Ternary Holey Carbon Nanohorns/TiO2/PVP Nanohybrids as Sensing Films for Resistive Humidity Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
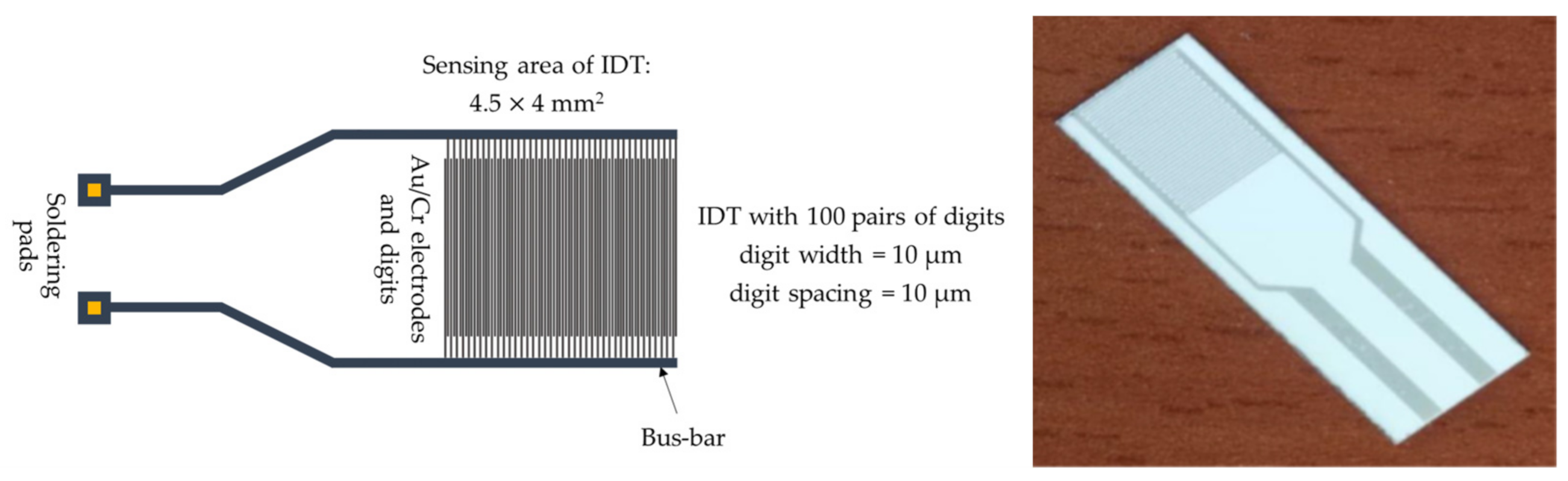
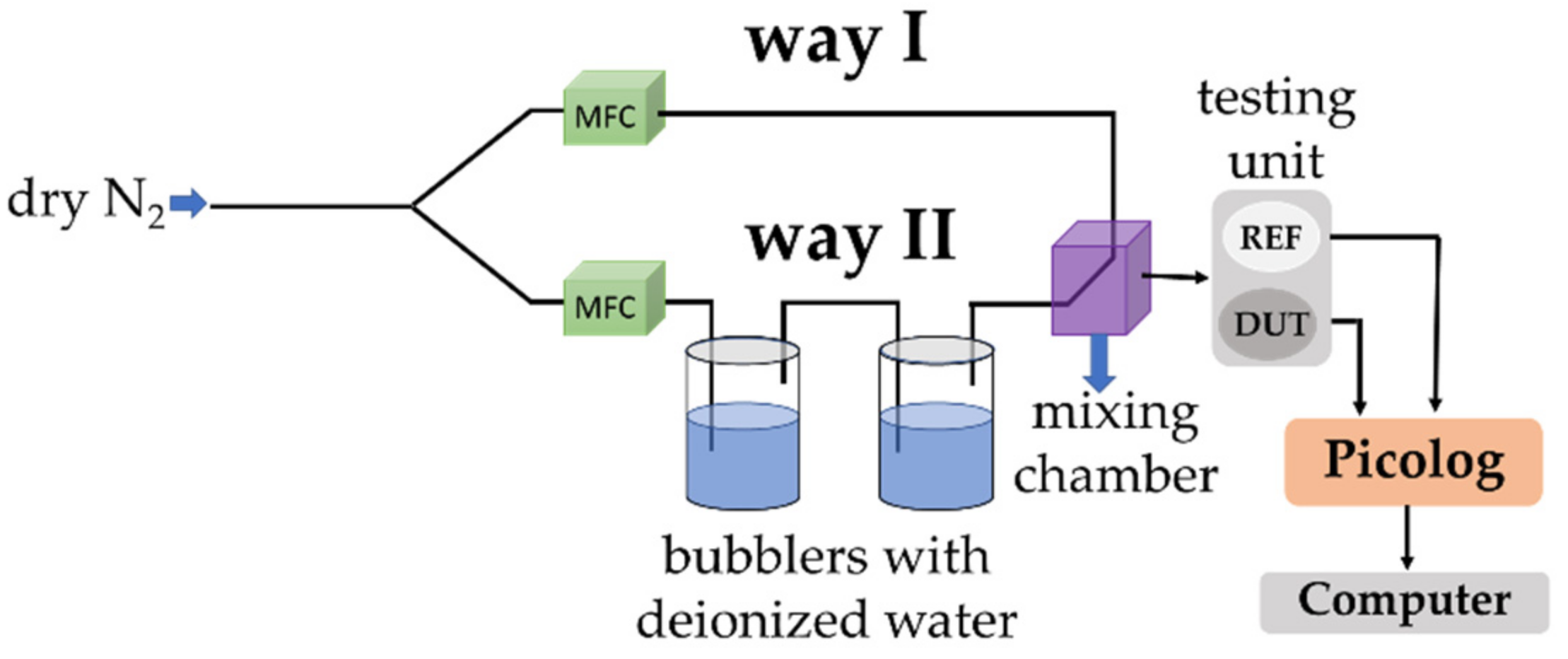
2.2. Methods
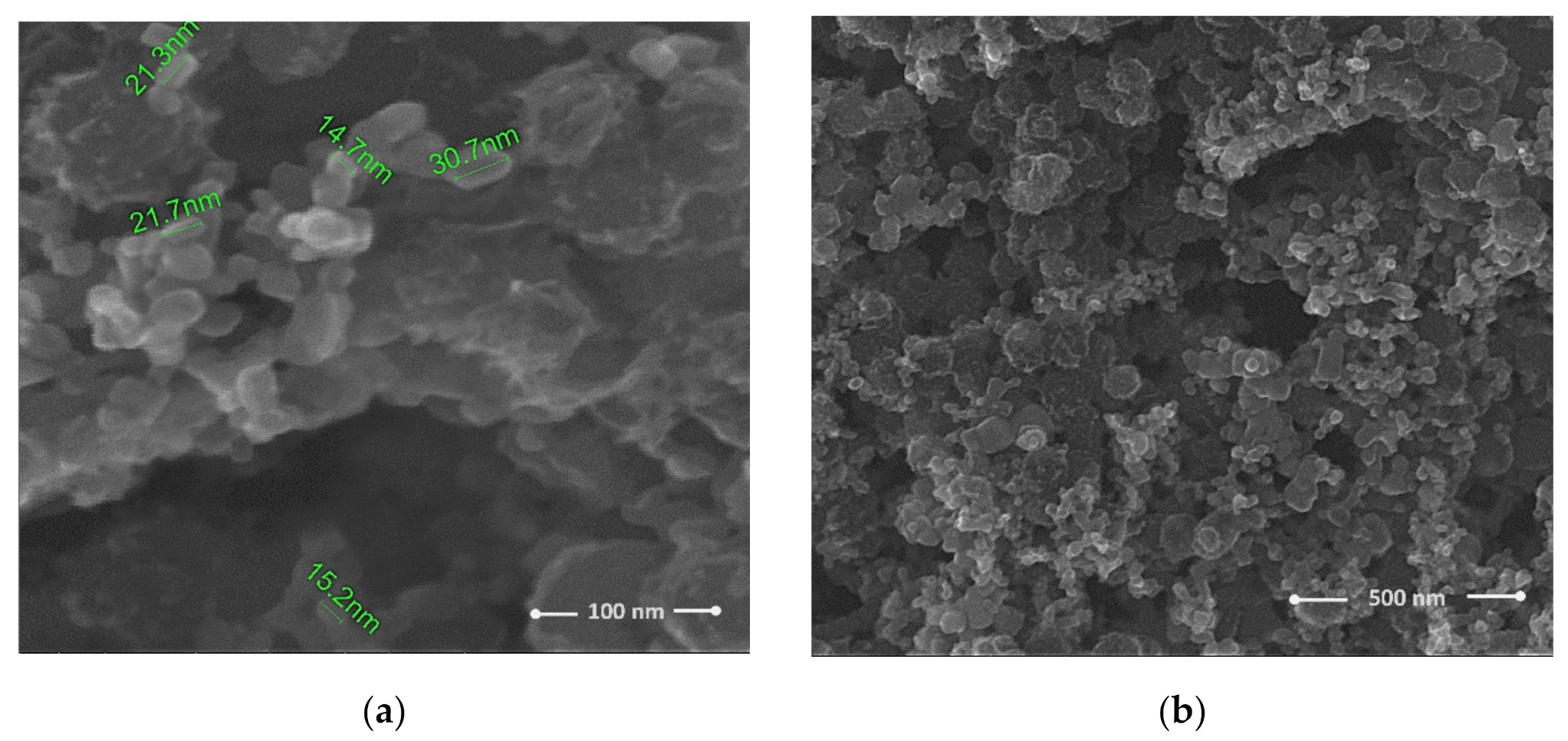
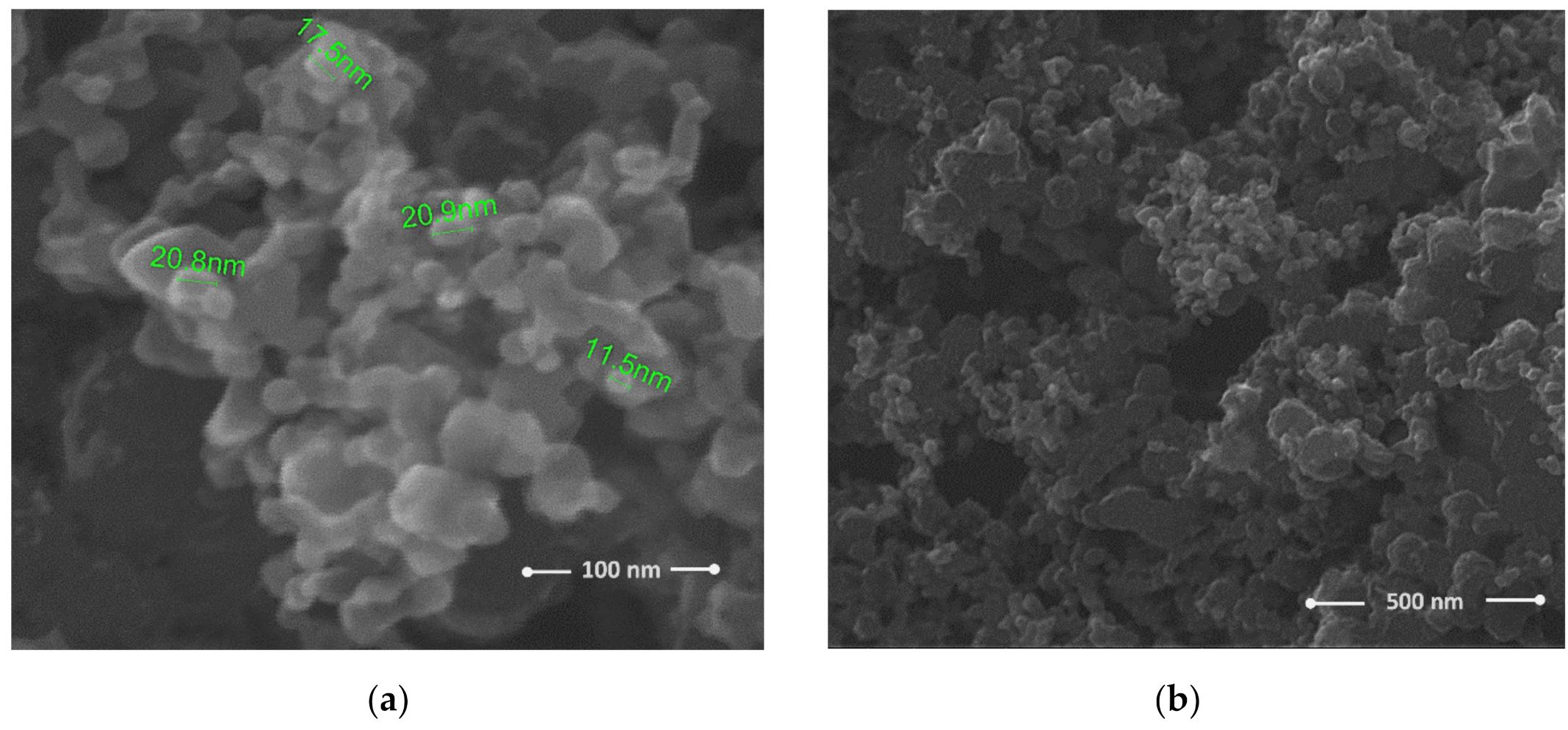
2.3. The Preparation of the Ternary Nanocarbonic Materials-Based Nanocomposite Sensing Films
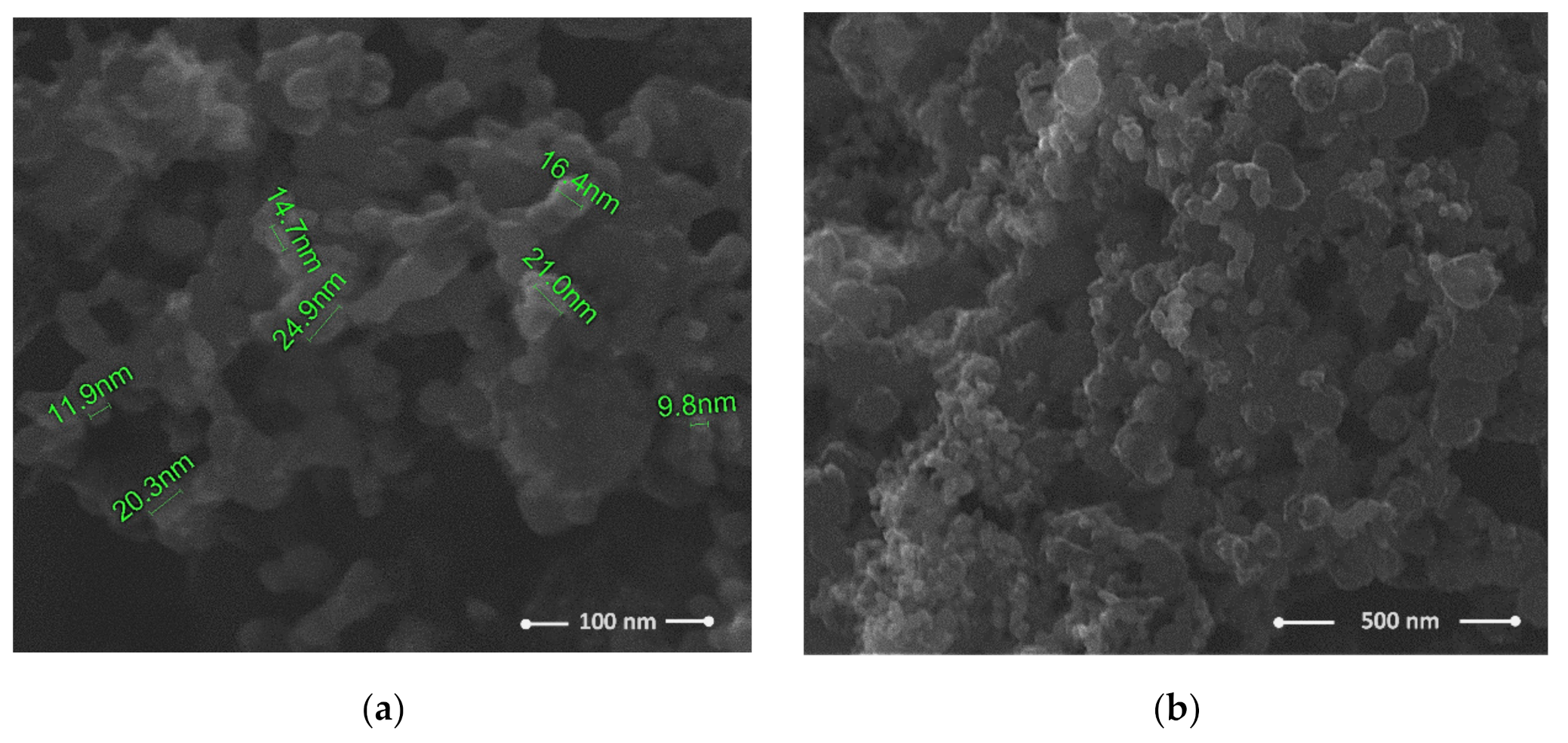
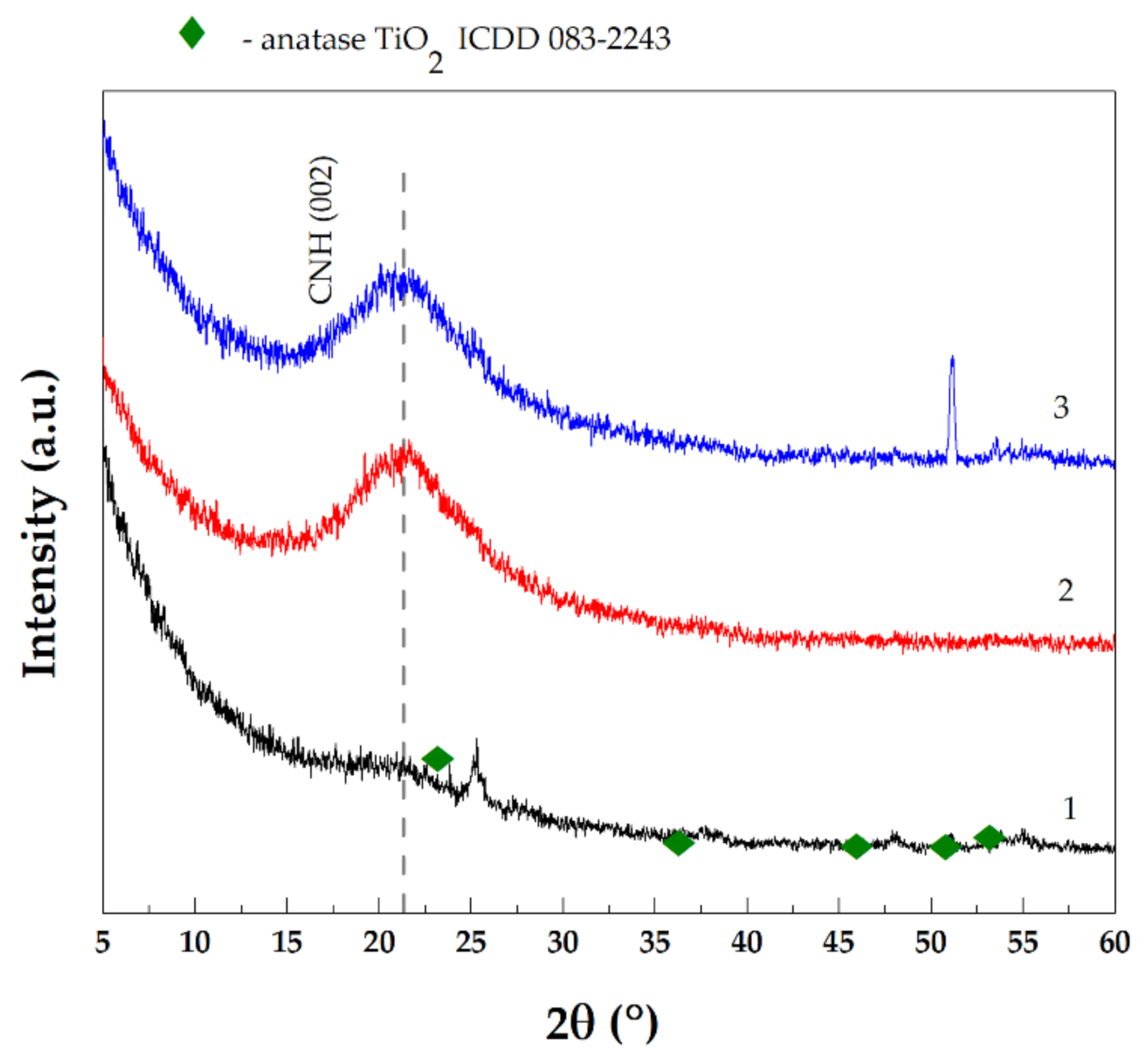
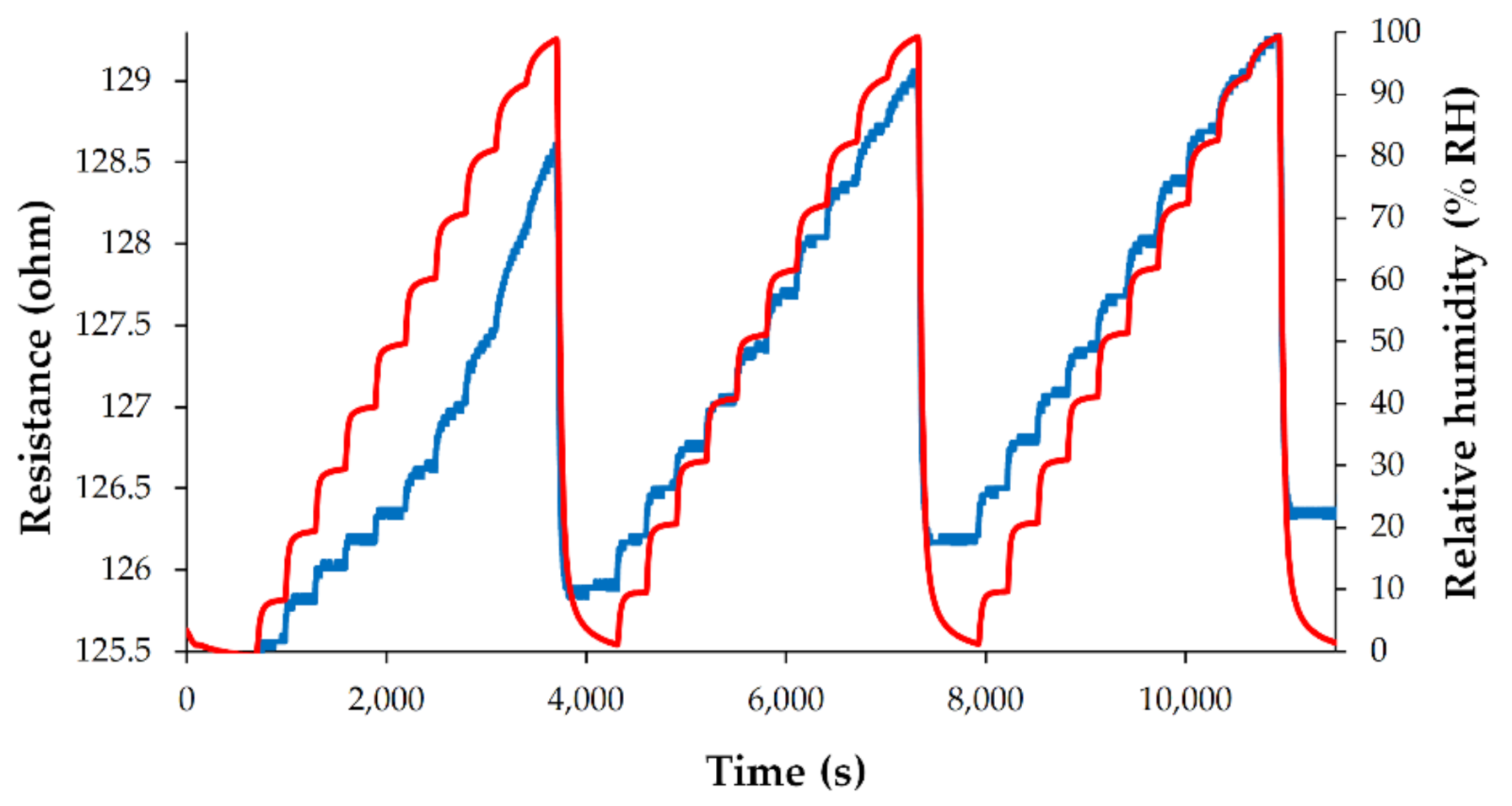
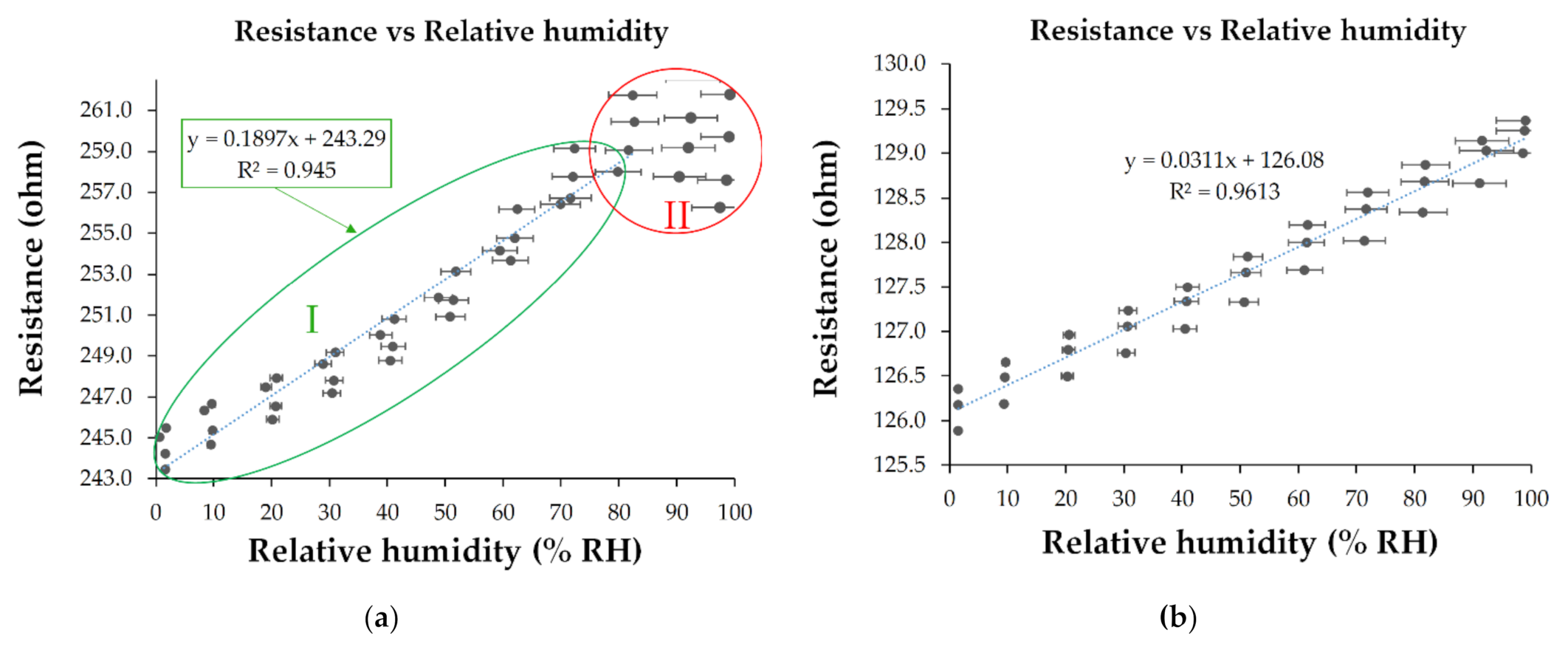
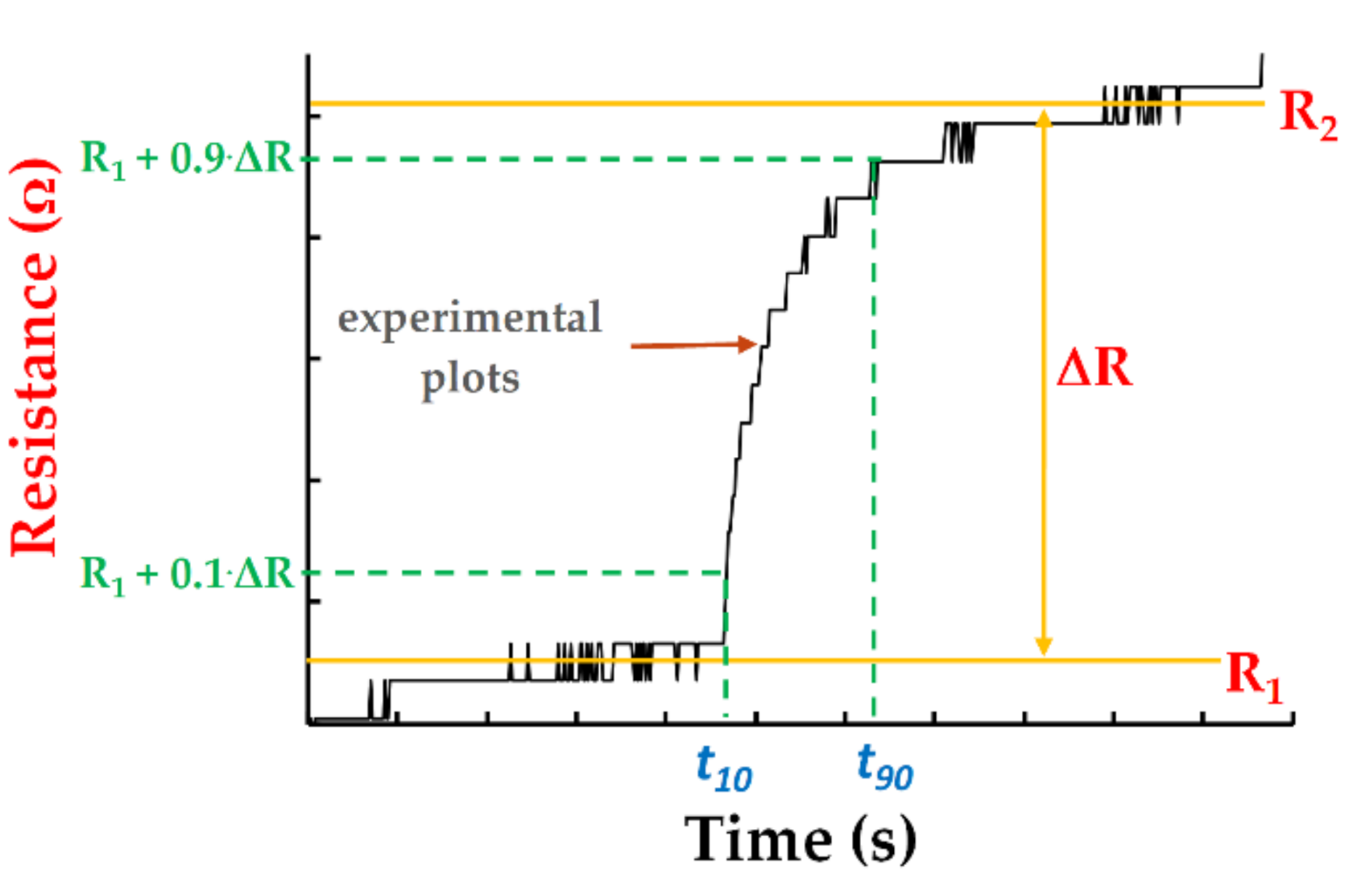
3. Results and Discussion
- the T1-chemiresisitive sensor, which employed the sensing layer based on CNHox/TiO2/PVP at the 1/1/1 ratio (w/w/w);
- the T2-chemiresisitive sensor, which employed the sensing layer based on CNHox/TiO2/PVP at the 2/1/1 ratio (w/w/w); and
- the T3-chemiresisitive sensor, which employed the sensing layer based on CNHox/TiO2/PVP at the 3/1/1 ratio (w/w/w).
Analysis of Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.Y.; Lee, G.B. Humidity sensors: A review. Sens. Lett. 2005, 3, 1–15. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Huang, Q.; Qin, M. A novel capacitive-type humidity sensor using CMOS fabrication technology. Sens. Actuators B 2004, 99, 491–498. [Google Scholar] [CrossRef]
- Penza, M.; Anisimkin, V.I. Surface acoustic wave humidity sensor using polyvinyl-alcohol film. Sens. Actuators A 1999, 76, 162–166. [Google Scholar] [CrossRef]
- Okcan, B.; Akin, T. A thermal conductivity-based humidity sensor in a standard CMOS process. In Proceedings of the 17th IEEE International Conference on Micro Electro Mechanical Systems, Maastricht MEMS 2004 Technical Digest, Maastricht, The Netherlands, 25–29 January 2004; pp. 552–555. [Google Scholar]
- Muto, S.; Suzuki, O.; Amano, T.; Morisawa, M. A plastic optical fibre sensor for real-time humidity monitoring. Meas. Sci. Technol. 2003, 14, 746. [Google Scholar] [CrossRef]
- Su, P.; Wang, C. Novel flexible resistive-type humidity sensor. Sens. Actuators B 2007, 123, 1071–1076. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fu, W.; Yang, H.; Qi, Q.; Zeng, Y.; Zhang, T.; Zou, G. Synthesis and characterization of TiO2 nanotubes for humidity sensing. Appl. Surf. Sci. 2008, 254, 5545–5547. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, L.; Wu, F.; Yuan, S.; Zhao, Y.; Zhang, M. The sol-gel template synthesis of porous TiO2 for a high performance humidity sensor. Nanotechnology 2011, 22, 275502. [Google Scholar] [CrossRef]
- Shinde, P.V.; Gagare, S.; Rout, C.S.; Late, D.J. TiO2 nanoflowers based humidity sensor and cytotoxic activity. RSC Adv. 2020, 10, 29378–29384. [Google Scholar] [CrossRef]
- Ghalamboran, M.; Saedi, Y. TiO2-TiO2 composite resistive humidity sensor: Ethanol cross sensitivity. In IOP Conference Series: Materials Science and Engineering, Volume 108, 5th International Conference on Materials and Applications for Sensors and Transducers (IC-MAST2015), Mykonos, Greece, 27–30 September 2015; IOP Publishing: Bristol, UK, 2016. [Google Scholar]
- Biju, K.P.; Jain, M.K. Effect of crystallization on humidity sensing properties of sol-gel derived nanocrystalline TiO2 thin films. Thin Solid Films 2008, 516, 2175–2180. [Google Scholar] [CrossRef]
- Dubourg, G.; Segkos, A.; Katona, J.; Radović, M.; Savić, S.; Niarchos, G.; Crnojević-Bengin, V. Fabrication and characterization of flexible and miniaturized humidity sensors using screen-printed TiO2 nanoparticles as sensitive layer. Sensors 2017, 17, 1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulliani, J.M.; Inserra, B.; Ziegler, D. Carbon-based materials for humidity sensing: A short review. Micromachines 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chethan, B.; Prakash, H.R.; Ravikiran, Y.T.; Vijayakumari, S.C.; Ramana, C.V.; Thomas, S.; Kim, D. Enhancing humidity sensing performance of polyaniline/water soluble graphene oxide composite. Talanta 2019, 196, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, X.; Li, M.; Fan, Y.; Sun, R. Humidity sensor based on reduced graphene oxide/lignosulfonate composite thin-film. Sens. Actuators B 2018, 255, 1569–1576. [Google Scholar] [CrossRef]
- Lin, W.; Chang, H.; Wu, R. Applied novel sensing material graphene/polypyrrole for humidity sensor. Sens. Actuators B 2013, 181, 326–331. [Google Scholar] [CrossRef]
- Das, M.; Ghosh, S.; Roy, S. Non-covalent functionalization of CNTs with polycarbazole: A chemiresistive humidity sensor with tunable chemo-electric attributes at room temperature. New J. Chem. 2018, 42, 6918–6931. [Google Scholar] [CrossRef]
- Zhang, X.; Maddipatla, D.; Bose, A.K.; Hajian, S.; Narakathu, B.B.; Williams, J.D.; Atashbar, M.Z. Printed carbon nanotubes-based flexible resistive humidity sensor. IEEE Sens. J. 2020, 20, 12592–12601. [Google Scholar] [CrossRef]
- Zhang, X.; Ming, H.; Liu, R.; Han, X.; Kang, Z.; Liu, Y.; Zhang, Y. Highly sensitive humidity sensing properties of carbon quantum dots films. Mater. Res. Bull. 2013, 48, 790–794. [Google Scholar] [CrossRef]
- Epeloa, J.; Repetto, C.E.; Gómez, B.J.; Nachez, L.; Dobry, A. Resistivity humidity sensors based on hydrogenated amorphous carbon films. Mater. Res. Express 2019, 6, 025604. [Google Scholar] [CrossRef]
- Afify, A.S.; Ahmad, S.; Khushnood, R.A.; Jagdale, P.; Tulliani, J.-M. Elaboration and characterization of novel humidity sensor based on micro-carbonized bamboo particles. Sens. Actuators B Chem. 2017, 239, 1251–1256. [Google Scholar] [CrossRef]
- Llobet, E.; Barberà-Brunet, R.; Etrillard, C.; Létard, J.F.; Debéda, H. Humidity sensing properties of screen-printed carbon-black an Fe(II) spin crossover compound hybrid films. Procedia Eng. 2014, 87, 132–135. [Google Scholar] [CrossRef] [Green Version]
- Selvam, K.P.; Nakagawa, T.; Marui, T.; Inoue, H.; Nishikawa, T.; Hayashi, Y. Synthesis of solvent-free conductive and flexible cellulose-carbon nanohorn sheets and their application as a water vapor sensor. Mater. Res. Express 2020, 7, 056402. [Google Scholar] [CrossRef] [Green Version]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Nicolescu, C.M. Oxidized carbon nanohorns as novel sensing layer for resistive humidity sensor. Acta Chim. Slovenica 2020, 67, 1–7. [Google Scholar]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Nicolescu, C.M. Oxidized carbon nanohorn-hydrophilic polymer nanocomposite as the resistive sensing layer for relative humidity. Anal. Lett. 2021, 54, 527–540. [Google Scholar] [CrossRef]
- Serban, B.C.; Cobianu, C.; Dumbravescu, N.; Buiu, O.; Avramescu, V.; Bumbac, M.; Brezeanu, M. Electrical Percolation Threshold In Oxidized Single Wall Carbon Nanohorn-Polyvinylpyrrolidone Nanocomposite: A Possible Application for High Sensitivity Resistive Humidity Sensor. In Proceedings of the International Semiconductor Conference (CAS), Sinaia, Romania, 7–9 October 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 239–242. [Google Scholar]
- Serban, B.C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Nicolescu, C.M.; Brezeanu, M.; Pachiu, C.; Craciun, G.; et al. Ternary nanocomposites based on oxidized carbon nanohorns as sensing layers for room temperature resistive humidity sensing. Materials 2021, 14, 2705. [Google Scholar] [CrossRef]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Avramescu, V.; Dumbrăvescu, N.; Brezeanu, M.; Marinescu, R. Ternary carbon-based nanocomposite as sensing layer for resistive humidity sensor. Multidiscip. Digit. Publ. Inst. Proc. 2019, 29, 114. [Google Scholar] [CrossRef] [Green Version]
- Șerban, B.C.; Cobianu, C.; Buiu, O.; Dumbrăvescu, N.; Avramescu, V.; Brezeanu, M.; Marinescu, M.R. Ternary oxidized carbon nanohorn-based nanohybrid as sensing layer for resistive humidity sensor. In Proceedings of the 3rd International Conference on Emerging Technologies in Materials Engineering, Bucharest, Romania, 29–30 October 2019; p. 83. [Google Scholar]
- Șerban, B.C.; Cobianu, C.; Buiu, O.; Dumbrăvescu, N.; Avramescu, V.; Brezeanu, M.; Marinescu, M.R. Ternary hydrophilic carbon nanohorn/ZnO/PVP nanohybrid structure for room temperature resistive humidity sensing. In Proceedings of the 3rd International Conference on Emerging Technologies in Materials Engineering, Bucharest, Romania, 29–30 October 2019; p. 84. [Google Scholar]
- Serban, B.C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Nicolescu, C.M.; Brezeanu, M.; Radulescu, C.; Craciun, G.; et al. Quaternary oxidized carbon nanohorns-based nanohybrid as sensing coating for room temperature resistive humidity monitoring. Coatings 2021, 11, 530. [Google Scholar] [CrossRef]
- Zhu, Z.; Lin, W. Evaluation humidity sensing properties of graphene/HS-TiO2 nanocomposites. J. Nanosci. Nanotechnol. 2021, 21, 5143–5149. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, X.; Wang, J.; Gaskov, A.M.; Akbar, S.A. Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens. Actuators B 2016, 230, 330–336. [Google Scholar] [CrossRef]
- Luo, L.; Yuan, M.; Sun, H.; Peng, T.; Xie, T.; Chen, Q.; Chen, J. Effect of calcination temperature on the humidity sensitivity of TiO2/graphene oxide nanocomposites. Mater. Sci. Semicond. Process. 2019, 89, 186–193. [Google Scholar] [CrossRef]
- Ghadiry, M.; Gholami, M.; Lai, C.; Ahmad, H.; Chong, W. Ultra-sensitive humidity sensor based on optical properties of graphene oxide and nano-anatase TiO2. PLoS ONE 2016, 11, e0153949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef] [PubMed]
- Serban, B.C.; Bumbac, M.; Buiu, O.; Cobianu, C.; Brezeanu, M.; Nicolescu, C. Carbon nanohorns and their nanocomposites: Synthesis, properties and applications. A concise review. Ann. Acad. Rom. Sci. Ser. Math. Appl. 2018, 11, 5–18. [Google Scholar]
- Cobianu, C.; Serban, B.C.; Dumbravescu, N.; Buiu, O.; Avramescu, V.; Pachiu, C.; Cobianu, C. Organic-inorganic ternary nanohybrids of single-walled carbon nanohorns for room temperature chemiresistive ethanol detection. Nanomaterials 2020, 10, 2552. [Google Scholar] [CrossRef]
- Cobianu, C.; Serban, B.C.; Dumbravescu, N.; Buiu, O.; Avramescu, V.; Bumbac, M.; Cobianu, C. Room temperature chemiresistive ethanol detection by ternary nanocomposites of oxidized single wall carbon nanohorn (ox-SWCNH). In 2020 International Semiconductor Conference (CAS), Sinaia, Romania, 7–9 October 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 13–16. [Google Scholar]
- Murata, K.; Hirahara, K.; Yudasaka, M.; Iijima, S.; Kasuya, D.; Kaneko, K. Nanowindow-induced molecular sieving effect in a single-wall carbon nanohorn. J. Phys. Chem. B. 2002, 106, 12668–12669. [Google Scholar] [CrossRef]
- Tanigaki, N.; Murata, K.; Hayashi, T.; Kaneko, K. Mild oxidation-production of subnanometer-sized nanowindows of single wall carbon nanohorn. J. Colloid Interface Sci. 2018, 529, 332–336. [Google Scholar] [CrossRef]
- Marinescu, M.R.; Serban, B.C.; Cobianu, C.; Dumbravescu, N.; Ionescu, O.; Buiu, O.; Ghiculescu, L.D. Mechanical properties of carbon-based nanocomposites for sensors used in biomedical applications. UPB Bull. B Chem. Mater. Sci. Ser. B 2021, 83, 31–42. [Google Scholar]
- Ding, M.; Sorescu, D.C.; Star, A. Photoinduced charge transfer and acetone sensitivity of single-walled carbon nanotube-titanium dioxide hybrids. J. Am. Chem. Soc. 2013, 135, 9015–9022. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Choi, H.C.; Jung, Y.M.; Kim, S.B. Size effects in the raman spectra of TiO2 nanoparticles. Vib. Spectrosc. 2005, 37, 33–38. [Google Scholar] [CrossRef]
- Mathpal, M.C.; Tripathi, A.K.; Singh, M.K.; Gairola, S.P.; Pandey, S.N.; Agarwal, A. Effect of annealing temperature on raman spectra of TiO2 nanoparticles. Chem. Phys. Lett. 2013, 555, 182–186. [Google Scholar] [CrossRef]
- Tan, X.; Wu, X.; Hu, Z.; Ma, D.; Shi, Z. Synthesis and catalytic activity of palladium supported on heteroatom doped single-wall carbon nanohorns. RSC Adv. 2017, 7, 29985–29991. [Google Scholar] [CrossRef] [Green Version]
- De Luca, A.; Santra, S.; Ghosh, R.; Ali, S.Z.; Gardner, J.W.; Guha, P.K.; Udrea, F. Temperature-modulated graphene oxide resistive humidity sensor for indoor air quality monitoring. Nanoscale 2016, 8, 4565–4572. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B. Humidity-sensing properties of chemically reduced graphene oxide/polymer nanocomposite film sensor on layer-by-layer nano self-assembly. Sens. Actuators B Chem. 2014, 197, 66–72. [Google Scholar] [CrossRef]
- Sun, L.; Haidry, A.A.; Fatima, Q.; Li, Z.; Yao, Z. Improving the humidity sensing below 30% RH of TiO2 with GO modification. Mater. Res. Bull. 2018, 99, 124–131. [Google Scholar] [CrossRef]
- Lin, W.; Liao, C.; Chang, T.; Chen, S.; Wu, R. Humidity sensing properties of novel graphene/TiO2 composites by sol-gel process. Sens. Actuators B 2015, 209, 555–561. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581. [Google Scholar] [CrossRef]
- Dai, H.; Feng, N.; Li, J.; Zhang, J.; Li, W. Chemiresistive humidity sensor based on chitosan/zinc oxide/single-walled carbon nanotube composite film. Sens. Actuators B 2019, 283, 786–792. [Google Scholar] [CrossRef]
- Nasab, S.S.; Zahmatkesh, S. Preparation, structural characterization, and gas separation properties of functionalized zinc oxide particle filled poly(ether-amide) nanocomposite films. J. Plast. Film Sheeting 2017, 33, 92–113. [Google Scholar] [CrossRef]
- Vural, S.; Koytepe, S.; Seckin, T.; Adiguzel, I. Synthesis, characterization and dielectric properties of rodlike zinc oxide-polyimide nanocomposites. Polym.-Plast. Technol. Eng. 2012, 51, 369–376. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part II: Underlying theories. J. Chem. Educ. 1968, 45, 643. [Google Scholar] [CrossRef]
- Serban, B.C.; Cobianu, C.; Dumbravescu, N.; Buiu, O.; Bumbac, M.; Nicolescu, C.M.; Serbanescu, M. Electrical percolation threshold and size effects in polyvinylpyrrolidone-oxidized single-wall carbon nanohorn nanocomposite: The impact for relative humidity resistive sensors design. Sensors 2021, 21, 1435. [Google Scholar] [CrossRef]
- Yildiz, A.; Lisesivdin, S.B.; Kasap, M.; Mardare, D. Electrical properties of TiO2 thin films. J. Non-Cryst. Solids 2008, 354, 4944–4947. [Google Scholar] [CrossRef]








| Sensing Layer | Sensitivity |
|---|---|
| CNHox [27] | 0.013–0.021 |
| CNHox-PVP 1/1 [28] | 0.020–0.058 |
| CNHox-PVP 2/1 [28] | 0.017–0.025 |
| CNHox-TiO2-PVP 1/1/1 * | 0.150–0.300 |
| CNHox-TiO2-PVP 2/1/1 | 0.010–0.040 |
| CNHox-TiO2-PVP 3/1/1 | 0.010–0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serban, B.-C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Brezeanu, M.; Radulescu, C.; Craciun, G.; Nicolescu, C.M.; Romanitan, C.; et al. Ternary Holey Carbon Nanohorns/TiO2/PVP Nanohybrids as Sensing Films for Resistive Humidity Sensors. Coatings 2021, 11, 1065. https://doi.org/10.3390/coatings11091065
Serban B-C, Buiu O, Bumbac M, Dumbravescu N, Avramescu V, Brezeanu M, Radulescu C, Craciun G, Nicolescu CM, Romanitan C, et al. Ternary Holey Carbon Nanohorns/TiO2/PVP Nanohybrids as Sensing Films for Resistive Humidity Sensors. Coatings. 2021; 11(9):1065. https://doi.org/10.3390/coatings11091065
Chicago/Turabian StyleSerban, Bogdan-Catalin, Octavian Buiu, Marius Bumbac, Niculae Dumbravescu, Viorel Avramescu, Mihai Brezeanu, Cristiana Radulescu, Gabriel Craciun, Cristina Mihaela Nicolescu, Cosmin Romanitan, and et al. 2021. "Ternary Holey Carbon Nanohorns/TiO2/PVP Nanohybrids as Sensing Films for Resistive Humidity Sensors" Coatings 11, no. 9: 1065. https://doi.org/10.3390/coatings11091065
APA StyleSerban, B.-C., Buiu, O., Bumbac, M., Dumbravescu, N., Avramescu, V., Brezeanu, M., Radulescu, C., Craciun, G., Nicolescu, C. M., Romanitan, C., & Comanescu, F. (2021). Ternary Holey Carbon Nanohorns/TiO2/PVP Nanohybrids as Sensing Films for Resistive Humidity Sensors. Coatings, 11(9), 1065. https://doi.org/10.3390/coatings11091065










