Preparation and Application of Polyurethane Coating Material Based on Epoxycyclohexane-Tetrahydrofuran in Paper Conservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CHO-THF Copolyether
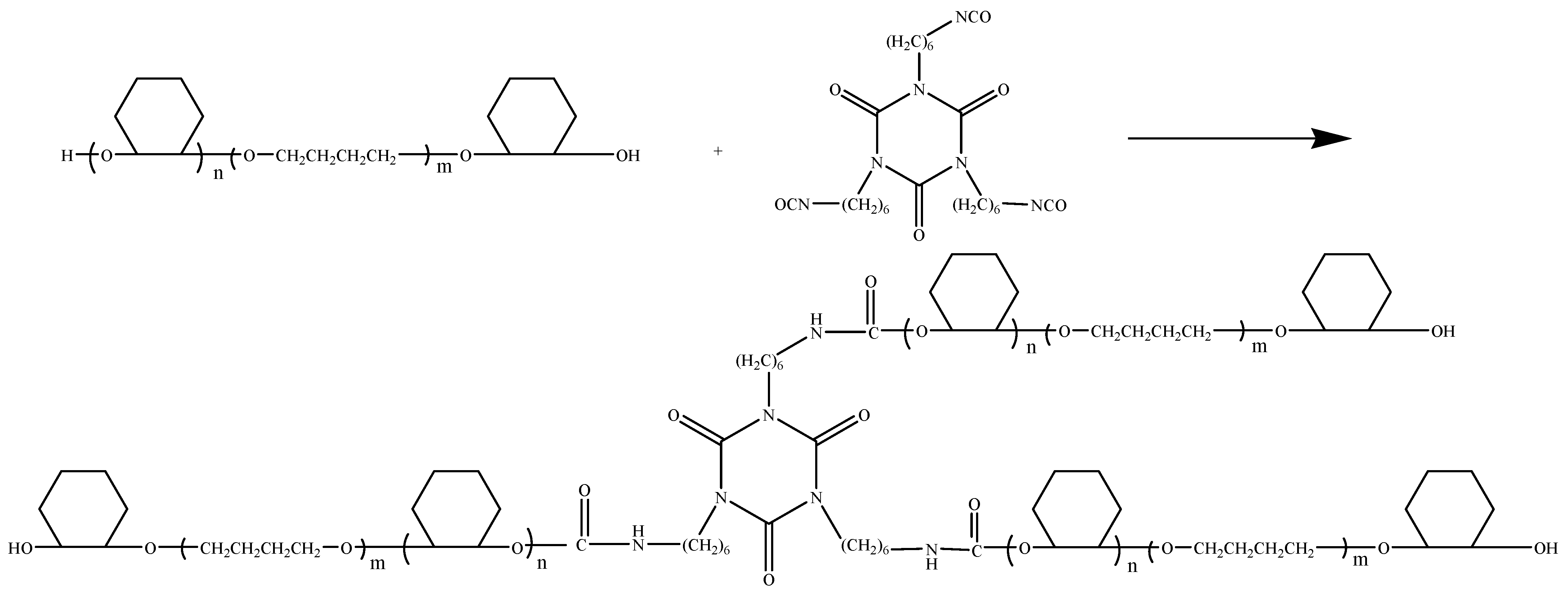
2.3. Synthesis of Polyurethane Based on CHO-THF Copolyether
2.4. Paper Coating
2.5. Analysis and Testing Methods
3. Results and Discussion
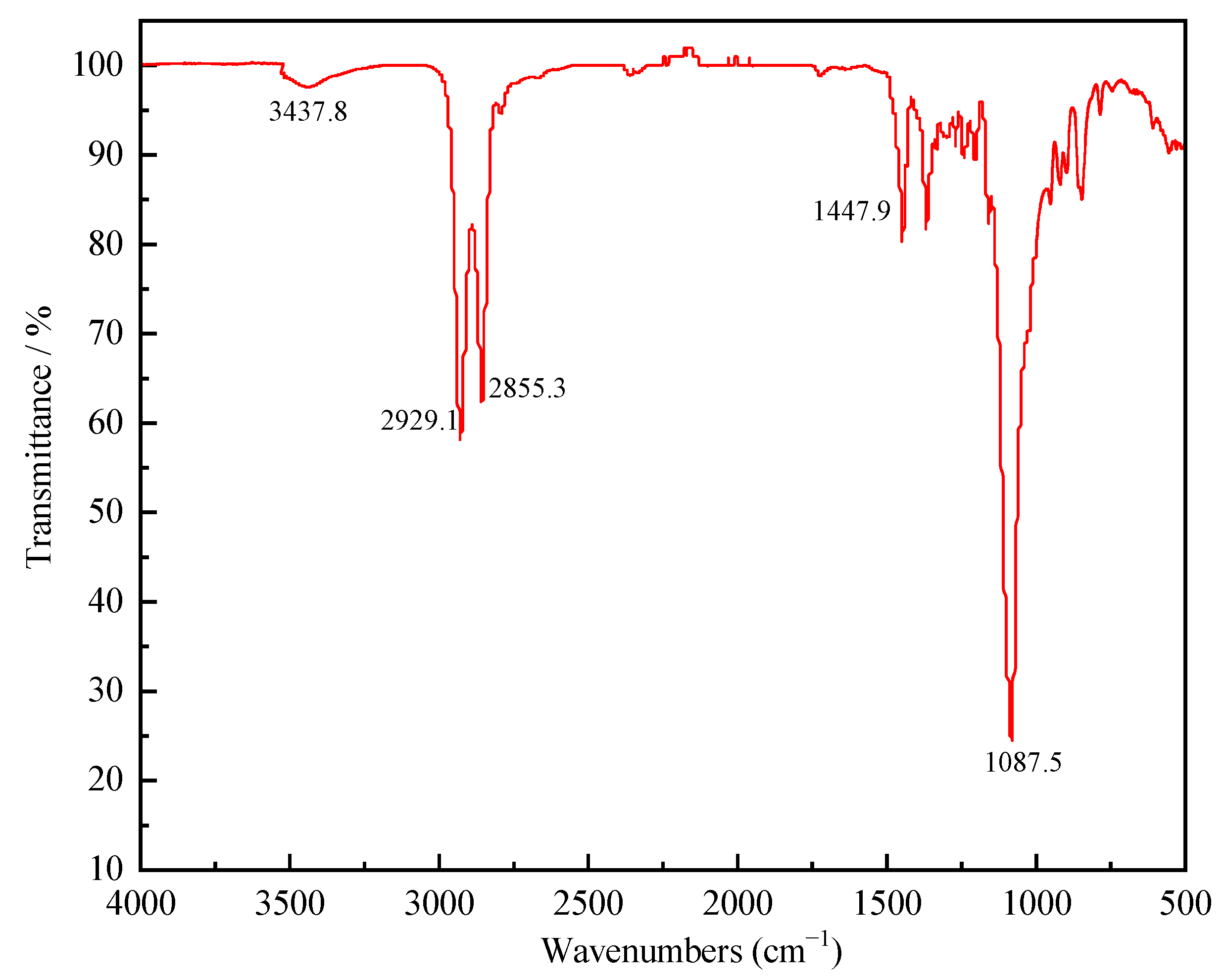
3.1. The Reaction Mechanism of CHO-THF Copolyether
3.2. Properties of Polyurethane Based on CHO-THF Copolyether
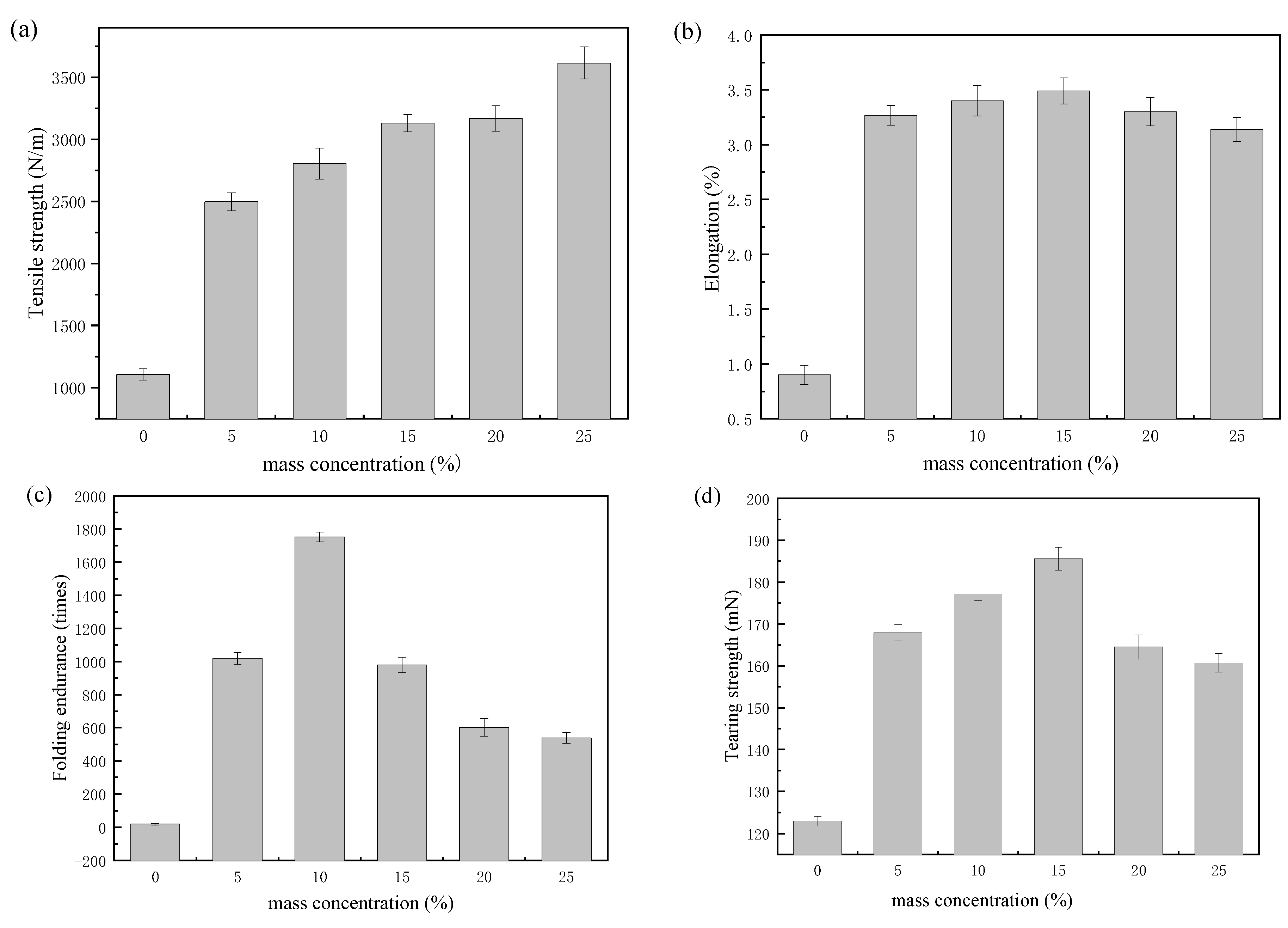
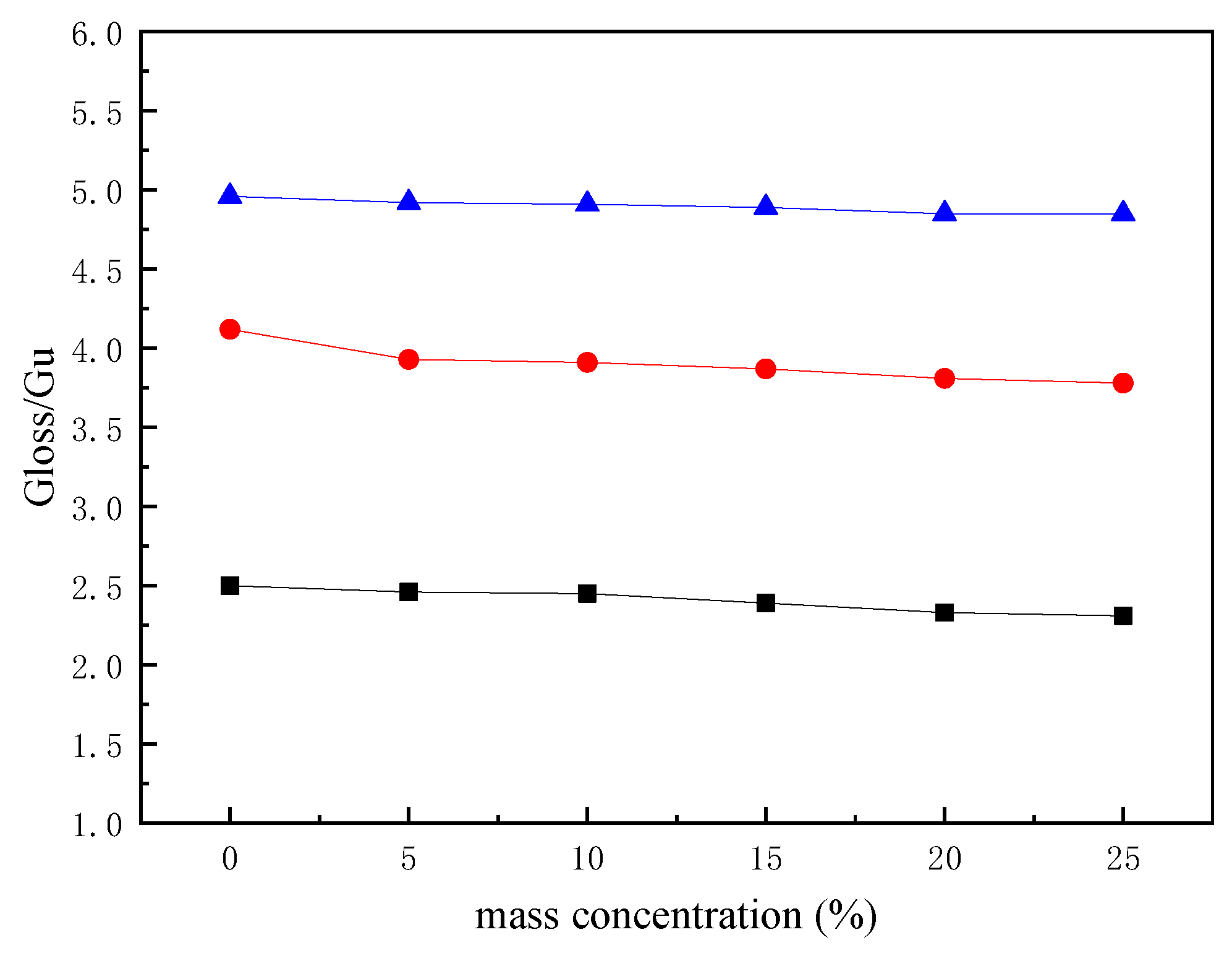
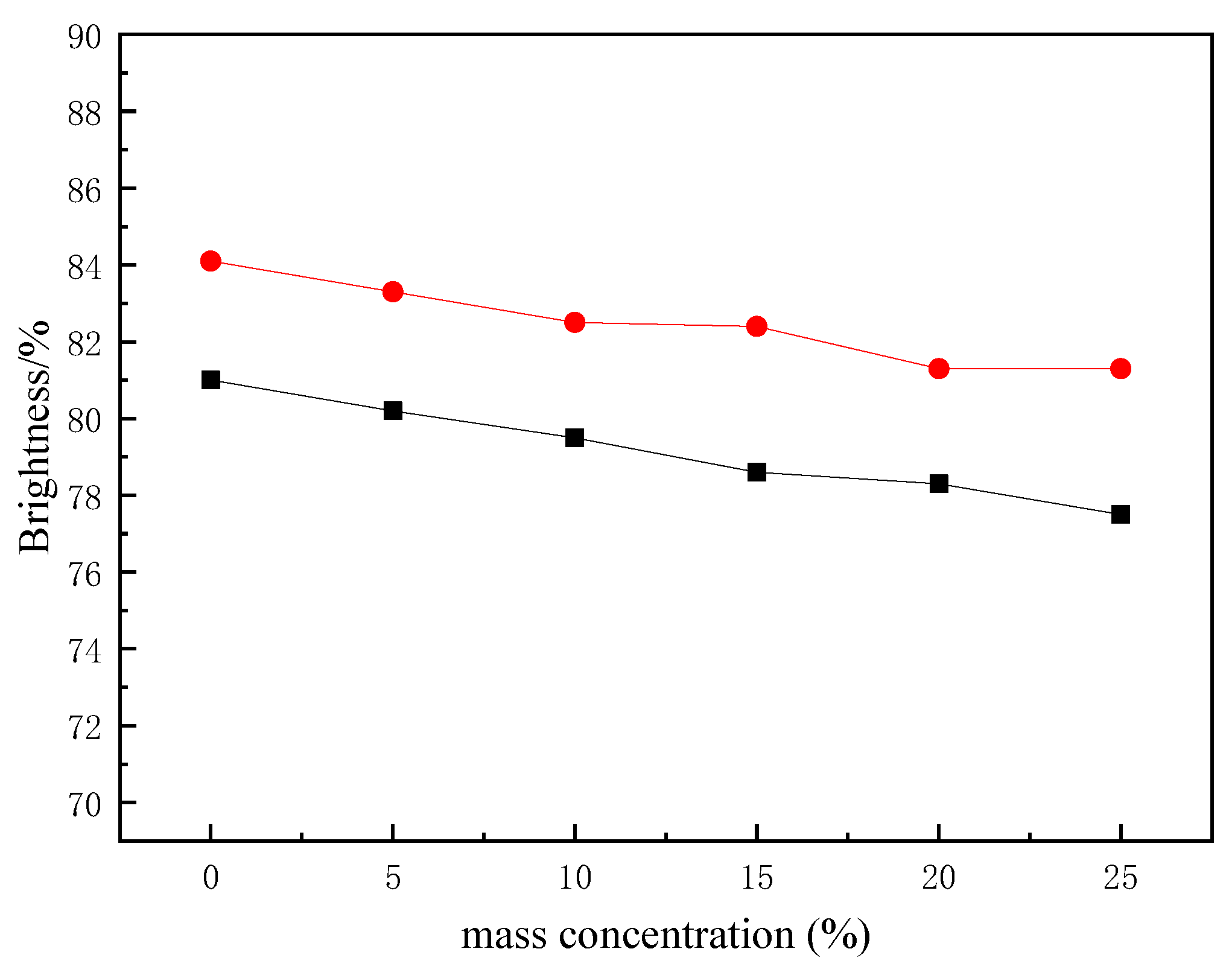
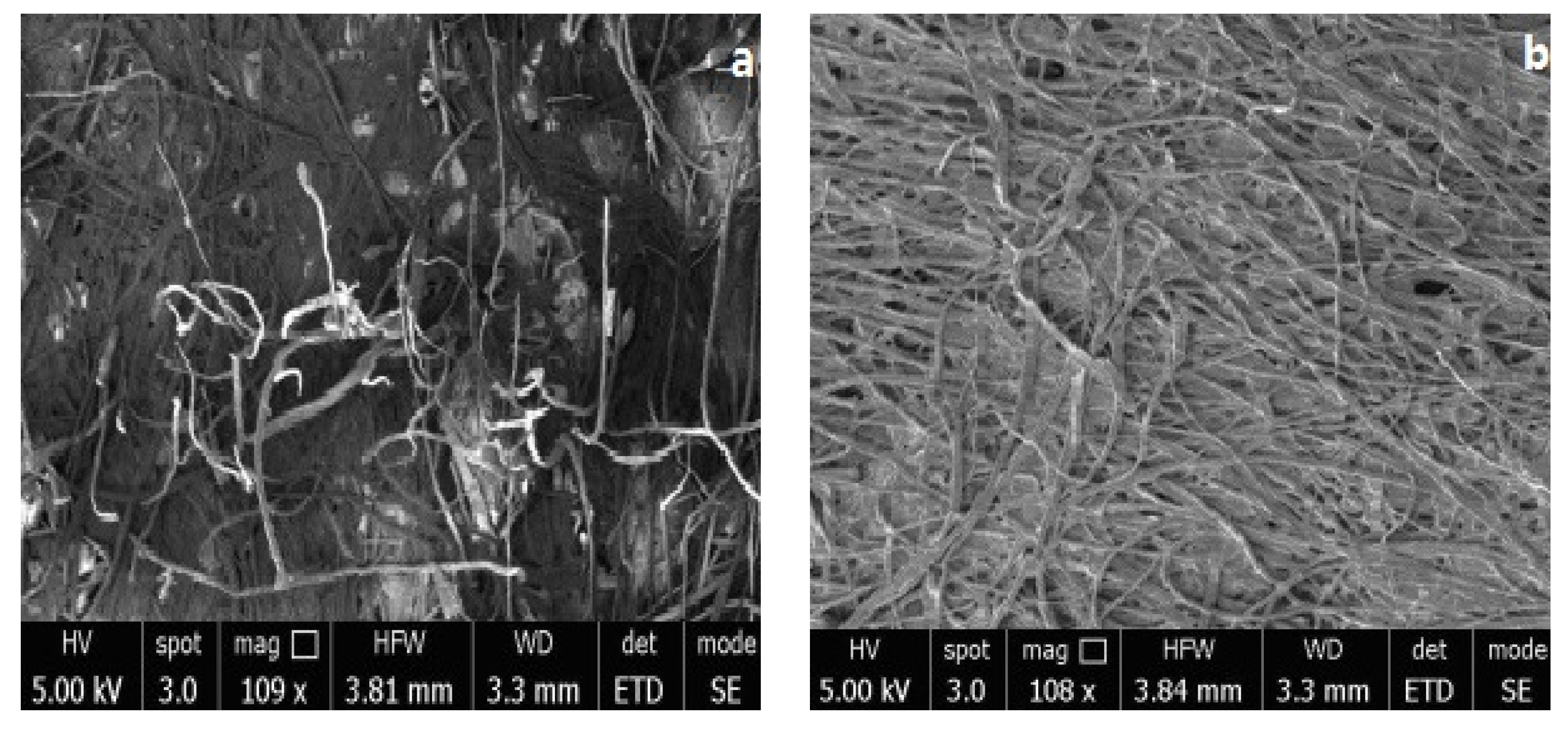
3.3. Application of Polyurethane Based on CHO-THF Copolyether in Paper
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Afsharpour, M.; Imani, S. Preventive protection of paper works by using nanocomposite coating of zinc oxide. J. Cult. Herit. 2017, 25, 142–148. [Google Scholar] [CrossRef]
- Qiao, L.; Chen, K.; Zhao, D.; Zhan, Y.; Min, W.; Huang, Q.; Shen, G.; Zhou, J. The application of poly(methyl methacrylate-co-butyl acrylate-co-styrene) in reinforcing fragile papers: Experiments and computer simulations. Cellulose 2017, 24, 5157–5171. [Google Scholar] [CrossRef]
- Camargos, C.H.M.; Figueiredo, J.C.D.; Pereira, F.V. Cellulose nanocrystal-based composite for restoration of lacunae on damaged documents and artworks on paper. J. Cult. Herit. 2017, 23, 170–175. [Google Scholar] [CrossRef]
- Jin, S.; Qi, Y.; Shen, Y.; Li, H. A transparent polyurethane based on nanosilica in reinforcing papers. Nord. Pulp Pap. Res. J. 2021, 36, 82–90. [Google Scholar] [CrossRef]
- Melo, D.; Sequeira, S.; Lopes, J.; Macedo, M.F. Stains versus colourants produced by fungi colonising paper cultural heritage: A review. J. Cult. Herit. 2019, 35, 161–182. [Google Scholar] [CrossRef]
- Wu, X.S. Classification of Plant Fibrous Material with Principal Component Analysis. Trans. China Pulp Pap. 2005, 20, 10–12. [Google Scholar]
- Xu, J.; Zhang, T.; Jiang, Y.; Chen, Q.; Yang, D.; Qiu, F.; Yu, Z. Synthesis of microcrystalline cellulose/TiO2/fluorine/styrene-acrylate coatings and the application for simulated paper cultural relic protection. Cellulose 2020, 27, 6549–6562. [Google Scholar] [CrossRef]
- Qiao, Y.H. Synthesis of Polyether Polyol and Preparation of Polyurethane Materials Based on Epoxy Cyclohexane and Epichlorohydrin. Master’s Thesis, Zhengzhou University, Zhengzhou, China, April 2018. [Google Scholar]
- Jin, S.; Qi, Y.P.; Shen, Y.F.; Li, H. Study on the application of oligomers in paper reinforcement protection. J. Chem. 2018, 1, 229–233. [Google Scholar]
- Krol, P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Mater. Sci. 2007, 52, 915–1015. [Google Scholar] [CrossRef]
- Chen, Q.; Wen, W.-Y.; Qiu, F.-X.; Xu, J.-C.; Yu, H.-Q.; Yang, D.-Y. Preparation and application of modified carboxymethyl cellulose Si/polyacrylate protective coating material for paper relics. Chem. Pap. 2016, 70, 946–959. [Google Scholar] [CrossRef]
- Totolin, M.I.; Neamţu, I. Positive findings for plasma polymer (meth)acrylate thin films in heritage protective applications. J. Cult. Herit. 2011, 12, 392–398. [Google Scholar] [CrossRef]
- Yang, B.; Lv, C.L.; Sheng, J.X. High Performance Polymeric Optical Materials; Chemical Industry Press: Beijing, China, 2005. [Google Scholar]
- Mu, C.S.; Hunag, K.L.; Li, K.X.; Luo, S.J.; Liu, Y.H.; Huang, S.S.; He, Y.L.; Li, W.G. Study progress in polyether polyol. Chem. Ind. Dev. 2009, 38, 13–18. [Google Scholar]
- Qian, B.Z.; Zhu, J.F. Development progress on polyether polyol at home and abroad. Fine Spec. Chem. 2010, 18, 5–12. [Google Scholar]
- Adhikari, R.; Gunatillake, P.A.; McCarthy, S.J.; Meijs, G.F. Mixed macrodiol-based siloxane polyurethanes: Effect of the comacrodiol structure on properties and morphology. J. Appl. Polym. 2015, 78, 1071–1082. [Google Scholar] [CrossRef]
- Gui, X. Synthesis of CHO-THF Polyether Transparent Polyurethane Materials. Master’s Thesis, Zhengzhou University, Zhengzhou, China, November 2017. [Google Scholar]
- Jin, S.S. Study on Conservation Materials of Silicon-Based Polyurethane for Paper Relics. Master’s Thesis, Zhengzhou University, Zhengzhou, China, August 2019. [Google Scholar]
- Liu, J.; Jin, S.-S.; Qi, Y.-P.; Shen, Y.-F.; Li, H. Preparation and Application of Polyurethane Coating Material Based on Epoxy Cyclohexane Protective for Paper. Coatings 2021, 11, 431. [Google Scholar] [CrossRef]
- Qi, Y.P.; Shen, Y.F.; Yang, Y.; Li, P.; Li, H.; Jin, S.S. A Reinforcing Fluid for Reinforcing Paper Artefacts. CN Patent CN201810081684.0, 14 August 2018. [Google Scholar]
- Jin, S.S. The application of organosilicon modifiedpolyurethane in reinforcing traditional paper. Nord. Pulp Pap. Res. J. 2019, 34, 485–494. [Google Scholar] [CrossRef]
- Yang, N.N. Study on Synthesis and Properties of Transparent Polyurethane Materials Based on Epoxy Cyclohexane. Master’s Thesis, Zhengzhou University, Zhengzhou, China, September 2016. [Google Scholar]
- Yan, L. Deteriorated Paper Conservation with Cellulose Solution Based on NaOH/Urea Solvent. Master’s Thesis, Zhengzhou University, Zhengzhou, China, August 2019. [Google Scholar]
- Zhang, J.K.; Cao, Y. Determination methods and research progress of NCO in polyurethane. Chem. Adhes. 2016, 38, 294–302. [Google Scholar]
- Liu, J.; Jin, S.-S.; Qi, Y.-P.; Shen, Y.-F.; Li, H. Preparation and Application of Polyurethane Coating Material Based on Epoxycyclohexane-Epoxychloropropane Protective for Paper. Coatings 2021, 11, 1005. [Google Scholar] [CrossRef]
- GB/T12914-2008. Paper and Board-Determination of Tensile Properties; Standards Press of China: Beijing, China, 2008. [Google Scholar]
- GB/T 457-2008. Paper and Board-Determination of Folding Endurance; Standards Press of China: Beijing, China, 2008. [Google Scholar]
- GB/T 455-2002. Paper and Board-Determination of Tearing Resistance; Standards Press of China: Beijing, China, 2002. [Google Scholar]
- GB/T 8941-2013. Paper and Board-Measurement of Specular Gloss; Standards Press of China: Beijing, China, 2013. [Google Scholar]
- GB/T 7974-2013. Paper, Board and Pulps-Measurement of Diffuse Blue Reflectance Factor-D65 Brightness; Standards Press of China: Beijing, China, 2013. [Google Scholar]
- Li, Y.; Li, Z.; Shen, G.; Zhan, Y. Paper conservation with an aqueous NaOH/urea cellulose solution. Cellulose 2019, 26, 4589–4599. [Google Scholar] [CrossRef]

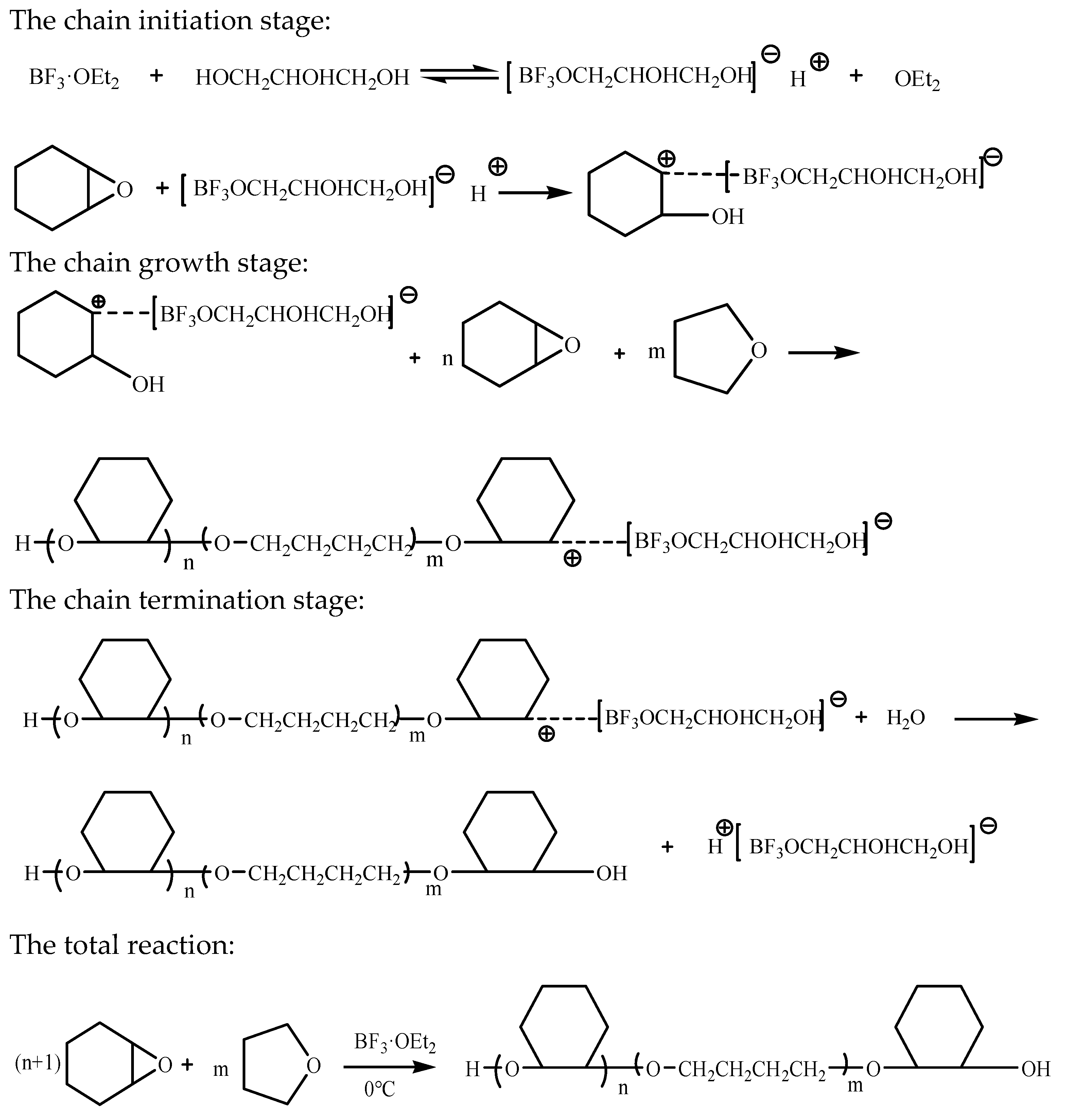



| Material | Specification | Manufacturer |
|---|---|---|
| CHO | industrial grade | Shenma Group (Pingdingshan, China) |
| THF | analytical grade | Tianjin Komeo Chemical Reagent Co., Ltd. (Tianjin, China) |
| glycerol | analytical grade | Tianjin Komeo Chemical Reagent Co., Ltd. (Tianjin, China) |
| dichloromethane | analytical grade | Tianjin Komeo Chemical Reagent Co., Ltd. (Tianjin, China) |
| boron trifluoride diethyl etherate | analytical grade | Tianjin Chemical Reagent Plant 3 (Tianjin, China) |
| ammonium carbonate | analytical grade | Tianjin Chemical Reagent Plant 3 (Tianjin, China) |
| ethyl acetate | analytical grade | Tianjin Komeo Chemical Reagent Co., Ltd. (Tianjin, China) |
| HDI trimer | industrial grade | Bayer (Leverkusen, Germany) |
| dibutyltin dilaurate | analytical grade | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| Xuan paper | – | Xuan Paper Company (Xuancheng, China) |
| Characteristic Functional Groups | Chemical Shift δ (ppm) |
|---|---|
| a | 3.1 |
| b | 3.5 |
| c | 3.3 |
| d | 1.9 |
| e | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Jin, S.-S.; Qi, Y.-P.; Shen, Y.-F.; Li, H. Preparation and Application of Polyurethane Coating Material Based on Epoxycyclohexane-Tetrahydrofuran in Paper Conservation. Coatings 2021, 11, 1143. https://doi.org/10.3390/coatings11091143
Liu J, Jin S-S, Qi Y-P, Shen Y-F, Li H. Preparation and Application of Polyurethane Coating Material Based on Epoxycyclohexane-Tetrahydrofuran in Paper Conservation. Coatings. 2021; 11(9):1143. https://doi.org/10.3390/coatings11091143
Chicago/Turabian StyleLiu, Juan, Shan-Shan Jin, Ying-Ping Qi, Yong-Feng Shen, and Hua Li. 2021. "Preparation and Application of Polyurethane Coating Material Based on Epoxycyclohexane-Tetrahydrofuran in Paper Conservation" Coatings 11, no. 9: 1143. https://doi.org/10.3390/coatings11091143
APA StyleLiu, J., Jin, S.-S., Qi, Y.-P., Shen, Y.-F., & Li, H. (2021). Preparation and Application of Polyurethane Coating Material Based on Epoxycyclohexane-Tetrahydrofuran in Paper Conservation. Coatings, 11(9), 1143. https://doi.org/10.3390/coatings11091143





