Abstract
The secondary aluminum ash is the black slag left after the primary aluminum ash is extracted from the metal aluminum. To address the environmental pollution and resource waste caused by the accumulation and landfill of aluminum ash, this study fabricated non-fired bricks by using secondary aluminum ash as the principal raw material, which was supplemented by cement, slaked lime, gypsum and engineering sand. The effects of mix proportions of various admixtures on the mechanical properties of non-fired bricks were investigated, and on this basis, the hydration mechanism was analyzed. The results showed that the mix proportions were 68.3% aluminum ash, 11.4% cement, 6.4% slaked lime, 4.2% gypsum and 9.7% engineering sand. The compressive strength of the fabricated bricks reached 22.19 MPa, and their quality indicators were in line with the MU20 requirements for Non-fired Rubbish Gangue Bricks. Evident hydration reaction occurred inside the non-fired bricks, with main products being calcium silicate hydrate (CSH), calcium aluminate hydrate (CAH) and ettringite (AFt). Besides, a dense structure was formed, which enhanced the brick strength.
1. Introduction
Aluminum ash is a kind of solid waste produced during industrial aluminum production. According to statistics, approximately 30–250 kg of aluminum ash is produced per ton of aluminum during electrolysis, melting and casting, as well as scrap recycling processes [1]. Depending on source, aluminum ash can be classified into the primary and secondary types. Primary aluminum ash refers to the waste slag produced during the electrolysis of primary aluminum or casting of aluminum, which has high elemental aluminum content and can be used as the raw material for ash frying [2,3]. Meanwhile, secondary aluminum ash refers to the hardly-disposable waste slag left after the ash frying with primary aluminum ash. Although the secondary aluminum ash contains less aluminum than the primary type, the total mass of aluminum-containing phase still exceeds 50%, which thus has great utilization value [4]. Currently, secondary aluminum ash is disposed in China mainly by accumulation and landfill, which causes environmental pollution and resource waste [5].
To date, research on the comprehensive utilization of aluminum ash is concentrated in the areas of building, refractory and other inorganic materials. Xu et al. [6] fabricated composite cement by utilizing waste aluminum ash from the production of aluminum sulfate water purifier, where the aluminum ash amounted to 8%–10%. Li et al. [7] utilized aluminum ash to fabricate a high-alumina refractory with MgAl2O4 as the main phase and CaAl2O4 as the secondary phase. Adeosun et al. [8] prepared insulating refractory bricks by incorporating aluminum ash into plastic clay and kaolin, which could withstand up to 1200 °C. Murayama et al. [9] prepared Mg-Zn, Ca-Al and Zn-Al hydrotalcites by dissolving aluminum ash (raw material) in HCl and NaOH solvents, which could serve as steelmaking deoxidizers. Zhang et al. [10] prepared sintered bricks with aluminum ash as raw materials, and obtained the best formula as follows: aluminum ash 50%, engineering soil 37.50%, coal gangue 12.50%, and the compressive strength of the sintered brick can reach 16.21 MPa. Ni and Zhang [11,12] prepared aluminum ash coating by plasma spraying. The aluminum ash coating prepared by plasma spraying has excellent properties and the strength of the coating can reach 606.54 HV. However, the existing techniques for secondary aluminum ash treatment consume only small amounts aluminum ash, which cannot thoroughly manage the long-accumulated aluminum ash or the newly produced aluminum ash every year. Hence, finding a comprehensive utilization method that enables substantial consumption of secondary aluminum ash is of profound significance to environmental and resource protection.
Since 2003, the production and use of solid clay bricks have been banned in China. Non-fired brick is an efficient substitute for solid clay brick, which contributes to protecting the environment and saving land resources. In this work, non-fired bricks were fabricated by using secondary aluminum ash from an aluminum company in Jiangsu as the principal raw material, which was supplemented by cement, slaked lime, gypsum and engineering sand. The effects of the four admixtures on the mechanical properties of the non-fired bricks were investigated, and fairly optimal mix proportions for making non-fired bricks from secondary aluminum ash were obtained. Further, the relevant hydration mechanism was analyzed. The findings of this study provide a theoretical basis for the application of aluminum ash for making non-fired bricks.
2. Materials and Methods
2.1. Raw Materials
Secondary aluminum ash was collected from Haiguang Metal Co., Ltd. in Suqian, China, which was produced after frying treatment of primary aluminum ash. Slaked lime (Ca(OH)2) and gypsum (CaSO4·2H2O) (analytical grade) were both from Zhiyuan Chemical Reagent Co., Ltd. in Tianjin, China. Engineering sand (fineness modulus: 2.6) was produced by Jiuqi Building Materials Co., Ltd. in Weifang, China and Portland cement PO 42.5 was produced by Yangchun Cement Co., Ltd. in Weifang, China.
2.2. Experimental Procedure
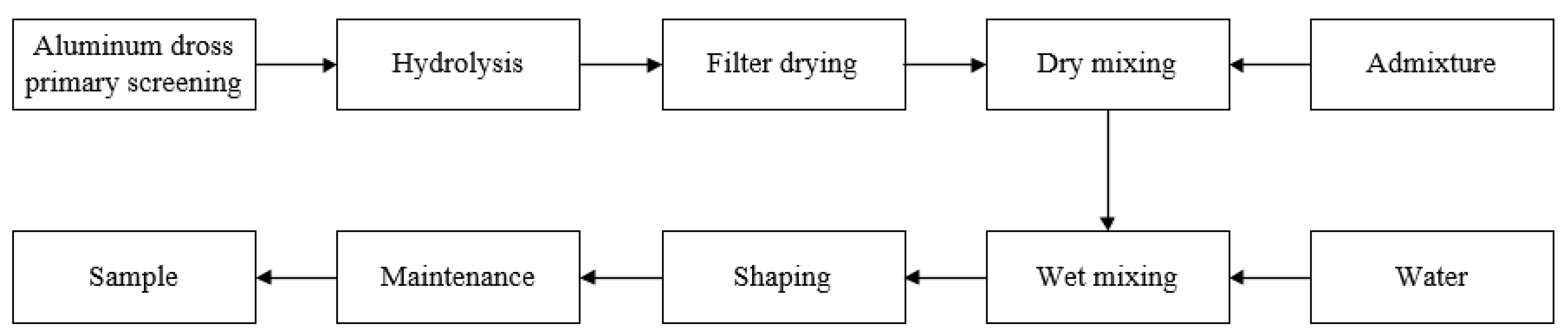
As a first step, the secondary aluminum ash was homogenized and sieved with a 50-mesh screen, and the aluminum ash that does not pass through the screen is regarded as impurity removal. Then, the primarily sieved aluminum ash was treated for 24 h with ultrapure water to remove nitrogen and salt, followed by thorough drying. Next, the dried aluminum ash and the admixtures were mixed uniformly according to proportions in the table, and then poured into an independently-prepared mold with a molding pressure of 15 MPa to form 40 mm × 40 mm × 160 mm specimens. Finally, the specimens were steam-cured at 80 °C for 18 h, and then sprinkle cured naturally for 3 d to obtain the experimental samples. Five samples were made in each group, and after the maximum and minimum values were removed, the average values of the remaining three data were taken as the experimental data. Figure 1 depicts the sample preparation flow.
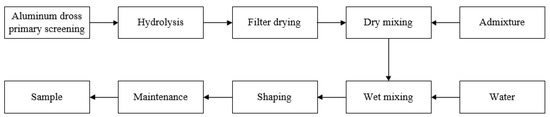
Figure 1.
Sample preparation flow chart.
2.3. Characterization Method
In accordance with the Chinese standard GB/T 17671-1999 “Test Method for Cement Mortar Strength” (ISO Method) [13], the compressive strength and flexural strength of the non-fired bricks was examined with a microcomputer-controlled compressive and flexural tester. The frost resistance and water absorption index were determined according to the JC/T 422-2007 standard “Non-fired Rubbish Gangue Bricks” [14]. The leaching toxicity test was carried out according to the Chinese standard GB 5085.3-2007 “Hazardous waste Identification Standard leaching toxicity Identification” [15]. Scanning electron microscope (SEM, HITACHI S-3400N, Hitachi Corporation, Tokyo, Japan) was employed to observe the microstructures of secondary aluminum ash and non-fired brick samples, while X-ray diffractometer (XRD, Rigaku D/max2550V, Rigaku Corporation, Tokyo, Japan) was utilized to analyze their phase compositions.
3. Results and Discussion
3.1. Aluminum Ash and Its Characteristics
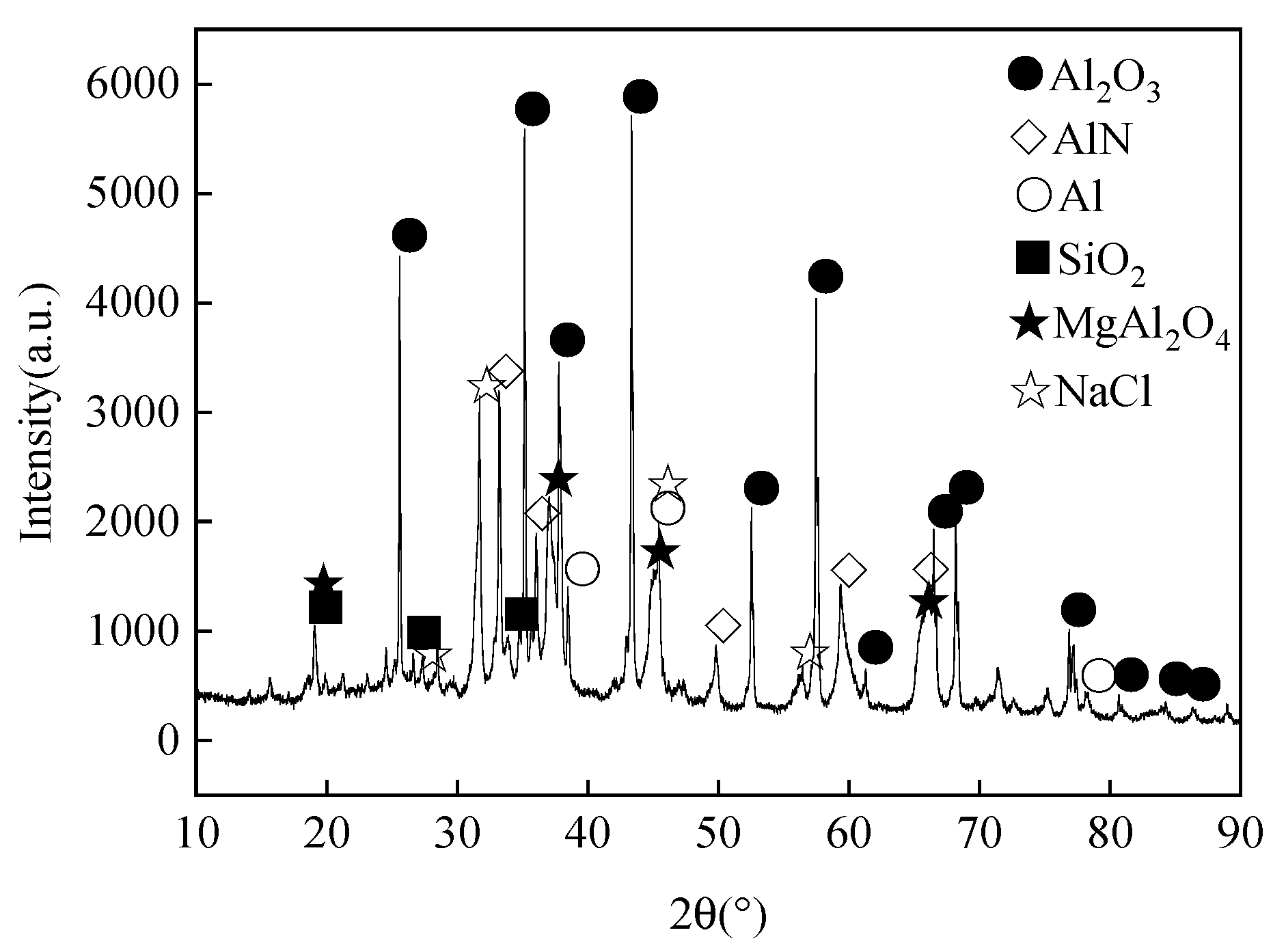
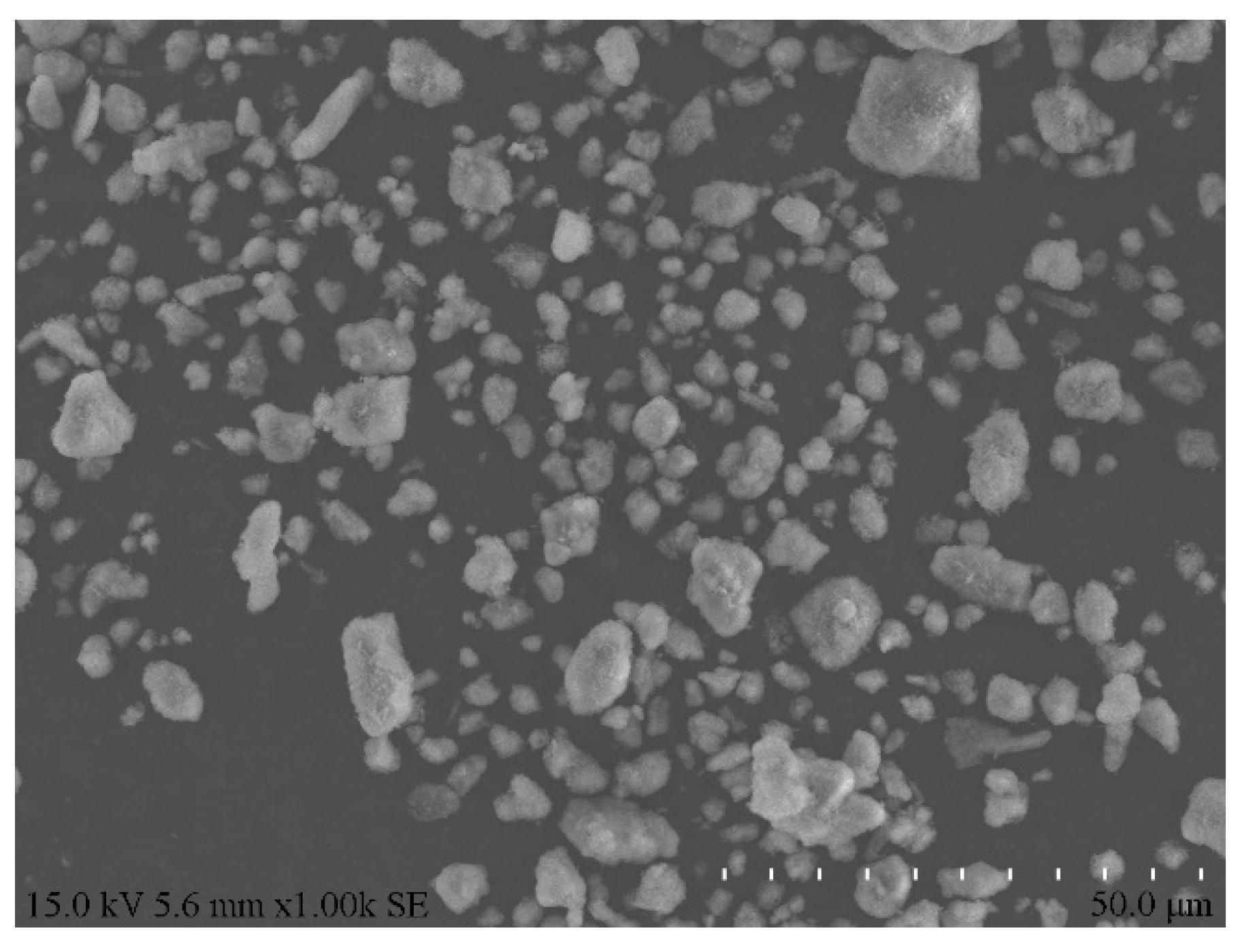
Table 1 details the chemical composition of aluminum ash, whereas Figure 2 and Figure 3 display its XRD pattern and particle morphology.

Table 1.
Major components of aluminum ash (wt.%).

Figure 2.
XRD pattern of aluminum ash.
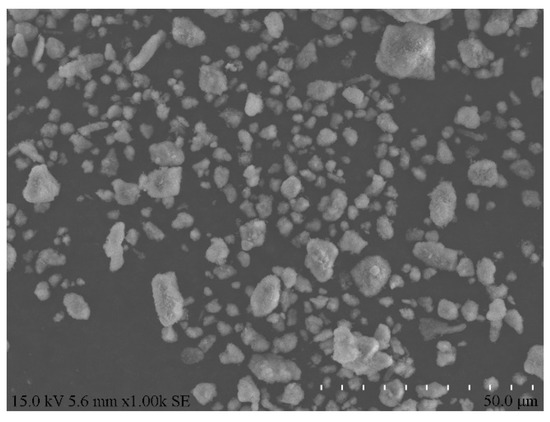
Figure 3.
Morphology of aluminum ash.
As is clear from Table 1 and Figure 2, the major chemical components of secondary aluminum ash were alumina (Al2O3), aluminum nitride (AlN) and Aluminum (Al), among which the content of AlN was very high. This was attributed to the chemical reaction between molten aluminum and nitrogen at high temperatures during the aluminum casting, which led to substantial generation of AlN. Figure 3 displays the morphology of aluminum ash, which was mostly scattered and loose, approximately spherical, and the particles were relatively independent. Such particle morphology could facilitate the subsequent hydration reaction.
3.2. Effects of Mix Proportions on Non-Fired Bricks
3.2.1. Effect of Cement–Aluminum Ash Mix Proportion
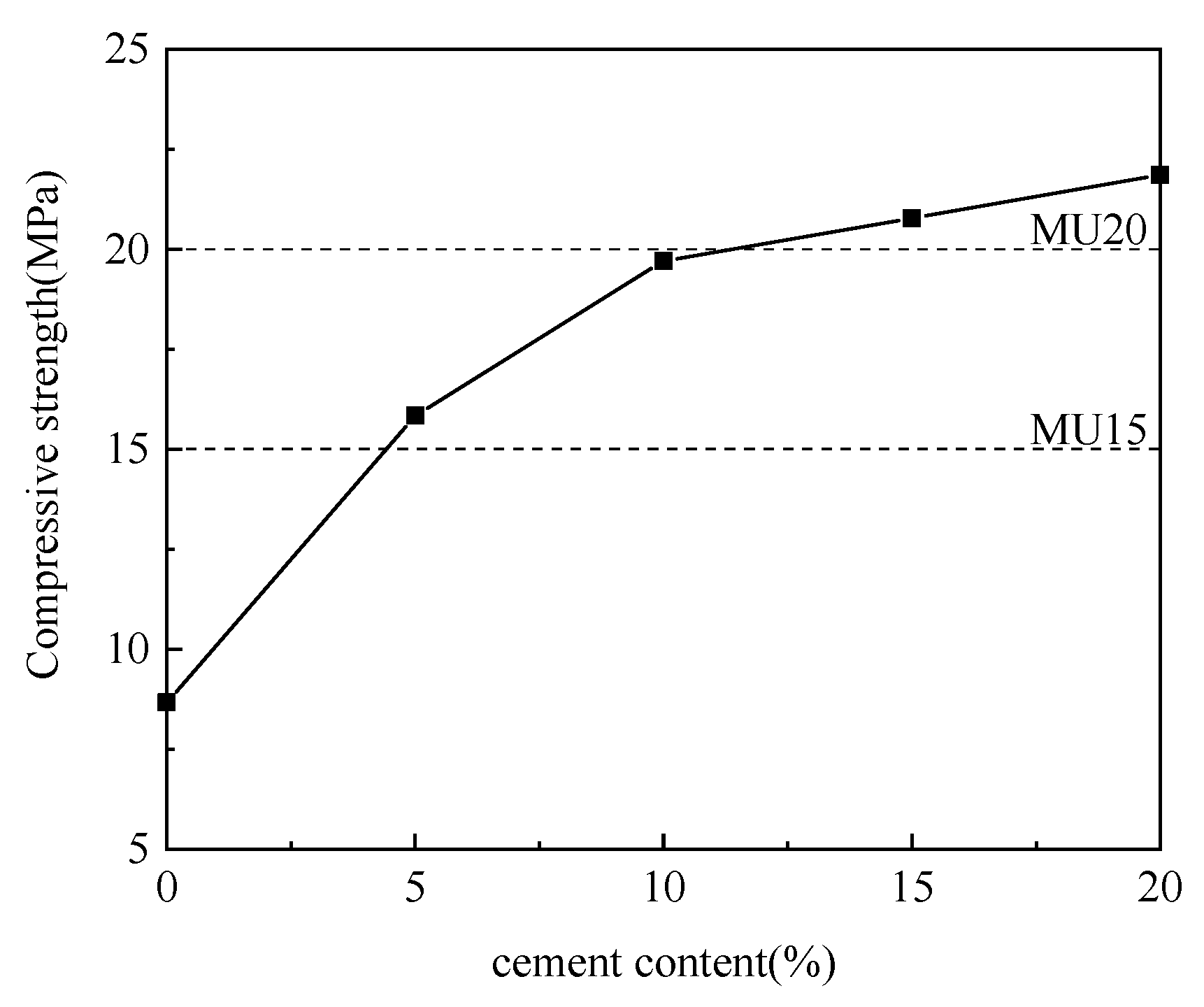
Using aluminum ash as the adjusting component, the cement was incorporated at different ratios for experimentation. Table 2 details the specific mix proportions, and Figure 4 illustrates the experimental results.

Table 2.
Cement and aluminum ash mix design. (wt.%).
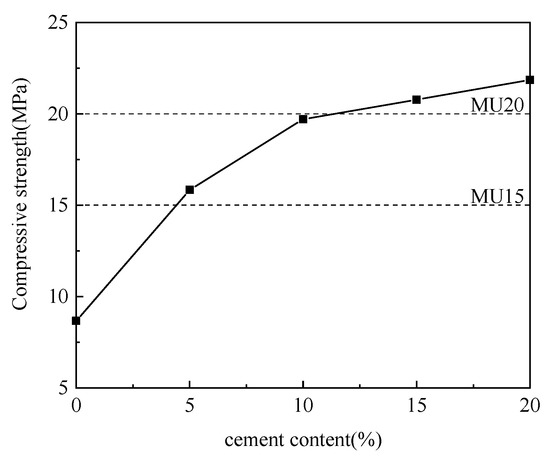
Figure 4.
Effect of cement content on compressive strength.
As is clear from Figure 4, with the increase in cement content and the decrease in aluminum ash content, the compressive strength of non-fired bricks increased gradually, which peaked (21.86 MPa) at a cement content of 20%. The cement itself underwent hydration reaction to produce calcium silicate hydrate (CSH) and other products. Meanwhile, Ca(OH)2 was produced during cement hydration, which further deepened the alkalinity to generate calcium aluminate hydrate (CAH), thereby enhancing the brick strength. The chemical reactions involved are formulated in (1)–(4) [16,17,18]. However, the amount of cement directly affected the production cost of non-fired bricks while reducing the utilization rate of aluminum ash. Accordingly, considering the effect of cement content on the compressive strength, the appropriate mix proportion between aluminum ash and cement was determined to be 60:10.
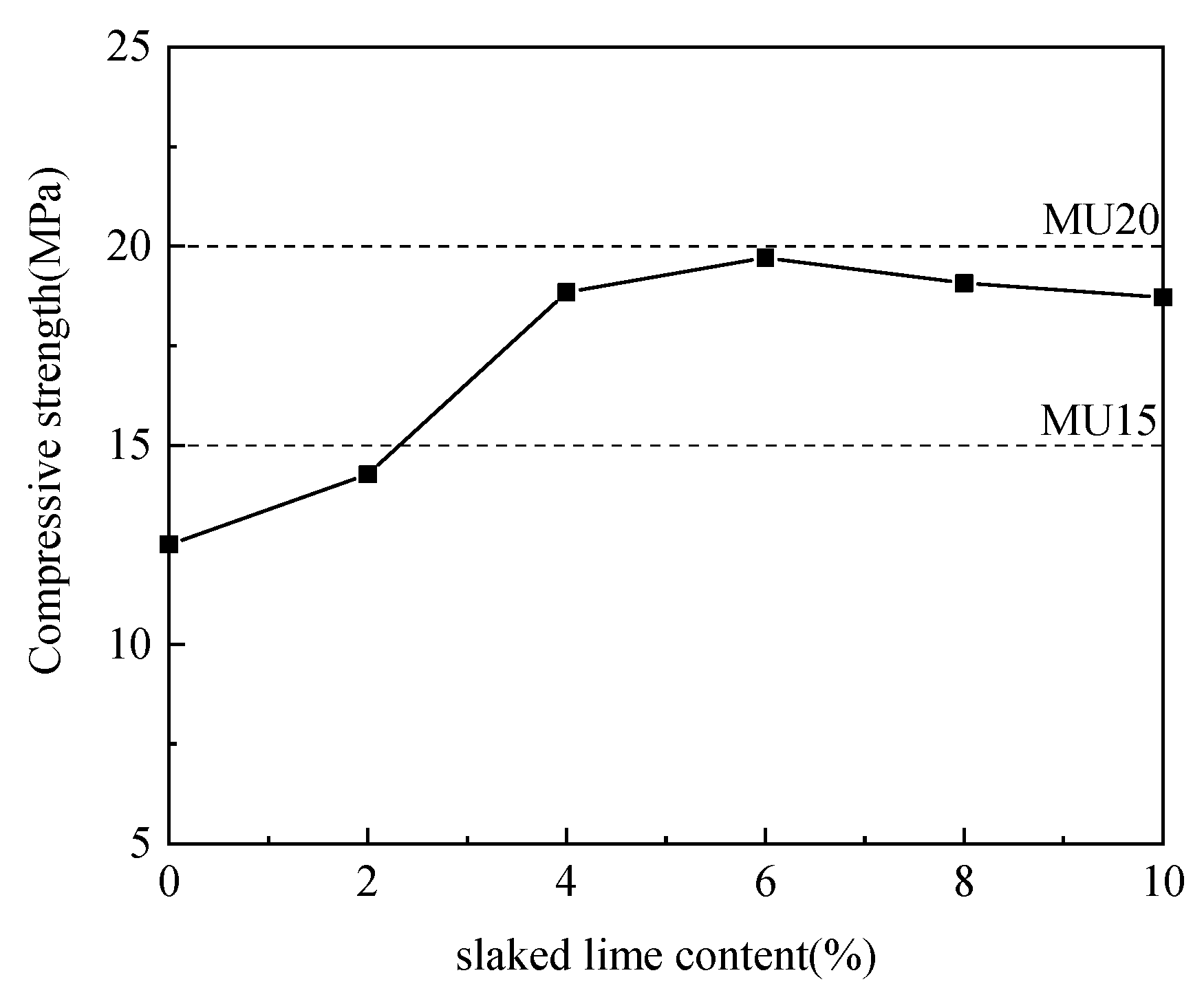
3.2.2. Effect of Slaked Lime–Aluminum Ash Mix Proportion
Using aluminum ash as the adjusting component, the slaked lime was incorporated at different ratios for experimentation. Table 3 details the specific mix proportions, and Figure 5 illustrates the experimental results.

Table 3.
Slaked lime and aluminum ash mix design. (wt.%).

Figure 5.
Effect of slaked lime content on compressive strength.
According to Figure 5, with the increase in slaked lime content and the decrease in aluminum ash content, the compressive strength of non-fired bricks increased initially and then decreased, which peaked (19.71 MPa) at a slaked lime content of 6%. The use of slaked lime as an activator enhanced the brick strength, and the reaction occurred was formulated in (4) [19]. However, given the insufficient activity of slaked lime, its further increase could not participate timely in the reaction, which was dispersed in the system and reacted with CO2 in the air to form expansive CaCO3, resulting in strength decrease. Thus, the mix proportion between aluminum ash and slaked lime was determined to be 64:6.
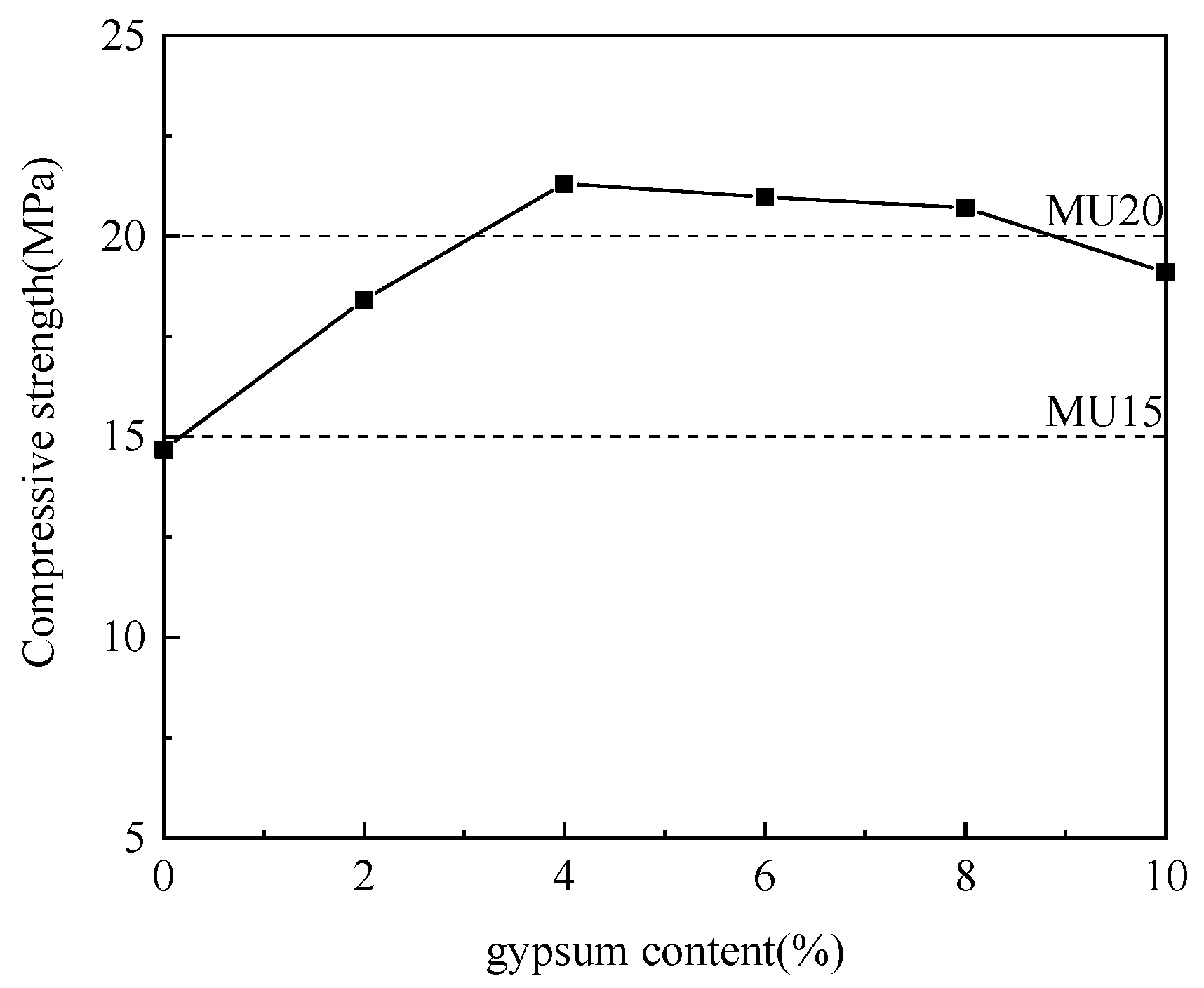
3.2.3. Effect of Gypsum–Aluminum Ash Mix Proportion
Using aluminum ash as the adjusting component, the gypsum was incorporated at different ratios for experimentation. Table 4 details the specific mix proportions, and Figure 6 illustrates the experimental results.

Table 4.
Gypsum and aluminum ash mix design. (wt.%).
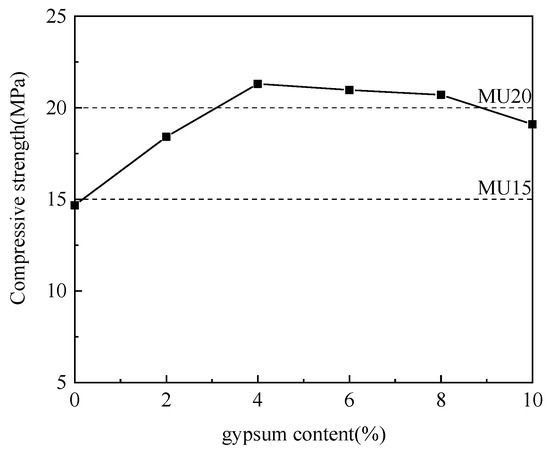
Figure 6.
Effect of gypsum content on compressive strength.
As is clear from Figure 6, with the increase in gypsum content and the decrease in aluminum ash content, the compressive strength of non-fired bricks increased initially and then decreased, which peaked (21.31 MPa) at a gypsum content of 4%. Gypsum, also as an activator, worked jointly with slaked lime to stimulate the active Al2O3 in the aluminum ash, thereby forming ettringite (AFt). The relevant reactions were formulated in (4) and (5) [19,20]. In the case of excessively large amount of gypsum, the calcium sulfate residue after the reaction was disorderly distributed in the system, reducing the strength of the non-fired bricks [21]. Thus, the mix proportion between aluminum ash and gypsum was determined to be 65:4.
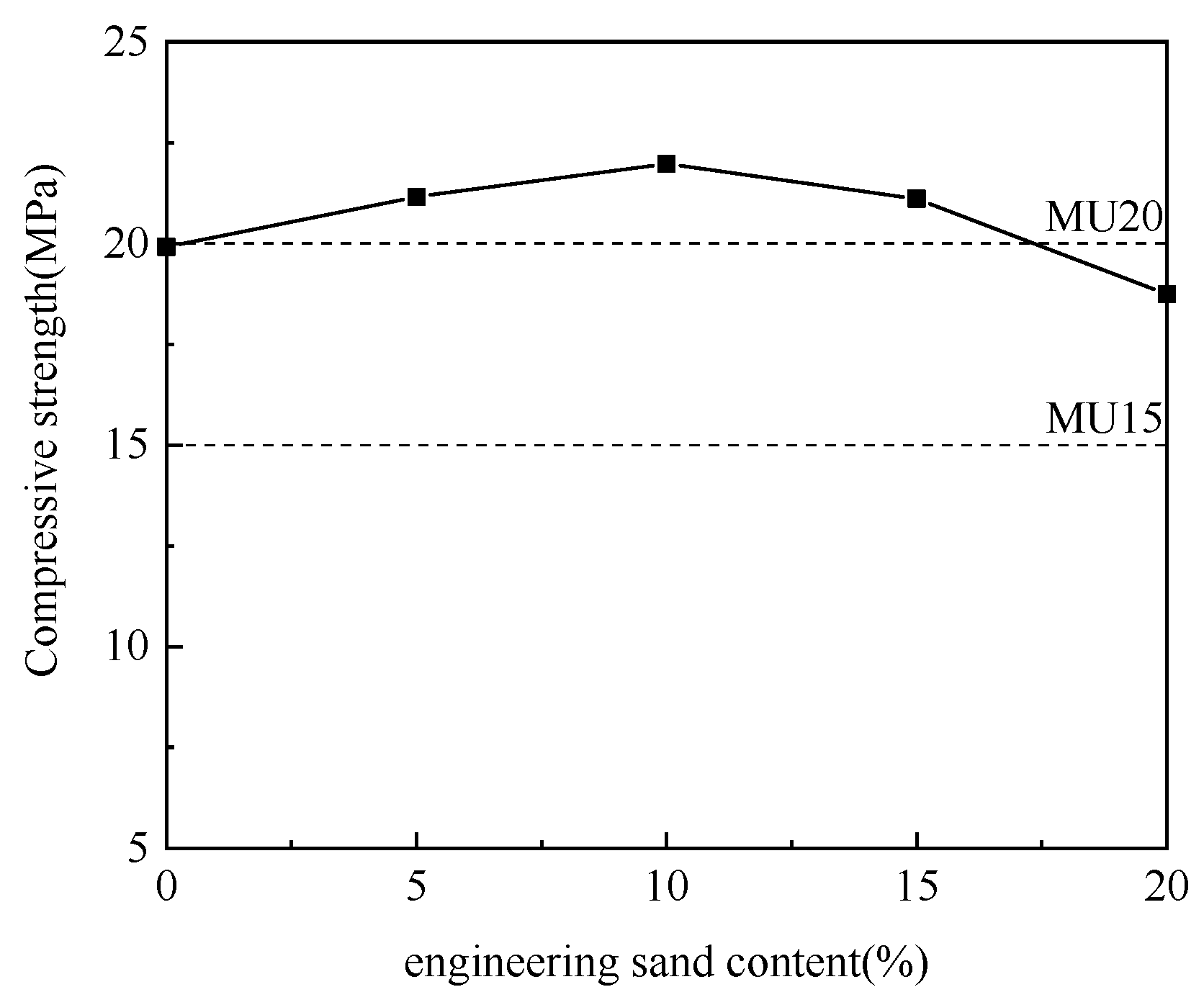
3.2.4. Effect of Engineering Sand–Aluminum Ash Mix Proportion
Using aluminum ash as the adjusting component, the engineering sand was incorporated at different ratios for experimentation. Table 5 details the specific mix proportions, and Figure 7 illustrates the experimental results.

Table 5.
Engineering sand and aluminum ash mix design. (wt.%).
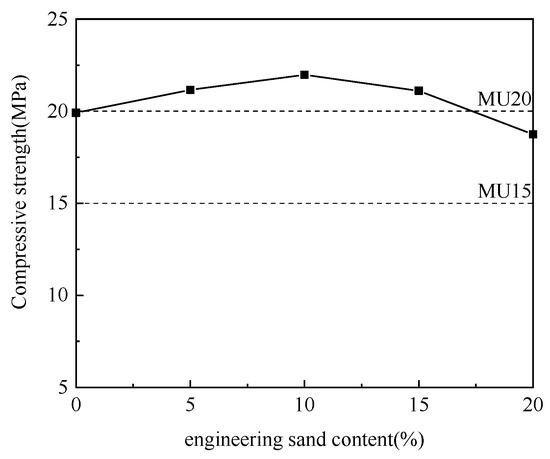
Figure 7.
Effect of engineering sand content on compressive strength.
According to Figure 7, with the increase in engineering sand content and the decrease in aluminum ash content, the compressive strength of non-fired bricks increased initially and then decreased, which peaked (21.97 MPa) at an engineering sand content of 10%. With further increase in engineering sand content, the brick compressive strength tended to decline. Engineering sand did not participate in the hydration reaction, which acted as aggregates to support the framework. Non-fired bricks are essentially a kind of concrete product. Adequate addition of aggregates could reduce the brick shrinkage and effectively prevent brick cracking. Meanwhile, the hydration products were attached to aggregates with larger particle sizes, which facilitated the enhancement of brick strength [22]. Thus, the mix proportion between aluminum ash and engineering sand was determined to be 70:10.
3.2.5. Validation of Optimal Mix Proportions
According to the aforedetermined mix proportions of aluminum ash and other components, the fairly optimal ratios of aluminum ash, cement, slaked lime, gypsum and engineering sand were calculated to be 68.3%, 11.4%, 6.4%, 4.2% and 9.7% (as percentages of total mass). Table 6 details the performance test results of the non-fired bricks (E1) made by the optimal mix proportions. Clearly, the compressive strength was 22.19 MPa, which was superior to the best experimental results of A5, B4, C3 and D3. The compressive strength, flexural strength and frost resistance of E1 samples conformed to the MU20 grade requirements, and the water absorption and leaching toxicity met the corresponding indicator.

Table 6.
Performance test results of E1 samples.
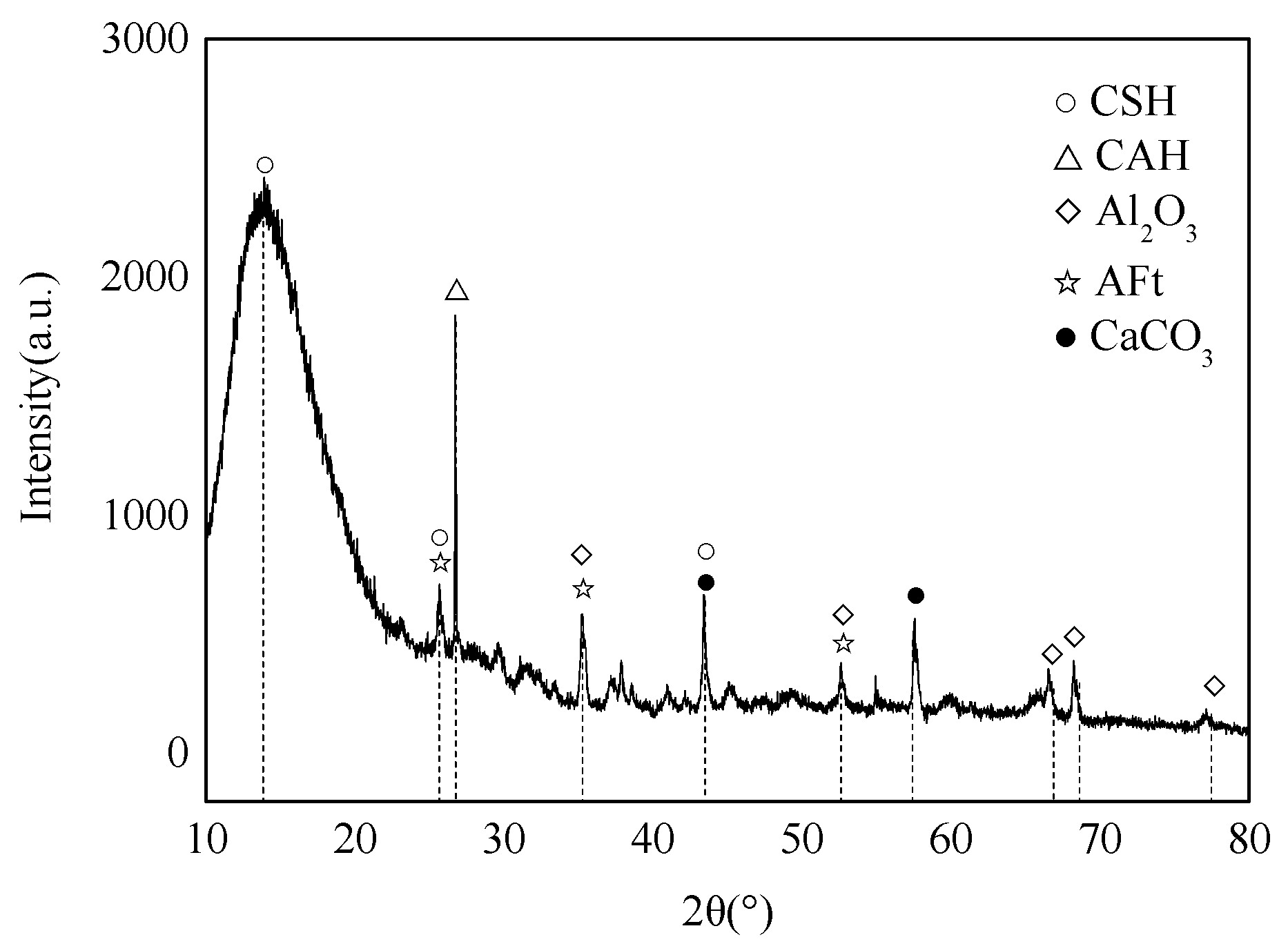
3.3. Sample Texture and Structure
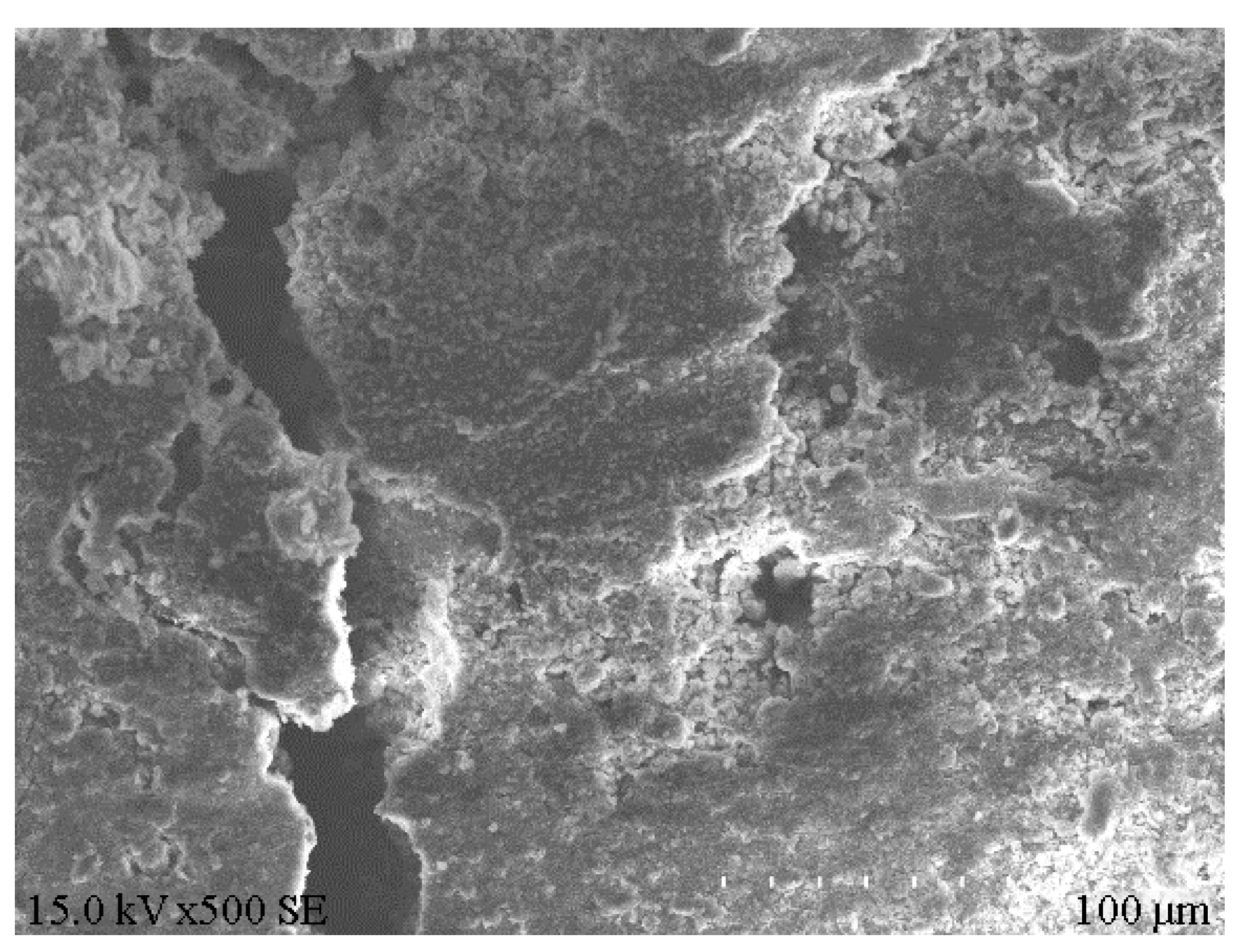
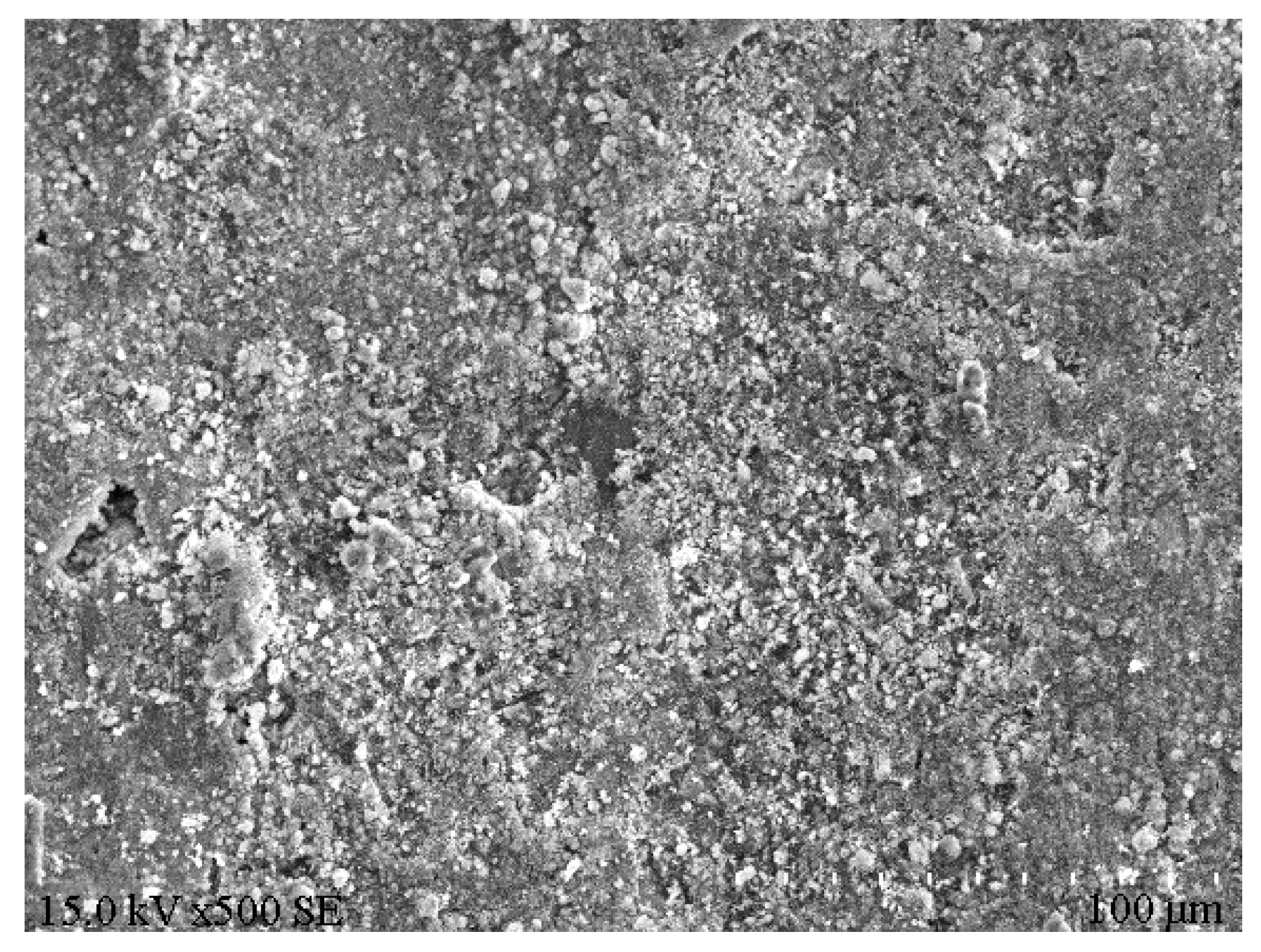
XRD analysis was performed on the e1 samples, which had the highest compressive strength, and the results are displayed in Figure 8. Meanwhile, SEM analysis was carried out on the E1 samples, as well as the A1 samples, which had the lowest compressive strength, and the results are depicted in Figure 9 and Figure 10. According to Figure 8, substantial hydration products were generated in the system following reaction, mainly AFt, CSH and CAH. Besides, some products were present as Al2O3 in the non-fired bricks, which were further hydrated during later curing. There was also some amount of CaCO3 in the samples, which was probably generated by the reaction of residual Ca(OH)2 with CO2 in the air. According to Figure 9, in the absence of cement and other cementitious materials, obvious voids or cracks were present on the sample surfaces, and the inter-particle binding was not tight. As is clear from Figure 10, the brick structure was denser, without obvious voids, where a hydraulically hardened slurry with a dense spatial structure was formed, thereby enhancing the sample strength [23].
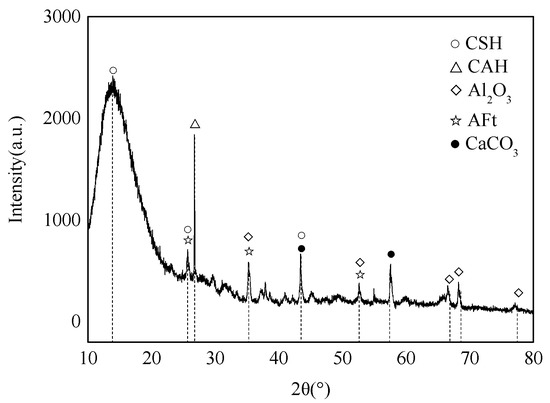
Figure 8.
XRD pattern of E1 samples.
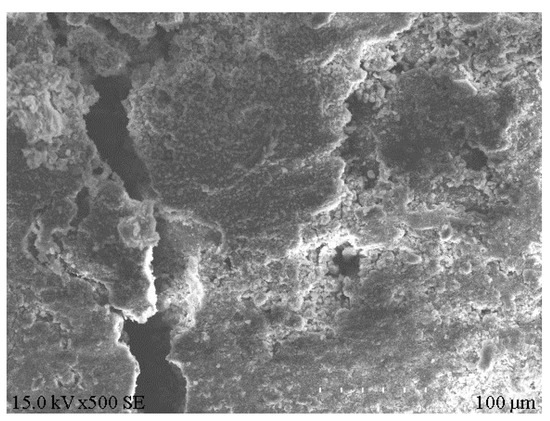
Figure 9.
Microstructure of A1 samples.
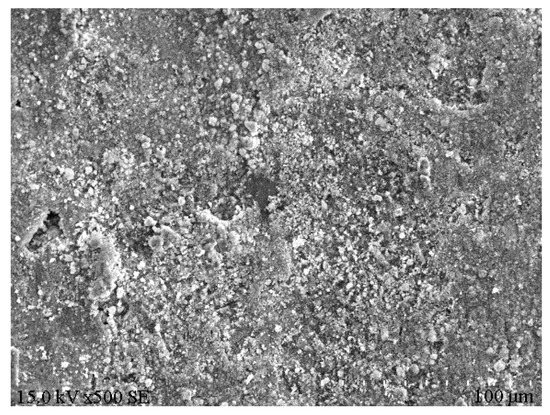
Figure 10.
Microstructure of E1 samples.
4. Conclusions
- (1)
- Preferred mix ratios for making non-fired bricks from secondary aluminum ash were 68.3% aluminum ash, 11.4% cement, 6.4% slaked lime, 4.2% gypsum and 9.7% engineering sand. The compressive strength, frost resistance and water absorption index of the resulting non-fired bricks all conform to the MU20 grade requirements in JC/T 422-2007 “Non-fired Rubbish Gangue Bricks”. The findings of this study provide a theoretical basis for the application of aluminum ash for making non-fired bricks, which can effectively reduce the inventory of aluminum ash and reduce environmental pollution.
- (2)
- Major hydration products of non-fired aluminum ash bricks are AFt, CSH and CAH, which are cemented together to form a dense interwoven and interfilled structure, thereby enhancing the brick strength.
Author Contributions
Conceptualization, H.N. and W.T.; methodology, W.W.; validation, W.W.; formal analysis, S.L. and H.N.; investigation, X.W. and S.L.; resources, H.N. and S.L.; writing—original draft, H.N.; writing—review and editing, S.L. and W.W.; visualization, H.N. and W.W.; super-vision, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was Supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Province Policy Guidance Program (International Science and Technology Cooperation) Project (BZ2021045), Key R&D Projects of Jiangsu Province (BE2019060).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, N.Y.; Ning, P.; Xie, T.J.; Duan, G. research progress on recycling and comprehensive utilization of aluminum dross. Bull. Chin. Ceram. Soc. 2017, 6, 23. [Google Scholar]
- Yang, Q.; Li, Q.; Zhang, G.F.; Shi, Q. Research and prospect of comprehensive utilization of aluminum dross. Light Met. 2019, 6, 1–5. [Google Scholar]
- Guo, R.; Liu, X.Z.; Li, Q.D.; Yi, X.M. Present situation of high value recycling technology of aluminum ash. Inorg. Chem. Ind. 2017, 49, 12–15, 25. [Google Scholar]
- Yang, H.; Shen, S.F.; Liu, H.Y.; Zheng, X.J.; Li, W.G.; Zhao, Q.C.; Wang, J.L.; Luo, Y.F. Study on mineralogical characteristics of secondary aluminum ash. Nonferrous Met. Eng. 2019, 9, 117–124. [Google Scholar]
- Hong, J.P.; Wang, J.; Chen, H.Y.; Sun, B.D.; Li, J.J.; Chen, C. Process of aluminum dross recycling and life cycle assessment for Al-Si alloys and brown fused alumina. Trans. Nonferrous Met. Soc. China 2010, 20, 2155–2161. [Google Scholar] [CrossRef]
- Xu, Z.F.; Song, W.G.; Xu, G.C. Successful practice of producing composite cement with aluminum slag. China Cem. 2004, 12, 76–77. [Google Scholar]
- Li, A.; Zhang, H.; Yang, H. Evaluation of aluminum dross as raw material for high-alumina refractory. Ceram. Int. 2014, 40, 12585–12590. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Akpan, E.I.; Dada, M.O. Refractory characteristics of aluminum dross-kaolin composite. JOM J. Miner. Met. Mater. Soc. 2014, 66, 2253–2261. [Google Scholar] [CrossRef]
- Murayama, N.; Maekawa, I.; Ushiro, H.; Miyoshi, T.; Shibata, J.; Valix, M. Synthesis of various layered double hydroxides using aluminum dross generated in aluminum recycling process. Int. J. Mineral. Process. 2012, 110, 46–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, H.J.; Lv, S.S.; Wang, X.X.; Li, S.Y.; Zhang, J.Q. Preparation of sintered brick with aluminum dross and optimization of process parameters. Coatings 2021, 11, 1039. [Google Scholar] [CrossRef]
- Ni, H.J.; Zhang, J.Q.; Lv, S.S.; Gu, T.; Wang, X.X. Performance optimization of original aluminum ash coating. Coatings 2020, 10, 831. [Google Scholar] [CrossRef]
- Ni, H.J.; Zhang, J.Q.; Lv, S.S.; Wang, X.X.; Zhu, Y.; Gu, T. Preparation and performance optimization of original aluminum ash coating based on plasma spraying. Coatings 2019, 9, 770. [Google Scholar] [CrossRef] [Green Version]
- GB/T 17671-1999; Test Method for Cement Mortar Strength. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 1999.
- JC/T 422-2007; Non-Fired Rubbish Gangue Bricks. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2007.
- GB 5085.3-2007; Hazardous Waste Identification Standard Leaching Toxicity Identification. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2007.
- Wang, Y.; Ni, W.; Zhang, S.Q.; Wang, Y.J.; Li, J. Research status of hydration mechanism of alumino-based cementitious system. Metal. Mines 2019, 4, 194–198. [Google Scholar]
- Kashef-Haghighi, S.; Shao, Y.X.; Ghoshal, S. Mathematical modeling of CO2 uptake by concrete during accelerated carbonation curing. Cem. Concr. Res. 2015, 67, 1–10. [Google Scholar] [CrossRef]
- Tang, M.Y.; Wang, Y.C.; Wang, Z.S. Production of building materials bricks with barium containing waste residue. Inorg. Chem. Ind. 1994, 2, 34–38. [Google Scholar]
- Hu, P.H.; Zhang, G.Z. Research on lightweight energy-saving unburned brick with large mixed fly ash. Bull. Chin. Ceram. Soc. 2012, 31, 984–987. [Google Scholar]
- Xing, J.; Hu, J.W.; Li, C.; Qiu, J.P.; Sun, X.G. The effect of gypsum on the cementitious performance of blast furnace slag stimulated by calcium oxide. China Min. Mag. 2019, 28, 166–171. [Google Scholar]
- Wang, D.Z.; He, Y.X. Study on effect of gypsum whisker on properties and structure of cement composite material. Inorg. Chem. Ind. 2015, 47, 65–68, 72. [Google Scholar]
- Feng, N.Q. New Practical Collection of Cement Concrete; Science Press: Beijing, China, 2005; pp. 78–125. [Google Scholar]
- Wu, H.; Lu, X.Y.; Luo, Z.J.; Yang, Y. Preparation and mechanism analysis of activated coal gangue unburned bricks. Non-Met. Mines 2018, 41, 30–33. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).