Impact of Zinc Oxide Nano Particles, Poly Vinyl Alcohol, and Natural Polymers on Quality Characteristics of Nanocomposite Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals Required for Film Formation
2.2. Preparation of Corn Starch/WPI/WPC/Carrageenan Composite Film
2.3. Preparation of Corn Starch/WPI/WPC/Carrageenan Composite Film with PVA
2.4. Preparation of Corn Starch/WPI/WPC/Carrageenan/PVA/ZnO nanocomposite Film
2.5. Characterization of Slurry Used for Film Formation
2.6. Characterization of Film
2.7. Mechanical Properties of Film
2.7.1. Scanning Electron Microscopy (SEM)
2.7.2. Statistical Analysis
3. Results and Discussions
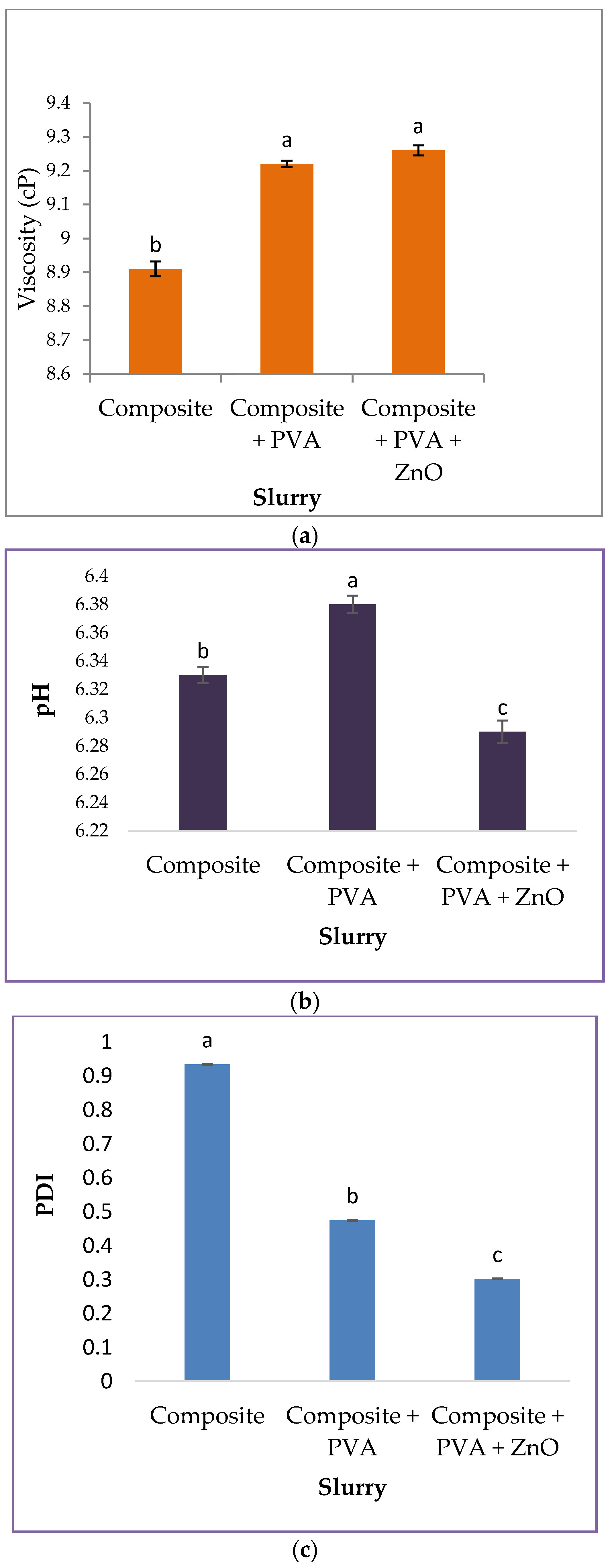
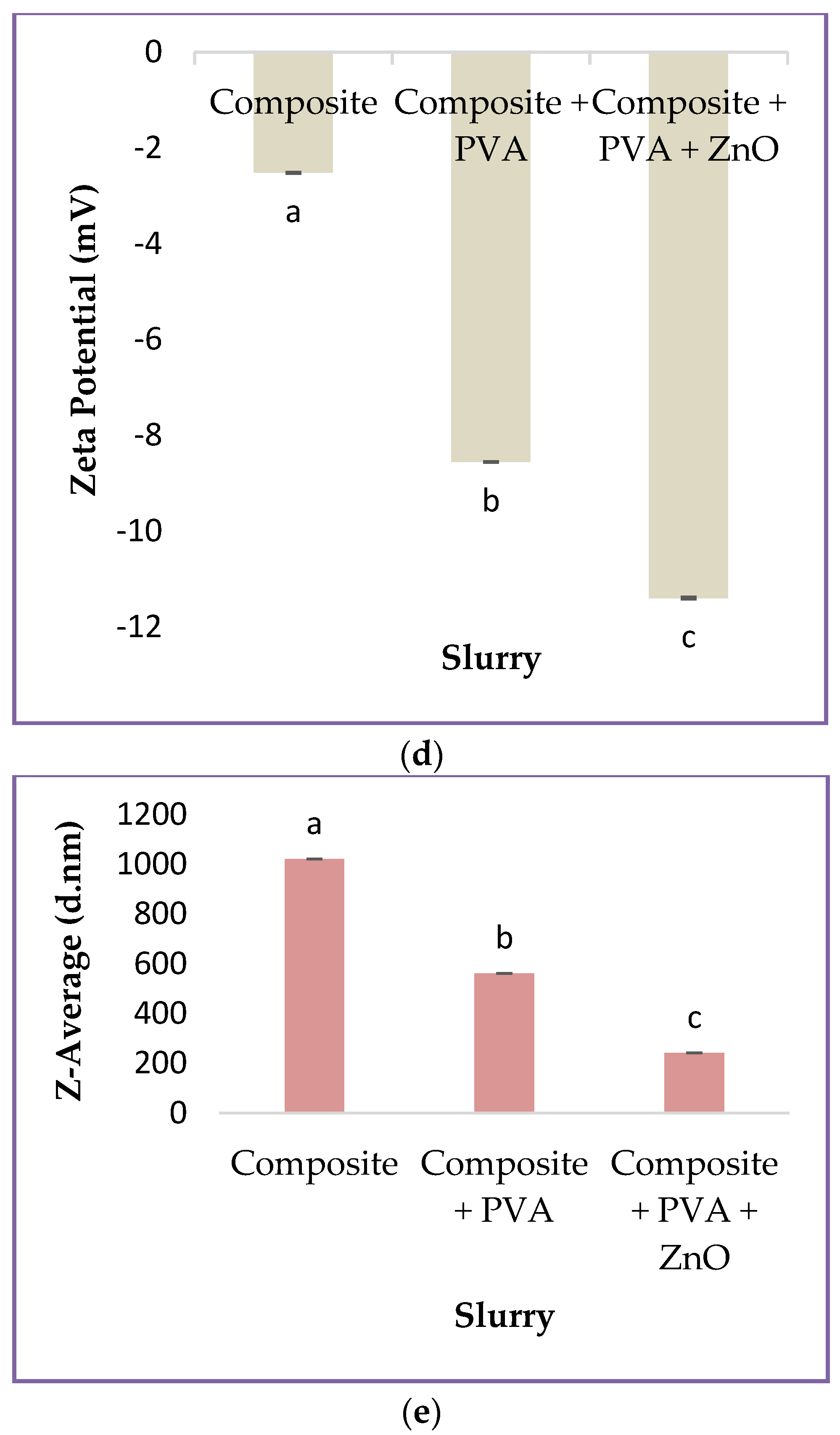
3.1. Properties of Different Type of Slurries
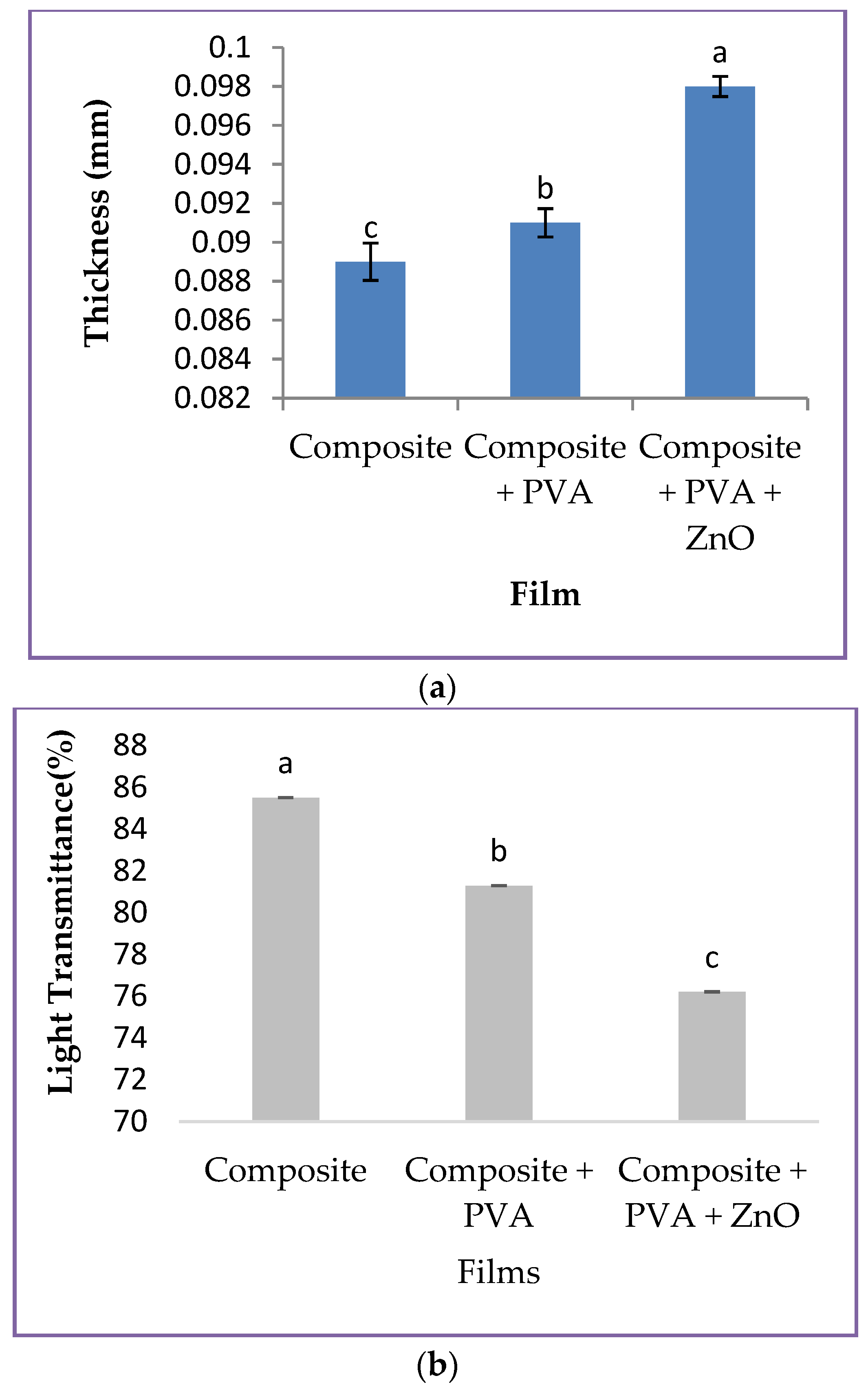
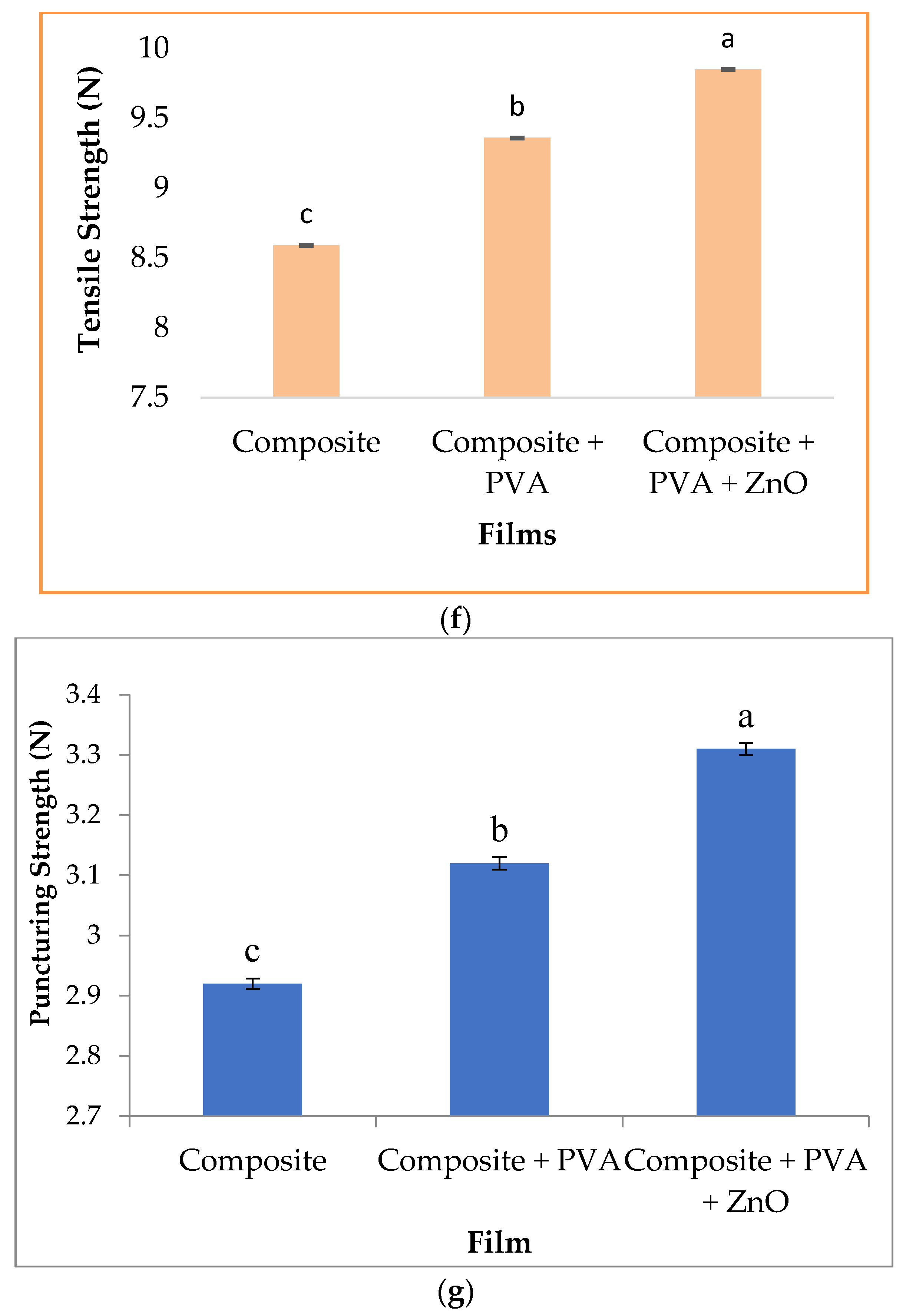
3.2. Properties of Different Type of Films
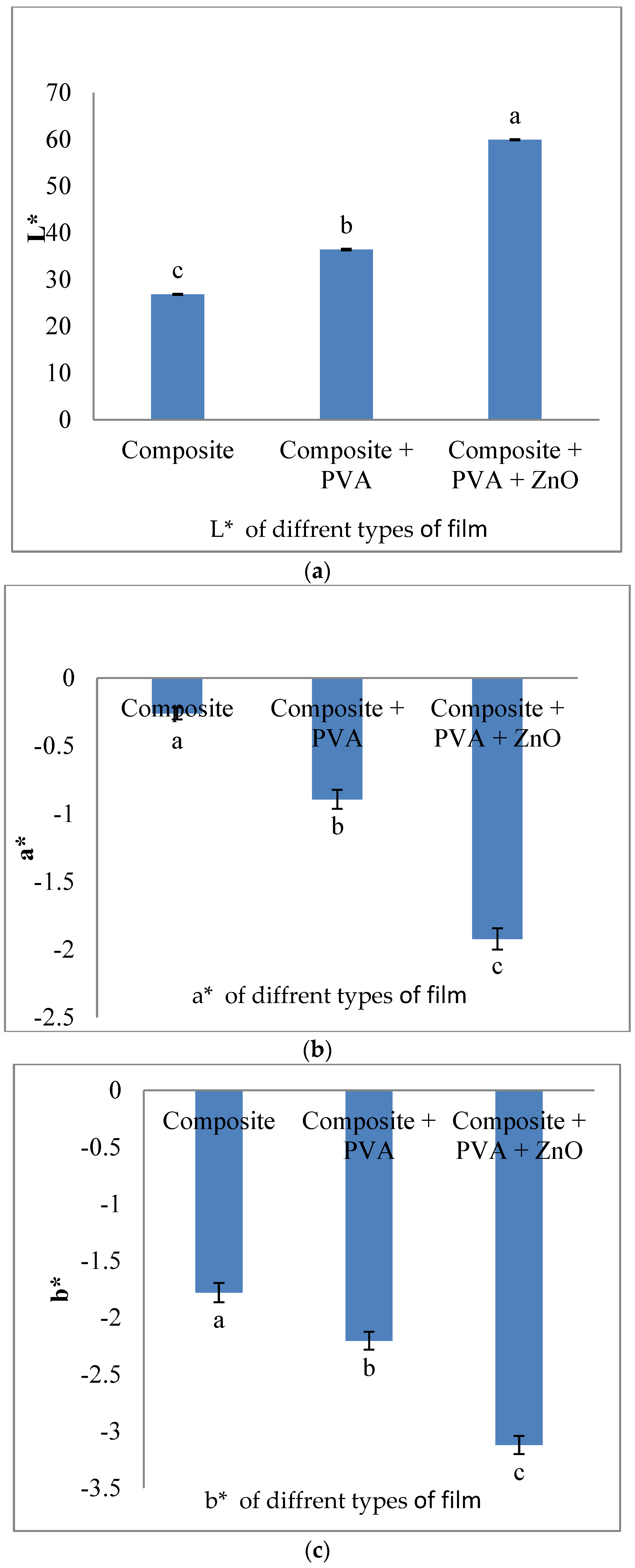
3.3. Colour
3.4. Scanning Electron Microscopy (SEM) of the Nanocomposite Film
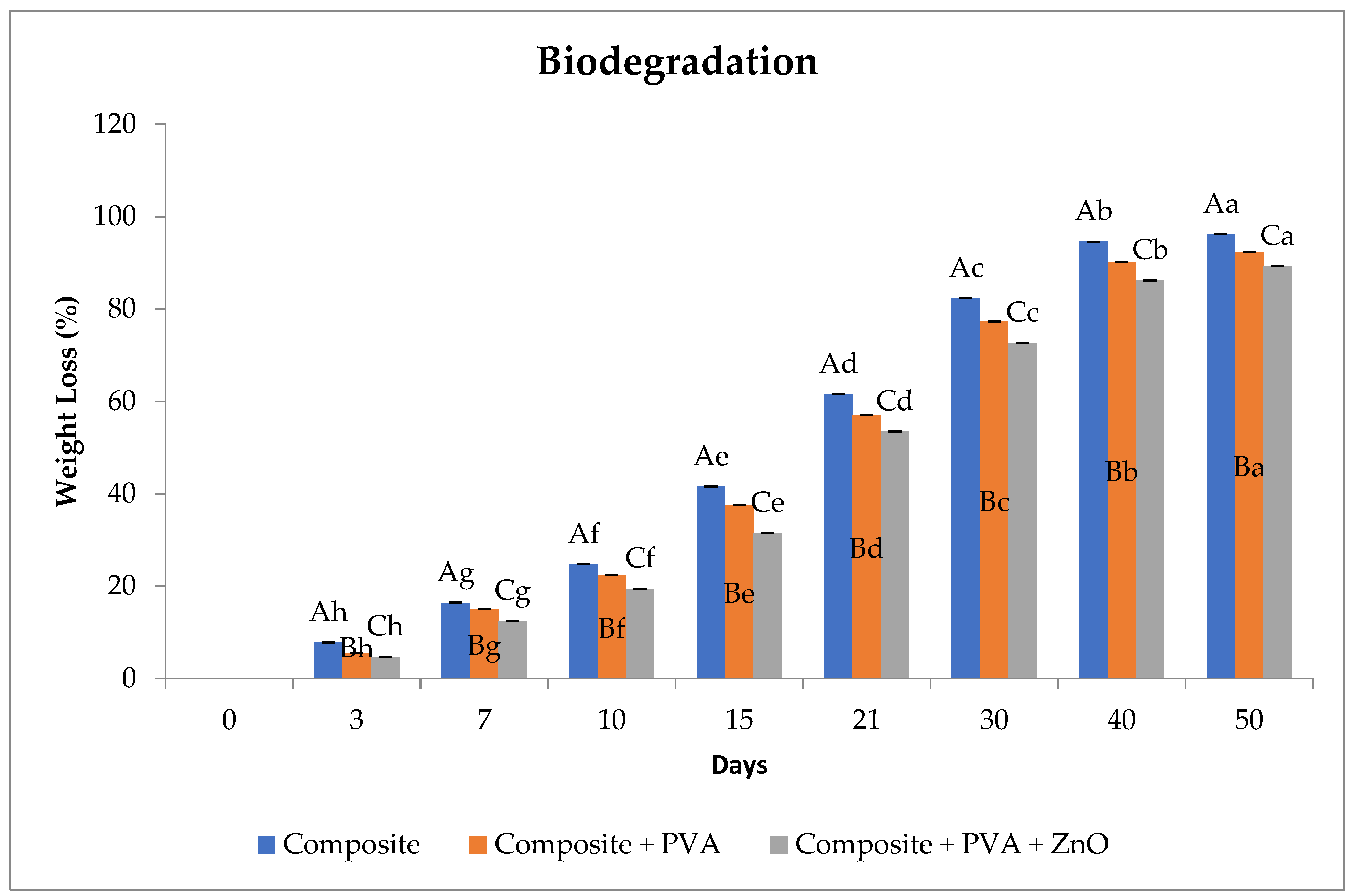
3.5. Biodegradation of Film
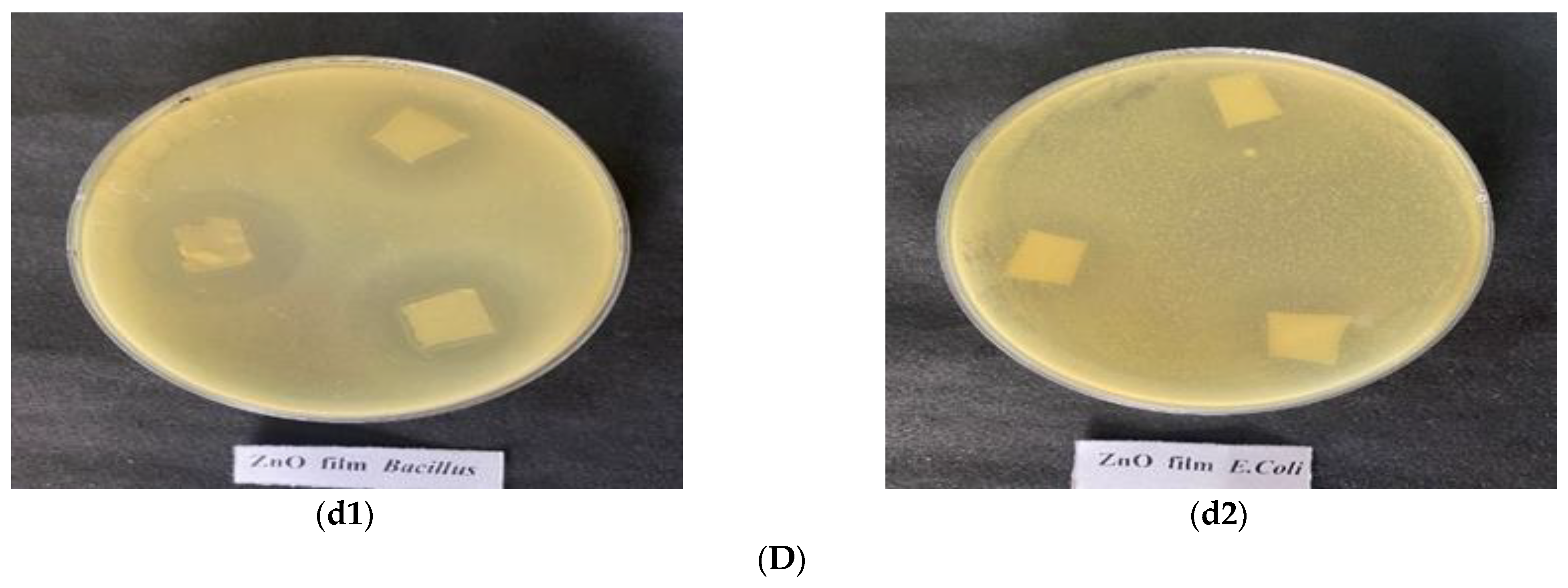
3.6. Antibacterial Activity of Nanoparticles Incorporated in Slurry and Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- De Azeredo, H.M. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, D.J. Plasticizer effect on oxygen permeability of β-lactoglobulin films. J. Agric. Food Chem. 2000, 48, 6298–6302. [Google Scholar] [CrossRef] [PubMed]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible films and coatings. In Innovations in Food Packaging; Academic Press: Cambridge, MA, USA, 2005; pp. 403–433. [Google Scholar]
- Han, Y.J.; Kim, S.S. Physical Properties of Mixed κ/λ- and κ/ι-carrageenan Films. Korean J. Food Sci. Technol. 2008, 40, 42–46. [Google Scholar]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of recrystallization on tensile, optical and water vapour barrier properties of corn starch films containing fatty acids. Food Hydrocoll. 2012, 26, 302–310. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan–Poly Vinyl Alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of Poly Vinyl Alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Karbowiak, T.; Hervet, H.; Léger, L.; Champion, D.; Debeaufort, F.; Voilley, A. Effect of plasticizers (water and glycerol) on the diffusion of a small molecule in iota-carrageenan biopolymer films for edible coating application. Biomacromolecules 2006, 7, 2011–2019. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A.; Hallaj, R. Influence of nano-ZnO on microbial growth, bioactive content and postharvest quality of strawberries during storage. Innov. Food Sci. Emerg. Technol. 2016, 35, 168–176. [Google Scholar] [CrossRef]
- Vasile, C.; Râpă, M.; Ştefan, M.; Stan, M.; Macavei, S.; Darie-Niţă, R.N.; Barbu-Tudoran, L.; Vodnar, D.C.; Popa, E.E.; Ştefan, R.; et al. New PLA/ZnO: Cu/Ag bionanocomposites for food packaging. Express Polym. Lett. 2017, 11, 531–544. [Google Scholar] [CrossRef]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed Mater. Res. A 2006, 78, 595–604. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Zhang, H.; Yuan, M.; Qin, Y. Evaluation of PLA nanocomposite films on physicochemical and microbiological properties of refrigerated cottage cheese. J. Food Process. Preser. 2018, 42, 13362. [Google Scholar] [CrossRef]
- Singh, G.; Sivakumar, S.; Chawla, R.; Viji, P.C. Development and Characterization of Environment Friendly Starch and Protein Based Packaging Materials for Food Applications. Int. J. Agric. Env. Biotechnol. 2022, 15, 637. [Google Scholar] [CrossRef]
- Singh, G. Process Optimization for the Development and Characterization of Nanobiocomposite Film Using Nanoparticles. Master’s Thesis, Guru Angad Dev Veterinary and Animal Sciences University, Punjab, India, 2019. [Google Scholar]
- Sivakumar, S.; Chawla, R.; Singh, N.; Singh, G. Effect of ultrasonication on properties of whey protein based nanobiocomposite film. In Proceedings of the National Conference on Emerging and Sustainable Technologies in Food Processing (ESTFP-2018), Longowal, India, 15–16 March 2018. [Google Scholar]
- Sivakumar, S.; Chawla, R.; Singh, N.; Singh, G. From Synthetics to Natural, Growing Era of Edible Films (Starch vs. Protein). In Proceedings of the Third National Conference on Contemporary Food Processing and Preservation Technologies, Solan, India, 12–13 April 2018. [Google Scholar]
- Singh, T.P.; Chatli, M.K.; Sahoo, J. Development of chitosan based edible films: Process optimization using response surface methodology. J. Food Sci. Technol. 2015, 52, 2530–2543. [Google Scholar] [CrossRef]
- ASTM International. ASTM E96-Standard Test Methods for Water Vapor Transmission of Materials; ASTM International: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Al-Sahlany, S.T. Production of biodegradable film from soy protein and essential oil of lemon peel and use it as cheese preservative. Basrah J. Agric. Sci. 2017, 30, 27–35. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Sieo, C.C.; Kalavathy, R.; Liang, J.B.; Alitheen, N.B.; Faseleh Jahromi, M.; Ho, Y.W. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. BioMed Res. Int. 2014, 2014, 927268. [Google Scholar] [CrossRef]
- Gurpreet, K.; Singh, S.K. Review of nanoemulsion formulation and characterization techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Zayyoun, N.; Bahmad, L.; Laânab, L.; Jaber, B. The effect of pH on the synthesis of stable Cu2O/CuO nanoparticles by sol–gel method in a glycolic medium. Appl. Phys. 2016, 122, 488. [Google Scholar] [CrossRef]
- Qi, J.; Ye, Y.Y.; Wu, J.J.; Wang, H.T.; Li, F.T. Dispersion and stability of titanium dioxide nanoparticles in aqueous suspension: Effects of ultrasonication and concentration. Water Sci. Technol. 2013, 67, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Marsalek, R. Particle size and zeta potential of ZnO. APCBEE Procedia 2014, 9, 13–17. [Google Scholar] [CrossRef]
- Nanocomposix. Zeta Potential Analysis of Nanoparticles; Nanocomposix: San Diego, CA, USA, 2012. [Google Scholar]
- Gharoy Ahangar, E.; Abbaspour Fard, M.H.; Shahtahmassebi, N.; Khojastehpour, M.; Maddahi, P. Preparation and characterization of PVA/ZnO nanocomposite. J. Food Process. Preserv. 2015, 39, 1442–1451. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Park, H.M.; Ng, P.K. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef]
- Ngo TM, P.; Dang TM, Q.; Tran, T.X.; Rachtanapun, P. Effects of zinc oxide nanoparticles on the properties of pectin/alginate edible films. Int. J. Polym. Sci. 2018, 2018, 5645797. [Google Scholar]
- Yang, M.; Shi, J.; Xia, Y. Effect of SiO2, PVA and glycerol concentrations on chemical and mechanical properties of alginate-based films. Int. J. Biol. Macromol. 2018, 107, 2686–2694. [Google Scholar] [CrossRef]
- Atta, A.; Abdel Reheem, A.M.; Abdeltwab, E. Ion beam irradiation effects on surface morphology and optical properties of ZnO/PVA composites. Surf. Rev. Lett. 2020, 27, 1950214. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Mahmud, S.; Robal, M. Antimicrobial, rheological, and physicochemical properties of sago starch films filled with nanorod-rich zinc oxide. J. Food Eng. 2012, 113, 511–519. [Google Scholar] [CrossRef]
- Saputri, A.E.; Praseptiangga, D.; Rochima, E.; Panatarani, C.; Joni, I.M. Mechanical and solubility properties of bio-nanocomposite film of semi refined kappa carrageenan/ZnO nanoparticles. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 1927, p. 030040. [Google Scholar]
- Akhavan, A.; Khoylou, F.; Ataeivarjovi, E. Preparation, and characterization of gamma irradiated Starch/PVA/ZnO nanocomposite films. Radiat. Phys. Chem. 2017, 138, 49–53. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Castro-Aguirre, E.; Auras, R. Mechanical, structural and thermal properties of Ag–Cu and ZnO reinforced polylactide nanocomposite films. Int. J. Biol. Macromol. 2016, 86, 885–892. [Google Scholar] [CrossRef]
- Wu, S.; Chen, X.; Yi, M.; Ge, J.; Yin, G.; Li, X.; He, M. Improving thermal, mechanical, and barrier properties of feather keratin/Poly Vinyl Alcohol/tris (hydroxymethyl) aminomethane nanocomposite films by incorporating sodium montmorillonite and TiO2. Nanomaterials 2019, 9, 298. [Google Scholar] [CrossRef]
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf-life extension of green grape. Heliyon 2019, 5, e01867. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S.H.; Roufegarinejad, L.; Hamishehkar, H. Application of reinforced ZnO nanoparticle-incorporated gelatin bionanocomposite film with chitosan nanofiber for packaging of chicken fillet and cheese as food models. Food Bioproc. Technol. 2019, 12, 1205–1219. [Google Scholar] [CrossRef]
- Ji, Z. Use of compositional and combinatorial nanomaterial libraries for biological studies. Sci. Bull. 2016, 61, 755–771. [Google Scholar] [CrossRef]
- Paula, M.; Diego, I.; Dionisio, R.; Vinhas, G.; Alves, S. Gamma irradiation effects on polycaprolactone/zinc oxide nanocomposite films. Polímeros 2019, 29, e2019014-21. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; Garcia, A.; Martino, M.N.; Zaritzky, N.E. Microstructural characterization of yam starch films. Carbohydr. Polym. 2002, 50, 379–386. [Google Scholar] [CrossRef]
- Jayakumar, A.; Heera, K.V.; Sumi, T.S.; Joseph, M.; Mathew, S.; Praveen, G.; Indu CNRadhakrishnan, E.K. Starch-PVA composite films with zinc-oxide nanoparticles and phytochemicals as intelligent pH sensing wraps for food packaging application. Int. J. Biol. Macromol. 2019, 136, 395–403. [Google Scholar] [CrossRef]
- Abbasi, Z. Water resistance, weight loss and enzymatic degradation of blends starch/Poly Vinyl Alcohol containing SiO2 nanoparticle. J. Taiwan Inst. Chem. Eng. 2012, 43, 264–268. [Google Scholar] [CrossRef]
- Azahari, N.A.; Othman, N.; Ismail, H. Biodegradation studies of Poly Vinyl Alcohol/corn starch Corn starch (Maize)blend films in solid and solution media. J. Phys. Sci. 2011, 22, 15–31. [Google Scholar]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; York, D.W. Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, G.; Shanmugam, S.; Chawla, R.; Goel, N.; Talwar, G.; Mishra, S.K.; Chatli, M.K. Impact of Zinc Oxide Nano Particles, Poly Vinyl Alcohol, and Natural Polymers on Quality Characteristics of Nanocomposite Film. Coatings 2023, 13, 420. https://doi.org/10.3390/coatings13020420
Singh G, Shanmugam S, Chawla R, Goel N, Talwar G, Mishra SK, Chatli MK. Impact of Zinc Oxide Nano Particles, Poly Vinyl Alcohol, and Natural Polymers on Quality Characteristics of Nanocomposite Film. Coatings. 2023; 13(2):420. https://doi.org/10.3390/coatings13020420
Chicago/Turabian StyleSingh, Gurpreet, Sivakumar Shanmugam, Rekha Chawla, Nitika Goel, Gopika Talwar, Santosh Kumar Mishra, and Manish Kumar Chatli. 2023. "Impact of Zinc Oxide Nano Particles, Poly Vinyl Alcohol, and Natural Polymers on Quality Characteristics of Nanocomposite Film" Coatings 13, no. 2: 420. https://doi.org/10.3390/coatings13020420




