The Effect of High-Temperature Water Vapour on Degradation and Failure of Hot Section Components of Gas Turbine Engines
Abstract
:1. Introduction
2. Effect of High Temperature Water Vapour on Substrate Alloys and Intermetallic Bond Coats
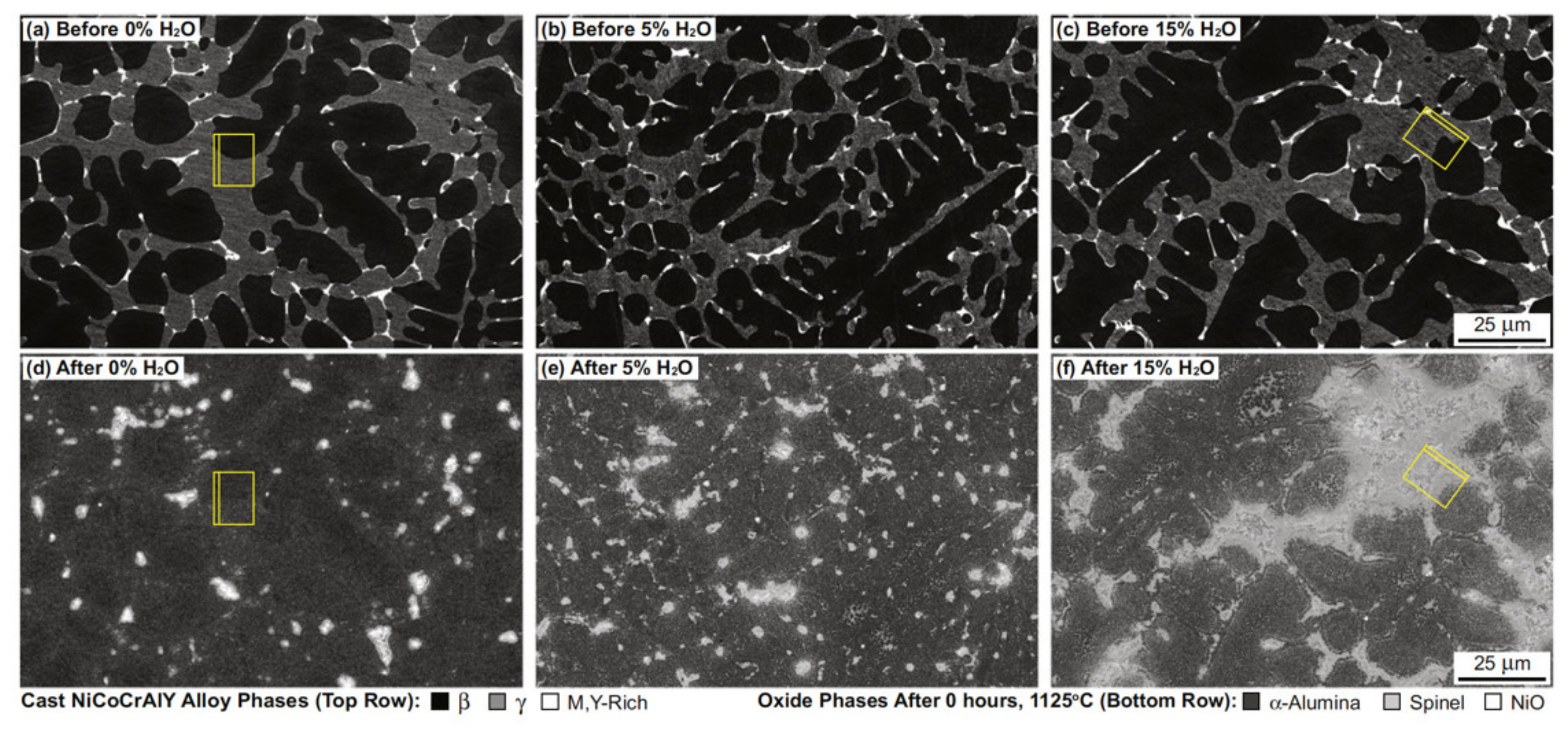
2.1. Effect of Water Vapour on Alloys
2.2. Effect of Water Vapour Effect Bond Coat
3. The Effect of Water Vapour on Thermal Barrier Coatings
3.1. Effect of Water Vapour on Topcoat Properties
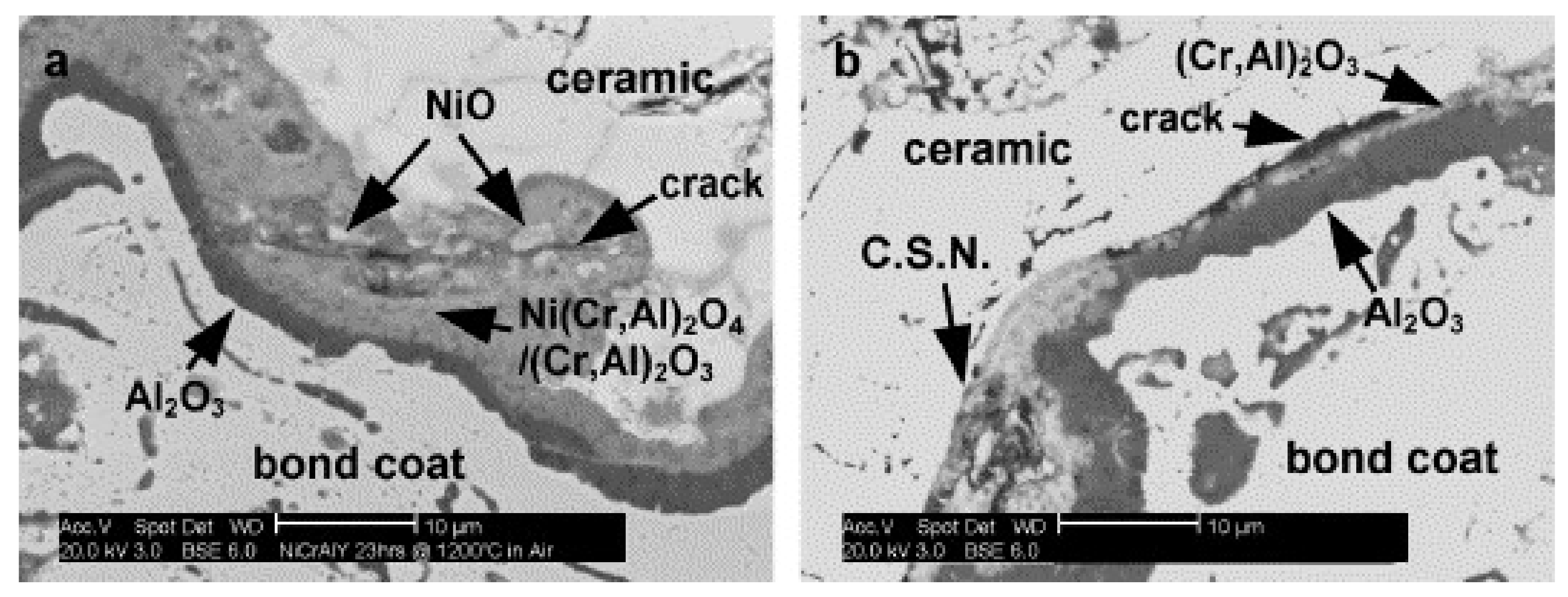
3.2. Effect of Water Vapour on Crack Nucleation and Propagation
3.3. Effect of Water Vapor on TGO
4. Effect of Water Vapor on Environmental Barrier Coatings (EBCs)
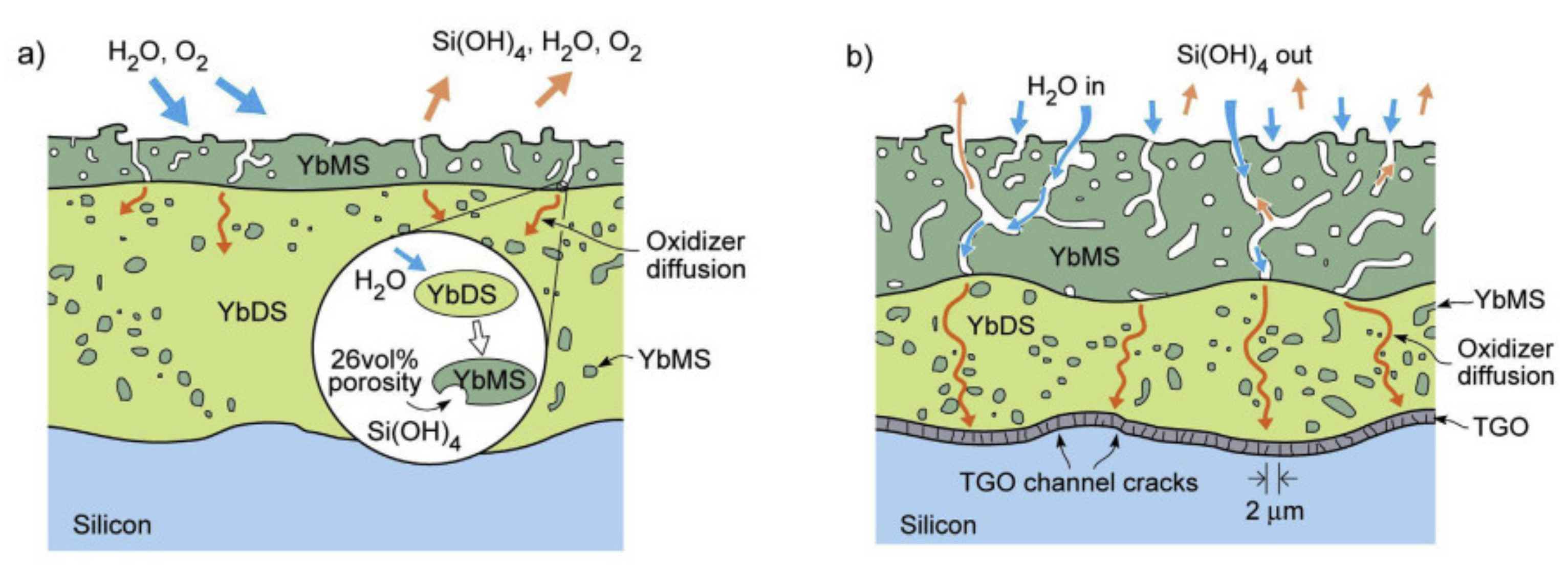
4.1. Water Vapour Reaction with Thermally Grown Oxides (TGO) Scale
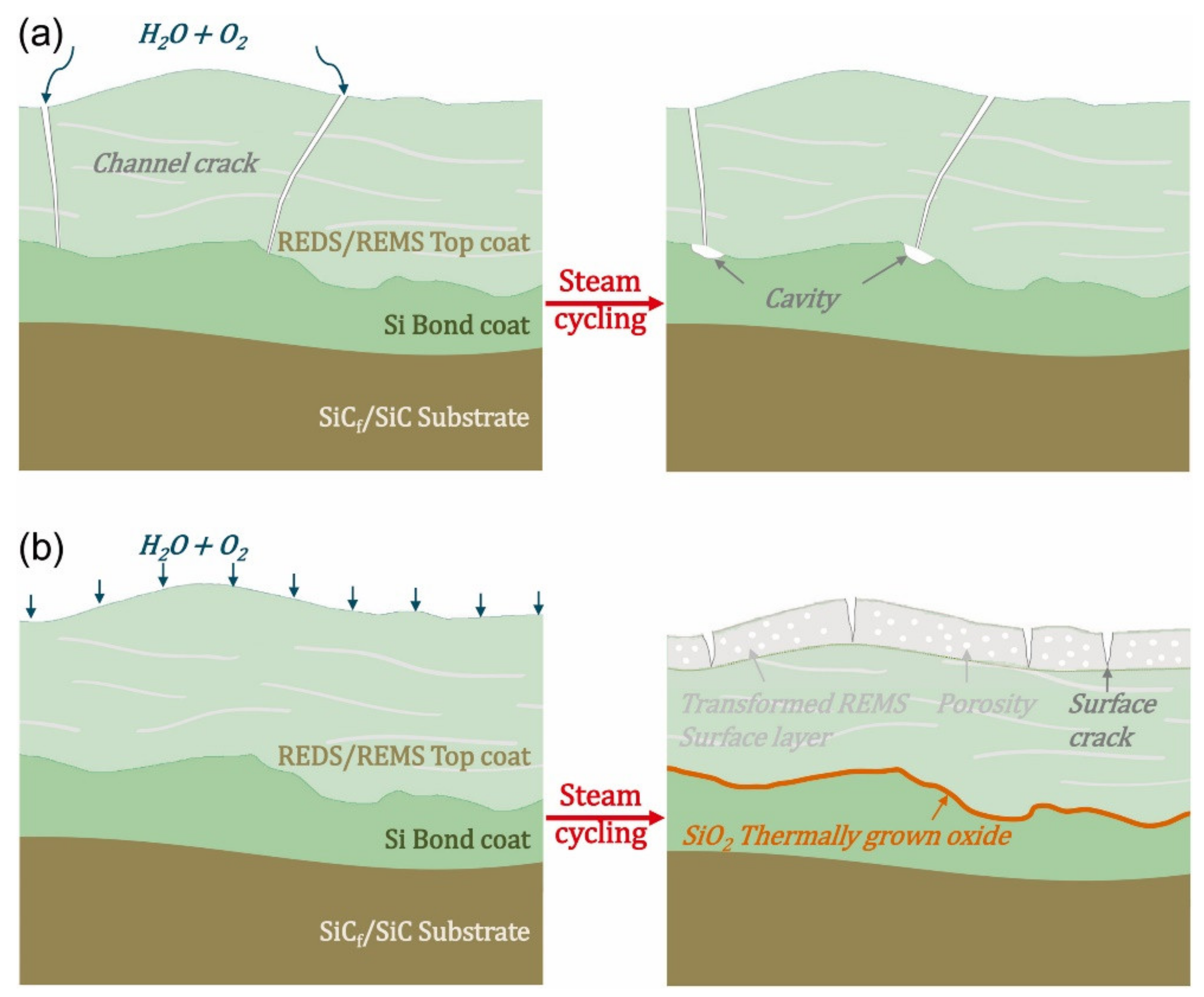
4.2. Surface Cracking due to Water Vapour
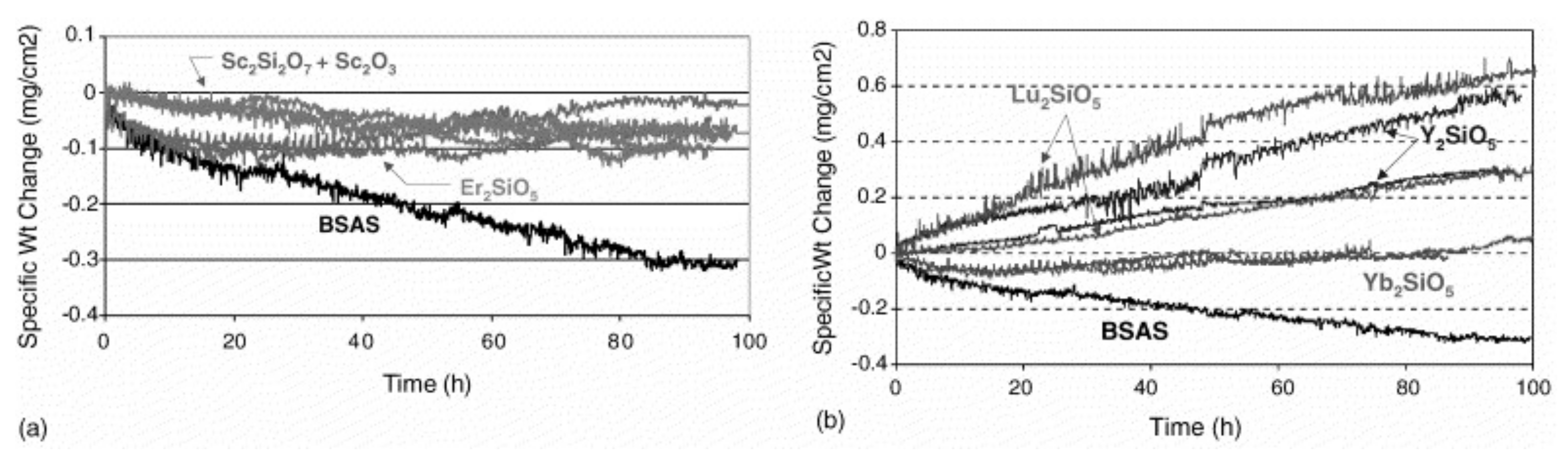
4.3. Recession of Environmental Barrier Coatings
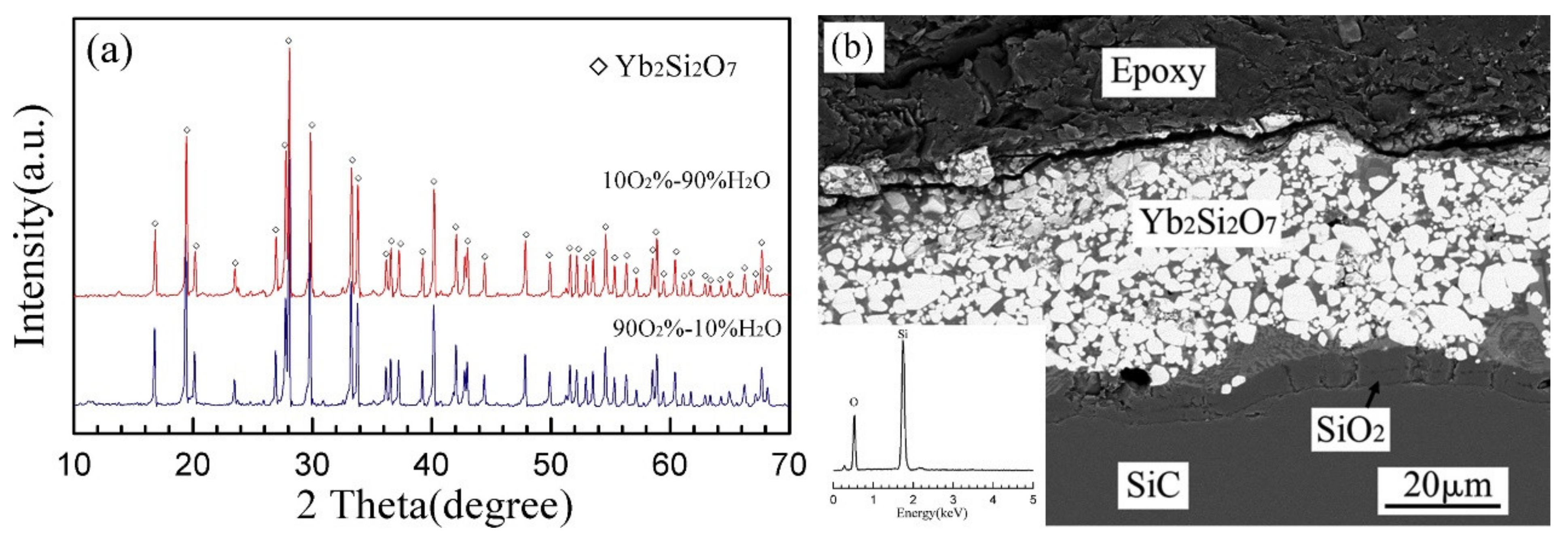
4.4. Recession Evaluation on EBC Topcoat and TGO Scale
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lefebvre, A.H.; Ballal, D.R. Gas Turbine Combustion; CRC Press/Taylor Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Braun-Unkhoff, M.; Riedel, U. Alternative fuels in aviation. CEAS Aeronaut. J. 2015, 6, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Kurzawska, P.; Jasiński, R. Overview of sustainable aviation fuels with emission characteristic and particles emission of the turbine engine fueled ATJ blends with different percentages of ATJ fuel. Energies 2021, 14, 1858. [Google Scholar] [CrossRef]
- Maris-Sida, M.C.; Meier, G.H.; Pettit, F.S. Some water vapor effects during the oxidation of alloys that are α-Al2O3 formers. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2003, 34, 2609–2619. [Google Scholar] [CrossRef]
- Saunders, S.R.J.; Monteiro, M.; Rizzo, F. The oxidation behaviour of metals and alloys at high temperatures in atmospheres containing water vapour: A review. Prog. Mater. Sci. 2008, 53, 775–837. [Google Scholar] [CrossRef]
- Sullivan, M.H.; Mumm, D.R. Vapor-Phase-Mediated Phenomena Associated with High Temperature, High Water Content Oxidation of MCrAlX Bond Coats. Oxid. Met. 2014, 82, 1–20. [Google Scholar] [CrossRef]
- Sullivan, M.H.; Mumm, D.R. Transient stage oxidation of MCrAlY bond coat alloys in high temperature, high water vapor content environments. Surf. Coat. Technol. 2014, 258, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al Alloys Between 1000° and 1200 °C. J. Electrochem. Soc. 1971, 118, 1782. [Google Scholar] [CrossRef]
- Wallwork, G.R.; Hed, A.Z. Some limiting factors in the use of alloys at high temperatures. Oxid. Met. 1971, 3, 171–184. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Kwakernaak, C.; Sloof, W.G. The effects of alloy microstructure refinement on the short-term thermal oxidation of NiCoCrAlY alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2006, 37, 683–693. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, B.; Kim, H.; Lee, C. Isothermal oxidation of air plasma spray NiCrAlY bond coatings. Surf. Coat. Technol. 2002, 150, 297–308. [Google Scholar] [CrossRef]
- Shillington, E.A.; Clarke, D. Spalling failure of a thermal barrier coating associated with aluminum depletion in the bond-coat. Acta Mater. 1999, 47, 1297–1305. [Google Scholar] [CrossRef]
- Angle, J.P.; Morgan, P.E.D.; Mecartney, M.L. Water vapor-enhanced diffusion in alumina. J. Am. Ceram. Soc. 2013, 96, 3372–3374. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, J.; Gong, S.; Xu, H. Influence of water vapor on the isothermal oxidation behavior of low pressure plasma sprayed NiCrAlY coating at high temperature. Surf. Coat. Technol. 2002, 161, 86–91. [Google Scholar] [CrossRef]
- Kaplin, C.; Brochu, M. Effects of water vapor on high temperature oxidation of cryomilled NiCoCrAlY coatings in air and low-SO2 environments. Surf. Coat. Technol. 2011, 205, 4221–4227. [Google Scholar] [CrossRef]
- Stolle, R. Conventional and advanced coatings for turbine airfoils. MTU Aero Engines 2010. [Google Scholar]
- Haynes, J.A.; Unocic, K.A.; Pint, B.A. Effect of water vapor on the 1100 °C oxidation behavior of plasma-sprayed TBCs with HVOF NiCoCrAlX bond coatings. Surf. Coat. Technol. 2013, 215, 39–45. [Google Scholar] [CrossRef]
- Rudolphi, M.; Renusch, D.; Zschau, H.E.; Schütze, M. The effect of moisture on the delayed spallation of thermal barrier coatings: VPS NiCoCrAlY bond coat + APS YSZ top coat. Mater. High Temp. 2009, 26, 325–329. [Google Scholar] [CrossRef]
- Déneux, V.; Cadoret, Y.; Hervier, S.; Monceau, D. Effect of Water Vapor on the Spallation of Thermal Barrier Coating Systems During Laboratory Cyclic Oxidation Testing. Oxid. Met. 2010, 73, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Smialek, J.L. Moisture-induced delayed spallation and interfacial hydrogen embrittlement of alumina scales. Jom 2006, 58, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Pint, B.A.; Garner, G.W.; Lowe, T.M.; Haynes, J.A.; Zhang, Y. Effect of increased water vapor levels on TBC lifetime with Pt-containing bond coatings. Surf. Coat. Technol. 2011, 206, 1566–1570. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, J.; Gong, S.; Xu, H. Influence of water vapor on the high temperature oxidation behavior of thermal barrier coatings. Mater. Sci. Eng. A 2003, 348, 327–332. [Google Scholar] [CrossRef]
- Haynes, J.A.; Unocic, K.A.; Lance, M.J.; Pint, B.A. Impact of superalloy composition, bond coat roughness and water vapor on TBC lifetime with HVOF NiCoCrAlYHfSi bond coatings. Surf. Coat. Technol. 2013, 237, 65–70. [Google Scholar] [CrossRef]
- Yanar, N.M.; Stiger, M.J.; Maris-Sida, M.; Pettit, F.S.; Meier, G.H. Effects of high temperature exposure on the durability of thermal barrier coatings. Key Eng. Mater. 2001, 197, 145–163. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Marple, B.R.; Patnaik, P.C. Oxidation and crack nucleation/growth in an air-plasma-sprayed thermal barrier coating with NiCrAlY bond coat. Surf. Coat. Technol. 2005, 197, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Goward, G. Progress in coatings for gas turbine airfoils. Surf. Coat. Technol. 1998, 108–109, 73–79. [Google Scholar] [CrossRef]
- Salazar, A.; Gómez-García, J.; Poza, P.; Utrilla, V. Microstructural Evolution of Thermal Barrier Coatings during Isothermal Oxidation. Key Eng. Mater. 2007, 333, 269–272. [Google Scholar] [CrossRef]
- Tamura, M.; Takahashi, M.; Ishii, J.; Suzuki, K.; Sato, M.; Shimomura, K. Multilayered thermal barrier coating for land-based gas turbines. J. Therm. Spray Technol. 1999, 8, 68–72. [Google Scholar] [CrossRef]
- Bennett, A. Properties of thermal barrier coatings. Mater. Sci. Technol. 1986, 2, 257–261. [Google Scholar] [CrossRef]
- Lance, M.J.; Unocic, K.A.; Haynes, J.A.; Pint, B.A. Effect of water vapor on thermally-grown alumina scales on Pt-modified and simple aluminide bond coatings. Surf. Coat. Technol. 2013, 237, 2–7. [Google Scholar] [CrossRef]
- Fontana, S.; Chevalier, S.; Caboche, G. Metallic interconnects for solid oxide fuel cell: Effect of water vapour on oxidation resistance of differently coated alloys. J. Power Sources 2009, 193, 136–145. [Google Scholar] [CrossRef]
- Reed, R.C. The Superalloys: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Unocic, K.A.; Pint, B.A. Effect of water vapor on thermally grown alumina scales on bond coatings. Surf. Coat. Technol. 2013, 215, 30–38. [Google Scholar] [CrossRef]
- Gleeson, B. Thermal barrier coatings for aeroengine applications. J. Propuls. Power 2006, 22, 375–383. [Google Scholar] [CrossRef]
- Pint, B.A.; Unocic, K.A.; Haynes, J.A. The effect of environment on TBC lifetime. In Ceramics, controls, diagnostics and instrumentation, education, manufacturing materials and metallurgy, honors and awards, Proceedings of ASME Turbo Expo 2015, Montreal, Quebec, Canada, 15–19 June 2015; GT2015-43762; ASME: New York, NY, USA, 2015; pp. 1–10. [Google Scholar] [CrossRef]
- Pint, B.A.; Haynes, J.A. Effect of water vapour content on thermal barrier coating lifetime. Mater. Sci. Technol. 2013, 29, 828–834. [Google Scholar] [CrossRef]
- Eriksson, R.; Yuan, K.; Li, X.H.; Lin Peng, R. Corrosion of NiCoCrAlY Coatings and TBC Systems Subjected to Water Vapor and Sodium Sulfate. J. Therm. Spray Technol. 2015, 24, 953–964. [Google Scholar] [CrossRef]
- Wu, Y.; Narita, T. The cyclic oxidation behavior of the single crystal TMS-82+ superalloy in humidified air. Mater. Corros. 2009, 60, 781–787. [Google Scholar] [CrossRef]
- Song, P.; He, X.; Xiong, X.; Ma, H.; Song, Q.; Lü, J.; Lu, J. Effect of water vapor on evolution of a thick Pt-layer modified oxide on the NiCoCrAl alloy at high temperature. Mater. Res. Express 2018, 5, 036514. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, H.; Gong, S. Influence of water vapor on the cyclic-oxidation behavior of a low-pressure plasma-sprayed NiCrAlY coating. Oxid. Met. 2004, 62, 195–206. [Google Scholar] [CrossRef]
- Duan, W.; Song, P.; Li, C.; Huang, T.; Ge, Z.; Feng, J.; Lu, J. Effect of water vapor on the failure behavior of thermal barrier coating with Hf-doped NiCoCrAlY bond coating. J. Mater. Res. 2019, 34, 2653–2663. [Google Scholar] [CrossRef]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Padture, N.P. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Langston, L.S. Anticipated but Unwelcome. Mech. Eng. 2018, 140, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.N.; Fox, D.S.; Bansal, N.P. Rare earth silicate environmental barrier coatings for SiC/SiC composites and Si3N4 ceramics. J. Eur. Ceram. Soc. 2005, 25, 1705–1715. [Google Scholar] [CrossRef]
- Ohji, T.; Singh, M. (Eds.) Engineered Ceramics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Jacobson, N.S. Corrosion of Silicon-Based Ceramics in Combustion Environments. J. Am. Ceram. Soc. 1993, 76, 3–28. [Google Scholar] [CrossRef] [Green Version]
- Opila, E.J.; Hann, R.E. Paralinear oxidation of CVD SiC in water vapor. J. Am. Ceram. Soc. 1997, 80, 197–205. [Google Scholar] [CrossRef]
- Richards, B.T.; Young, K.A.; De Francqueville, F.; Sehr, S.; Begley, M.R.; Wadley, H.N.G. Response of ytterbium disilicate-silicon environmental barrier coatings to thermal cycling in water vapor. Acta Mater. 2016, 106, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Golden, R.A.; Mueller, K.; Opila, E.J. Thermochemical stability of Y2Si2O7 in high-temperature water vapor. J. Am. Ceram. Soc. 2020, 103, 4517–4535. [Google Scholar] [CrossRef]
- Zhu, D.; Lee, K.N.; Miller, R.A. Cyclic Failure Mechanisms of Thermal and Environmental Barrier Coating Systems under Thermal Gradient Test Conditions. In 26th Annual International Conference on Advanced Ceramics and Composite; NASA/TM: Cleveland, OH, USA, 2002. [Google Scholar]
- Garcia, E.; Sotelo-Mazon, O.; Poblano-Salas, C.A.; Trapaga, G.; Sampath, S. Characterization of Yb2Si2O7–Yb2SiO5 composite environmental barrier coatings resultant from in situ plasma spray processing. Ceram. Int. 2020, 46, 21328–21335. [Google Scholar] [CrossRef]
- Lv, B.; Qu, Z.; Xu, B.; Wang, Y.; Fang, D. Water vapor volatilization and oxidation induced surface cracking of environmental barrier coating systems: A numerical approach. Ceram. Int. 2021, 47, 16547–16554. [Google Scholar] [CrossRef]
- Robinson, R.C.; Smialek, J.L. SiC recession caused by SiO2 scale volatility under combustion conditions: I. Experimental results and empirical model. J. Am. Ceram. Soc. 1999, 82, 1817–1825. [Google Scholar] [CrossRef]
- Opila, E.J. Oxidation and volatilization of silica formers in water vapor. J. Am. Ceram. Soc. 2003, 86, 1238–1248. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.N. Key Durability Issues With Mullite-Based Environmental Barrier Coatings for Si-Based Ceramics. J. Eng. Gas Turbines Power 2000, 122, 632–636. [Google Scholar] [CrossRef] [Green Version]
- Ueno, S.; Jayaseelan, D.D.; Ohji, T. Development of oxide-based EBC for silicon nitride. Int. J. Appl. Ceram. Technol. 2004, 1, 362–373. [Google Scholar] [CrossRef]
- Ueno, S.; Ohji, T.; Lin, H.T. Recession behavior of Yb2Si2O7 phase under high speed steam jet at high temperatures. Corros. Sci. 2008, 50, 178–182. [Google Scholar] [CrossRef]
- Al Nasiri, N.; Patra, N.; Jayaseelan, D.D.; Lee, W.E. Water vapour corrosion of rare earth monosilicates for environmental barrier coating application. Ceram. Int. 2017, 43, 7393–7400. [Google Scholar] [CrossRef] [Green Version]
- Eaton, H.E.; Linsey, G.D. Accelerated oxidation of SiC CMC’s by water vapor and protection via environmental barrier coating approach. J. Eur. Ceram. Soc. 2002, 22, 2741–2747. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Hu, F.; Yang, J.; Cheng, L.; Wang, Y. Polymer-derived yttrium silicate coatings on 2D C/SiC composites. J. Eur. Ceram. Soc. 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Ueno, S.; Jayaseelan, D.D.; Ohji, T. Comparison of water vapor corrosion behavior of silicon nitride with various EBC layers. J. Ceram. Process. Res. 2004, 5, 355–359. [Google Scholar]
- Kennedy, G.C.; Wasserburg, G.J.; Heard, H.C.; Newton, R.C. The upper three-phase region in the system SiO2-H2O. Am. J. Sci. 1962, 260, 501–521. [Google Scholar] [CrossRef]
- Maier, N.; Nickel, K.G.; Rixecker, G. High temperature water vapour corrosion of rare earth disilicates (Y,Yb,Lu)2Si2O7 in the presence of Al(OH)3 impurities. J. Eur. Ceram. Soc. 2007, 27, 2705–2713. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y. Formation and growth of silica layer beneath environmental barrier coatings under water-vapor environment. J. Alloys Compd. 2018, 739, 817–826. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Feng, J.; Zhang, X.; Deng, C.; Zhou, K.; Zeng, D.; Guo, S.; Zhao, R.; Li, S. Water vapor corrosion behavior of Yb2SiO5 environmental barrier coatings prepared by plasma spray-physical vapor deposition. Coatings 2020, 10, 392. [Google Scholar] [CrossRef]
- Sun, L.; Luo, Y.; Tian, Z.; Du, T.; Ren, X.; Li, J.; Hu, W.; Zhang, J.; Wang, J. High temperature corrosion of (Er0.25Tm0.25Yb0.25Lu0.25)2Si2O7 environmental barrier coating material subjected to water vapor and molten calcium–magnesium–aluminosilicate (CMAS). Corros. Sci. 2020, 175, 108881. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Y.; Cao, X.; Huang, X.; Li, Y. Thermal shock resistance and bonding strength of tri-layer Yb2SiO5/mullite/Si coating on SiCf/SiC composites. Ceram. Int. 2020, 46, 27292–27298. [Google Scholar] [CrossRef]
- Ruggles-Wrenn, M.B.; Williams, T.M. Fatigue of a SiC/SiC ceramic composite with an ytterbium-disilicate environmental barrier coating at elevated temperature. Int. J. Appl. Ceram. Technol. 2020, 17, 2074–2082. [Google Scholar] [CrossRef]
- Kowalski, B.; Harder, B. Thermally grown oxide in water vapor on coated and uncoated SiC. J. Am. Ceram. Soc. 2020, 103, 5978–5989. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Liu, R.; Jiang, D. Study on water vapor corrosion resistance of rare earth monosilicates RE2SiO5 (RE = Lu, Yb, Tm, Er, Ho, Dy, Y, and Sc) from first-principles calculations. Heliyon 2018, 4, e00857. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, J. First-principles investigation on the corrosion resistance of rare earth disilicates in water vapor. J. Eur. Ceram. Soc. 2009, 29, 2163–2167. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J. Corrosion of barium aluminosilicates by water-vapour: An investigation from first principles. Corros. Sci. 2009, 51, 2126–2129. [Google Scholar] [CrossRef]
- Smialek, J.L.; Robinson, R.C.; Opila, E.J.; Fox, D.S.; Jacobson, N.S. SiC and Si3N4 recession due to SiO2 scale volatility under combustor conditions. Adv. Compos. Mater. 1999, 8, 33–45. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Seo, D.; Canteenwalla, P. The Effect of High-Temperature Water Vapour on Degradation and Failure of Hot Section Components of Gas Turbine Engines. Coatings 2021, 11, 1061. https://doi.org/10.3390/coatings11091061
Chen K, Seo D, Canteenwalla P. The Effect of High-Temperature Water Vapour on Degradation and Failure of Hot Section Components of Gas Turbine Engines. Coatings. 2021; 11(9):1061. https://doi.org/10.3390/coatings11091061
Chicago/Turabian StyleChen, Kuiying, Dongyi Seo, and Pervez Canteenwalla. 2021. "The Effect of High-Temperature Water Vapour on Degradation and Failure of Hot Section Components of Gas Turbine Engines" Coatings 11, no. 9: 1061. https://doi.org/10.3390/coatings11091061
APA StyleChen, K., Seo, D., & Canteenwalla, P. (2021). The Effect of High-Temperature Water Vapour on Degradation and Failure of Hot Section Components of Gas Turbine Engines. Coatings, 11(9), 1061. https://doi.org/10.3390/coatings11091061




