Abstract
Consumer demands for biocompatible, minimally processed and eco-friendly foods have increased drastically and are currently trending. Polysaccharides derived from various plant seeds exhibiting structure conformational diversity are among such foods and used for the development of edible films. In this study, the physical properties of flaxseed, proximate characterization and rheological, mechanical and thermal features of flaxseed meal-based edible films were investigated. A development strategy worked through adding pectin + flaxseed meal to a plasticizer (glycerol) with a ratio of 7:3 w/v, whereas, in the control group, the flaxseed meal remained unaltered. The rheological results showed the non-Newtonian behavior of film-forming solutions and data were well fitted into the power law model. The developed film (flaxseed + pectin) was slightly brownish and exhibited a transparency of 17.78, which is clear enough to be used as see-through packaging material, whereas the control sample had a transparency of 38.25, indicating its fair transparency. The water vapor permeability of the test sample was also beneficial (0.992 g/cm2/24 h) and was competitively close to the control (0.981 g/cm2/24 h). The developed blended films were 98–99% soluble in water and acid, indicating their usefulness when applied as a coating. The mechanical properties, tensile strength and elongation value of the test sample were less than the control. This study will be helpful in the development of a novel biodegradable film for extending the life of different foods.
1. Introduction
Current eating patterns, problems associated with the restricted consumption of eatables and shelf-life extension issues for fresh harvests direct the research for some alternatives to work toward. Working toward using flaxseed (Linum Usitatissimum) is advantageous, owing to its healthy nutritional attributes, long back cultivation history and its functional potential. Among other things, it heightens our gastrointestinal microflora with its prebiotic expressions [1]. Promising biological activities viz; high ALA (alpha-linolenic acid), flavonoids, lignans and phenolic acids in flaxseed [2] necessitate its application expansion under today’s demanding category within the heading of “functional foods”. Its consumption after pre-processing tactics minimizes the anti-nutrients (cyanogenic glycosides, trypsin inhibitor and phytic acid) and also give thrust to the bioavailability features for its nutrients [3].
The continuous demand for ready-to-eat foods with an elevated shelf life through long-lasting freshness is the foremost reason for its consumer preference. Packaging plays an essential role by providing physical protection to food and selective environmental conditions necessary to have an excellent keeping quality. Edible films are generally produced from lipids, polysaccharides or proteins [4,5], bearing in mind the need to arrest gaseous and moisture migration both in and out of the food. Edible films can also be incorporated with additives viz. antioxidants and antimicrobials, and flavors to improve their physical intactness, facilitate handling and preserve the food quality. These barriers can be applied to ready-to-eat and fresh produce, such as fruits and vegetables [6,7]. Every film-forming additive has its own significance viz; glycerol as a plasticizer is of utmost importance, owing to its role in the enhancement of the flexibility attributes of films. In contrast, they alter the barrier, water solubility and mechanical properties of the resulting films to a varying degree [8,9].
Plant-based edible coatings and films are a sustainable alternative to plastic packaging [10]. Pectin, owing to its polymerized structure and cementing attributes, works well as a base ingredient for developing an edible package [11,12]. It is the chief component among plant cells, and chemically constituted of poly α-1,4-galacturonic acids. It is generally recognized as safe (GRAS) by the United States Food and Drug Administration, and is preferably utilized as a gelling, stabilizing or thickening agent in foods viz; fruity drinks, frozen desserts and jams [4]. Recently, Al-Asmar et al. [13] prepared and characterized a pectin-based film for strawberry packaging. Giosafatto et al. [14] also developed a polysaccharide-based edible film with the addition of mesoporous silica nanoparticles. Hence, various studies showed the compatibility of pectin-based edible and biodegradable films [15,16,17]. The easy access of film-forming traits among pectin and its good polymerization capacity, in combination with flaxseed extract (mucilage—a gelatinous substance comprising mostly polysaccharides), is one of the unique combinations presented herein. Moreover, working on these polysaccharide ingredients governs the health-promoting and environment friendly bio-packaging solutions. Thus, in lieu of all of these headed attributes, and in order to serve for the betterment of a healthy community, process parameters were herein standardized and presented through highlighted physico-chemical, rheological and mechanical terms for an edible film.
2. Materials and Methods
Brown flaxseed was procured from the local grocery shop of Sector-23D in Chandigarh (India). Pure pectin was procured from Sisco research laboratory Pvt Ltd., Mumbai, India. It was labeled as 80% galacturonic acid with a minimum of 7% of methoxyl content. Glycerol was obtained under the brand name LobaChemie (Mumbai, India). All of the glassware utilized were of Borosil (Mumbai, India) make.
2.1. Physical Characteristics of Flaxseed
Physical characteristics, such as length, width, thickness, thousand kernel weight, bulk density, tapped density, true density, angle of repose, Carr’s index, Hausner’s ratio and porosity of the flaxseed were determined using the AOAC Method [18].
2.2. Proximate Analysis of Flaxseed
The AOAC method was undertaken to characterize the flaxseeds for their % moisture, ash, crude protein, crude fat, crude fiber and carbohydrates under the proximate heading for flaxseed [18]. Fibroplus and Soxplus of Pelican Chennai were selected to determine crude fiber and crude fat values, respectively. The carbohydrate proportion was measured by the formula: total carbohydrate = 100 − (moisture + crude fat + ash + crude protein).
2.3. Film Formation and Casting
Flaxseeds were thoroughly washed under potable water to remove any extraneous matter. Given seeds were allowed to soak in water overnight at room temperature and followed through pulverization with the help of grinder (Morphy Richards). This pulverized mass was subjected to centrifugation after dilution with water at ratio of 3:1 (water:pulverized mass) with the assistance of magnetic stirrer at an elevated temperature of 45 °C at 130 rpm for approximately 2.5 h. Next, the extracted mass was centrifuged at 10,000 rpm for 8 min for complete extraction and the supernatant was separated out for the purposes of the pectin and glycerol mix. Pectin and glycerol addition demanded one more agitation through magnetic stirrer to avoid lumping, and homogenous mass was maintained at 300 rpm at 85 °C for 30 min. The given proportions of mix were varied in order to reveal test and control samples for better prediction of casted films by selected set of test parameters. The mix was then dried in a convection oven at 30 2 °C for 25 to 30 h in the form of thin film placed on large size glass petri dishes to eradicate moisture content [14]. The ratio of flaxseed meal (FM) + pectin:plasticizer (glycerol) was maintained at 7:3 (Table 1). Blended suspension (flaxseed mucilage, pectin and glycerol with water) agitation, as suggested previously, is the key concept to having a glossy, transparent and well-finished film. The pectin film prepared without flaxseed meal was used as a control sample.

Table 1.
Casting composition of films.
2.4. Rheological Analysis
Rheological analysis of different film compositions was carried out by using stainless steel plate and plate (PP 50) geometry (MCR 74 rheometer, Anton Paar GmbH, Ostfildern, Germany). 1 g of each sample was compressed between a 1-mm gap size. The steady shear (viscosity vs. shear rate) and (shear stress vs. shear rate) experiments were conducted at shear rate ranging from 1 to 30 s−1. The data were fitted into power model (Equation (1)) using SPSS 18.0 statistical software [19].
σ = K γn
2.5. Film Characterization
2.5.1. Thickness
The thickness of the casted mass was measured using a digital micrometer (HyperTrex Tungsten Carbide Steel micrometer, Mumbai, India) (0.01 mm sensitivity) by placing it randomly among five positions across the film, and the mean value was calculated for each sample.
2.5.2. Moisture Content of the Film
The moisture content of the film was determined through a given standardized operation [18], wherein small film pieces were subjected to drying in a ventilated oven at 105 °C for 3 h.
2.5.3. Color
Color values for the standardized film were observed with the aid of a Color Flex meter (Hunter Lab Color Flex 150 Hunter Associates Inc., Salem, OH, USA) using 45°/0° geometry and pre-calibration tactics through a given set of black and green tiles. The L* (0–100, dark to light), a* (±, green/red) and b* (±, blue/yellow) values were calculated.
2.5.4. Light Transmission and Transparency
The light transmission and transparency of the edible film were measured using a UV-Vis spectrophotometer (PerkinElmer, Waltham, MA, USA) [20]. The sample was cut into a rectangular shape with an approximate area of 8035 mm2 and inserted into the cuvette. The light transmission and transparency were determined against the air as a standard. The obtained absorbance was used to calculate the transparency value of the sample using the equation
where A600 is the absorbance at 600 nm and x is the thickness (mm) of the film [21].
Transparency value = A600/x
2.5.5. Water Vapor Permeability
The permeability index of the film samples was evaluated gravimetrically with slight modifications to the standardized protocol. Silica gel was placed in a glass jar, and then a 5-cm diameter sample of film with an almost equal circumference to the glass jar was stuck to it with parafilm. This complete setup was then monitored at 80% RH with the aid of a saturated salt solution of ammonium sulfate at 20 °C. After every 24 h, the jar was weighed until a constant increase in weight was achieved.
2.5.6. Solubility Index
The solubility index was measured with water and acid solubility of the films through slight amendment in the existing setup, as suggested by Zhang et al. [22]. Film samples were cut into 2 × 2 cm2 in duplicates, weighed and dried at 105 for 24 h. The dried films were weighed and immersed in 10 mL of distilled water for 24 h at 25, under stirring (150 rpm). The film pieces were removed after 24 h of incubation by filtration through Whatman filter paper No. 4 and re-dried for 24 h at 105 °C to determine the weight of the dry matter dispersed in water. The water-soluble matter weight was calculated by subtracting the weight of the insoluble dry matter from the weight of the initial dry matter, and was expressed as a percentage of the initial dry matter content.
Similarly, to reveal acid solubility extent, films were cut into 2 × 2 cm2 in duplicates. They were weighed and dried at 105 °C for 24 h followed by weighing of the dried films and immersing in 1 M hydrochloric acid for 24 h at 25 °C with continuous stirring (150 rpm). After 24 h of incubation, the liquefied mass was filtered through Whatman filter paper No. 4 and re-dried for 24 h at 105 °C to determine the weight of the dry matter dispersed in 1 M hydrochloric acid. The calculation was performed by subtracting the weight of the insoluble dry matter from the weight of the initial dry matter and was expressed as a percentage of the initial dry matter content.
2.6. Mechanical Properties
2.6.1. Tensile Strength and Elongation at Break
A Texture Analyzer (TA.XT2i, M/s Stable Micro System, Surrey, UK) fitted with a 25-kg load cell was used to determine the tensile strength and elongation at breaking point (ASTM, 1992). The samples were cut into the required dimensions (2.54 cm × 10 cm) and were acclimatized for 48 h (at room temperature with 50 ± 2% relative humidity) before analysis. For the tensile strength, the initial grip separation and crosshead speed were set at 50 mm and 2 mm/sec, respectively. The tensile strength (TS) was calculated as TS (pa) = F/(w × d),where F is force (N), d is film thickness (µm) and w is film width (m). The elongation at breaking point was measured directly from the instrument [23].
2.6.2. Seal Strength
In order to determine seal strength, the samples were cut into 2.54 cm × 5.00 cm strips. Later, two strips were joined together and sealed by an impulse heat sealer at a temperature of 110 ± 10 °C and a sealing time of 1–2 s. The unsealed ends of the strips were attached between the two jaws of the texture analyzer, where the distance between the jaws was set 5 cm apart and the crosshead speed was 2 mms−1 [24]. The tests were repeated with five film samples.
2.7. Thermal Analysis
Thermal estimation for the film was performed by hermetically sealing 5-mg film samples in aluminized pans using a differential scanning calorimeter (DSC) of TA Instruments (New Castle, DE, USA). Before the experiment, the films were conditioned for 48 h at 25 °C and 0% RH with silica gel to obtain the maximum dehydrated film samples [25].
2.8. Sensory Analysis
The sensory analysis of the edible films was performed through 30 semi-trained panelists (23 female and 7 male) at department level. The samples were presented to a panelist and plain water was given to them to rinse their mouth in between the evaluation of samples. Sensory evaluation was performed at 30 ± 2 °C, and the mean score of all attributes was used to estimate overall acceptability of the product. The overall acceptability was analyzed in terms of appearance, color, taste and flavor. The film samples (2 cm × 2 cm) were used for analysis. Sensory evaluation of films was performed using a hedonic scale (0–9): 1 = disliked extremely; 2 = disliked very much; 3 = disliked moderately; 4 = disliked slightly; 5 = liked/disliked; 6 = liked slightly; 7 = liked moderately; 8 = liked very much; 9 = liked extremely.
2.9. Statistical Analysis
A statistical study in triplicate was analyzed and compiled by statistical means using ANOVA (analysis of variation) and significant form (p < 0.05).
3. Results and Discussion
3.1. Physical and Proximate Analysis of Flaxseed
The results of the physical and proximate analysis of the flaxseed are shown in Table 2.

Table 2.
Physical characterization and proximate composition of the flaxseed.
3.1.1. Physical Characteristics
The flaxseed was characterized mainly in order to determine the operation processes, such as the machinability and feasibility of the process, during the processing, transportation and storage efficiency steps [2]. The length, width and thickness of the flaxseed were found to be 5.74 ± 0.01, 2.28 ± 0.15 and 2.28 ± 0.15 mm, respectively (Table 2). Similarly, the thousand kernel weight of the flaxseed was observed at 5.56 ± 0.25 g. The gravimetric properties, such as the bulk, tapped and true density of the flaxseed, were also studied (Table 2), and were found to be 650 ± 0.2, 670 ± 0.1 and 1110 ± 0.2 kg/m3, respectively. The flow properties of the flaxseed were determined using various parameters, such as the angle of repose, Hausner’s ratio (HR), Carr’s index (CI) and porosity, as illustrated in Table 2. Knowledge of the flow properties is essential for understanding the uniformity of weight and the content transfer from one place to another. Low CI and HR values signify better flow properties. A CI value of less than 10 and HR value of less than 1.11 is considered to be an excellent flow, whereas a CI value greater than 30 or HR value greater than 1.60 is considered to be a low flow. The intermediate flow has CI values ranging from 10 to 15. A HR value of 1.12 to 1.18 is a good flow, whereas a CI value of 16–20 or HR value of 1.23–1.25 is a proper flow [26]. In this study, the CI value of the flaxseed was 2.98 and the HR value was 1.030, therefore showing good flow properties.
3.1.2. Proximate Composition
The proximate analysis showed that the flaxseed meal had 2.25 ± 0.04% moisture, 18.39 ± 0.14% ash, 4.17 ± 0.11% crude protein, 39.78 ± 0.06% crude fat, 26.29 ± 0.14% fiber content and 35.50 ± 0.04% carbohydrates (Table 2). Bekhit et al. [27] reported 7.7% moisture, 4% ash, 20% crude protein, 41% crude fat and 28% total dietary fiber. Herchi et al. [20] observed 5.22% moisture, 2.90% ash, 22.65% protein, 35.10% fat and 34.12% total carbohydrates. The variation in the above-cited proximate values may be due to the traditional plant breeding practices and geographical conditions. Gathered data also govern the nutraceutical traits embedded within the seeds, owing to their high crude fiber and crude protein framework; additionally, their high level of ash somehow reflects their divalent cations proportion too, which is necessary for healthy and immune-boosted longevity [28,29].
3.2. Rheological Behavior
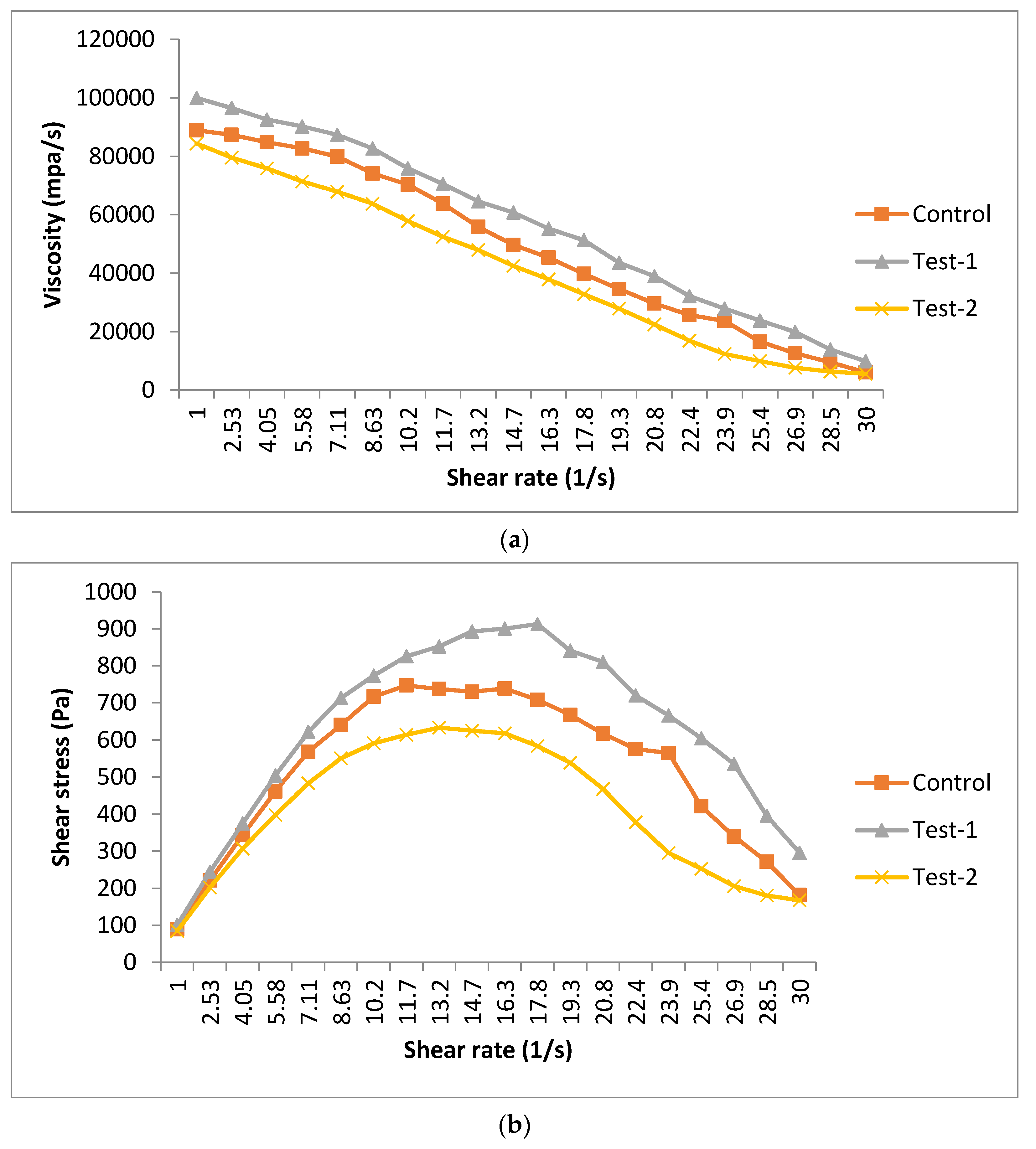
The rheological behavior of different film-forming solutions is represented in Figure 1. These results represented the change in viscosity of film-forming solutions as a function of shear rate. Different solutions (control, test-1 and test-2) showed a variation in the viscosity. The higher viscosity was observed in the test-1 sample (99,994.5 mpa/s), followed by the control (88,932.5 mpa/s) and test-2 (84,373.3 mpa/s). The higher viscosity found in the test-1 sample was related to the combination of flaxseed and pectin, which makes a thick solution. The test-2 sample had a lower viscosity than the control, indicating that the thin solution was formed with only the flaxseed meal. Figure 1a shows that the viscosity decreases as the shear rate increases from 1 to 30/s, irrespective of the sample. It also shows the shear thinning behavior of all of the samples. Figure 1b shows the curve of the shear stress, with respect to the shear rate. As the shear rate increased, the shear stress first increased and then decreased, indicating the non-linear relationship between shear rate and shear stress.
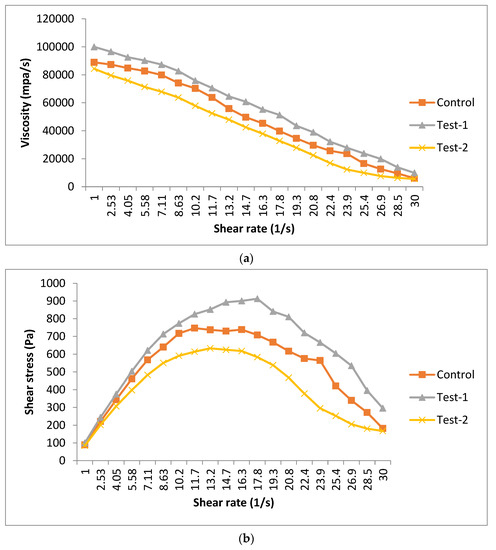
Figure 1.
Rheological characteristics—(a) steady shear (viscosity vs. shear rate) and (b) (shear stress vs. shear rate)—of different edible films. Control (pectin+ glycerol); test-1 (flaxseed + pectin + glycerol); test-2 (flaxseed + glycerol).
The data of different sample solutions were adjusted to the power law model shown, which was a goodness of fit with correlation coefficients R2 = 0.98 (control), R2 = 0.99 (test-1) and R2 = 0.95 (test-2), as summarized in Table 3. The flow behavior (n) was observed as less than 1 in all of the sample solutions, confirming the pseudo-plastic behavior of the solutions. These results were in concordance with the study of other authors for pectin- or starch-based films [30,31]. The consistency index (k) was higher for highly viscous solutions. This is related to the increased intermolecular interactions, which may resist the molecule movement. On the other hand, the control and test-2 sample showed less viscous behavior, which may be related to the lower concentration of polysaccharides, or not having the appropriate thickening and gelling agent [32].

Table 3.
Rheological parameters of edible films fitted to power law model.
3.3. Film Characterization
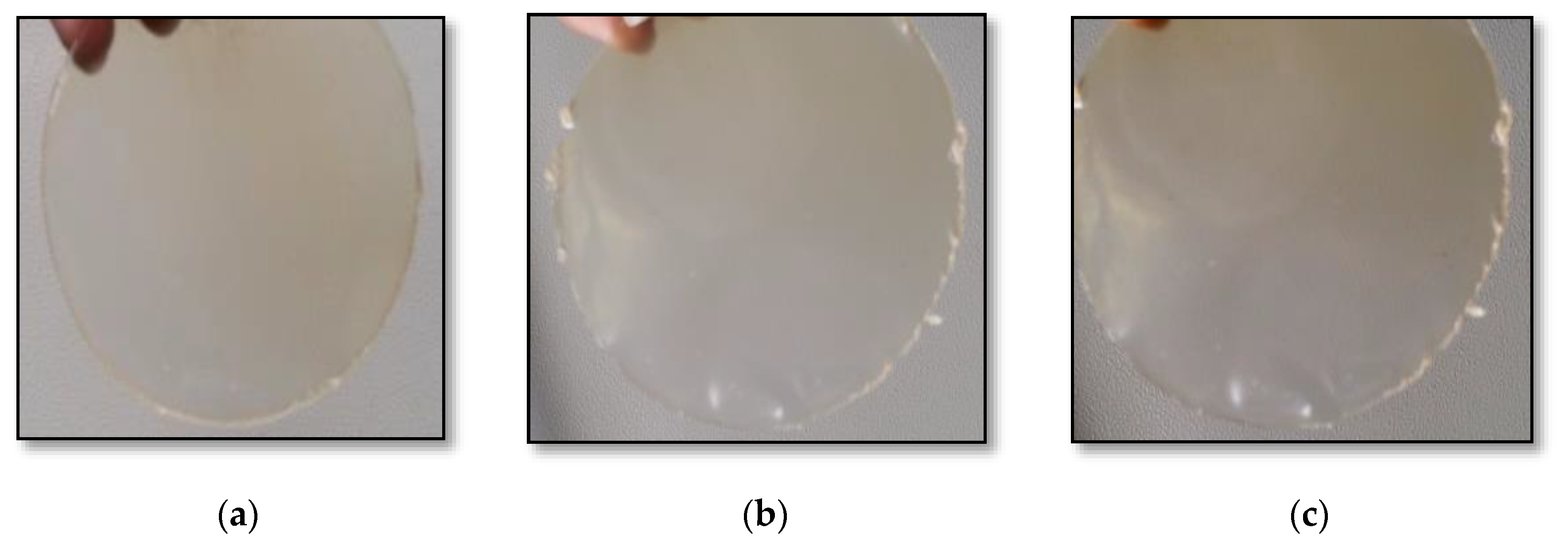
The casted films (control, test-1 and test-2 in triplicate) were packed carefully and stored under ambient conditions. They were then (Figure 2) analyzed for various parameters, as summarized in Table 4. The pectin-free film (test-2) was brittle and required care in peeling from the casted surface, whereas pectin incorporation (test-1) elevated its flexibility and made it easier to handle. Therefore, the pectin incorporated into the film-forming solutions enhanced the flexibility of the film. The film prepared without the flaxseed meal was taken as the control. The experiment was performed to determine the best combination required for film formation. It was recognized that high-quality film-forming solutions can be obtained using 2% flaxseed meal and 5% pectin.
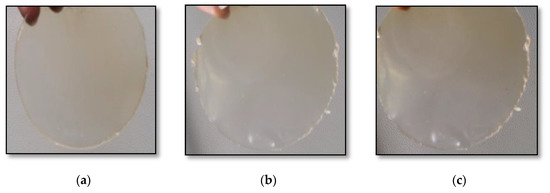
Figure 2.
Images of edible films: (a) test-1; (b) test-2; (c) control.

Table 4.
Physical, chemical and mechanical properties of the prepared films.
3.3.1. Thickness
Table 4 reveals the impact of flaxseed and pectin addition directly on the thickness of the films. There was a significant (p < 0.05) difference in the thickness of the three films. Flexibility among films is governed by variations in thickness; hence, it is suited to a long range of commodities, such as the packaging system. In addition to this, thickness finds many other ways of suitability through the given attributes, such as tensile strength, elongation and water vapor permeability [33]. The lowest thickness was observed in the test-2 sample (0.241 mm), compared to the control (0.625 mm) and test-1 (0.773 mm). The increase in the thickness of the test-1 sample may be due to the pectin forming cross-links with that of other constituents under its molecular framework, which serve to enhance the physical and chemical properties of the edible film [34,35]. The thickness of the control sample was less than the test-1 sample. This may be due to the absence of flaxseed gum in this sample, as the addition of flaxseed gum during preparation of the film significantly improved (p > 0.05) the thickness of the resulting films. These results were in concordance with a study by Namratha et al. [36], which reported that films are more stable and more complex with the addition of pectin. The various authors reported the non-significant effect of different concentration of glycerol on the thickness of the film [37].
3.3.2. Moisture Content
The variation in the additive proportion for the test and control samples was easily depicted through the observed percentage of moisture content (Table 4). The incorporation of pectin to the test samples provided many soluble solid fractions and increased the moisture of the developed films. The moisture content of the films ranged from 35.53 to 35.92%. This high percentage of moisture mass observed among the flaxseed- and pectin-loaded film samples (test-1) is directly linked to the high water-holding capacity of pectin and flaxseeds [38]. The presence of glycerol also contributes, wherein the water-holding capacity rises due to the hydrogen bonding that develops between the hydroxyl groups of glycerol and flaxseeds [39].In biopolymer films, the OH groups along the plasticizer chains build up polymer–plasticizer hydrogen bonds, which replace the polymer–polymer interactions [40], resulting in the high moisture content of the film.
3.3.3. Color
Appearance and color are the foremost characteristics that add to the acceptability of a product. Customer attraction is a phenomenon based on appearance and helps to drive a product’s market adaptability. In relation to this, clear edible films are typically more desirable. Visually, the edible films had a slightly dull appearance (Figure 2). Table 4 shows the L* (lightness/darkness), a* (redness/greenness) and b* (yellowness/blueness) CIE Lab color values of the films. A color change was observed after the incorporation of flaxseed. The L*, a* and b*values of the film with the flaxseed (test-1) were 59.47 ± 0.14, 4.98 ± 0.09 and 14.36 ± 0.17, respectively. A light difference in the color value was also observed compared to the film without flaxseed (53.26 ± 0.15, 4.10 ± 0.18 and 19.54 ± 0.11, respectively) and the control (65.62 ± 0.12, 5.10 ± 0.14 and 9.68 ± 0.09, respectively). It was observed that the addition of the flaxseed meal resulted in decrease in the L* and a* value, and an increase in the b* value. Hence, the flaxseed-based edible films became slightly brownish; however, they remained transparent (Figure 2).
3.3.4. Light Transmission and Transparency
Concerning the optical properties, transparency is the promising feature to have both acceptability terms and consumer preferences. Present trends and scenarios demand transparent packaging material for food, allowing product visibility. The edible flaxseed-based pectin sample (test-1) had a transparency of 17.78; therefore, the film is clear enough to be used as see-through packaging material. Similar tests for the control sample had a transparency of 38.25, indicating that the film was reasonably transparent (Table 4).This increased value for the control sample may be attributed to pectin agglomeration below the film, or due to the uneven casting of the film. Pectin significantly affects the transparency of the edible film, as reported by other authors [10,12]. In contrast, the film prepared with only flaxseed (test-2) showed a poor transparency, with a value of 44.63. Therefore, the results indicated that films incorporated with a combination of flaxseed gum, pectin and glycerol became more transparent and clearer enough; hence, they can be used as transparent coating materials or packaging.
3.3.5. Water Vapor Permeability
The deterioration of food in packaging depends on the water transfer between the internal products and their surroundings. Therefore, there is a requirement for the water barrier characteristics of packaging material. The increased film thickness provides resistance to mass transfer across the film [41].Water vapor permeability values for the test-1 sample and the control were competitively close to each other, and were found to be 0.992 and 0.981 mg mm day−1 cm−2, respectively, whereas test-2 showed a very poor water permeability (1.035 mg mm day−1 cm−2) (Table 4). This indicated that pectin had a significant effect on the water vapor permeability of the film. The water vapor permeability of the films was lesser than synthetic films, such as polyester films (3791 mg mm day−1 cm−2) and high-density polyethylene films (HDPE) (500 mg mm day−1 cm−2) [42].An elevated water vapor pressure equilibrium on the inner surface of the film increases its permeability characteristics; therefore, a relative humidity gradient between the film and its surroundings comes into consideration. Tee et al. [43] also reported the excellent barrier properties of flaxseed-based films. The differences in results between the films may be related to the presence of different combinations of the flaxseed meal, pectin and glycerol. The results showed the superior water barrier properties of the film and its possible use as edible packaging for different products.
3.3.6. Solubility Index
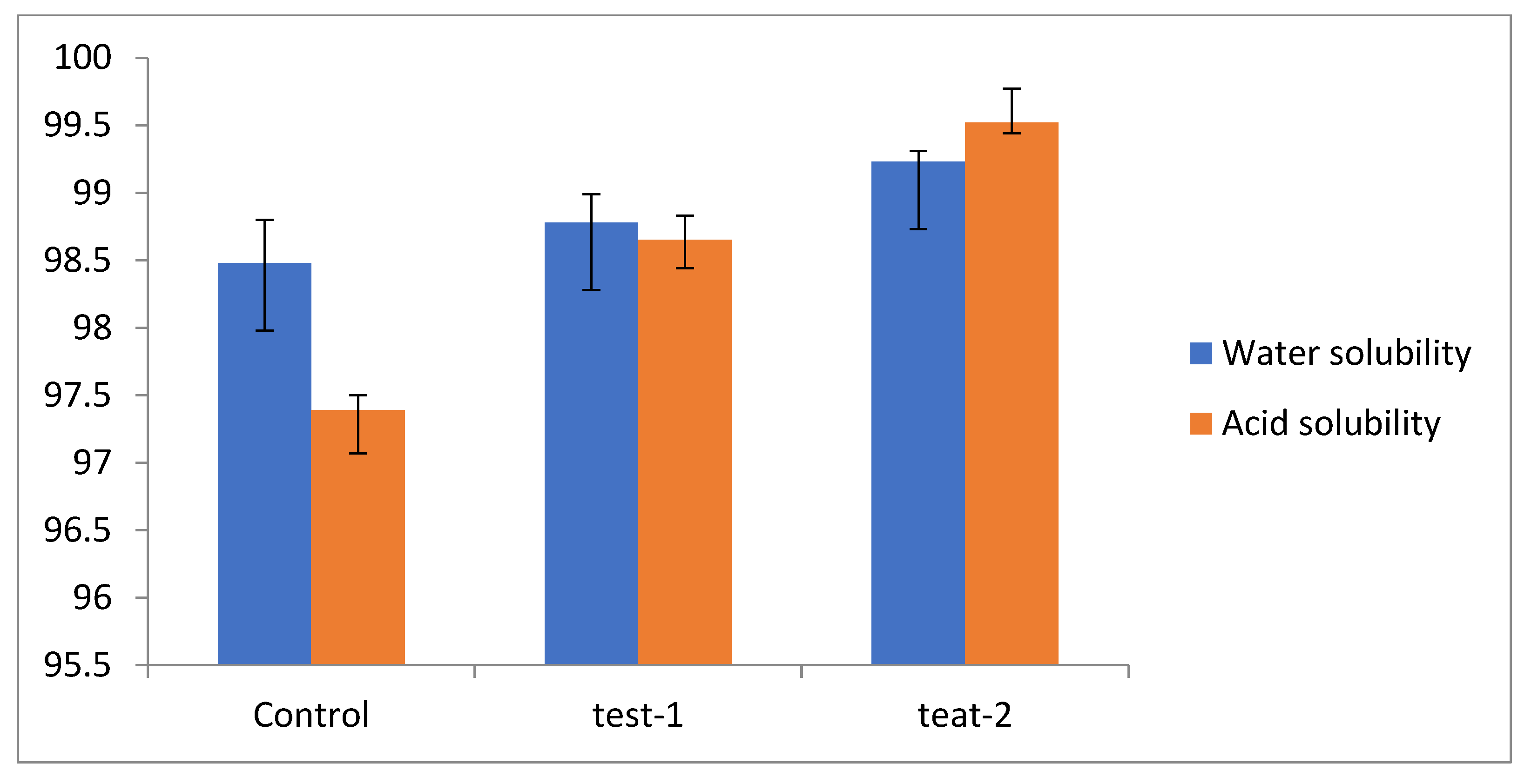
Solubility is an essential aspect of edible films, as it governs the consumers’ preferential-cum-selection attributes, in addition to its main role as a food protectant [44]. Solubility is a notably vital feature, especially when a film is in contact with water. Generally, a higher solubility would invite broader application appropriateness. In addition, solubility can be addressed in terms of the convenience of edible films among ready-to-eat foods as they dissolve in boiled water and the consumer’s mouth [45,46]. Biodegradability may also be a possible advantage when considering environmental concerns. As the solubility value of the test films in acid and water is very high, when compared to the control (Figure 3), this indicates that the films are readily soluble in the mouth and, therefore, will not stick to the inside of the stomach. The high solubility of flaxseed-meal-based films may be due to the hydrophilic nature of the polysaccharide and slightly branched structure of flaxseed. In view of the hydrophilic nature of the flaxseed meal, the film was dissolved in water and lost its strength with time. On the other hand, the high-soluble films may be beneficial in some applications; for instance, in ready-to-eat products, in which the film could melt in boiling water during preparation [47].
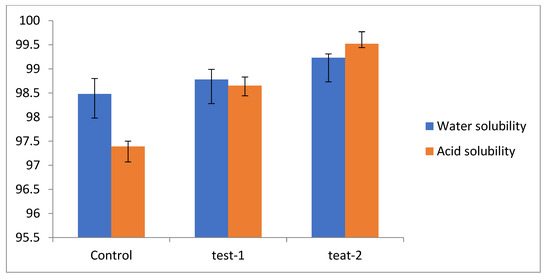
Figure 3.
Solubility index of different films: control (pectin + glycerol); test-1 (flax + pectin + glycerol); test-2 (flaxseed + glycerol). The error bars indicate the standard deviation of means n = 3 (p ≤ 0.05).
3.4. Mechanical Properties of Films
Mechanical properties indicate the durability of the films and, to a greater extent, govern their mechanical integrity, along with the food’s structure. The mechanical behavior of the film, along with the incorporated varied proportion of flaxseed meal and pectin, is presented in Table 4.
3.4.1. Tensile Strength and Elongation at Break
The effect of pectin and flaxseed on the tensile strength and percentage of elongation values of the films is shown in Table 4. The resulting tensile strength (3.97 ± 0.14) of the test-1 sample was close to the control sample (3.74 ± 0.10).The pectin-containing films showed a high tensile strength. Here, the pectin network plays a significant role in its strong bonding with other compounds by interfacial hydrogen and ionic connections [48]. Flexibility can be seen through the elongation at break values of the samples (Table 4). It was observed that the addition of glycerol (plasticizer) during the preparation of the films does not impact their extensibility or flexibility in any way. Small glycerol molecules may have penetrated the mucilage polymer matrix, thereby causing a cross-linking effect in the matrix [20].
3.4.2. Seal Strength
Seal strength also reflects the mechanical strength of the films (Table 4). It was observed that the seal strength was slightly lower in the test samples compared to the control. Usually, a high seal strength value is advantageous, as it triggers a high separation force and is required for food safety. In contrast, a low seal strength may be desirable in some applications where trouble-free opening is required [49]. It was observed that the strong interactions between functional groups of pectin molecules and the flaxseed meal through hydrogen and ionic interactions enhanced the mechanical properties of the edible film.
3.5. Thermal Properties
It is essential to know the thermal stability of the film. The DSC showed a single Tg value, which was different depending on the film composition. The thermal analysis characterization of the developed films revealed their processing temperature limits and application concerns. Table 4 shows the DSC results for the film samples (tests and control). Thermal analysis also provides insight towards the mechanical strengths of the films. The Tg value was highest for the control film, due to its high pectin content (72.35 ± 0.10). The lowest Tg value (62.10 ± 0.10) was observed for the flaxseed film (test-2). Highly methoxylated pectin showed higher Tg values (150 °C), where the degree of methylation in the pectin was over 60%, which explains its thermal stability compared to the flaxseed film [50].
3.6. Sensory Analysis
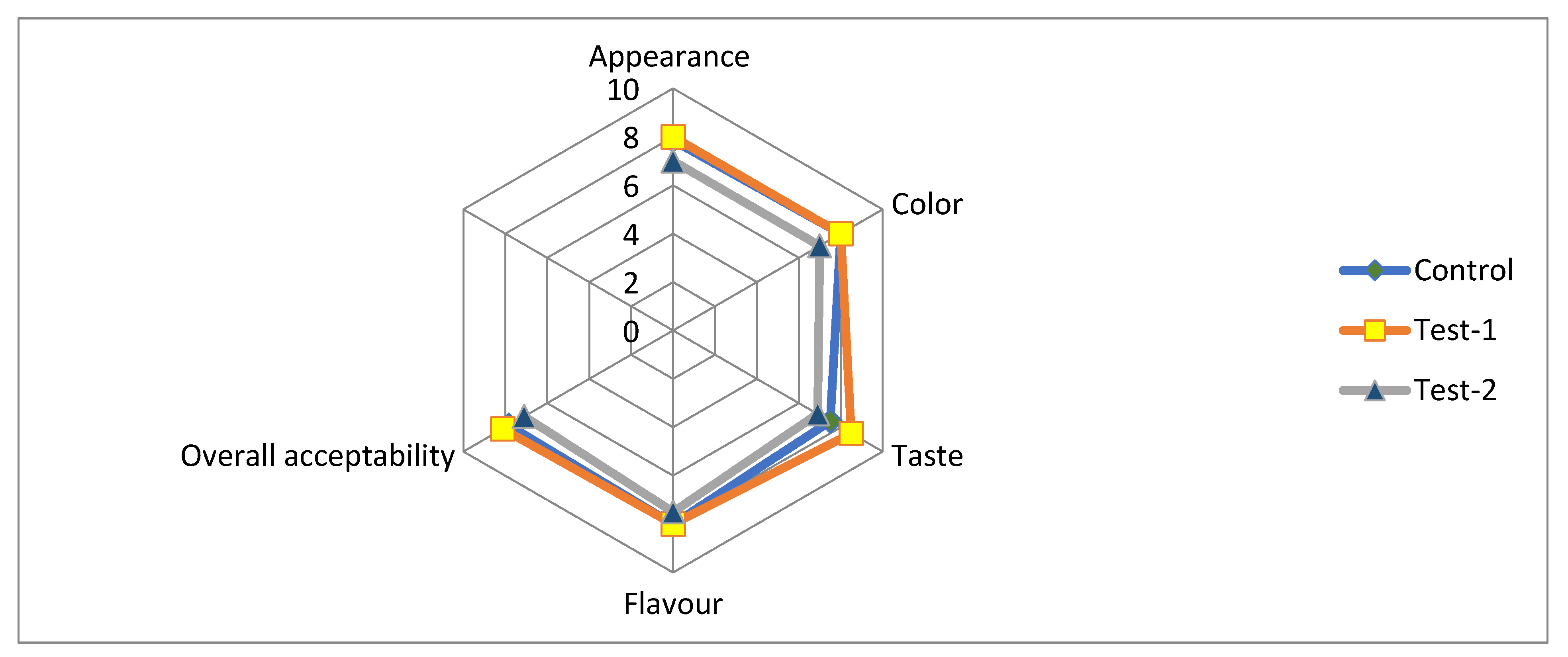
Casted samples of film (three sample) were evaluated for sensory attributes (appearance, color, taste, flavor and overall acceptability), as presented in Figure 4. The scores of different sensory attributes were between six (liked slightly) to eight (liked very much), irrespective of the film composition. The sensory score of the test-1 film was higher than the control and test-2. The scores of the control sample were little-varied and significantly different to the test-1 sample.
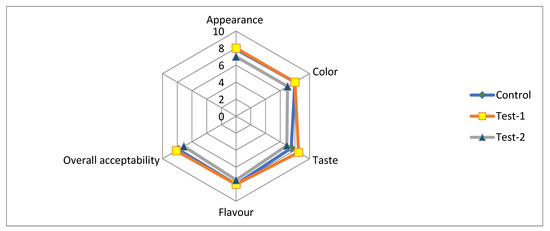
Figure 4.
Sensory analysis for all of the film specimens: control (pectin + glycerol); test-1 (flaxseed + pectin + glycerol); test-2 (flaxseed + glycerol).
4. Conclusions
The plant-based films were prepared using pectin and flaxseed, with glycerol as a plasticizer. The results showed that the resultant film had good rheological and mechanical properties. Therefore, this study utilized a biomaterial-based approach to obtain a biodegradable and sustainable edible film for various foods and feed industrial applications. The extension of application summarizes its intended use as a sustainable alternative for raw and processed food commodities, including fresh produce, in order to stretch their longevity. The studied concept of maximum elongation at break values for the investigated glycerol was sufficient in providing an optimum ductility, transparency and color. The pectin + flaxseed-based film was almost transparent, with significant water solubility features. Its application may be found in food, pharma, cosmetics and toiletry industries, as well as in agricultural practices. Coating by means of edible packaging is also a capable method for diversifying and adding value to foods by reducing food package waste.
Author Contributions
Conceptualization, S.P.B. and A.S.; methodology, A.S.; Software, M.K.; validation, S.P.B. and P.K.; Formal analysis, A.S.; Investigation, N.K.; resources, M.T.; data curation, A.S. and R.K.; wtiting-review and editing, R.K. and N.K.; Visualiztion, P.K.; supervision, A.S.; project administration, A.S.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We extend our thanks to our Director-Principal, Kashmir Singh (Mata Gujri College, Fatehgarh Sahib, India), for providing us with easy access to resources and the platform to carry out this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deluca, J.A.; Garcia-Villatoro, E.L.; Allred, C.D. Flaxseed bioactive compounds and colorectal cancer prevention. Curr. Oncol. Rep. 2018, 20, 59. [Google Scholar] [CrossRef]
- Ganorkar, P.M.; Jain, R.K. Flaxseed—A nutritional punch. Int. Food Res. J. 2013, 20, 519–525. [Google Scholar]
- Khare, B.; Sangwan, V.; Rani, V. Influence of sprouting on proximate composition, dietary fiber, nutrient availability, antinutrient, and antioxidant activity of flaxseed varieties. J. Food Process. Preserv. 2021, 45, e15344. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.X.; de Jesús Avena-Bustillos, R.; Soares, N.D.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties-A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Trif, M.; Vodnar, D.C.; Mitrea, L.; Rusu, A.V.; Socol, C.T. Design and development of oleoresins rich in carotenoids coated microbeads. Coatings 2019, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-a corn starch-eugenol edible film: Physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef]
- Rusu, A.V.; Criste, F.L.; Mierliţă, D.; Socol, C.T.; Trif, M. formulation of lipoprotein microencapsulated beadlets by ionic complexes in algae-based carbohydrates. Coatings 2020, 10, 302. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.A.; Martino, M.N.; Zaritzky, N.E. Microstructural characterization of plasticized starch-based films. Starch-Stärke 2000, 52, 118–124. [Google Scholar] [CrossRef]
- Farooq, M.; Azadfar, E.; Rusu, A.; Trif, M.; Poushi, M.K.; Wang, Y. improving the shelf life of peeled fresh almond kernels by edible coating with mastic gum. Coatings 2021, 11, 618. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutierrez, G.; Vioque, B.; Rubio-Senent, F.; Fernandez Bolanos, J. Physical and functional properties of pectin-fish gelatin films containing the olive phenols hydroxyl tyrosol and 3,4-dihydroxyphenylglycol. Carbohydr. Polym. 2017, 178, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural pectin polysaccharides as edible coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef] [Green Version]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Sanchez, A.; Villalonga Santana, R.; Mariniello, L. Effect of mesoporous silica nanoparticles on the physicochemical properties of pectin packaging material for strawberry wrapping. Nanomaterials 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giosafatto, C.V.L.; Sabbah, M.; Al-Asmar, A.; Esposito, M.; Sanchez, A.; Villalonga Santana, R.; Cammarota, M.; Mariniello, L.; Di Pierro, P.; Porta, R. Effect of mesoporous silica nanoparticles on glycerol-plasticized anionic and cationic polysaccharide edible films. Coatings 2019, 9, 172. [Google Scholar] [CrossRef] [Green Version]
- Luna-Sosa, B.; Martínez-Ávila, G.C.; Rodríguez-Fuentes, H.; Azevedo, A.G.; Pastrana, L.M.; Rojas, R.; Cerqueira, M.A. Pectin-Based Films Loaded with Hydroponic Nopal Mucilages: Development and Physicochemical Characterization. Coatings 2020, 10, 467. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Characteristics and antimicrobial properties of active edible films based on pectin and nanochitosan. Int. J. Mol. Sci. 2020, 21, 2224. [Google Scholar] [CrossRef] [Green Version]
- Tumbarski, Y.; Petkova, N.; Todorova, M.; Ivanov, I.; Deseva, I.; Mihaylova, D.; Ibrahim, S.A. Effects of pectin-based edible coatings containing a bacteriocin of Bacillus methylotrophicus BM47 on the quality and storage life of fresh blackberries. Ital. J. Food Sci. 2020, 32, 420–437. [Google Scholar]
- AOAC. Official Methods of Analyses, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2012. [Google Scholar]
- Kaur, R.; Kaur, K. Effect of processing on color, rheology and bioactive compounds of different sweet pepper purees. Plant Foods Hum. Nutr. 2012, 75, 369–375. [Google Scholar] [CrossRef]
- Tee, Y.B.; Wong, J.; Tan, M.C.; Talib, R.A. Development of edible film from flaxseed mucilage. BioResources 2016, 11, 10286–10295. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H.; Floros, J.D. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Y.; Shi, Q. Characterization of a novel edible film based on gum ghatti: Effect of plasticizer type and concentration. Carbohydr. Polym. 2016, 153, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Pirsa, S.; Mohtarami, F.; Kalantari, S. Preparation of biodegradable composite starch/tragacanth gum/nanoclay film and study of its physicochemical and mechanical properties. Chem. Rev. Lett. 2020, 3, 98–103. [Google Scholar]
- Das, M.; Chowdhury, T. Heat sealing property of starch basedself-supporting edible films. Food Packag. Shelf Life 2016, 9, 64–68. [Google Scholar] [CrossRef]
- Jridi, M.; Abdelhedi, O.; Salem, A.; Kechaou, H.; Nasri, M.; Menchari, Y. Physicochemical, antioxidant and antibacterial properties of fish gelatin-based edible films enriched with orange peel pectin: Wrapping application. Food Hydrocoll. 2020, 103, 105688. [Google Scholar] [CrossRef]
- Shah, R.B.; Tawakkul, M.A.; Khan, M.A. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech 2008, 9, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Bekhit, A.E.D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Herchi, W.; Bahashwan, S.; Sebei, K.; Saleh, H.B.; Kallel, H.; Boukhchina, S. Effects of germination on chemical composition and antioxidant activity of flaxseed (Linum usitatissimum L.) oil. Grasas y Aceites 2015, 66, 057. [Google Scholar] [CrossRef] [Green Version]
- Forstner, S.; Rusu, A. Development of personalised food for the nutrition of elderly consumers. In Know Your Food: Food Ethics and Innovation; Academic Publishers: Wageningen, The Netherlands, 2015; pp. 24–27. [Google Scholar]
- Rusu, A.; Randriambelonoro, M.; Perrin, C.; Valk, C.; Álvarez, B.; Schwarze, A.-K. Aspects influencing food intake and approaches towards personalising nutrition in the elderly. J. Popul. Ageing 2020, 13, 239–256. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.K.; Chaturvedi, K.; Yadav, S.K. Evaluation of structural integrity and functionality of commercial pectin based edible films incorporated with corn flour, beetroot, orange peel, muesli and rice flour. Food Hydrocoll. 2019, 91, 127–135. [Google Scholar]
- Meza, B.E.; Peralta, J.M.; Zorrilla, S.E. Rheological properties of a commercial food glaze material and their effect on the film thickness obtained by dip coating. J. Food Process Eng. 2015, 38, 510–516. [Google Scholar] [CrossRef]
- Ni, X.; Wang, K.; Wu, K.; Corke, H.; Nishinari, K.; Jiang, F. Stability, microstructure and rheological behavior of konjac glucomannan- zein mixed systems. Carbohydr. Polym. 2018, 188, 260–267. [Google Scholar] [CrossRef]
- Arham, R.; Salengke, S.; Metusalach, M.; Mulyati, M.T. Optimization of agar and glycerol concentration in the manufacture of edible film. Int. Food Res. J. 2018, 25, 1845–1851. [Google Scholar]
- Wu, H.; Lei, Y.; Zhu, R.; Zhao, M.; Lu, J.; Xiao, D.; Jiao, C.; Zhang, Z.; Shen, G.; Li, S. Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll. 2019, 90, 41–49. [Google Scholar] [CrossRef]
- Namratha, S.; Sreejit, V.; Preetha, R. Fabrication and evaluation of physicochemical properties of probiotic edible film based on pectin–alginate–casein composite. Int. J. Food Sci. Technol. 2020, 55, 1497–1505. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A.; Yarmand, M.S. Development and characterization of new biodegradable edible film made from kefiran, an exopolysaccharide obtained from kefir grains. Food Chem. 2011, 127, 1496–1502. [Google Scholar] [CrossRef]
- Salehi, F. Characterization of new biodegradable edible films and coatings based on seeds gum: A review. J. Packag. Technol. Res. 2019, 3, 193–201. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; de Oliveira Rios, A.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Paulson, A.T. Mechanical and water vapor barrier properties of edible gellan films. Food Res. Int. 2000, 33, 563–570. [Google Scholar] [CrossRef]
- Pierro, P.; Di Mariniello, L.; Giosafatto, C.V.L.; Masi, P.; Porta, R. Solubility and permeability properties of edible pectin-soy flour films obtained in the absence or presence of transglutaminase. Food Biotechnol. 2006, 19, 37–49. [Google Scholar] [CrossRef]
- McHugh, T.H.; Avena-Bustillas, R.; Krochta, J.M. Hydrophilic edible films: Modified procedure for water vapor permeability and explanation of thickness effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Tee, Y.B.; Tee, L.T.; Daengprok, W.; Talib, R.A. Chemical, physical, and barrier properties of edible film from flaxseed mucilage. BioResources 2017, 12, 6656–6664. [Google Scholar] [CrossRef] [Green Version]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Physical, mechanical, barrier, and thermal properties of polyol-plasticized biodegradable edible film made from kefiran. Carbohydr. Polym. 2011, 84, 477–483. [Google Scholar] [CrossRef]
- Kumar, N. Polysaccharide-based component and their relevance in edible film/coating: A review. Nutr. Food Sci. 2019, 50, 793–823. [Google Scholar] [CrossRef]
- Dietrich, T.; Velasco, M.V.; Echeverría, P.; Pop, B.; Rusu, A. Crop and plant biomass as valuable material for BBB. Alternatives for valorization of green wastes. In Biotransformation of Agricultural Waste and by-Products: The Food, Feed, Fibre, Fuel (4F) Economy; Elsevier: San Diego, CA, USA, 2016. [Google Scholar]
- Pitak, N.; Rakshit, S.K. Physical and antimicrobial properties of banana flour/chitosan biodegradable and self sealing films used for preserving Fresh-cut vegetables. LWT-Food Sci. Technol. 2011, 44, 2310–2315. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Voon, H.C.; Bhat, R.; Easa, A.M.; Liong, M.T.; Karim, A.A. Effect of addition of halloysitenanoclay and SiO2 nanoparticles on barrier and mechanical properties of bovine gelatin films. Food Bioprocess Technol. 2012, 5, 1766–1774. [Google Scholar] [CrossRef]
- Iijima, M.; Hatakeyama, T.; Nakamura, K.; Hatakeyama, H. Thermomechanical analysis of polysaccharide hydrogels in water. J. Therm. Anal. Calorim. 2001, 64, 617–627. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).