Carbon Dot/Polymer Composites with Various Precursors and Their Sensing Applications: A Review
Abstract
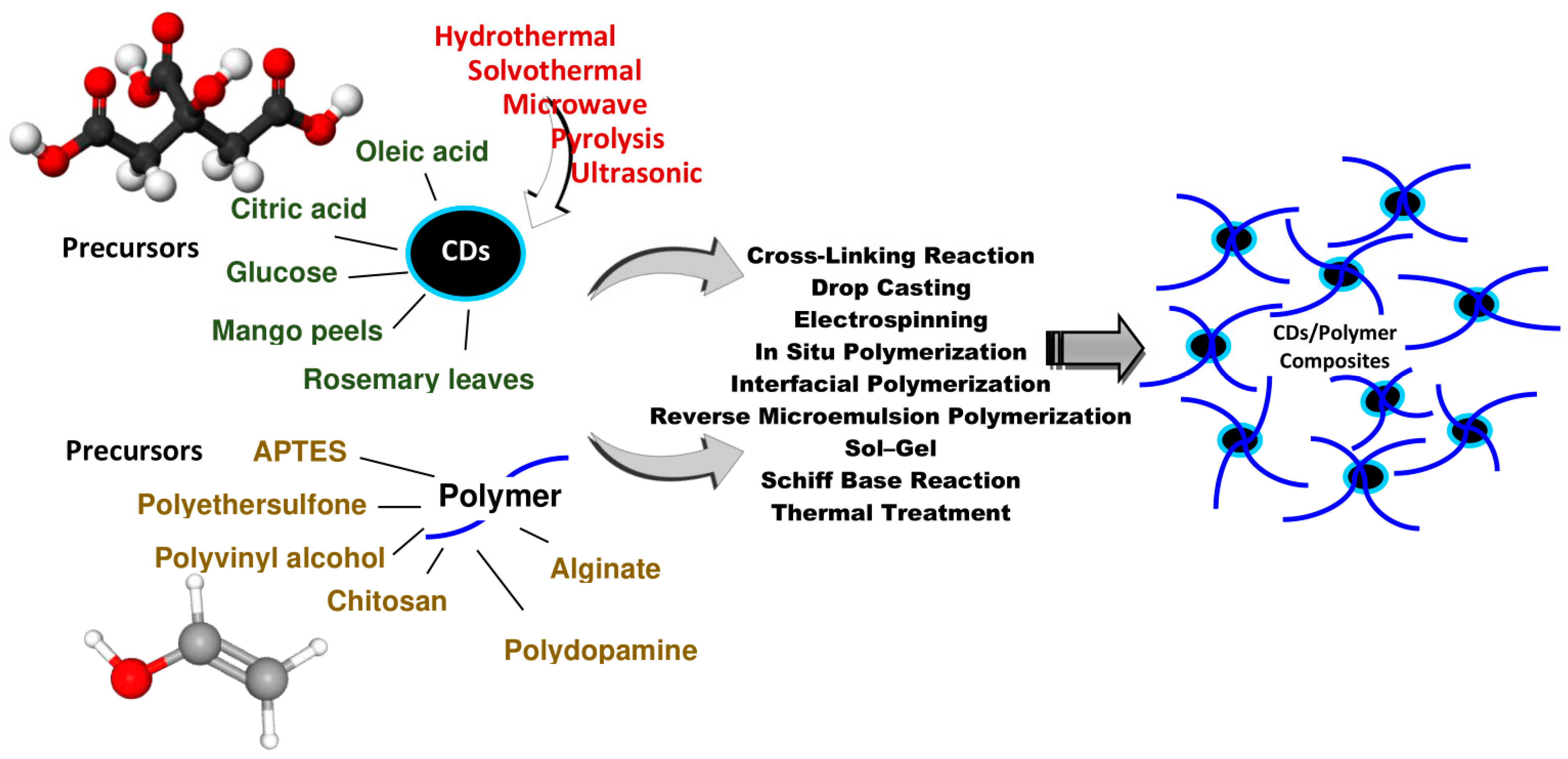
:1. Introduction
2. Precursors for CD/Polymer Composites
3. Synthesis Methods for CD/Polymer Composites
3.1. Hydrothermal Treatment
3.2. Solvothermal Treatment
3.3. Microwave Treatment
3.4. Pyrolysis/Thermal Decomposition
3.5. Ultrasonic Treatment
4. Optical Properties of CD/Polymer Composites
5. Coating Characteristics of CD/Polymer Composites
6. Sensing Applications of CD/Polymer Composites
6.1. Chemical Sensors
6.2. Biological Sensors
6.3. Physical Sensors
7. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Semeniuk, M.; Yi, Z.; Poursorkhabi, V.; Tjong, J.; Jaffer, S.; Lu, Z.H.; Sain, M. Future Perspectives and Review on Organic Carbon Dots in Electronic Applications. ACS Nano 2019, 13, 6224–6255. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Jia, X.; Li, J.; Wang, E. One-pot green synthesis of optically pH-sensitive carbon dots with upconversion luminescence. Nanoscale 2012, 4, 5572–5575. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, C.; Wu, G.; Chen, S. The Rapid and Large-Scale Production of Carbon Quantum Dots and their Integration with Polymers. Angew. Chem. 2021, 133, 8668–8678. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization. Adv. Mater. 2017, 30, 1704740. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Banerjee, S.; Das, N.C. Advancement in science and technology of carbon dot-polymer hybrid composites: A review. Funct. Compos. Struct. 2019, 1, 022001. [Google Scholar] [CrossRef]
- Kausar, A. Polymer/carbon-based quantum dot nanocomposite: Forthcoming materials for technical application. J. Macromol. Sci. Part. A Pure Appl. Chem. 2019, 56, 341–356. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Yu, S.; Jiang, C. Fluorescent carbon dots: Rational synthesis, tunable optical properties and analytical applications. RSC Adv. 2017, 7, 40973–40989. [Google Scholar] [CrossRef] [Green Version]
- Meng, W.; Bai, X.; Wang, B.; Liu, Z.; Lu, S.; Yang, B. Biomass-Derived Carbon Dots and Their Applications. Energy Environ. Mater. 2019, 2, 172–192. [Google Scholar] [CrossRef]
- Zulfajri, M.; Abdelhamid, H.N.; Sudewi, S.; Dayalan, S.; Rasool, A.; Habib, A.; Huang, G.G. Plant Part-Derived Carbon Dots for Biosensing. Biosensors 2020, 10, 68. [Google Scholar] [CrossRef]
- Feng, H.; Qian, Z. Functional Carbon Quantum Dots: A Versatile Platform for Chemosensing and Biosensing. Chem. Rec. 2018, 18, 491–505. [Google Scholar] [CrossRef]
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm. 2019, 564, 308–317. [Google Scholar] [CrossRef]
- Stepanidenko, E.A.; Ushakova, E.V.; Fedorov, A.V.; Rogach, A.L. Applications of carbon dots in optoelectronics. Nanomaterials 2021, 11, 364. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Rasal, A.S.; Yadav, S.; Yadav, A.; Kashale, A.A.; Manjunatha, S.T.; Altaee, A.; Chang, J.-Y. Carbon Quantum Dots for Energy Applications: A Review. ACS Appl. Nano Mater. 2021. [Google Scholar] [CrossRef]
- Zhao, B.; Tan, Z. Fluorescent Carbon Dots: Fantastic Electroluminescent Materials for Light-Emitting Diodes. Adv. Sci. 2021, 8, 2001977. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural carbon-based quantum dots and their applications in drug delivery: A review. Biomed. Pharmacother. 2020, 132, 110834. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhao, Z.; Liang, K.; Nan, F.; Li, Y.; Wang, J.; Ge, J.; Wang, P. Recent advances and prospects of carbon dots in cancer nanotheranostics. Mater. Chem. Front. 2020, 4, 449–471. [Google Scholar] [CrossRef]
- Zulfajri, M.; Kao, Y.T.; Huang, G.G. Retrieve of residual waste of carbon dots derived from straw mushroom as a hydrochar for the removal of organic dyes from aqueous solutions. Sustain. Chem. Pharm. 2021, 22, 100469. [Google Scholar] [CrossRef]
- Zulfajri, M.; Rasool, A.; Huang, G.G. A fluorescent sensor based on oyster mushroom-carbon dots for sensing nitroarenes in aqueous solutions. New J. Chem. 2020, 44, 10525–10535. [Google Scholar] [CrossRef]
- Zulfajri, M.; Liu, K.C.; Pu, Y.H.; Rasool, A.; Dayalan, S.; Huang, G.G. Utilization of carbon dots derived from Volvariella volvacea mushroom for a highly sensitive detection of Fe3+ and Pb2+ ions in aqueous solutions. Chemosensors 2020, 8, 47. [Google Scholar] [CrossRef]
- Polatoğlu, B.; Bozkurt, E. Green synthesis of fluorescent carbon dots from Kumquat (Fortunella margarita) for detection of Fe3+ ions in aqueous solution. Res. Chem. Intermed. 2021, 47, 1865–1881. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Sundramoorthy, A.K.; Babu, R.S.; Lee, Y.R. Leftover Kiwi Fruit Peel-Derived Carbon Dots as a Highly Selective Fluorescent Sensor for Detection of Ferric Ion. Chemosensors 2021, 9, 166. [Google Scholar] [CrossRef]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Luong, J.H.T.; Gedanken, A. Green synthesis of multifunctional carbon dots with antibacterial activities. Nanomaterials 2021, 11, 369. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Y.; Li, S.; Lu, B.; Liu, C.; Yang, H.; Ren, X.; Hou, Y. Hydrothermal growth of nitrogen-rich carbon dots as a precise multifunctional probe for both Fe3+ detection and cellular bio-imaging. Opt. Mater. 2019, 89, 92–99. [Google Scholar] [CrossRef]
- Lesani, P.; Ardekani, S.M.; Dehghani, A.; Hassan, M.; Gomes, V.G. Excitation-independent carbon dot probes for exogenous and endogenous Fe3+ sensing in living cells: Fluorescence lifetime and sensing mechanism. Sens. Actuators B Chem. 2019, 285, 145–155. [Google Scholar] [CrossRef]
- Chen, X.; Bai, J.; Ma, Y.; Yuan, G.; Mei, J.; Zhang, L.; Ren, L. Multifunctional sensing applications of biocompatible N-doped carbon dots as pH and Fe3+ sensors. Microchem. J. 2019, 149, 103981. [Google Scholar] [CrossRef]
- Feng, Z.; Adolfsson, K.H.; Xu, Y.; Fang, H.; Hakkarainen, M.; Wu, M. Carbon dot/polymer nanocomposites: From green synthesis to energy, environmental and biomedical applications. Sustain. Mater. Technol. 2021, 29, e00304. [Google Scholar]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, R.; Liu, Y.; Huang, H.; Yu, H.; Ming, H.; Lian, S.; Lee, S.T.; Kang, Z. Carbon quantum dots/Cu2O composites with protruding nanostructures and their highly efficient (near) infrared photocatalytic behavior. J. Mater. Chem. 2012, 22, 17470–17475. [Google Scholar] [CrossRef]
- Bui, T.T.; Park, S.Y. A carbon dot-hemoglobin complex-based biosensor for cholesterol detection. Green Chem. 2016, 18, 4245–4253. [Google Scholar] [CrossRef]
- Kausar, A. Advances in Polymer/Fullerene Nanocomposite: A Review on Essential Features and Applications. Polym. Plast. Technol. Eng. 2017, 56, 594–605. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. Review of the mechanical properties of carbon nanofiber/polymer composites. Compos. Part. A Appl. Sci. Manuf. 2011, 42, 2126–2142. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube-polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Pinto, A.M.; Magalhães, F.D. Graphene-Polymer Composites. Polymers 2021, 13, 685. [Google Scholar] [CrossRef]
- Zhou, Y.; Sharma, S.K.; Peng, Z.; Leblanc, R.M. Polymers in carbon dots: A review. Polymers 2017, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- De, B. Carbon Dots and Their Polymeric Nanocomposites. In Nanomaterials and Polymer Nanocomposites: Raw Materials to Applications; Karak, N., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 217–260. ISBN 9780128146163. [Google Scholar]
- Konwar, A.; Gogoi, N.; Majumdar, G.; Chowdhury, D. Green chitosan-carbon dots nanocomposite hydrogel film with superior properties. Carbohydr. Polym. 2015, 115, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zhang, L.; Lu, J.; Xu, C.; Cai, C.; Lin, H. Triple-Mode Emission of Carbon Dots: Applications for Advanced Anti-Counterfeiting. Angew. Chem. Int. Ed. 2016, 55, 7231–7235. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Y.; Jiang, Y.; Miao, M.; Cao, S.; Fang, J. Use of carbon dots to enhance UV-blocking of transparent nanocellulose films. Carbohydr. Polym. 2017, 161, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Zhong, Z.F.; Rong, M.Z.; Zhou, X.; Chen, X.D.; Zhang, M.Q. An easy approach of preparing strongly luminescent carbon dots and their polymer based composites for enhancing solar cell efficiency. Carbon N. Y. 2014, 70, 190–198. [Google Scholar] [CrossRef]
- Permatasari, F.A.; Irham, M.A.; Bisri, S.Z.; Iskandar, F. Carbon-based quantum dots for supercapacitors: Recent advances and future challenges. Nanomaterials 2021, 11, 91. [Google Scholar] [CrossRef]
- Hao, Y.; Gan, Z.; Xu, J.; Wu, X.; Chu, P.K. Poly(ethylene glycol)/carbon quantum dot composite solid films exhibiting intense and tunable blue-red emission. Appl. Surf. Sci. 2014, 311, 490–497. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.L.; Huang, C.Z. Carbon dot-based composites for catalytic applications. Green Chem. 2020, 22, 4034–4054. [Google Scholar] [CrossRef]
- Shauloff, N.; Bhattacharya, S.; Jelinek, R. Elastic carbon dot/polymer films for fluorescent tensile sensing and mechano-optical tuning. Carbon N. Y. 2019, 152, 363–371. [Google Scholar] [CrossRef]
- Sui, B.; Li, Y.; Yang, B. Nanocomposite hydrogels based on carbon dots and polymers. Chin. Chem. Lett. 2020, 31, 1443–1447. [Google Scholar] [CrossRef]
- Jani, M.; Arcos-Pareja, J.A.; Ni, M. Engineered Zero-Dimensional Fullerene/Carbon Dots-Polymer Based Nanocomposite Membranes for Wastewater Treatment. Molecules 2020, 25, 4934. [Google Scholar] [CrossRef] [PubMed]
- Kováčová, M.; Špitalská, E.; Markovic, Z.; Špitálský, Z. Carbon Quantum Dots As Antibacterial Photosensitizers and Their Polymer Nanocomposite Applications. Part. Part. Syst. Charact. 2020, 37, 1–11. [Google Scholar] [CrossRef]
- Zulfajri, M.; Dayalan, S.; Li, W.Y.; Chang, C.J.; Chang, Y.P.; Huang, G.G. Nitrogen-doped carbon dots from averrhoa carambola fruit extract as a fluorescent probe for methyl orange. Sensors 2019, 19, 5008. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Zhang, Y.; Wang, P.; Yang, Y.; Wang, Y.; Xu, J.; Wang, Y.; Yu, W.W. Synthesis of Nitrogen and Sulfur Co-doped Carbon Dots from Garlic for Selective Detection of Fe3+. Nanoscale Res. Lett. 2016, 11, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putro, P.A.; Yudasari, N.; Isnaeni; Maddu, A. Spectroscopy study of Polyvinyl alcohol/carbon dots composite films. Walailak J. Sci. Technol. 2021, 18, 9184. [Google Scholar] [CrossRef]
- Carvalho, J.; Santos, L.R.; Germino, J.C.; Terezo, A.J.; Moreto, J.A.; Quites, F.J.; Freitas, R.G. Hydrothermal Synthesis to Water-stable Luminescent Carbon Dots from Acerola Fruit for Photoluminescent Composites Preparation and its Application as Sensors. Mater. Res. 2019, 22, e20180920. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Liu, Y.; Niu, N.; Chen, L. Synthesis of molecularly imprinted fluorescent probe based on biomass-derived carbon quantum dots for detection of mesotrione. Anal. Bioanal. Chem. 2019, 411, 5519–5530. [Google Scholar] [CrossRef]
- Shariati, R.; Rezaei, B.; Jamei, H.R.; Ensafi, A.A. Application of coated green source carbon dots with silica molecularly imprinted polymers as a fluorescence probe for selective and sensitive determination of phenobarbital. Talanta 2019, 194, 143–149. [Google Scholar] [CrossRef]
- Kazemifard, N.; Ensafi, A.A.; Rezaei, B. Green synthesized carbon dots embedded in silica molecularly imprinted polymers, characterization and application as a rapid and selective fluorimetric sensor for determination of thiabendazole in juices. Food Chem. 2020, 310, 125812. [Google Scholar] [CrossRef]
- Safaei, B.; Youssefi, M.; Rezaei, B.; Irannejad, N. Synthesis and Properties of Photoluminescent Carbon Quantum Dot/Polyacrylonitrile Composite Nanofibers. Smart Sci. 2018, 6, 117–124. [Google Scholar] [CrossRef]
- Demir, B.; Lemberger, M.M.; Panagiotopoulou, M.; Medina Rangel, P.X.; Timur, S.; Hirsch, T.; Tse Sum Bui, B.; Wegener, J.; Haupt, K. Tracking Hyaluronan: Molecularly Imprinted Polymer Coated Carbon Dots for Cancer Cell Targeting and Imaging. ACS Appl. Mater. Interfaces 2018, 10, 3305–3313. [Google Scholar] [CrossRef]
- Wang, M.; Gao, M.; Deng, L.; Kang, X.; Yang, L.; Quan, T.; Xia, Z.; Gao, D. Composite Material Based on Carbon Dots and Molecularly Imprinted Polymers: A Facile Probe for Fluorescent Detection of 4-Nitrophenol. Nano 2020, 15, 2050105. [Google Scholar] [CrossRef]
- Jalili, R.; Amjadi, M. Bio-inspired molecularly imprinted polymer–green emitting carbon dot composite for selective and sensitive detection of 3-nitrotyrosine as a biomarker. Sens. Actuators B Chem. 2018, 255, 1072–1078. [Google Scholar] [CrossRef]
- Rimal, V.; Shishodia, S.; Srivastava, P.K. Novel synthesis of high-thermal stability carbon dots and nanocomposites from oleic acid as an organic substrate. Appl. Nanosci. 2020, 10, 455–464. [Google Scholar] [CrossRef]
- Fernandes, D.; Heslop, K.A.; Kelarakis, A.; Krysmann, M.J.; Estevez, L. In situ generation of carbon dots within a polymer matrix. Polymer 2020, 188, 122159. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Rasaki, S.A.; Thomas, T.; Wang, C.; Zhang, C.; Yang, M. Yellow-emitting carbon-dots-impregnated carboxy methyl cellulose/poly-vinyl-alcohol and chitosan: Stable, freestanding, enhanced-quenching Cu2+-ions sensor. J. Mater. Chem. C 2018, 6, 4508–4515. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.Z.; Weng, Y.; Tan, H. Pyrophosphate ion-responsive alginate hydrogel as an effective fluorescent sensing platform for alkaline phosphatase detection. Chem. Commun. 2019, 55, 11450–11453. [Google Scholar] [CrossRef]
- Xu, X.; Xu, G.; Wei, F.; Cen, Y.; Shi, M.; Cheng, X.; Chai, Y.; Sohail, M.; Hu, Q. Carbon dots coated with molecularly imprinted polymers: A facile bioprobe for fluorescent determination of caffeic acid. J. Colloid Interface Sci. 2018, 529, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Koulivand, H.; Shahbazi, A.; Vatanpour, V.; Rahmandoust, M. Development of carbon dot-modified polyethersulfone membranes for enhancement of nanofiltration, permeation and antifouling performance. Sep. Purif. Technol. 2020, 230, 115895. [Google Scholar] [CrossRef]
- Yuan, Z.; Wu, X.; Jiang, Y.; Li, Y.; Huang, J.; Hao, L.; Zhang, J.; Wang, J. Carbon dots-incorporated composite membrane towards enhanced organic solvent nano filtration performance. J. Memb. Sci. 2018, 549, 1–11. [Google Scholar] [CrossRef]
- Zhao, D.L.; Das, S.; Chung, T.S. Carbon Quantum Dots Grafted Antifouling Membranes for Osmotic Power Generation via Pressure-Retarded Osmosis Process. Environ. Sci. Technol. 2017, 51, 14016–14023. [Google Scholar] [CrossRef]
- Wang, C.; Hu, T.; Chen, Y.; Xu, Y.; Song, Q. Polymer-Assisted Self-Assembly of Multicolor Carbon Dots as Solid-State Phosphors for Fabrication of Warm, High-Quality, and Temperature-Responsive White-Light-Emitting Devices. ACS Appl. Mater. Interfaces 2019, 11, 22332–22338. [Google Scholar] [CrossRef]
- Kumar, V.B.; Sahu, A.K.; Mohsin, A.S.M.; Li, X.; Gedanken, A. Refractive-Index Tuning of Highly Fluorescent Carbon Dots. ACS Appl. Mater. Interfaces 2017, 9, 28930–28938. [Google Scholar] [CrossRef]
- Wang, M.; Gao, M.; Deng, L.; Kang, X.; Zhang, K.; Fu, Q.; Xia, Z.; Gao, D. A sensitive and selective fluorescent sensor for 2,4,6-trinitrophenol detection based on the composite material of magnetic covalent organic frameworks, molecularly imprinted polymers and carbon dots. Microchem. J. 2020, 154, 104590. [Google Scholar] [CrossRef]
- Wang, M.; Fu, Q.; Zhang, K.; Wan, Y.; Wang, L.; Gao, M.; Xia, Z.; Gao, D. A magnetic and carbon dot based molecularly imprinted composite for fluorometric detection of 2,4,6-trinitrophenol. Microchim. Acta 2019, 186, 86. [Google Scholar] [CrossRef]
- Gai, W.; Zhao, D.L.; Chung, T.S. Thin film nanocomposite hollow fiber membranes comprising Na+-functionalized carbon quantum dots for brackish water desalination. Water Res. 2019, 154, 54–61. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, D.L.; Chung, T.S. Na+ functionalized carbon quantum dot incorporated thin-film nanocomposite membranes for selenium and arsenic removal. J. Memb. Sci. 2018, 564, 483–491. [Google Scholar] [CrossRef]
- Gai, W.; Zhao, D.L.; Chung, T.S. Novel thin film composite hollow fiber membranes incorporated with carbon quantum dots for osmotic power generation. J. Memb. Sci. 2018, 551, 94–102. [Google Scholar] [CrossRef]
- Seyedpour, S.F.; Rahimpour, A.; Shamsabadi, A.A.; Soroush, M. Improved performance and antifouling properties of thin-film composite polyamide membranes modified with nano-sized bactericidal graphene quantum dots for forward osmosis. Chem. Eng. Res. Des. 2018, 139, 321–334. [Google Scholar] [CrossRef]
- Xu, S.; Li, F.; Su, B.; Hu, M.Z.; Gao, X.; Gao, C. Novel graphene quantum dots (GQDs)-incorporated thin film composite (TFC) membranes for forward osmosis (FO) desalination. Desalination 2019, 451, 219–230. [Google Scholar] [CrossRef]
- Sun, H.; Wu, P. Tuning the functional groups of carbon quantum dots in thin film nanocomposite membranes for nanofiltration. J. Memb. Sci. 2018, 564, 394–403. [Google Scholar] [CrossRef]
- Dai, J.; Dong, X.; Cortalezzi, M.F. De Molecularly imprinted polymers labeled with amino-functionalized carbon dots for fluorescent determination of 2,4-dinitrotoluene. Microchim. Acta 2017, 184, 1369–1377. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Song, X.; Su, B.; Mandal, B.; Prasad, B.; Gao, X.; Gao, C. Graphene Quantum Dots-Doped Thin Film Nanocomposite Polyimide Membranes with Enhanced Solvent Resistance for Solvent-Resistant Nanofiltration. ACS Appl. Mater. Interfaces 2019, 11, 6527–6540. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Kebria, M.R.S.; Rahimpour, A.; Bakeri, G. Graphene quantum dots modified polyvinylidenefluride (PVDF) nanofibrous membranes with enhanced performance for air Gap membrane distillation. Chem. Eng. Process. Process. Intensif. 2018, 126, 222–231. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, Q.; Zhang, R.; Su, Y.; Jiang, Z. Thin film nanocomposite membranes incorporated with graphene quantum dots for high flux and antifouling property. J. Memb. Sci. 2018, 553, 17–24. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, R.; Shen, J.; Liu, Y.N.; He, M.; You, X.; Su, Y.; Jiang, Z. Graphene quantum dots engineered nanofiltration membrane for ultrafast molecular separation. J. Memb. Sci. 2019, 572, 504–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Ma, X.T.; He, X.W.; Li, W.Y.; Zhang, Y.K. Carbon dots-embedded epitope imprinted polymer for targeted fluorescence imaging of cervical cancer via recognition of epidermal growth factor receptor. Microchim. Acta 2020, 187, 187–228. [Google Scholar] [CrossRef]
- Hu, T.; Wen, Z.; Thomas, T.; Wang, C.; Song, Q.; Yang, M.; Wang, C. Temperature-controlled spectral tuning of full-color carbon dots and their strongly fluorescent solid-state polymer composites for light-emitting diodes. Nanoscale Adv. 2019, 1, 1413–1420. [Google Scholar] [CrossRef] [Green Version]
- Devadas, B.; Imae, T. Effect of Carbon Dots on Conducting Polymers for Energy Storage Applications. ACS Sustain. Chem. Eng. 2018, 6, 127–134. [Google Scholar] [CrossRef]
- Tian, Z.; Li, D.; Ushakova, E.V.; Maslov, V.G.; Zhou, D.; Jing, P.; Shen, D.; Qu, S.; Rogach, A.L. Multilevel Data Encryption Using Thermal-Treatment Controlled Room Temperature Phosphorescence of Carbon Dot/Polyvinylalcohol Composites. Adv. Sci. 2018, 5, 1800795. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhao, L.; Xu, Y.; Zhou, T.; Liu, H.; Huang, N.; Ding, J.; Li, Y.; Ding, L. Single-hole hollow molecularly imprinted polymer embedded carbon dot for fast detection of tetracycline in honey. Talanta 2018, 185, 542–549. [Google Scholar] [CrossRef]
- Lei, S.; Zeng, M.; Huang, D.; Wang, L.; Zhang, L.; Xi, B.; Ma, W.; Chen, G.; Cheng, Z. Synergistic high-flux oil-saltwater separation and membrane desalination with carbon quantum dots functionalized membrane. ACS Sustain. Chem. Eng. 2019, 7, 13708–13716. [Google Scholar] [CrossRef]
- Li, X.; Hu, S.; Lin, Z.; Yi, J.; Liu, X.; Tang, X.; Wu, Q.; Zhang, G. Dual-responsive mesoporous silica nanoparticles coated with carbon dots and polymers for drug encapsulation and delivery. Nanomedicine 2020, 15, 2447–2458. [Google Scholar] [CrossRef]
- Kurt, S.B.; Sahiner, N. Chitosan based fibers embedding carbon dots with anti-bacterial and fluorescent properties. Polym. Compos. 2021, 42, 872–880. [Google Scholar] [CrossRef]
- Bai, J.; Ren, W.; Wang, Y.; Li, X.; Zhang, C.; Li, Z.; Xie, Z. High-performance thermoplastic polyurethane elastomer/carbon dots bulk nanocomposites with strong luminescence. High. Perform. Polym. 2020, 32, 857–867. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhang, K. Influence of hydrophilic carbon dots on polyamide thin film nanocomposite reverse osmosis membranes. J. Memb. Sci. 2017, 537, 42–53. [Google Scholar] [CrossRef]
- Shao, D.-D.; Yang, W.-J.; Xiao, H.-F.; Wang, Z.-Y.; Zhou, C.; Cao, X.-L.; Sun, S.-P. Self-Cleaning Nanofiltration Membranes by Coordinated Regulation of Carbon Quantum Dots and Polydopamine. ACS Appl. Mater. Interfaces 2020, 12, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yi, J.; Yu, Y.; Zhou, S. NIR upconversion fluorescence glucose sensing and glucose-responsive insulin release of carbon dot-immobilized hybrid microgels at physiological pH. Nanoscale 2017, 9, 509–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Shao, D.; Zhou, Z.; Xia, Q.; Chen, J. Carbon quantum dots (CQDs) nano filtration membranes towards efficient biogas slurry valorization. Chem. Eng. J. 2020, 385, 123993. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, A.; Gao, S.; Yan, D.; Guo, W.; Xu, Y.; Meng, Y.; Wang, C.; Shan, G. Enhancing photoluminescence of carbon quantum dots doped PVA films with randomly dispersed silica microspheres. Sci. Rep. 2020, 10, 5710. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z. Sustainable development of enhanced luminescence polymer-carbon dots composite film for rapid Cd2+ removal from wastewater. Molecules 2020, 25, 3541. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Phatake, R.S.; Barnea, S.N.; Zerby, N.; Zhu, J.; Shikler, R.; Lemcoff, N.G.; Jelinek, R. Fluorescent Self-Healing Carbon Dot/Polymer Gels. ACS Nano 2019, 13, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ding, G.; Wang, H.; Cui, G.; Zhang, P. One-step hydrothermal synthesis of carbon dots-polymer composites with solid-state photoluminescence. Mater. Lett. 2019, 238, 22–25. [Google Scholar] [CrossRef]
- Ma, S.; Zheng, H.; Chen, Y.; Zou, J.; Zhang, C.; Wang, Y. Nanocomposite Polymer Hydrogels Reinforced by Carbon Dots and Hectorite Clay. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 287–292. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Su, B.; Hu, M.Z.; Gao, X.; Gao, C. Amino-functionalized graphene quantum dots (aGQDs)-embedded thin film nanocomposites for solvent resistant nano filtration (SRNF) membranes based on covalence interactions. J. Memb. Sci. 2019, 588, 117212. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.Q.; Tang, Z.R.; Xu, Y.J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Salmeron, J.F.; Murru, F.; Lapresta-Fernández, A.; Rodríguez, N.; Capitan-Vallvey, L.F.; Morales, D.P.; Salinas-Castillo, A. Carbon dots as sensing layer for printed humidity and temperature sensors. Nanomaterials 2020, 10, 2446. [Google Scholar] [CrossRef]
- Zulfajri, M.; Gedda, G.; Chang, C.J.; Chang, Y.P.; Huang, G.G. Cranberry Beans Derived Carbon Dots as a Potential Fluorescence Sensor for Selective Detection of Fe3+ Ions in Aqueous Solution. ACS Omega 2019, 4, 15382–15392. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Wei, J.; Qiang, L.; Chen, X.; Meng, X. Fluorescent carbon dots for bioimaging and biosensing applications. J. Biomed. Nanotechnol. 2014, 10, 2677–2699. [Google Scholar] [CrossRef]
- Chan, K.K.; Yap, S.H.K.; Yong, K.T. Biogreen Synthesis of Carbon Dots for Biotechnology and Nanomedicine Applications. Nano-Micro Lett. 2018, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, B.; Gogoi, S.; Thakur, S.; Karak, N. Bio-based waterborne polyurethane/carbon dot nanocomposite as a surface coating material. Prog. Org. Coat. 2016, 90, 324–330. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sarkar, R.; Chakraborty, B.; Porgador, A.; Jelinek, R. Nitric Oxide Sensing through Azo-Dye Formation on Carbon Dots. ACS Sens. 2017, 2, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Farshbaf, M.; Davaran, S.; Rahimi, F.; Annabi, N.; Salehi, R.; Akbarzadeh, A. Carbon quantum dots: Recent progresses on synthesis, surface modification and applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1331–1348. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, M.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Review of Natural-Product-Derived Carbon Dots: From Natural Products to Functional Materials. ChemSusChem 2018, 11, 11–24. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.T.; Kang, Z. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon N. Y. 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason. Sonochem. 2020, 64, 105009. [Google Scholar] [CrossRef]
- Ma, Z.; Ming, H.; Huang, H.; Liu, Y.; Kang, Z. One-step ultrasonic synthesis of fluorescent N-doped carbon dots from glucose and their visible-light sensitive photocatalytic ability. New J. Chem. 2012, 36, 861–864. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Shiral Fernando, K.A.; Lecroy, G.E.; Maimaiti, H.; Harruff-Miller, B.A.; Lewis, W.K.; Bunker, C.E.; Hou, Z.L.; Sun, Y.P. Enhanced fluorescence properties of carbon dots in polymer films. J. Mater. Chem. C 2016, 4, 6967–6974. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, Y.; Zhang, F.; Chen, L.; Yang, Y.; Liu, X. Fluorescent polyvinyl alcohol films based on nitrogen and sulfur co-doped carbon dots towards white light-emitting devices. N J. Chem. 2016, 40, 8710–8716. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Geng, W.; Liu, H. Rapid detection of tryptamine by optosensor with molecularly imprinted polymers based on carbon dots-embedded covalent-organic frameworks. Sens. Actuators B Chem. 2019, 285, 546–552. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, X.; Li, D.; Zhou, D.; Jing, P.; Shen, D.; Qu, S.; Zboril, R.; Rogach, A.L. Full-Color Inorganic Carbon Dot Phosphors for White-Light-Emitting Diodes. Adv. Opt. Mater. 2017, 5, 1700416. [Google Scholar] [CrossRef]
- Zhao, Q.; Song, W.; Zhao, B.; Yang, B. Spectroscopic studies of the optical properties of carbon dots: Recent advances and future prospects. Mater. Chem. Front. 2020, 4, 472–488. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Feng, X.; Müllen, K. Bottom-up fabrication of photoluminescent graphene quantum dots with uniform morphology. J. Am. Chem. Soc. 2011, 133, 15221–15223. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Nie, S. Semiconductor nanocrystals: Structure, properties, and band gap engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in vivo Imaging, and Diagnostics. Science 2016, 307, 538–544. [Google Scholar]
- Yang, F.; Zhao, M.; Zheng, B.; Xiao, D.; Wu, L.; Guo, Y. Influence of pH on the fluorescence properties of graphene quantum dots using ozonation pre-oxide hydrothermal synthesis. J. Mater. Chem. 2012, 22, 25471–25479. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, S.J.; Wang, H.Y.; Qu, S.N.; Zhang, Y.L.; Zhang, J.H.; Chen, Q.D.; Xu, H.L.; Han, W.; Yang, B.; et al. Common origin of green luminescence in carbon nanodots and graphene quantum dots. ACS Nano 2014, 8, 2541–2547. [Google Scholar] [CrossRef]
- Anilkumar, P.; Wang, X.; Cao, L.; Sahu, S.; Liu, J.H.; Wang, P.; Korch, K.; Tackett, K.N.; Parenzan, A.; Sun, Y.P. Toward quantitatively fluorescent carbon-based “quantum” dots. Nanoscale 2011, 3, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, L.; Yang, S.-T.; Lu, F.; Meziani, M.J.; Tian, L.; Sun, K.W.; Bloodgood, M.A.; Sun, Y.-P. Bandgap-Like Strong Fluorescence in Functionalized Carbon Nanoparticles. Angew. Chem. 2010, 49, 5310–5314. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Suzuki, K.; Malfatti, L.; Takahashi, M.; Carboni, D.; Messina, F.; Tokudome, Y.; Takemoto, M.; Innocenzi, P. Design of Carbon Dots Photoluminescence through Organo-Functional Silane Grafting for Solid-State Emitting Devices. Sci. Rep. 2017, 7, 5469. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, D.; Karak, N. Biodegradable tough waterborne hyperbranched polyester/carbon dot nanocomposite: Approach towards an eco-friendly material. Green Chem. 2016, 18, 5200–5211. [Google Scholar] [CrossRef]













| Composite Name | CDs Precursor | Type | Polymer Precursor | Type | Ref. |
|---|---|---|---|---|---|
| CDs@MIPs | Citric acid | S | (3-aminopropyl) triethoxysilane | S | [63] |
| CDs@MIPs | Citric acid | S | (3-aminopropyl) triethoxysilane | S | [69] |
| MCOFs@MIPs@CDs | Citric acid | S | (3-aminopropyl) triethoxysilane | S | [75] |
| CDs/Fe3O4@MIPs | Citric acid | S | (3-aminopropyl) triethoxysilane | S | [76] |
| PES/CDs | Citric acid | S | Polyethersulfone | S | [70] |
| TFN-(Na-CQDs) | Citric acid | S | Polyethersulfone | S | [77] |
| Na-CQD-TFN | Citric acid | S | Polyethersulfone | S | [78] |
| TFC(Na-CQDs) | Citric acid | S | Polyethersulfone | S | [79] |
| GQD-TFN membrane | Citric acid | S | Polyethersulfone | S | [80] |
| PAN/PEI-CDs | Citric acid | S | Polyethyleneimine | S | [71] |
| PEI/GQDs-TFC | Citric acid | S | Polyethyleneimine | S | [81] |
| TFN-CQD membrane | Citric acid | S | Polysulfone | S | [82] |
| AC-dots-DNT-MIPs | Citric acid | S | Methyl acrylate | S | [83] |
| GQDs-TFN SRNF | Citric acid | S | Polyimide | S | [84] |
| TFC-PES-PDA-CQD | Citric acid | S | Polydopamine | S | [72] |
| GQDs/PVDF | Citric acid | S | Polyvinylidene fluoride | S | [85] |
| GQDs/PIP-TMC TFN | Citric acid | S | Piperazine/trymesoyl chloride | S | [86] |
| PES/GQDs-TMC | Citric acid | S | Trymesoyl chloride | S | [87] |
| C-MIP | Citric acid, Urea | S | Acrylamide | S | [88] |
| CD/PVA films | Citric acid, Urea | S | Polyvinyl alcohol | S | [89] |
| WCDs@PS | Citric acid, Urea | S | Polystyrene | S | [73] |
| PPy@Cdots | Citric acid, Urea | S | Pyrrole | S | [90] |
| PANI@Cdots | Citric acid, ED | S | Aniline | S | [90] |
| CDs@PVA | Citric acid, NH3 | S | Polyvinyl alcohol | S | [91] |
| HMIP@CDs | Ammonium citrate, Cysteine | S | Styrene | S | [92] |
| C18-CQD membrane | Citric acid, ODA | S | Tolylene-2,4-diisocyanate | S | [93] |
| CDs/PNVCL@MSNs | Citric acid, carbamide | S | Poly(N-vinylcaprolactam) | S | [94] |
| CS/PVA/CDs | Citric acid, PEI | S | Chitosan, Polyvinyl alcohol | N, S | [95] |
| TPU/CDs | Citric acid, 2AT | S | Poly(tetramethylene glycol) | S | [96] |
| BMIP@CDs | Glucose | S | Dopamine | S | [64] |
| CD-TFN membrane | Glucose | S | Polysulfone | S | [97] |
| PDA-CQDs | Glucose | S | Polydopamine | S | [98] |
| Poly(VPBA-AAm)-CDs | Glucose | S | 4-vinylphenylboronic acid, Acrylamide | S | [99] |
| CDs-PEI/PES | Glucose | S | Polyethyleneimine | S | [100] |
| PVA-N@C-dots | PEG-400 | S | Polyvinyl alcohol | S | [74] |
| y-CD/PVA | o-phenylenediamine | S | Polyvinyl alcohol | S | [67] |
| CD/PVA films | Urea, PPD | S | Polyvinyl alcohol | S | [101] |
| PVA/CDs | Carboxymethylcellulose, PEI | N, S | Polyvinyl alcohol | S | [102] |
| PVA/CD films | Inner cassava peels | N | Polyvinyl alcohol | S | [56] |
| C-dots/PVA | Acerola fruit | N | Polyvinyl alcohol | S | [57] |
| CQDs@MIPs | Mango peels | N | (3-aminopropyl) triethoxysilane | S | [58] |
| MIPs-GSCDs | Cedrus | N | (3-aminopropyl) triethoxysilane | S | [59] |
| CDs@SiO2@MIPs | Rosemary leaves | N | (3-aminopropyl) triethoxysilane | S | [60] |
| PAN/CQD nanofibers | Chitosan | N | Polyacrylonitrile | S | [61] |
| CD-MIPGlcA | Starch, Tryptophan | N, S | AB1, methacrylamide | S | [62] |
| y-CDs/CS | o-phenylenediamine | S | Chitosan | N | [67] |
| CDs-polymer | Oleic acid | S | Cellulose acetate | N | [65] |
| CDs@Cu/Alg | Cetylpyridinium chloride | S | Alginate | N | [68] |
| C-dot/PEI gel | Aldehyde | S | Polyethyleneimine | S | [103] |
| CDs-polymer | Hexamethylenetetramine | S | Polycarbonate | S | [104] |
| PE/CDs | Ethanolamine | S | Polyethylene | S | [66] |
| PP/CDs | Ethanolamine | S | Polypropylene | S | [66] |
| PEG/CDs | Ethanolamine | S | Polyethylene glycol | S | [66] |
| CDs/clay/NIPAm | β-cyclodextrin | S | N-isopropylacrylamide | S | [105] |
| C-dot/PVB film | Cetylpyridinium chloride/Daaq | S | Polyvinyl butyral, Polyvinyl alcohol | S | [50] |
| aGQDs-TFN OSN | Graphene oxide | S | Polyimide | S | [106] |
| Composite Name | Synthesis Methods | Ref. | ||
|---|---|---|---|---|
| CDs | Temp/Heat, Time | CD/Polymer Composites | ||
| PVA/CDs | Hydrothermal | 260 °C, 2 h | Stirring | [102] |
| CDs@MIPs | Hydrothermal | 200 °C, 2 h | Sol–Gel | [63] |
| CDs@MIPs | Hydrothermal | 210 °C, 5 h | Sol–Gel | [69] |
| BMIP@CDs | Hydrothermal | 90 °C, 0.5 h | Sol–Gel | [64] |
| CQDs@MIPs | Hydrothermal | 200 °C, 4 h | Sol–Gel Hydrolysis | [58] |
| Y-CD/PVA, Y-CD/CS | Hydrothermal | 130 °C, 2 h | Drop casting | [67] |
| C-dots/PVA | Hydrothermal | 160 °C, 18 h | Conventional solution casting | [57] |
| PPy@Cdots/PANI@Cdots | Hydrothermal | 230 °C, 4 h | In situ chemical polymerization | [90] |
| MCOFs@MIPs@CDs | Hydrothermal | 200 °C, 2 h | Reverse microemulsion polymerization | [75] |
| MIPs-GSCDs | Hydrothermal | 180 °C, 12 h | Reverse microemulsion polymerization | [59] |
| CDs@SiO2@MIPs | Hydrothermal | 180 °C, 12 h | Reverse microemulsion polymerization | [60] |
| aGQDs-TFN OSN | Hydrothermal | 120 °C, 5 h | Interfacial polymerization | [106] |
| PDA-CQDs | Hydrothermal | 180 °C, 20 h | Interfacial polymerization | [98] |
| CD-TFN membrane | Hydrothermal | 200 °C, 12 h | Interfacial polymerization | [97] |
| TFC(Na-CQDs) | Hydrothermal | 180 °C, 3 h | Interfacial polymerization | [79] |
| PEI/CDs/PES | Hydrothermal | 180 °C, 20 h | Cross-linking | [100] |
| CDs/clay/NIPAm | Hydrothermal | 70 °C, 4 h | In situ polymerization | [105] |
| TPU/CDs | Hydrothermal | 170 °C, 3 h | In situ polymerization | [96] |
| CDs-polymer | Hydrothermal | 150 °C, 12 h | Hydrothermal | [104] |
| CD-MIPGlcA | Hydrothermal | 160 °C, 12 h | Photopolymerization | [62] |
| WCDs@PS | Solvothermal | 180 °C, 6 h | Polymer-assisted self-assembly | [73] |
| CD/PVA films | Solvothermal | 120, 150, 180 °C, 6 h | Stirring | [89] |
| CDs/Fe3O4@MIPs | Solvothermal | 240 °C, 2 h | Reverse microemulsion polymerization | [76] |
| C-MIP | Solvothermal | 180 °C, 4 h | Reverse microemulsion polymerization | [88] |
| C-dot/PEI gel | Solvothermal | 150 °C, 2 h | Schiff base reaction | [103] |
| AC-dots-DNT-MIPs | Solvothermal | 170 °C, 2 h | Stirring/Bulk polymerization | [83] |
| PAN/CQD nanofibers | Solvothermal | 180 °C, 16 h | Electrospinning | [61] |
| C-dot/PVB film | NaOH reaction/Solvothermal | 25 °C, 12 h/200 °C, 24 h | Stirring | [50] |
| CDs/PNVCL@MSNs | Microwave | 3 min | Stirring | [94] |
| CDs@PVA | Microwave | 650 W, 6 min | Thermal treatment | [91] |
| HMIP@CDs | Microwave | 750 W, 2.5 min | Sol–Gel | [92] |
| PAN/PEI-CDs | Microwave | 750 W, 5 min | Interfacial polymerization | [71] |
| CDs-PET film | Microwave | 850 W, 5 min | Drop casting | [108] |
| PES/CDs | Pyrolysis | 160 °C, 55 min | Nonsolvent Induced phase inversion | [70] |
| PE/CDs, PP/CDs, PEG/CDs | Pyrolysis | 160, 180 °C, 60 min | Thermal treatment | [66] |
| CDs-polymer | Pyrolysis | 230–260 °C, a few min | Magnetic stirring | [65] |
| GQDs-TFN SRNF | Pyrolysis | 200 °C, 30 min | Interfacial polymerization | [84] |
| C18-CQDs membrane | Pyrolysis | 200 °C, 4 h | Cross-linking reaction | [93] |
| TFN-(Na-CQDs) | Pyrolysis | 180 °C, 3 h | Interfacial polymerization | [77] |
| PEI/GQDs-TFC | Pyrolysis | 200 °C, 30 min | Interfacial polymerization | [81] |
| TFN-CQD membrane | Pyrolysis | 200 °C, 2 h | Interfacial polymerization | [82] |
| GQD-TFN membrane | Pyrolysis | 200 °C, 30 min | Interfacial polymerization | [80] |
| Na-CQD-TFN | Pyrolysis | 180 °C, 3 h | Interfacial polymerization | [78] |
| GQDs/PIP-TMC TFN | Pyrolysis | 200 °C, 15 min | Interfacial polymerization | [86] |
| TFC-PES-PDA-CQD | Pyrolysis | 180 °C, 3 h | Interfacial polymerization | [72] |
| PES/GQDs-TMC | Pyrolysis | 200 °C, 15 min | Interfacial polymerization/thermal | [87] |
| GQDs/PVDF | Pyrolysis | 200 °C, 30 min | Electrospinning | [85] |
| CDS@Cu/Alg | Ultrasonic | 25 °C, 30 min | Cross-linking | [68] |
| PVA-N@C-dots | Ultrasonic | 60 °C, 3 h | Stirring/drop-cast | [74] |
| Poly(VPBA-AAm)-CDs | Ultrasonic and hydrothermal | 8 h/200 °C, 24 h | Free radical dispersion polymerization | [99] |
| Composites | λEx (nm) | λEm (nm) | QY (%) | Abs. (nm) | FL Color | Ref. |
|---|---|---|---|---|---|---|
| CD/PVA film | 532 | 585 | N/A | 489 | N/A | [101] |
| PVA/CD film | 420 | 530 | N/A | ~220, ~290 | G | [56] |
| CDs@MIPs | 370 | 470 | 51.8 | 370 | B | [63] |
| CS/PVA/CDs | 360 | 436 | N/A | 360 | C | [95] |
| PVA/CDs | 360 | ~470 | 47 | 294/340 | B | [102] |
| PE/CDs, PP/CDs, PEG/CDs | 350, 380 | ~450–470 | 2.5–38 | N/A | B, G, R | [66] |
| C-MIP | 540 | 610 | N/A | N/A | R | [88] |
| MCOFs@MIPs@CDs | 370 | 470 | N/A | N/A | C | [75] |
| TPU/CDs | 400 | 470 | 68 | 335, 399 | B | [96] |
| CD-polymer | 455 | 550 | 14.86 | 200–500 | Y | [104] |
| CDs/Fe3O4@MIPs | 370 | 470 | N/A | N/A | B | [76] |
| CD/PVA films | 365, 450, 420, 400 | 444,520, 550,585 | 5.3–12.4 | 365, 450, 420, 400 | B, G, Y, O | [89] |
| CDS@Cu/Alg | 400 | 513 | N/A | N/A | G | [68] |
| CQDs@MIPs | 360 | 453 | N/A | ~260 | N/A | [58] |
| C-dots/PVB film | 400 | 550 | N/A | 353, 410, 500 | G-B, O-R | [50] |
| MIPs-GSCDs | 340 | 410 | 18.6 | ~275 | N/A | [59] |
| CD-polymer | N/A | 470 | 50 | 250, 300 | G | [65] |
| CDs@SiO2@MIPs | 380 | >450 | N/A | 288 | N/A | [60] |
| WCDs@PS | 380 | ~590 | 10.7, 15.2 | N/A | O, B | [73] |
| CDs@MIPs | 360 | 450 | N/A | 250–300 | B | [69] |
| C-dot/PEI gel | 470 | ~565 | 1.9–4 | 290, 340, 380 | C | [103] |
| Y-CD/PVA, Y-CDs/CS | 420 | 550 | 6 | 234, 259, 285, 420 | Y | [67] |
| CDs@PVA | 365 | 420–440 | N/A | ~350 | B | [91] |
| C-dots/PVA | 360 | 459 | 8.64 | 282, 341 | G | [57] |
| HMIP@CDs | 390 | 503 | N/A | ~300 | N/A | [92] |
| BMIP@CDs | 425 | 520 | N/A | N/A | N/A | [64] |
| CD-MIPGlcA | 445 | ~500 | 0.97 | ~350 | B | [62] |
| Poly(VPBA-AAm)-CDs | 900 | 515 | N/A | 241 | B | [99] |
| PAN/CQD nanofibers | 350, 477, 530 | 560, 598, 660 | N/A | 314, 316, 318 | R, G, B | [61] |
| PVA-N@C-dots | 390 | ~460 | 44 | 286, 355 | C | [74] |
| Composites | Applications | Analytes | Linear Range | R2 | LOD | Ref. |
|---|---|---|---|---|---|---|
| CDs@MIPs | Chemical Sensor | 4-NP | 0.025–5 mg/L | 0.9883 | 35 nM | [63] |
| MCOFs@MIPs@CDs | Chemical Sensor | TNP | 0.0003–100 µM | 0.9920 | 0.1 nM | [75] |
| TPU/CDs | Chemical Sensor | Ag+ | 0.05–5.0 mM | 0.976 | 12 µM | [96] |
| CDs/Fe3O4@MIPs | Chemical Sensor | TNP | 1 nM-100 µM | 0.9975 | 0.5 nM | [76] |
| CQDs@MIPs | Chemical Sensor | Mesotrione | 15–3000 nmol/L | 0.991 | 4.7 nmol/L | [58] |
| Y-CD/PVA and CS | Chemical Sensor | Cu2+ | 0.0001–0.1 mM | 0.99 | 10 nM | [67] |
| C-dots/PVA | Chemical Sensor | Fe3+ | 0.001–0.012 mol/L | 0.9925 | N/A | [57] |
| AC-dots-DNT-MIPs | Chemical Sensor | DNT | 1–15 ppm | 0.998 | 0.28 ppm | [83] |
| C-MIP | Biological Sensor | EGFR epitopes | 2.0–15.0 µg/mL | 0.99396 | 0.73 µg/mL | [88] |
| CDs@Cu/Alg | Biological Sensor | ALP | 2–100 mU/mL | 0.9955 | 0.55 mU/mL | [68] |
| MIPs-GSCDs | Biological Sensor | Phenobarbital | 0.4–34.5 nmol/L | 0.997 | 0.1 nmol/L | [59] |
| CDs@SiO2@MIPs | Biological Sensor | TBZ | 0.03–1.73 µg/mL | - | 8 ng/mL | [60] |
| CDs@MIPs | Biological Sensor | Caffeic acid | 0.5–200 µM | 0.9973 | 0.11 µM | [69] |
| HMIP@CDs | Biological Sensor | Tetracycline | 10–200 µg/L | 0.9972 | 3.1 µg/L | [92] |
| BMIP@CDs | Biological Sensor | 3-NT | 0.05–1.85 µM | 0.9978 | 17 nM | [64] |
| Poly(VPBA-Aam)-CDs | Biological Sensor | Glucose | 0–20 mM | 0.9952 | N/A | [99] |
| WCDs@PS | Physical Sensor | Temp. | 20–80 °C | 0.979 | N/A | [73] |
| CDs-PET film | Physical Sensor | Humidity | <55%, >55% | 0.9579, 0.9050 | N/A | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulfajri, M.; Sudewi, S.; Ismulyati, S.; Rasool, A.; Adlim, M.; Huang, G.G. Carbon Dot/Polymer Composites with Various Precursors and Their Sensing Applications: A Review. Coatings 2021, 11, 1100. https://doi.org/10.3390/coatings11091100
Zulfajri M, Sudewi S, Ismulyati S, Rasool A, Adlim M, Huang GG. Carbon Dot/Polymer Composites with Various Precursors and Their Sensing Applications: A Review. Coatings. 2021; 11(9):1100. https://doi.org/10.3390/coatings11091100
Chicago/Turabian StyleZulfajri, Muhammad, Sri Sudewi, Sri Ismulyati, Akhtar Rasool, Muhammad Adlim, and Genin Gary Huang. 2021. "Carbon Dot/Polymer Composites with Various Precursors and Their Sensing Applications: A Review" Coatings 11, no. 9: 1100. https://doi.org/10.3390/coatings11091100
APA StyleZulfajri, M., Sudewi, S., Ismulyati, S., Rasool, A., Adlim, M., & Huang, G. G. (2021). Carbon Dot/Polymer Composites with Various Precursors and Their Sensing Applications: A Review. Coatings, 11(9), 1100. https://doi.org/10.3390/coatings11091100








