Investigations of Thermal Stability and Spectroscopic Features of Sm3+ Doped Strontium Aluminate Glasses
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Structural Properties
3.2. Density and Vicker’s Hardness
3.3. Thermal Properties
3.4. Absorption Spectra and Nephelauxetic Effect
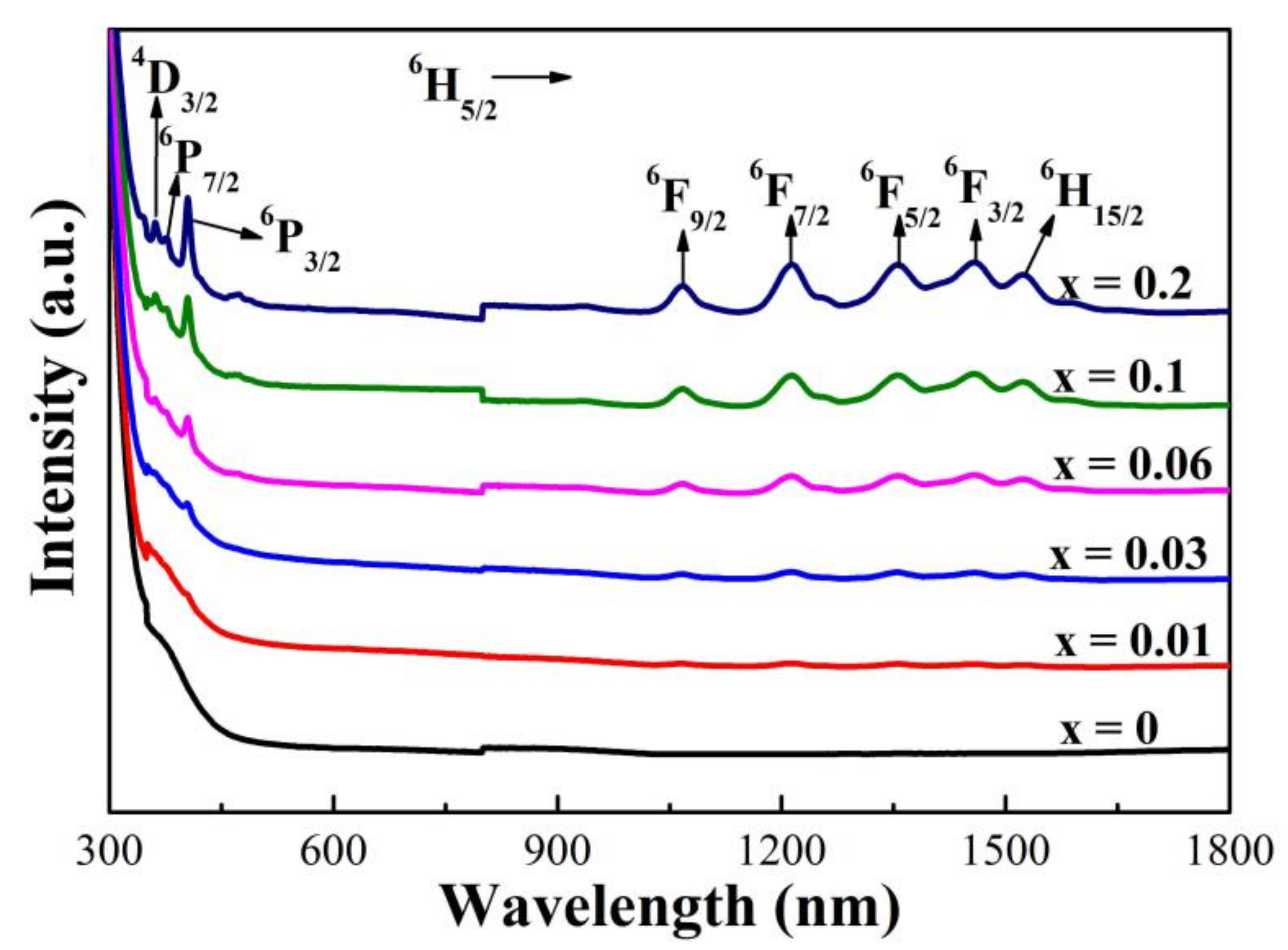
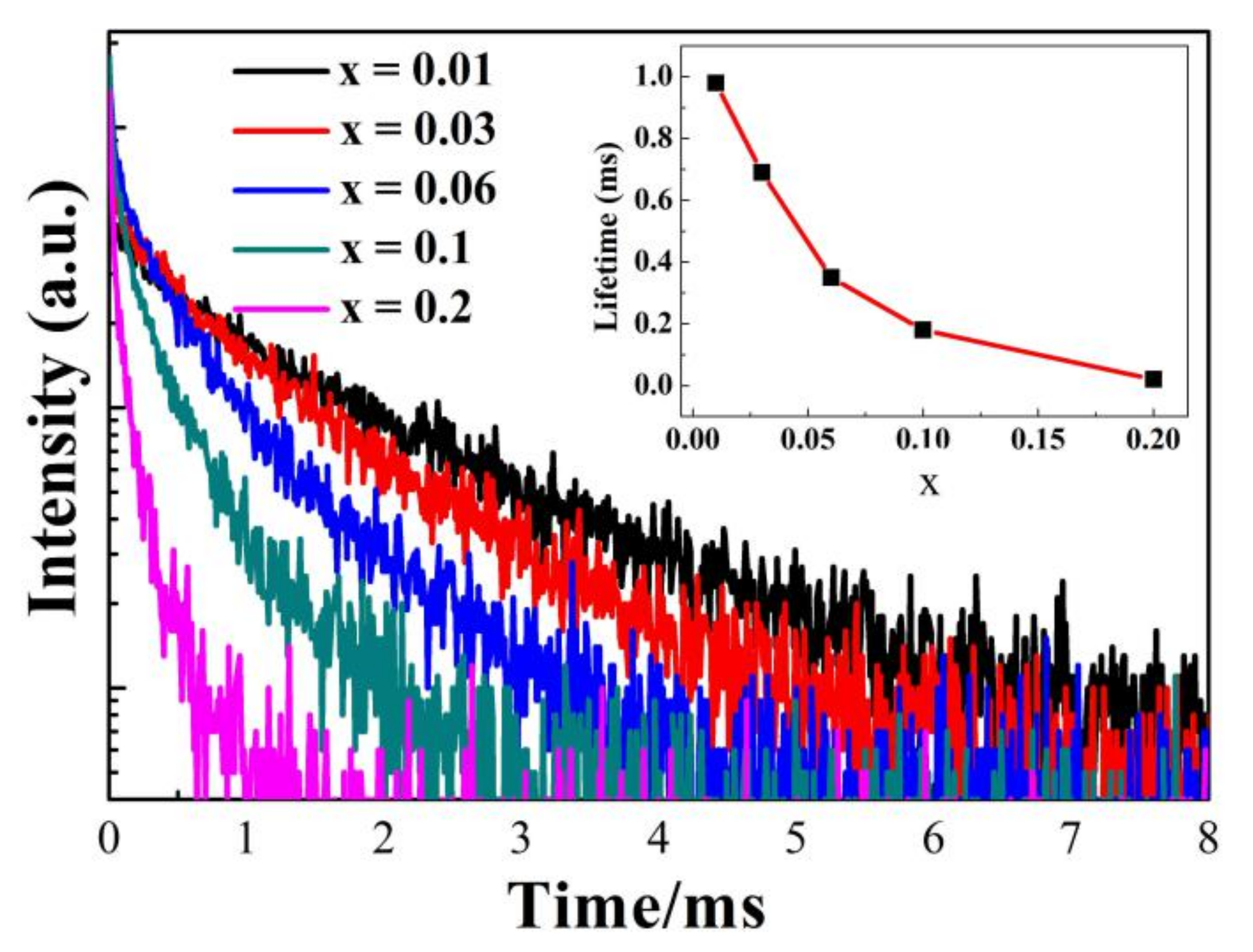
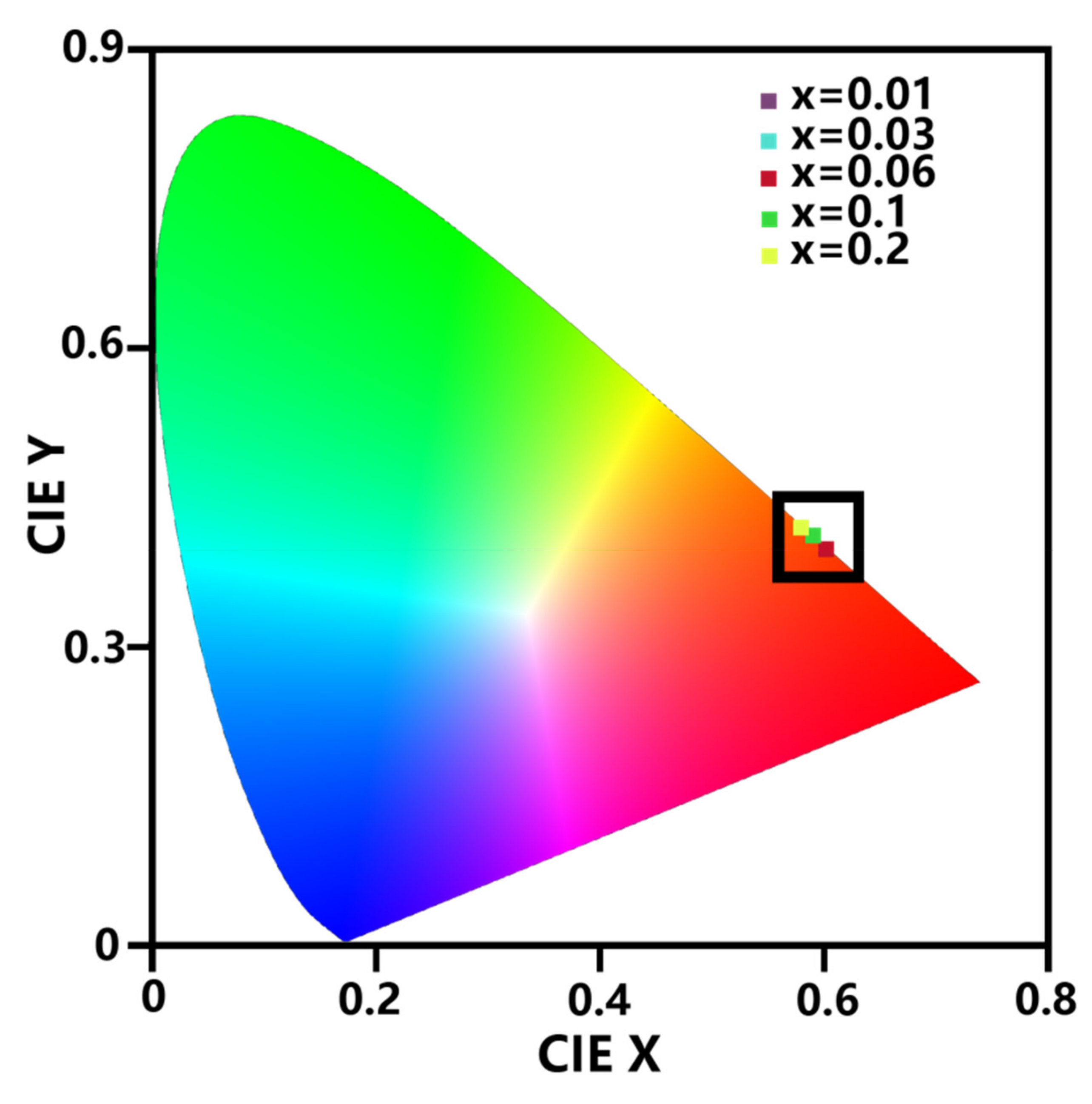
3.5. Photoluminescence Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, J.S.; Messaddeq, Y. Photoluminescence study of Eu3+ doped zinc-tungsten-antimonite glasses for red LED applications. J. Lumin. 2020, 228, 117608. [Google Scholar] [CrossRef]
- Subrahmanyam, T.; Gopal, K.R.; Suvarna, R.P.; Jamalaiah, B.C.; Kumar, M.V.V. Luminescent properties of Tb3+-doped TeO2-WO3-GeO2 glasses for green laser applications. Opt. Mater. 2018, 80, 154–159. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Baki, S.O.; Lira, A.; Kityk, I.V.; Caldiño, U.; Kaky, K.M.; Mahdi, M.A. Structural, thermal and optical investigations of Dy3+-doped B2O3-WO3-ZnO-Li2O- Na2O glasses for warm white light emitting applications. J. Lumin. 2017, 186, 283–300. [Google Scholar] [CrossRef]
- Khattab, T.A.; Rehan, M.; Shaheen, Y.H.T.I. Facile development of photoluminescent textile fabric via spray coating of Eu (II)-doped strontium aluminate. Ind. Eng. Chem. Res. 2018, 57, 11483–11492. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Binyaseen, A.M.; Aljuhani, E.; Aljohani, M.; Alzahrani, H.K.; Shah, R.; El-Metwaly, N.M. Production of smart nanocomposite for glass coating toward photochromic and long-persistent photoluminescent smart windows. Ceram. Int. 2022, 48, 903–912. [Google Scholar] [CrossRef]
- Mohan, S.; Kaur, S.; Singh, D.P.; Kaur, P. Structural and luminescence properties of samarium doped lead alumino borate glasses. Opt. Mater. 2017, 73, 223–233. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Tian, Y.F.; Fei, M.; He, L.R.; Zhang, P.J.; Zhang, W.H. Origin of the red luminescence in Sr3Al2O6: Eu phosphor-from the synergetic effects of Eu2+ and Eu3+. J. Rare Earths 2017, 35, 127–134. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yang, W.C.; Zhang, J.R.; Li, J.S.; Jiang, L.H.; Qi, X.W. High hardnesses of Tm3+-doped La2O3-Al2O3 luminescent glasses fabricated by containerless solidification. J. Non-Cryst. Solids 2019, 525, 119599. [Google Scholar] [CrossRef]
- Ma, X.G.; Li, X.Y.; Li, J.Q.; Genevois, C.; Ma, B.Q.; Etienne, A.; Wan, C.L.; Véron, E.; Peng, Z.J.; Allix, M. Pressureless glass crystallization of transparent yttrium aluminum garnet-based nanoceramics. Nat. Commun. 2018, 9, 1175. [Google Scholar] [CrossRef] [Green Version]
- Basavapoornima, C.; Linganna, K.; Kesavulu, C.; Ju, S.; Kim, B.; Han, W.-T.; Jayasankar, C. Spectroscopic and pump power dependent upconversion studies of Er3+-doped lead phosphate glasses for photonic applications. J. Alloys Compd. 2017, 699, 959–968. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, M.; Zhang, J.R.; Li, Y.; Gu, Y.H.; Qi, X.W. Photoluminescence, transmittance and electrical properties of Eu3+-doped Al2O3-SrO glasses synthesized by an aerodynamic levitation technique. J. Lumin. 2019, 206, 79–83. [Google Scholar] [CrossRef]
- Devi, C.B.A.; Swapna, K.; Mahamuda, S.; Venkateswarlu, M.; Prasad, M.V.V.K.S.; Reddy, K.S.R.K.; Deopa, N.; Rao, A.S. Spectroscopic studies and lasing potentialities of Sm3+ ions doped single alkali and mixed alkali fluoro tungstentellurite glasses. Opt. Laser Technol. 2019, 111, 176–183. [Google Scholar] [CrossRef]
- Jlassi, I.; Mnasri, S.; Elhouichet, H. Concentration dependent spectroscopic behavior of Sm3+-doped sodium fluoro-phosphates glasses for orange and reddish-orange light emitting applications. J. Lumin. 2018, 199, 516–527. [Google Scholar] [CrossRef]
- Kirdsiri, K.; Ramakrishna, R.R.; Damdee, B.; Kim, H.J.; Kaewjaeng, S.; Kothan, S.; Kaewkhao, J. Investigations of optical and luminescence features of Sm3+ doped Li2O-MO-B2O3 (M = Mg/Ca/Sr/Ba) glasses mixed with different modifier oxides as an orange light emitting phosphor for WLED’s. J. Alloys Compd. 2018, 749, 197–204. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Marimuthu, K.; Sudarsan, V. Concentration dependent spectroscopic behavior of Sm3+ doped lead fluoro-borophosphate glasses for laser and LED applications. J. Alloys Compd. 2015, 647, 209–220. [Google Scholar] [CrossRef]
- Deopa, N.; Rao, A.S.; Choudhary, A.; Saini, S.; Navhal, A.; Jayasimhadria, M.; Haranath, D.; Prakash, G.V. Photoluminescence investigations on Sm3+ ions doped borate glasses for tricolor w-LEDs and lasers. Mater. Res. Bull. 2018, 100, 206–212. [Google Scholar] [CrossRef]
- Ahmadi, F.; Asgari, A. Spectroscopic investigation of Sm3+ doped sulfophosphate glasses for visible photonic applications. J. Non-Cryst. Solids 2019, 505, 406–413. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhang, H.B.; Wang, T.; Lv, H.M.; Zou, X.Y.; Wei, Y.L.; Hu, W.H.; Su, C.H. Synthesis and luminescence properties of Sm3+ doped molybdate glass ceramic. J. Alloys Compd. 2020, 823, 153822. [Google Scholar] [CrossRef]
- Rudramamba, K.S.; Reddy, D.V.K.; Rao, T.S.; Taherunnisa, S.K.; Veeraiah, N.; Reddy, M.R. Optical properties of Sm3+ doped strontium bismuth borosilicate glasses for laser applications. Opt. Mater. 2019, 89, 68–79. [Google Scholar] [CrossRef]
- Zhu, M.G.; Wang, L. Study on the relationship between the melting point the hardness of the alkaline earth metal oxide and the electron structure. J. Ningbo Univ. 2004, 17, 482–484. [Google Scholar]
- Prabhu, N.S.; Hegde, V.; Wagh, A.; Sayyed, M.I.; Agar, O.; Kamath, S.D. Physical, structural and optical properties of Sm3+ doped lithium zinc alumino borate glasses. J. Non-Cryst. Solids 2019, 515, 116–124. [Google Scholar] [CrossRef]
- Li, P.F.; Qi, X.W.; Wang, L.M. A comprehensive study of lead telluride (PbTe)-based amorphous alloys: Glass formation and thermoelectric properties. J. Non-Cryst. Solids 2021, 571, 121057. [Google Scholar] [CrossRef]
- Zhang, M.H.; Wen, H.Q.; Pan, X.H.; Yu, J.D.; Shao, H.; Tang, M.B.; Gai, L.J.; Ai, F. Study on novel high refractive index La2O3-Lu2O3-TiO2 glasses prepared by aerodynamic levitation method. Mater. Lett. 2018, 222, 5–7. [Google Scholar] [CrossRef]
- Rasool, S.N.; Moorthy, L.R.; Jayasankar, C.K. Spectroscopic Investigation of Sm3+ doped phosphate based glasses for reddish-orange emission. Opt. Commun. 2013, 311, 156–162. [Google Scholar] [CrossRef]
- Yamusa, Y.A.; Hussin, R.; Shamsuri, W.N.W. Physical, optical and radiative properties of CaSO4-B2O3-P2O5 glasses doped with Sm3+ ions. Chin. J. Phys. 2018, 56, 932–943. [Google Scholar] [CrossRef]
- Mohan, S.; Kaur, S.; Kaur, P.; Singh, D.P. Spectroscopic investigations of Sm3+-doped lead alumino-borate glasses containing zinc, lithium and barium oxides. J. Alloys Compd. 2018, 763, 486–495. [Google Scholar] [CrossRef]
- Shamshad, L.; Ali, N.; Kaewkhao, J.; Rooh, G.; Ahmad, T.; Zaman, F. Luminescence characterization of Sm3+-doped sodium potassium borate glasses for laser application. J. Alloys Compd. 2018, 766, 828–840. [Google Scholar] [CrossRef]
- Boehm, L.; Reisfeld, R.; Spector, N. Optical transitions of Sm3+ in oxide glasses. J. Solid State Chem. 1979, 28, 75–78. [Google Scholar] [CrossRef]
- Jorgensen, C.K. Oxidation Numbers and Oxidation States; Springer Science & Business Media: New York, NY, USA, 1969; Volume 6, pp. 72–140. [Google Scholar]
- Shamshad, L.; Rooh, G.; Kirdsiri, K.; Srisittipokakun, N.; Damdee, B.; Kim, H.J. Photoluminescence and white light generation behavior of lithium gadolinium silicoborate glasses. J. Alloys Compd. 2017, 695, 2347–2355. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic energy levels in the trivalent lanthanide aquo ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, Tm3+. J. Chem. Phys. 1968, 49, 4424–4442. [Google Scholar] [CrossRef]
- Sreedhar, V.B.; Basavapoornima, C.; Jayasankar, C.K. Spectroscopic and fluorescence properties of Sm3+ doped zinc fluorophosphate glasses. J. Rare Earths 2014, 32, 918–926. [Google Scholar] [CrossRef]
- Swapna, K.; Mahamuda, S.; Rao, A.S.; Shakya, S.; Sasikala, T.; Haranath, D.; Prakash, G.V. Optical studies of Sm3+ ions doped zinc alumino bismuth borate glasses. Spectrochim. Acta A 2014, 125, 53–60. [Google Scholar] [CrossRef]
- Talewar, R.A.; Mahamuda, S.; Swapna, K.; Venkateswarlu, M.; Rao, A.S. Spectroscopic studies of Sm3+ ions doped alkaline-earth chloro borate glasses for visible photonic applications. Mater. Res. Bull. 2018, 105, 45–54. [Google Scholar] [CrossRef]
- Basavapoornima, C.; Jayasankar, C.K. Spectroscopic and photoluminescence properties of Sm3+ ions in Pb-K-Al-Na phosphate glasses for efficient visible lasers. J. Lumin. 2014, 153, 233–241. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Y.; Zhu, C.; Yuan, S.; Chen, G. Luminescence properties of Tb3+-Sm3+ codoped glasses for white light emitting diodes. Appl. Phys. Lett. 2007, 91, 091104. [Google Scholar] [CrossRef] [Green Version]
- Mariyappan, M.; Arunkumar, S.; Marimuthu, K. Concentration effect on the structural and spectroscopic investigations of Sm3+ ions doped B2O3-Bi2O3-CaF2-Na2O glasses. J. Lumin. 2018, 196, 151–160. [Google Scholar] [CrossRef]
- Suthanthirakumar, P.; Arunkumar, S.; Marimuthu, K. Spectroscopic properties and excited state dynamics of Sm3+ ions in zinc telluro-flfluoroborate glasses. J. Lumin. 2018, 202, 289–300. [Google Scholar] [CrossRef]
- Puchalska, M.; Zych, E. The effffect of charge compensation by means of Na+ ions on the luminescence behavior of Sm3+-doped CaAl4O7 phosphor. J. Lumin. 2012, 132, 826–831. [Google Scholar] [CrossRef]
- Min, X.; Fang, M.H.; Huang, Z.H.; Liu, Y.A.; Tang, C.; Zhu, H.K.; Wu, X.W. Preparation and luminescent properties of orange reddish emitting phosphor LaMgAl11O19:Sm3+. Opt. Mater. 2014, 37, 110–114. [Google Scholar] [CrossRef]
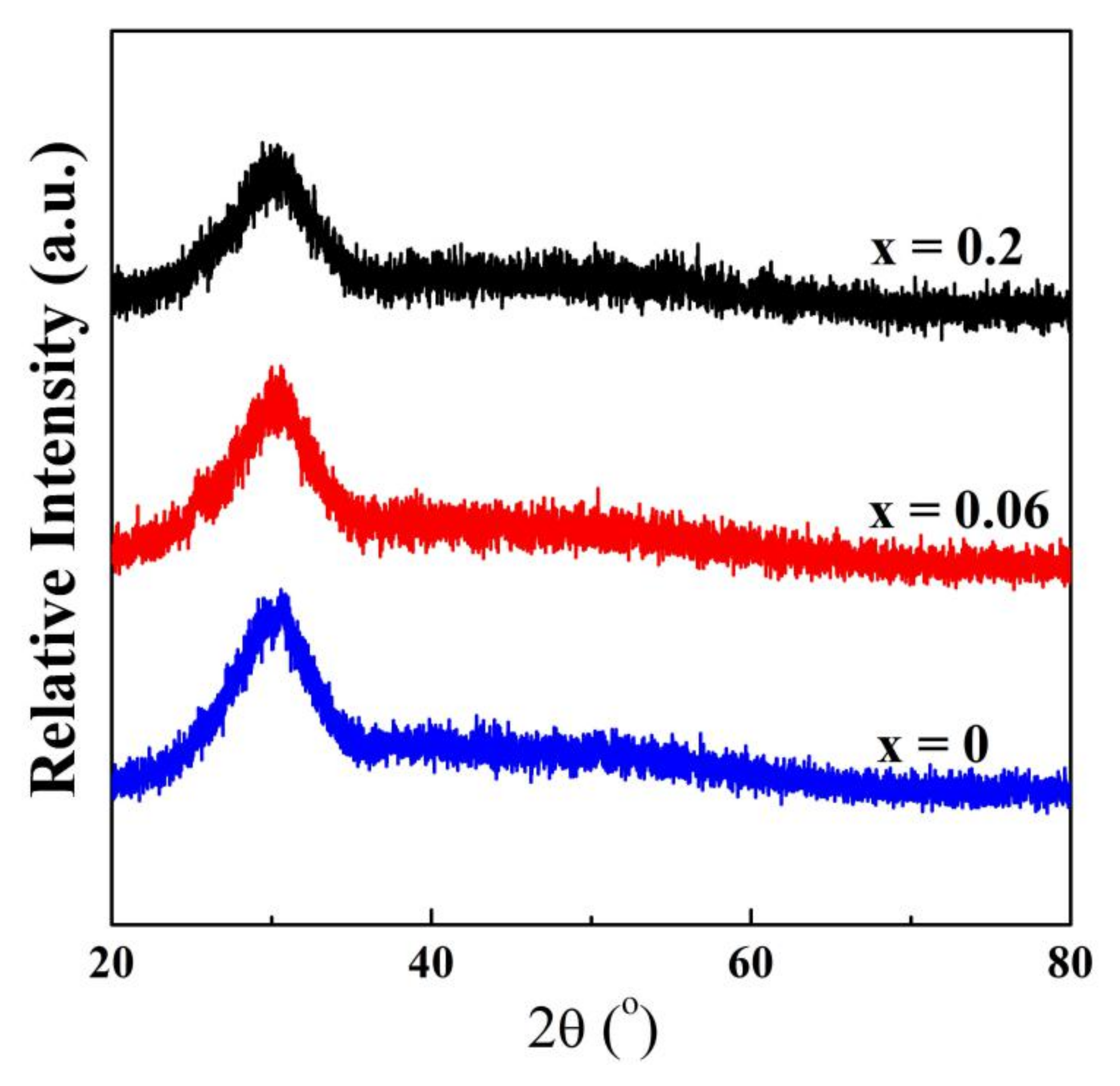
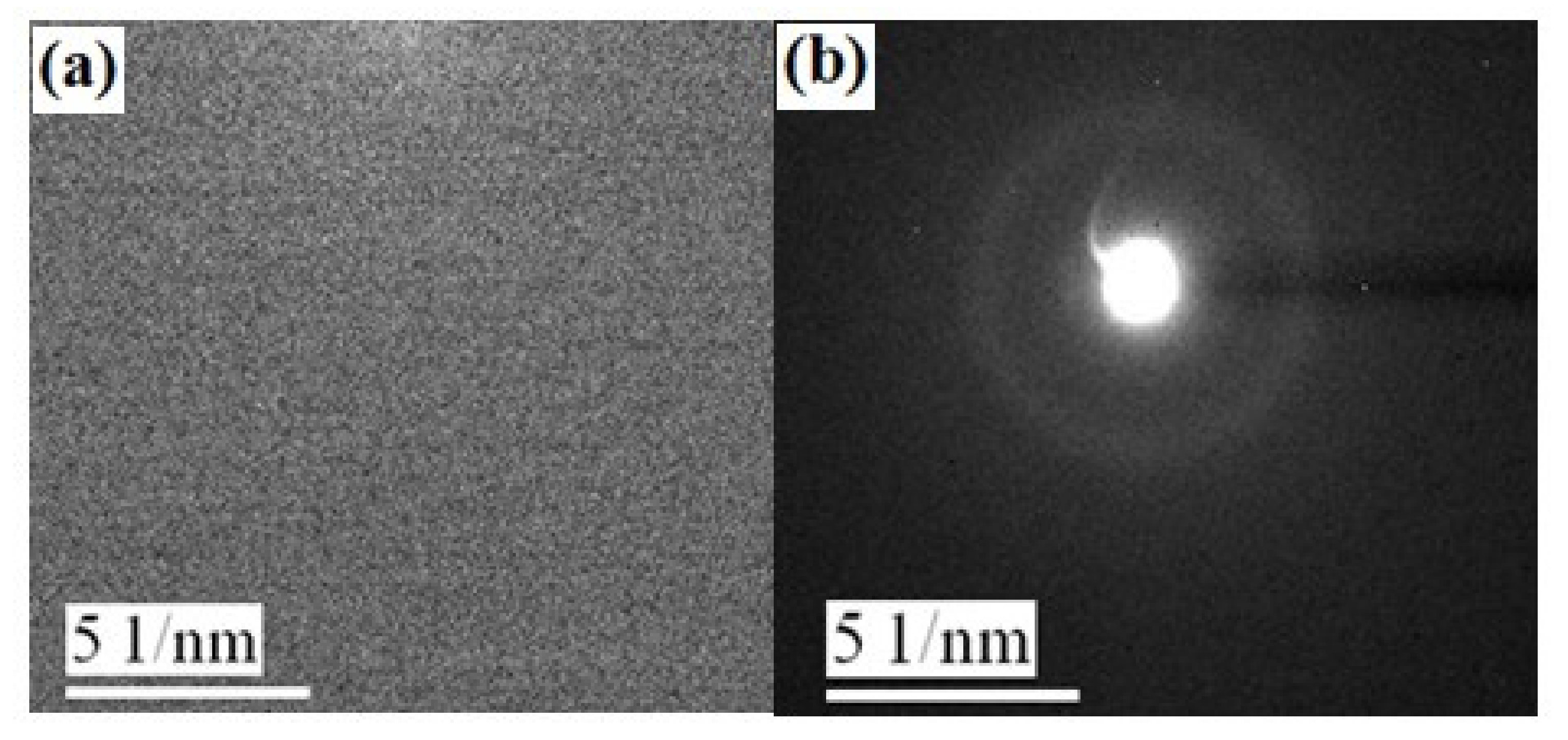
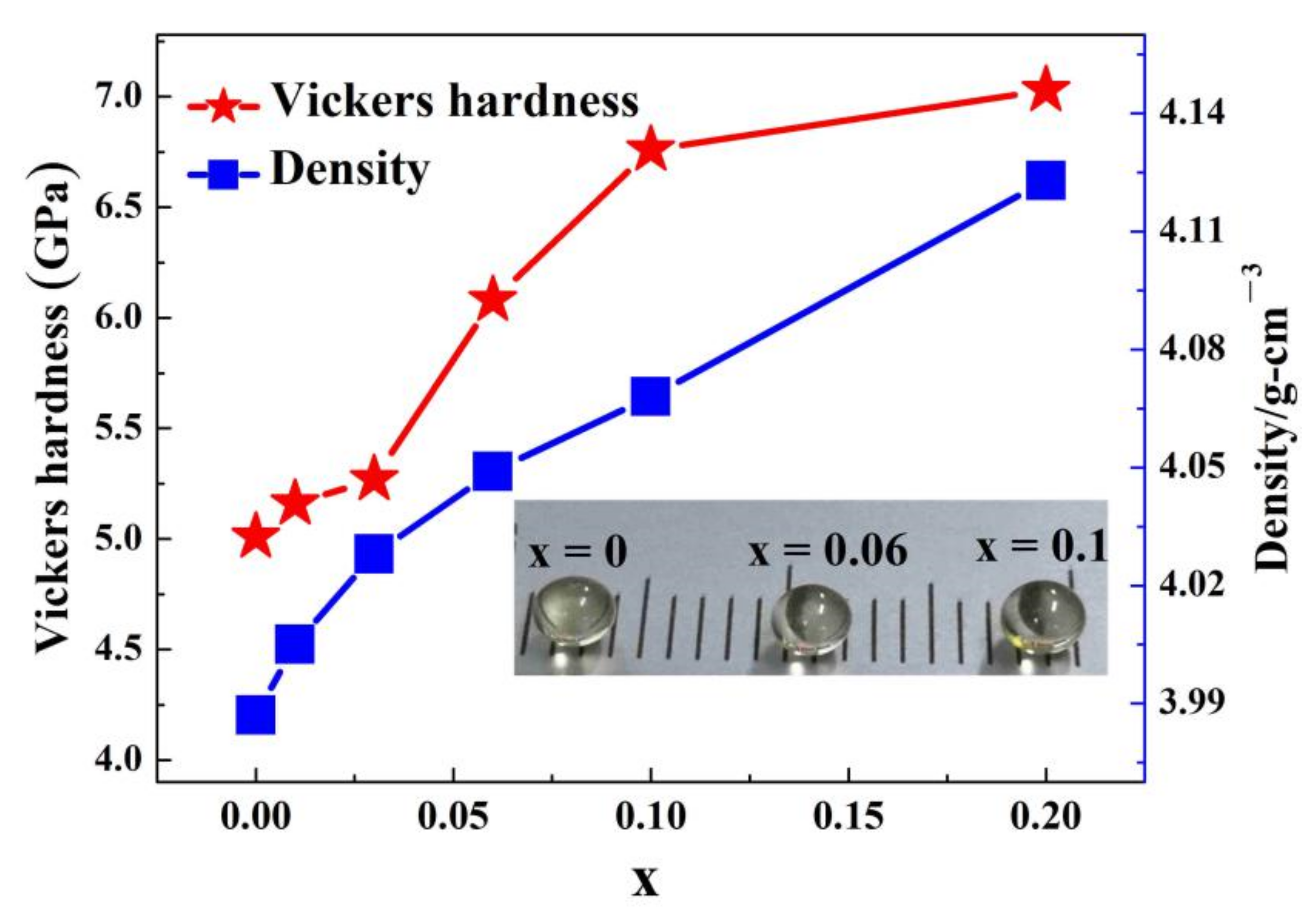
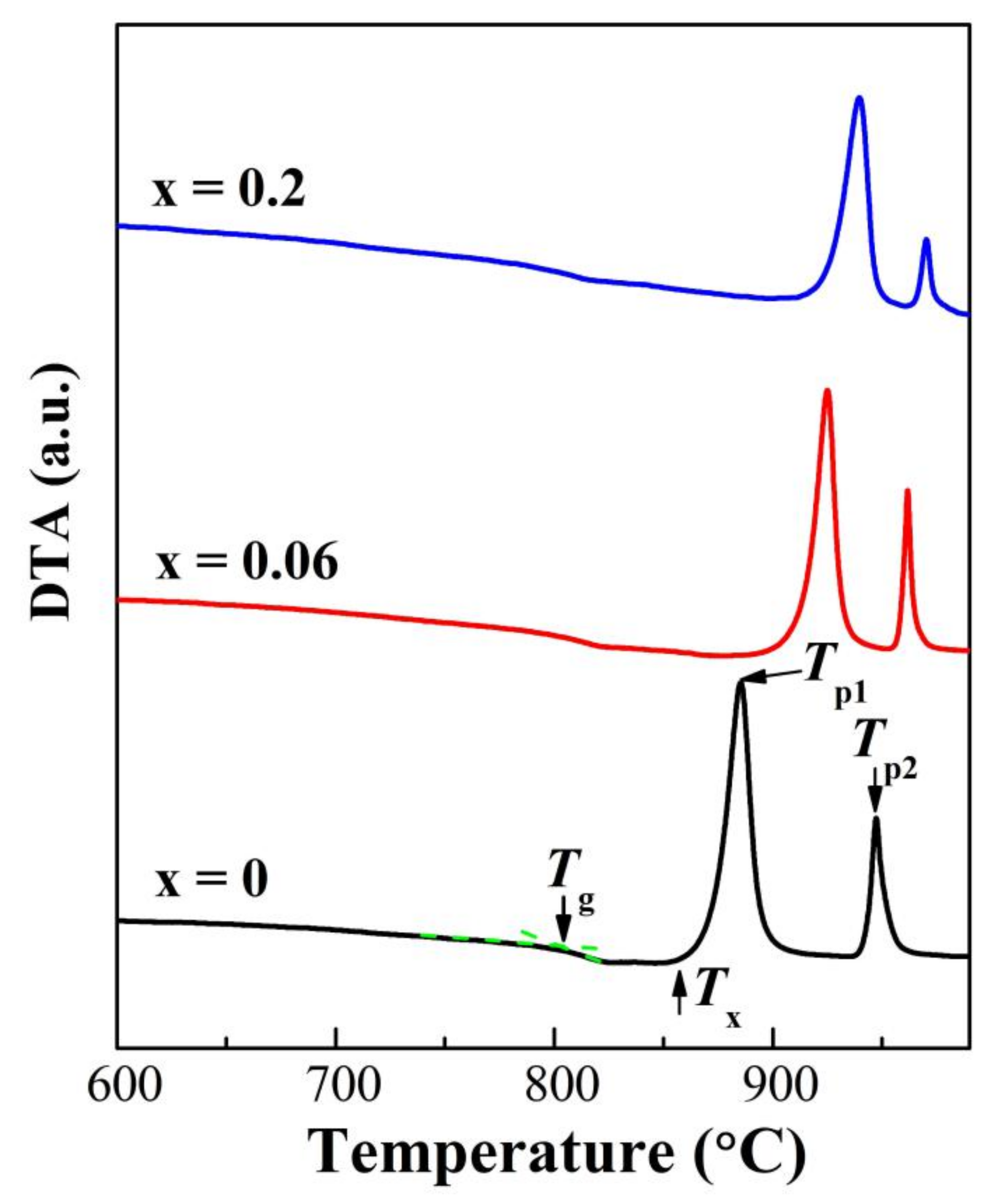


| x | Tg (°C) | Tx (°C) | Tp1 (°C) | Tp2 (°C) | ΔT (°C) |
|---|---|---|---|---|---|
| 0 | 804 | 858 | 885 | 947 | 54 |
| 0.01 | 800 | 854 | 884 | 950 | 54 |
| 0.03 | 798 | 854 | 882 | 963 | 56 |
| 0.06 | 798 | 897 | 925 | 962 | 99 |
| 0.1 | 797 | 918 | 938 | 964 | 121 |
| 0.2 | 792 | 919 | 940 | 971 | 127 |
| Transition 6H5/2 → | νc (x = 0.01) | νc (x = 0.03) | νc (x = 0.06) | νc (x = 0.1) | νc (x = 0.2) | νa [29] |
|---|---|---|---|---|---|---|
| 4D3/2 | 27,778 | 27,778 | 27,701 | 27,701 | 27,701 | 27,700 |
| 6P7/2 | 26,667 | 26,667 | 26,667 | 26,667 | 26,525 | 26,750 |
| 6P3/2 | 24,814 | 24,752 | 24,691 | 24,691 | 24,691 | 24,950 |
| 6F9/2 | 9390 | 9363 | 9372 | 9372 | 9381 | 9200 |
| 6F7/2 | 8244 | 8230 | 8251 | 8237 | 8237 | 8000 |
| 6F5/2 | 7380 | 7375 | 7375 | 7375 | 7375 | 7100 |
| 6F3/2 | 6849 | 6854 | 6849 | 6854 | 6854 | 6630 |
| 6H15/2 | 6566 | 6570 | 6562 | 6566 | 6566 | 6508 |
| 1.01585 | 1.01504 | 1.01459 | 1.01454 | 1.01400 | - | |
| −1.56021 | −1.48135 | −1.43759 | −1.43296 | −1.38035 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhang, X.; Zhang, J.; Qi, X.; Liu, X. Investigations of Thermal Stability and Spectroscopic Features of Sm3+ Doped Strontium Aluminate Glasses. Coatings 2022, 12, 3. https://doi.org/10.3390/coatings12010003
Li P, Zhang X, Zhang J, Qi X, Liu X. Investigations of Thermal Stability and Spectroscopic Features of Sm3+ Doped Strontium Aluminate Glasses. Coatings. 2022; 12(1):3. https://doi.org/10.3390/coatings12010003
Chicago/Turabian StyleLi, Pengfei, Xiaoyan Zhang, Jinrong Zhang, Xiwei Qi, and Xin Liu. 2022. "Investigations of Thermal Stability and Spectroscopic Features of Sm3+ Doped Strontium Aluminate Glasses" Coatings 12, no. 1: 3. https://doi.org/10.3390/coatings12010003






