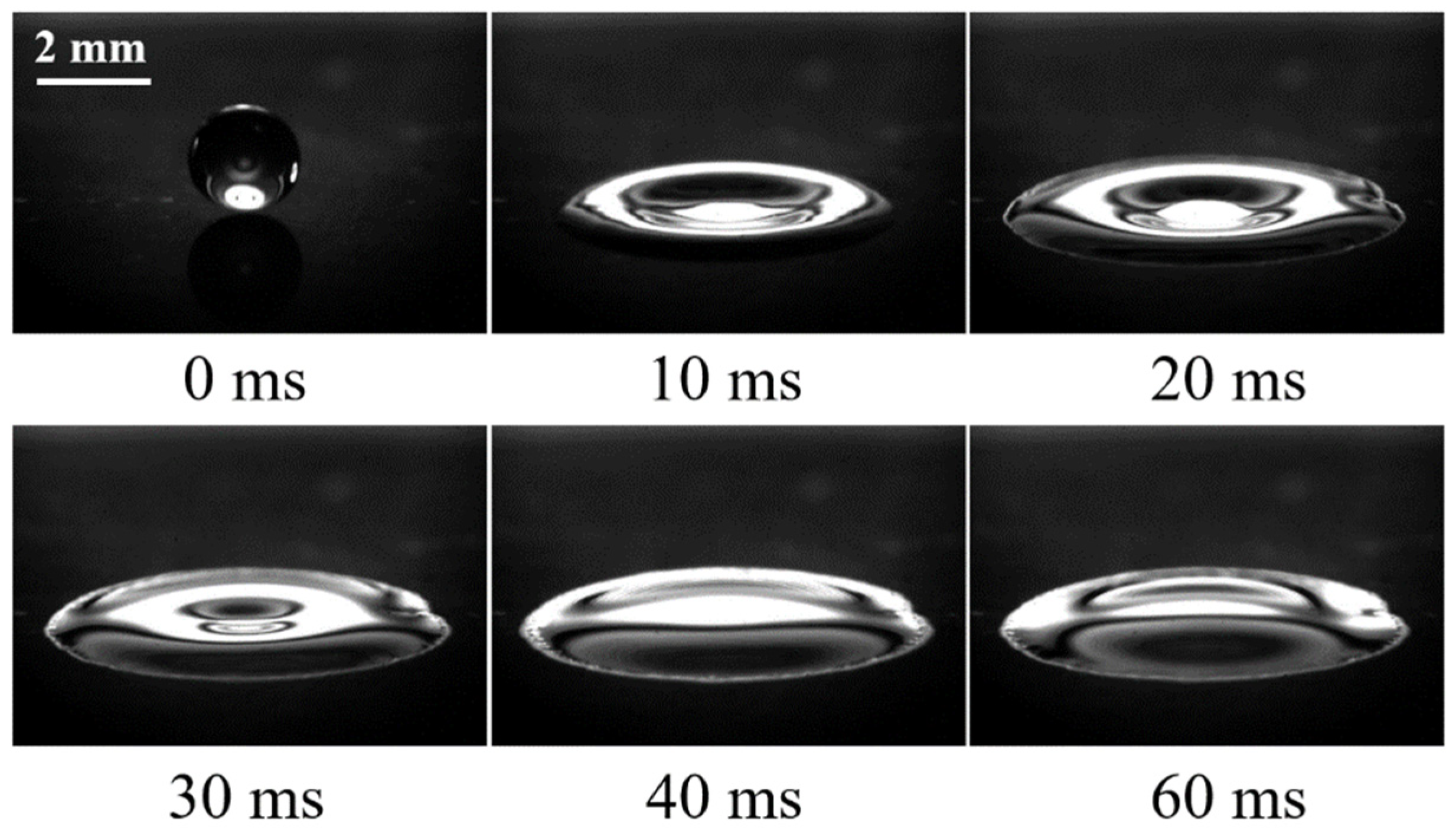
3.1. The Low-Temperature Ring on the Heated Superhydrophilic Surface
3.2. Temperature Evolution of the Impacting Droplet on the Heated Superhydrophilic Surface
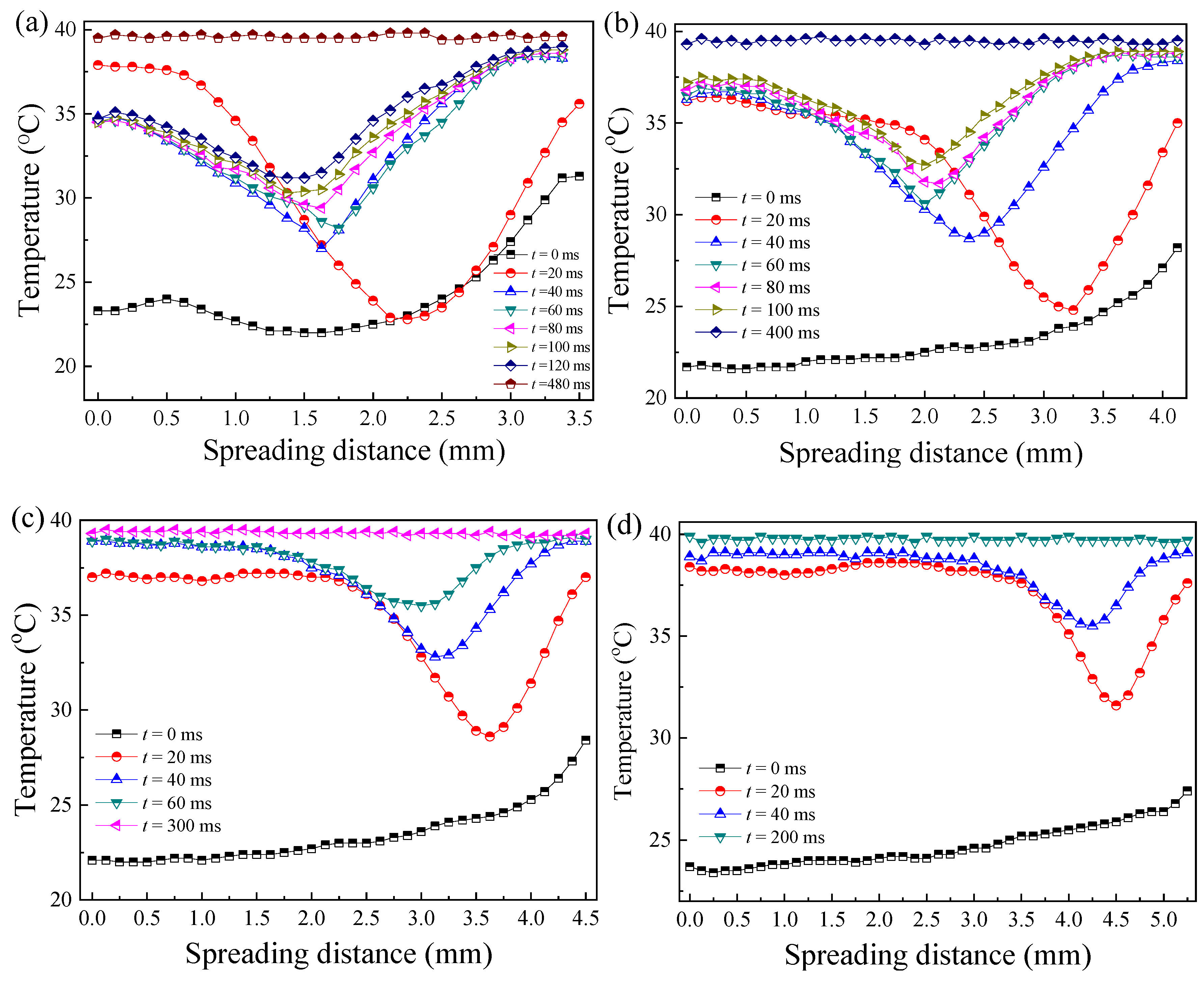
3.2.1. Effect of Weber Number
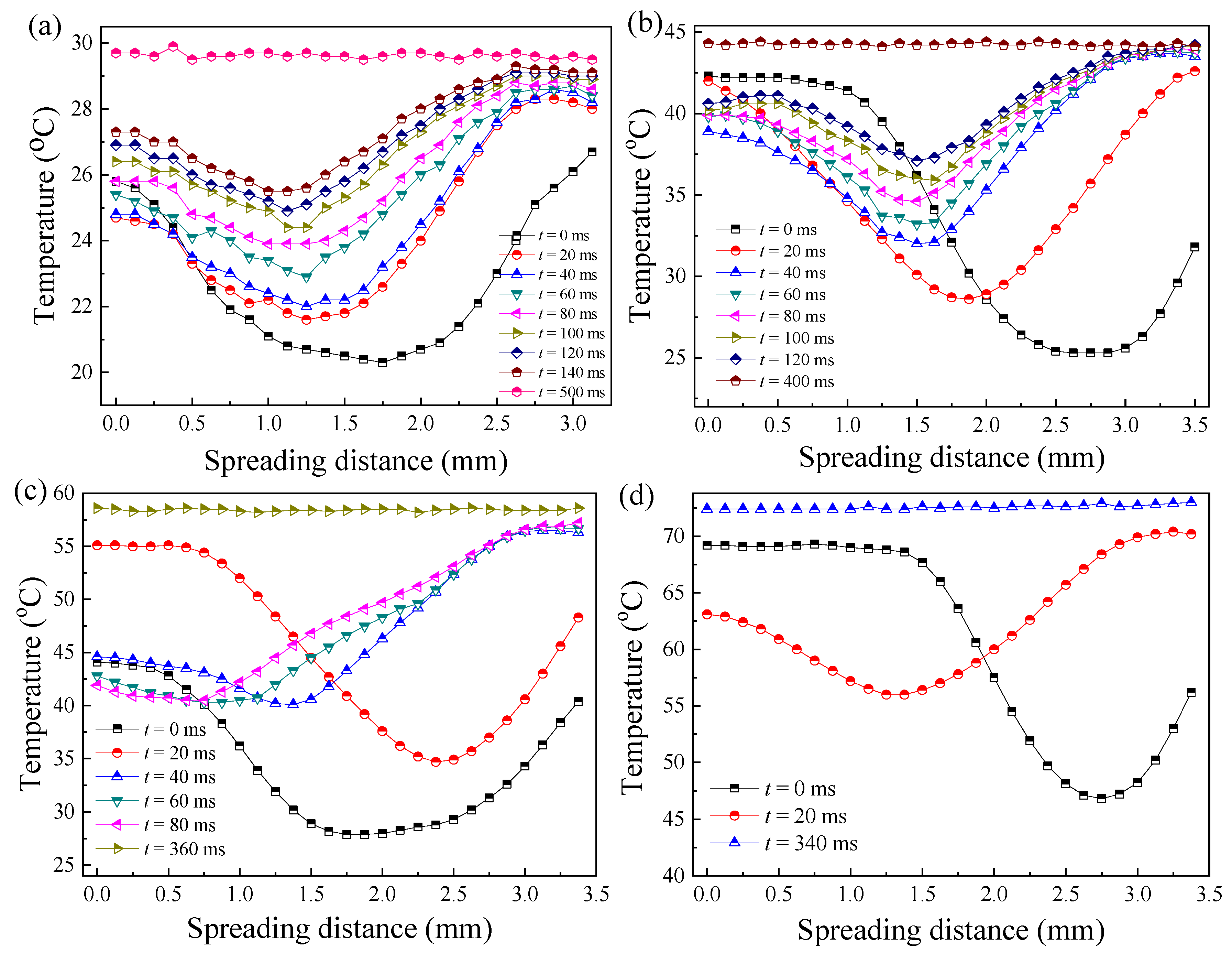
3.2.2. Effect of Wall Temperature
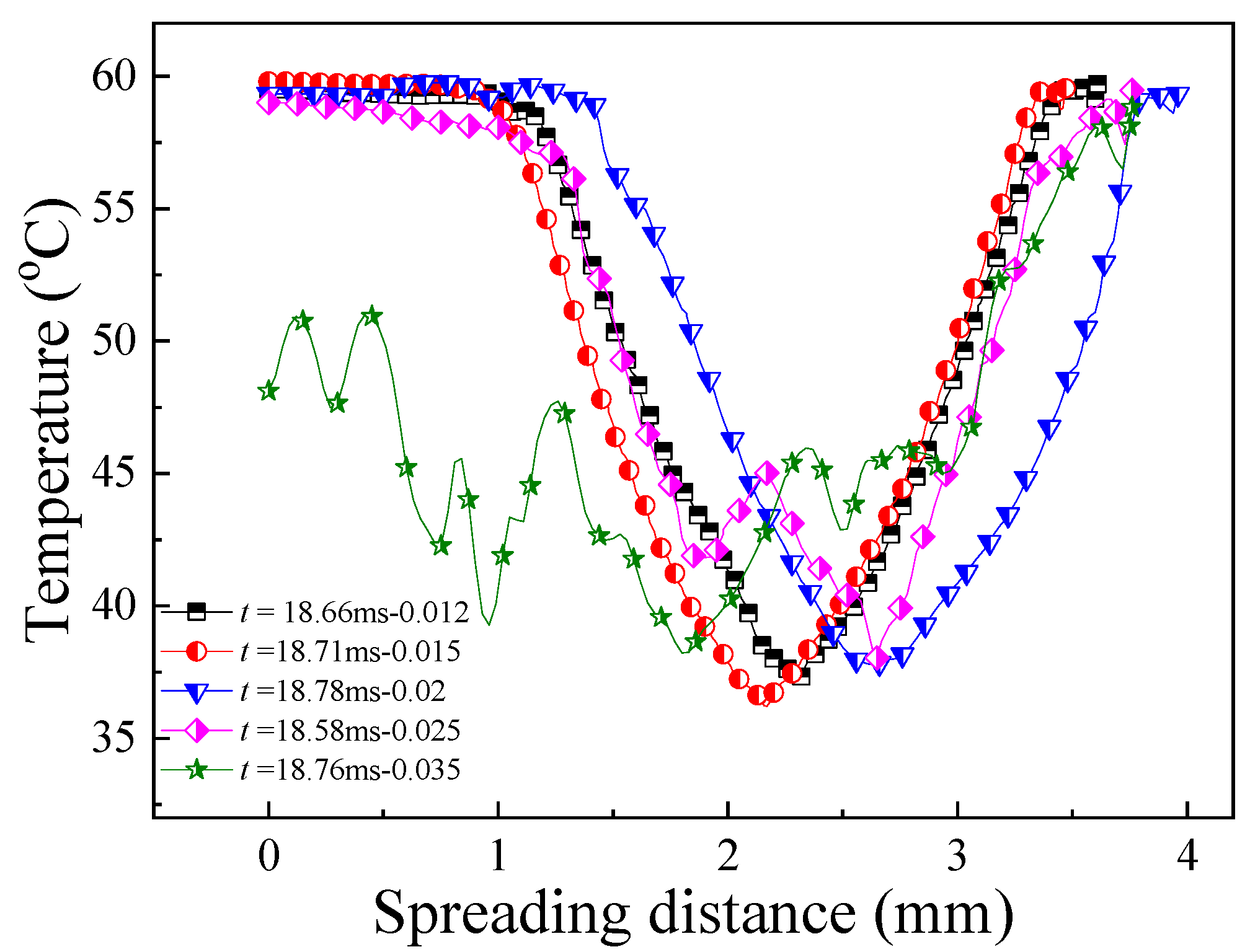
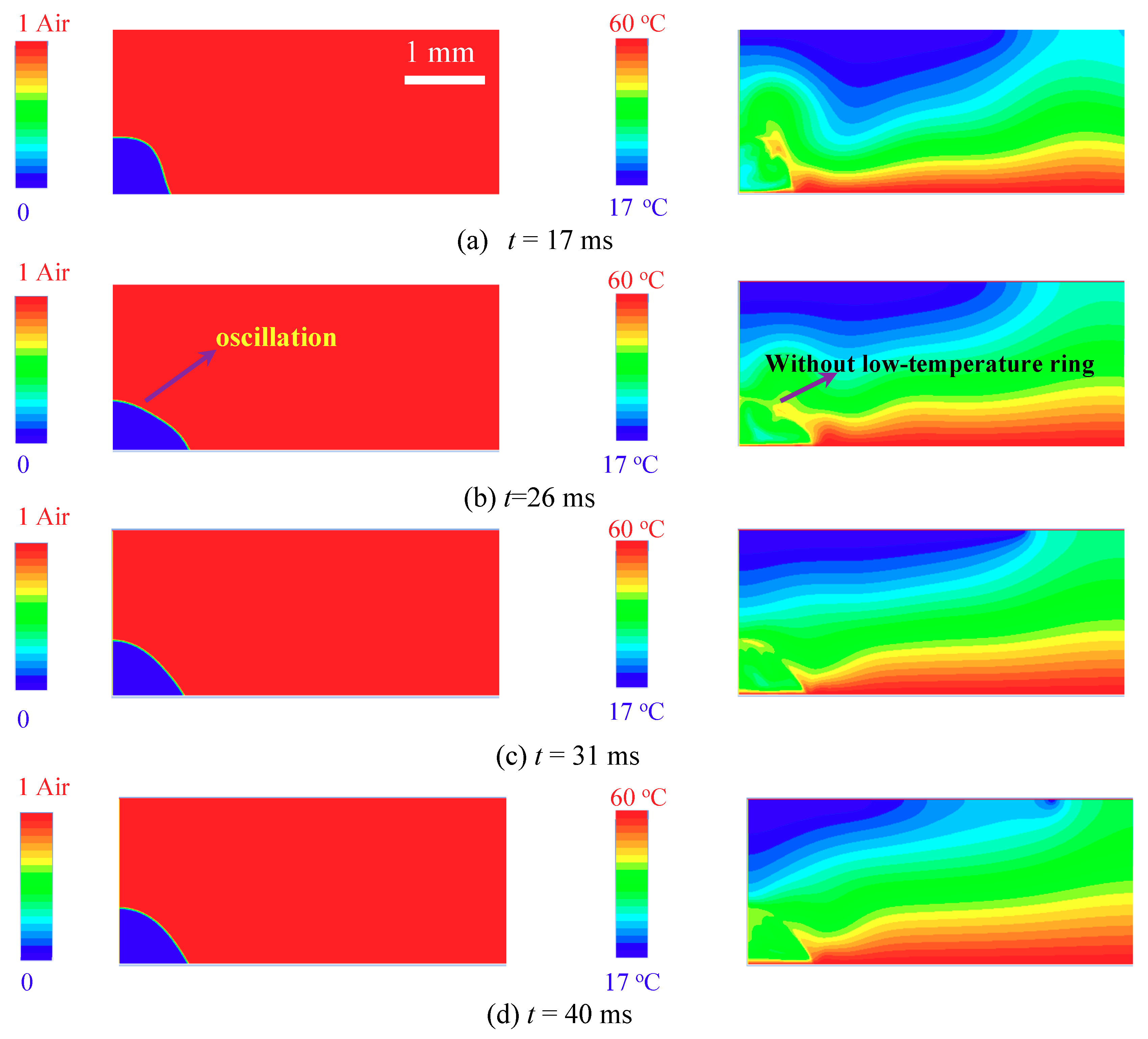
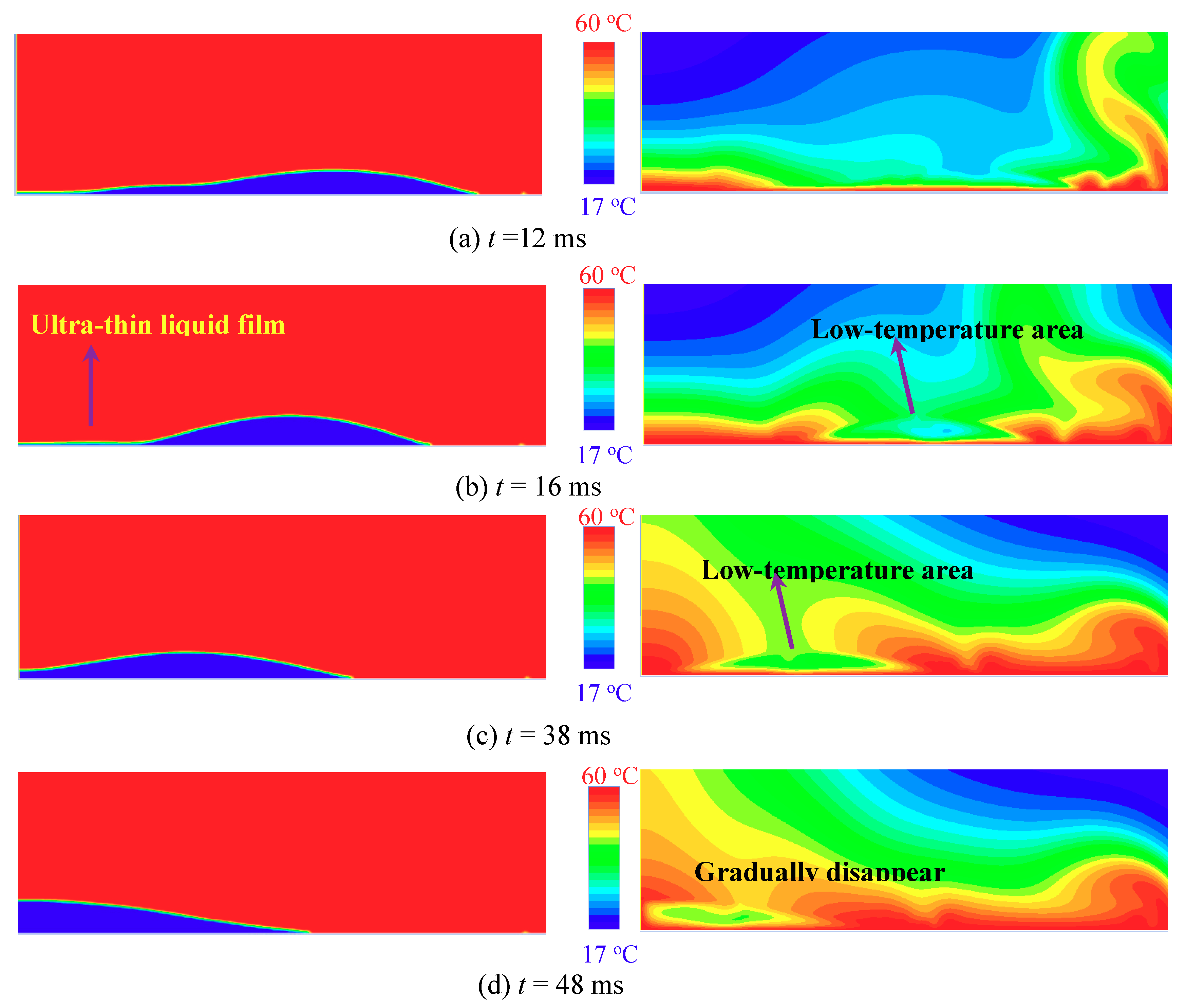
3.3. Mechanism of The Low-Temperature Ring on the Superhydrophilic Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Jiang, P.-X.; Christopher, D.M. Experimental investigation of spray cooling on micro-nano and hybrid-structured surfaces. Int. J. Heat Mass Transf. 2015, 80, 26–37. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, X.; Li, Y. Experimental study of falling film evaporation heat transfer on superhydrophilic horizontal-tubes at low spray density. Appl. Therm. Eng. 2017, 111, 1548–1556. [Google Scholar] [CrossRef]
- Worthington, A.M. On the forms assumed by drops of liquids falling vertically on a horizontal plate. Proc. R. Soc. Lond. 1876, 25, 261–272. [Google Scholar]
- Yarin, A.L. Drop impact dynamics: Splashing, spreading, receding, bouncing…. Annu. Rev. Fluid Mech. 2006, 38, 159–192. [Google Scholar] [CrossRef]
- Rioboo, R.; Marengo, M.; Tropea, C. Time evolution of liquid drop impact onto solid, dry surfaces. Exp. Fluids 2002, 33, 112–124. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of drop impact on heated walls. Int. J. Heat Mass Transf. 2017, 106, 103–126. [Google Scholar] [CrossRef]
- Wang, X.; Sun, D.; Wang, X.; Yan, W. Dynamics of droplets impacting hydrophilic surfaces decorated with a hydrophobic strip. Int. J. Heat Mass Transf. 2019, 135, 235–246. [Google Scholar] [CrossRef]
- Tang, C.; Qin, M.; Weng, X. Dynamics of droplet impact on solid surface with different roughness. Int. J. Multiph. Flow 2017, 96, 56–69. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, Z.; Sui, D. The impact and freezing processes of a water droplet on different inclined cold surfaces. Int. J. Heat Mass Transf. 2016, 97, 211–223. [Google Scholar] [CrossRef]
- Seol, H.K.A.; Gicheol, L.B.; Hyung, M.K.C.; Moo, H.K. Leidenfrost point and droplet dynamics on heated micropillar array surface. Int. J. Heat Mass Transf. 2019, 139, 1–9. [Google Scholar]
- Pasandideh-Fard, M.; Aziz, S.D.; Chandra, S. Cooling effectiveness of a water drop impinging on a hot surface. Int. J. Heat. Fluid. Flow 2001, 22, 201–210. [Google Scholar] [CrossRef]
- Fujimoto, H.; Oku, Y.; Ogihara, T. Hydrodynamics and boiling phenomena of water droplets impinging on hot solid. Int. J. Multiph. Flow 2010, 36, 620–642. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.; Bahadur, V.; Zhong, S. Temperature dependent droplet impact dynamics on flat and textured surfaces. App. Phy. Lett. 2012, 11, 7699. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, H.; Yang, Z. Experimental investigation of the impact and freezing processes of a water droplet on an ice surface. Int. J. Heat Mass Transf. 2017, 109, 716–724. [Google Scholar] [CrossRef]
- Jin, Z.; Sui, D.; Yang, Z. The impact, freezing, and melting processes of a water droplet on an inclined cold surface. Int. J. Heat Mass Transf. 2015, 90, 439–453. [Google Scholar] [CrossRef]
- Xiao, R.; Chu, K.H.; Wang, E.N. Multilayer liquid spreading on superhydrophilic nanostructured surfaces. App. Phy. Lett. 2009, 94, 988. [Google Scholar] [CrossRef]
- Lee, J.B.; Derome, D.; Carmeliet, J. Drop impact on natural porous stones. J. Colloid. Interf. Sci. 2016, 469, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Moon, M.; Lee, K. Liquid spreading on superhydrophilic micropillar arrays. J. Fluid Mech. 2011, 680, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, J.; Moon, M.W. Experimental study of drop spreading on textured superhydrophilic surfaces. Phys. Fluids. 2013, 25, 092110. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.S.; Park, G.; Kim, J. Wicking and spreading of water droplets on nanotubes. Langmuir 2012, 28, 2614–2619. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Q.; Wang, R. Visualization study on capillary-spreading behavior of liquid droplet in vertically aligned carbon nanotube array. Int. J. Heat Mass Transf. 2018, 120, 1055–1064. [Google Scholar] [CrossRef]
- Frank, X.; Perré, P.; Li, H.Z. Lattice Boltzmann investigation of droplet inertial spreading on various porous surfaces. Phys. Rev. E 2015, 91, 052405. [Google Scholar] [CrossRef]
- Hengyi, K.; Lourenço, S.D.N.; Yan, W.M. Lattice Boltzmann simulation of droplet dynamics on granular surfaces with variable wettability. Phys. Rev. E 2018, 98, 012902. [Google Scholar]
- Frank, X.; Perré, P. Droplet spreading on a porous surface: A lattice Boltzmann study. Phys. Fluids 2012, 24, 338. [Google Scholar] [CrossRef]
- Choi, M.; Son, G.; Shim, W. A level-set method for droplet impact and penetration into a porous medium. Comput. Fluids 2017, 145, 153–166. [Google Scholar] [CrossRef]
- Masumeh, F.S.; Mahmood, F.; Farshad, E. Contact angle hysteresis and motion behaviors of a water nano-droplet on suspended graphene under temperature gradient. Phys. Fluids 2018, 30, 052101. [Google Scholar]
- Song, F.H.; Li, B.Q.; Liu, C. Molecular dynamics simulation of nanosized water droplet spreading in an electric field. Langmuir 2013, 29, 4266–4274. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Ouyang, X. Water droplet spreading and wicking on nanostructured surfaces. Langmuir 2017, 33, 6701. [Google Scholar] [CrossRef]
- Wemp, C.K.; Carey, V.P. Water wicking and droplet spreading on randomly structured thin nanoporous layers. Langmuir 2017, 33, 14513. [Google Scholar] [CrossRef]
- Wang, Z.; Espín, L.; Bates, F.S. Water droplet spreading and imbibition on superhydrophilic poly(butylene terephthalate) melt-blown fiber mats. Chem. Eng. Sci. 2016, 146, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Espin, L.; Kumar, S. Droplet spreading and absorption on rough, permeable substrates. J. Fluid Mech. 2015, 784, 465–486. [Google Scholar] [CrossRef]
- Girard, F.; Antoni, M.; Sefiane, K. Infrared thermography investigation of an evaporating sessile water droplet on heated substrates. Langmuir 2010, 26, 4576. [Google Scholar] [CrossRef]
- Gao, X.; Kong, L.; Li, R.; Han, J. Heat transfer of single drop impact on a film flow cooling a hot surface. Int. J. Heat Mass Transf. 2017, 108, 1068–1077. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Lan, Z. The evolution of droplet impacting on thin liquid film at superhydrophilic surface. App. Phys. Lett. 2017, 111, 231601. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Lan, Z. The exact regulation of temperature evolutions for droplet impact on ultrathin cold films at superhydrophilic surface. Chem. Eng. Sci. 2017, 193, 205–216. [Google Scholar] [CrossRef]
- Zheng, Y.; Lan, Z.; Cao, K. A new insight of temperature distribution on superhydrophilic surface horizontal tubes falling film at low spray density. Int. Comm. Heat Mass Transf. 2018, 91, 17–22. [Google Scholar] [CrossRef]
- Youngsuk, N.Y.; Sungtaek, J. A comparative study of the morphology and wetting characteristics of micro/nanostructured Cu surfaces for phase change heat transfer applications. J. Adhes. Sci. Technol. 2013, 27, 2163–2176. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Chun, J.; Zhou, F.; Qiao, K.; Jiang, R.; Zhang, S.; Hao, T. The Low-Temperature Ring during Droplet Impact on a Superhydrophilic Surface. Coatings 2021, 11, 1043. https://doi.org/10.3390/coatings11091043
Ma H, Chun J, Zhou F, Qiao K, Jiang R, Zhang S, Hao T. The Low-Temperature Ring during Droplet Impact on a Superhydrophilic Surface. Coatings. 2021; 11(9):1043. https://doi.org/10.3390/coatings11091043
Chicago/Turabian StyleMa, Huixia, Jiang Chun, Feng Zhou, Kai Qiao, Rui Jiang, Shumei Zhang, and Tingting Hao. 2021. "The Low-Temperature Ring during Droplet Impact on a Superhydrophilic Surface" Coatings 11, no. 9: 1043. https://doi.org/10.3390/coatings11091043
APA StyleMa, H., Chun, J., Zhou, F., Qiao, K., Jiang, R., Zhang, S., & Hao, T. (2021). The Low-Temperature Ring during Droplet Impact on a Superhydrophilic Surface. Coatings, 11(9), 1043. https://doi.org/10.3390/coatings11091043





