Effect of Cerium and Magnesium on Surface Microcracks of Al–20Si Alloys Induced by High-Current Pulsed Electron Beam
Abstract
:1. Introduction
2. Experimental Details
2.1. Specimen Preparation
2.2. HCPEB Processing
2.3. Characterisation
2.4. Corrosion Analysis
3. Results and Discussion
3.1. Microstructure Analysis without HCPEB Irradiation
3.2. Microstructure Analysis under HCPEB Irradiation
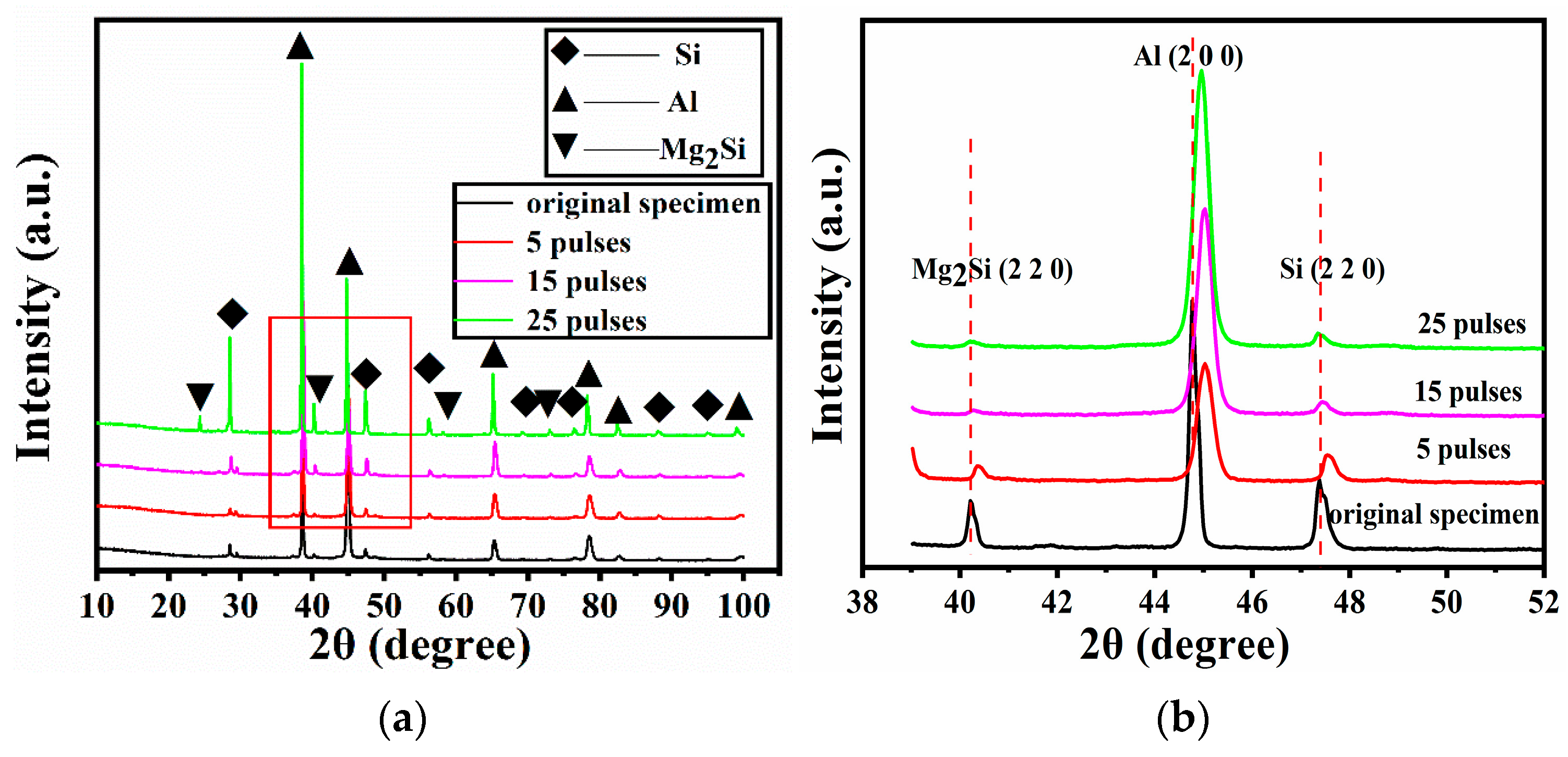
3.3. Phase Identification
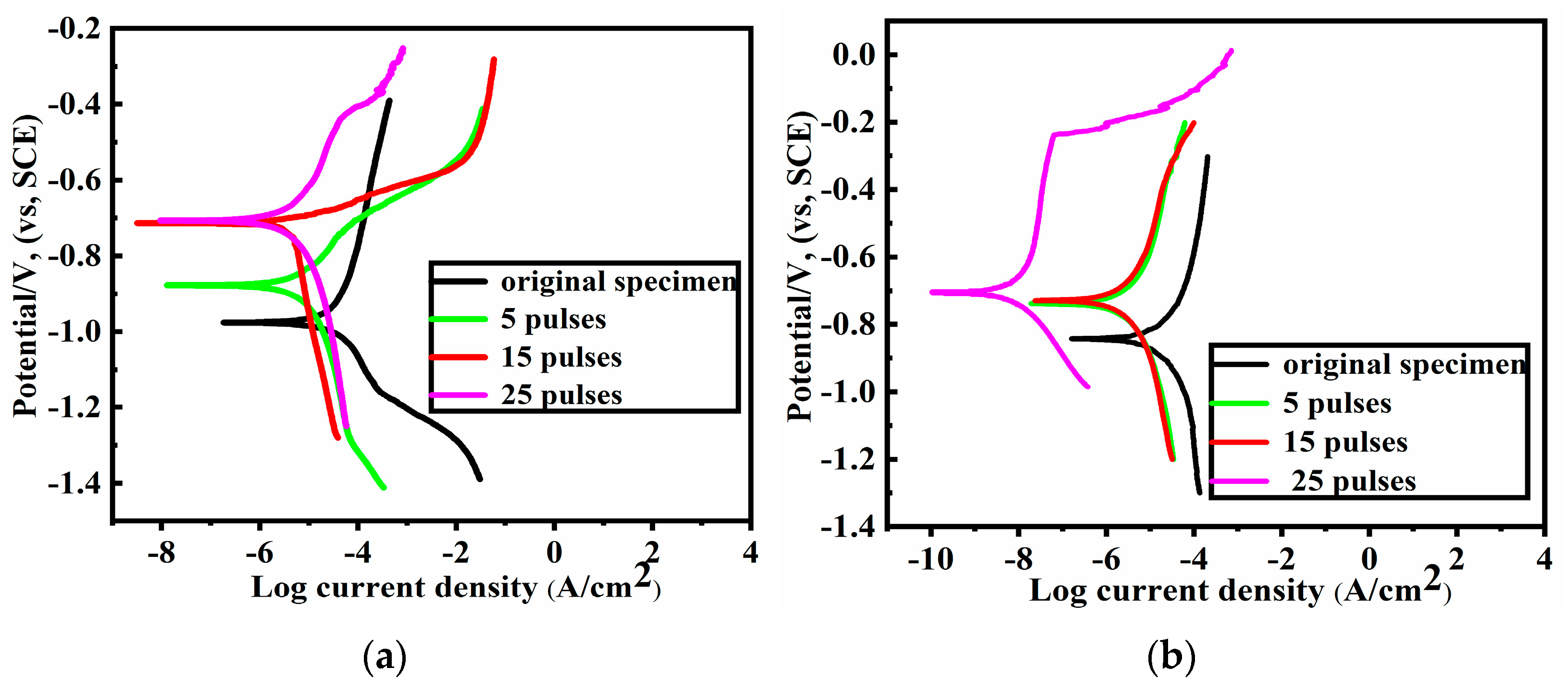
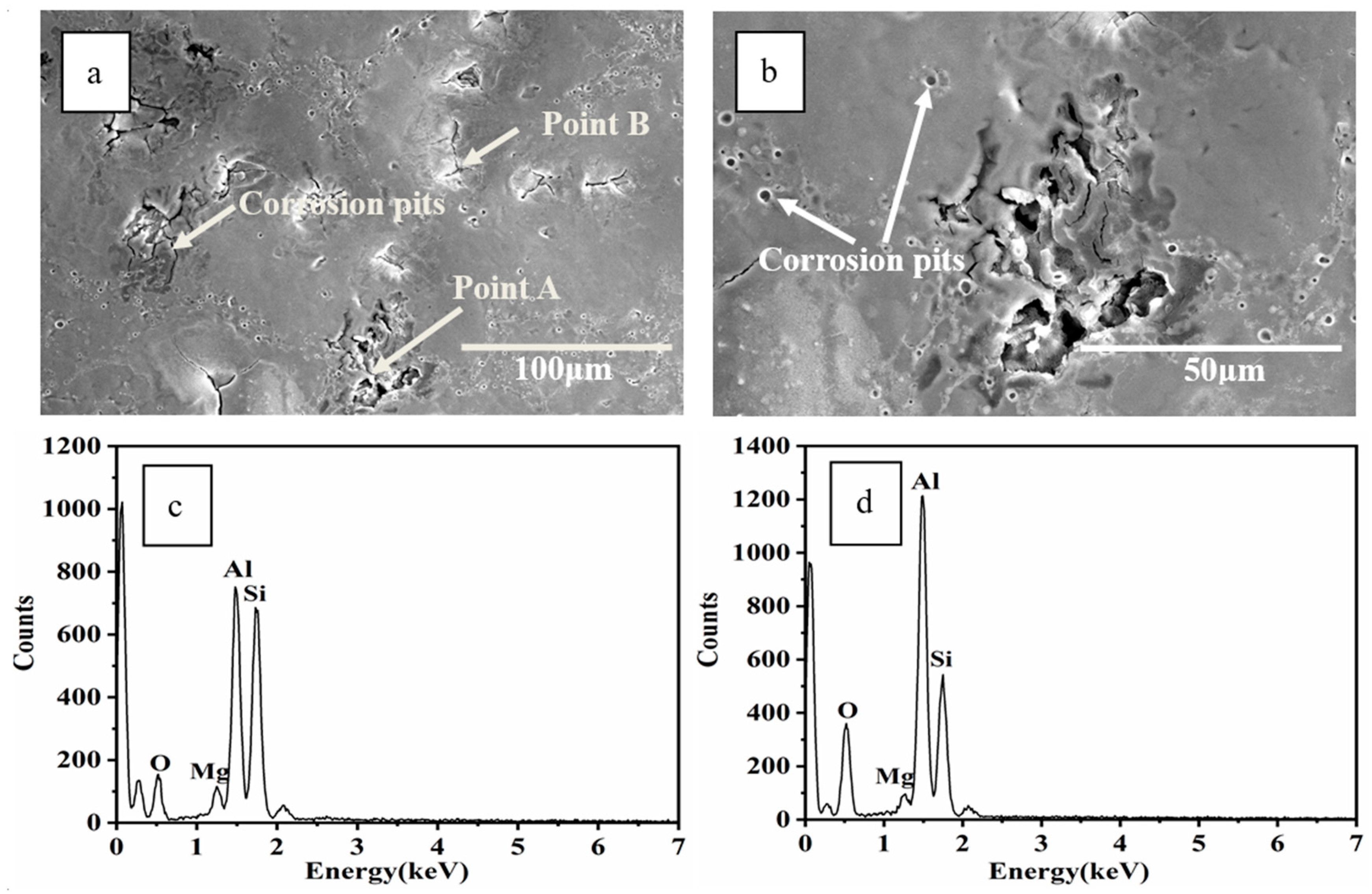
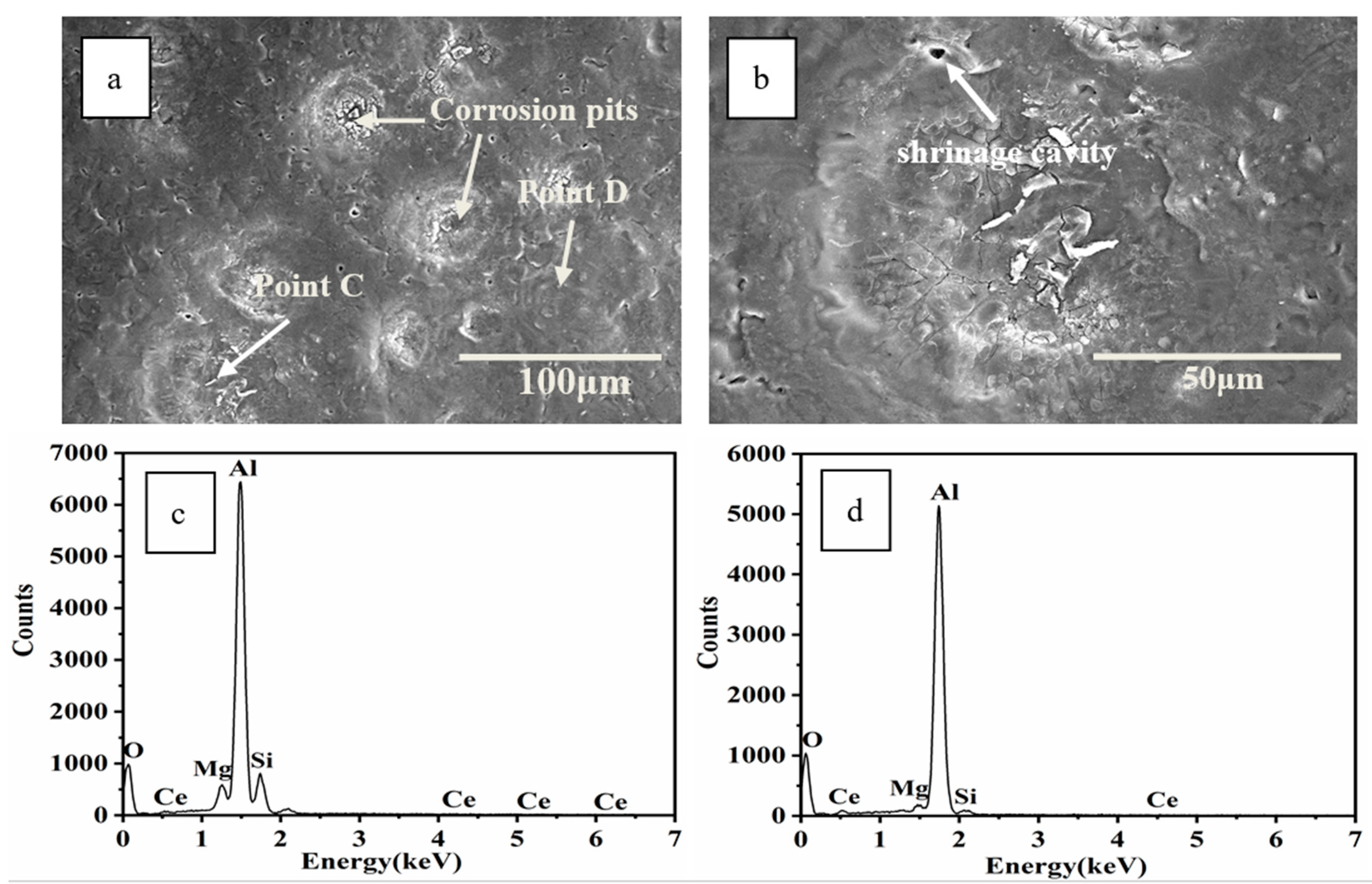
3.4. Corrosion Analysis
4. Conclusions
- (1)
- SEM analysis revealed that microcracks in the HCPEB-irradiated Al–20Si alloys were eliminated by the synergistic effect of Ce and Mg. The microcrack elimination was attributed to grain refinement effect of two elements and reduction of the local stress concentration in the brittle phase induced by rare earth Ce.
- (2)
- TEM analysis demonstrated that the metastable structures arose because of the rapid heating and solidification effects associated with HCPEB irradiation, which included a nano-primary Si phase, a nano-cellular aluminium structure, and a nano-Mg2Si phase. The formation of these metastable structures implied the grain refinement of the alloy surface, thus resulting in enhanced resistance to microcrack propagation, thereby delaying the initiation of microcracks.
- (3)
- EPMA analysis confirmed the uniform distribution of Ce in the Al matrix on the HCPEB-irradiated alloy surface, implying that Ce was completely dissolved in the phase, thereby significantly contributing to microcrack elimination. Moreover, the Mg and Si exhibited a certain degree of aggregation in the Mg2Si phase.
- (4)
- The phase transformation was absent on the HCPEB-irradiated Al–20Si–5Mg–0.7Ce alloy surface, as characterised by XRD analysis, and the phases mainly consisted of Al, Si, and Mg2Si phases. Broadening and shifting of the diffraction peaks was observed because of the formation of metastable structures and the presence of residual stress induced by HCPEB irradiation.
- (5)
- Compared with the alloy specimens containing Mg, the Al–20Si–5Mg–0.7Ce alloy specimens exhibited a superior anticorrosion property after HCPEB irradiation mainly due to the combined effects of the grain refinement and microcrack elimination.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, L.; Gao, B.; Zhu, G.L.; Hao, Y.; Sun, S.C.; Tu, G.F. The effect of neodymium on the microcracks generated on the Al–17.5Si alloy surface treated by high current pulsed electron beam. Appl. Surf. Sci. 2016, 364, 490–497. [Google Scholar] [CrossRef]
- Cai, J.; Li, C.; Yao, Y.M.; Lyu, P.; Guan, Q.F.; Li, Y.X.; Lu, J.Z. Microstructural modifications and high-temperature oxidation resistance of arc ion plated NiCoCrAlYSiHf coating via high-current pulsed electron beam. Corros. Sci. 2021, 182, 109281–109295. [Google Scholar] [CrossRef]
- Zhang, C.L.; Tian, N.N.; Li, L.; Yang, Z.R.; Lv, P.; Jin, Y.X.; Zhu, H.J.; Guan, Q.F. The effect of high-current pulsed electron beam on phase formation and surface properties of chromium/copper system. Vacuum 2020, 174, 109222–109232. [Google Scholar] [CrossRef]
- Sun, P.P.; Zhang, C.L.; Cai, J.; Lyu, P.; Jin, Y.X.; Guan, Q.F. Properties of a rapidly solidified Ni–Nb layer prepared using a high-current pulsed electron beam. Vacuum 2020, 177, 109362–109370. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Z.X.; Zhao, L.; Zhao, L.; Guan, Q.F.; Liu, Y.H. Surface microstructures and high-temperature high-pressure corrosion behavior of N18 zirconium alloy induced by high current pulsed electron beam irradiation. Appl. Surf. Sci. 2019, 484, 453–460. [Google Scholar] [CrossRef]
- Zhang, C.L.; Cai, J.; Lv, P.; Zhang, Y.W.; Xia, H.; Guan, Q.F. Surface microstructure and properties of Cu–C powder metallurgical alloy induced by high-current pulsed electron beam. J. Alloys Compd. 2017, 697, 96–103. [Google Scholar] [CrossRef]
- Fu, Y.L.; Hu, J.; Zhao, W.S.; Peng, F.J.; Huo, W.J.; Cao, X.T. Microstructure modification and corrosion improvement of AISI1045 steel induced by pseudospark electron beam treatment. Nucl. Instrum. Meth. B 2020, 469, 10–18. [Google Scholar] [CrossRef]
- Zhang, K.M.; Ma, J.X.; Zou, J.X.; Liu, Y.R. Surface microstructure and property modifications in a duplex stainless steel induced by high current pulsed electron beam treatments. J. Alloys Compd. 2017, 707, 178–183. [Google Scholar] [CrossRef]
- Yan, P.; Zou, J.X.; Zhang, C.L.; Grosdidier, T. Surface modifications of a cold rolled 2024 Al alloy by high current pulsed electron beams. Appl. Surf. Sci. 2020, 504, 144382–144396. [Google Scholar] [CrossRef]
- Konovalov, S.; Zaguliaev, D.; Ivanov, Y.; Gromov, V.; Abaturova, A. Modification of Al–10Si–2Cu alloy surface by intensive pulsed electron beam. J. Mater. Res. Technol. 2020, 9, 5591–5598. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.M.; Zou, J.X.; Yan, P.; Song, L.X.; Liu, Y.R. Surface microstructure modifications and in-vitro corrosion resistance improvement of a WE43 Mg alloy treated by pulsed electron beams. Vacuum 2020, 173, 109132–109140. [Google Scholar] [CrossRef]
- Morini, F.; Bestetti, M.; Franz, S.; Vicenzo, A.; Markov, A.; Yakovlev, E. Surface properties modification of magnesium alloys by low energy high current pulsed electron beam. Surf. Coat. Technol. 2021, 420, 127351–127362. [Google Scholar] [CrossRef]
- Meisner, L.L.; Semin, V.O.; Mironov, Y.P.; Meisner, S.N.; D’yachenko, F.A. Cross-sectional analysis of the graded microstructure and residual stress distribution in a TiNi alloy treated with low energy high-current pulsed electron beam. Mater. Today Commun. 2018, 17, 169–179. [Google Scholar] [CrossRef]
- Semin, V.O.; Meisner, L.L. Atomic structure of an amorphous Ti–Ta-based surface alloy synthesized on a TiNi substrate by an electron-beam method. Appl. Surf. Sci. 2019, 491, 411–419. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Z.X.; Zhao, L.; Guan, Q.F.; Zhao, L.; Liu, Y.H. Microstructures and corrosion resistance of Zircaloy-4 after surface alloying with copper by high-current pulsed electron beam. Appl. Surf. Sci. 2020, 501, 144222–144230. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Z.X.; Zhang, M.; Guan, Q.F.; Jin, Y.X.; Liu, Y.H. Effect of high current pulsed electron beam surface irradiation on the microstructure and electrochemical behavior of zircaloy-4. Nucl. Instrum. Meth. B 2018, 434, 81–87. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Chai, L.J.; Xiao, Z.P.; Tu, J.; Wang, Y.P.; Huang, W.J. Surface modification of Cu–25Cr alloy induced by high current pulsed electron beam. Trans. Nonferr. Metal. Soc. 2015, 25, 1935–1943. [Google Scholar] [CrossRef]
- Peng, W.H.; Hao, S.Z.; Zhao, L.M.; Chen, J.; Li, W. Formation mechanism of graphite nanospheres in W–C–Co system under high current pulsed electron beam irradiation. Mater. Lett. 2019, 244, 207–210. [Google Scholar] [CrossRef]
- Zhang, F.G. Friction and wear behavior of WC/Ni cemented carbide tool material irradiated by high-intensity pulsed electron beam. Ceram. Int. 2019, 45, 15327–15333. [Google Scholar] [CrossRef]
- Cai, J.; Yao, Y.M.; Wei, J.Z.; Guan, Q.F.; Lyu, P.; Ye, Y.X.; Li, Y.X. Microstructure and transient oxidation behavior of NiCoCrAlYSiHf coating modified via high-current pulsed electron beam. Surf. Coat. Technol. 2021, 422, 127499–127510. [Google Scholar] [CrossRef]
- Cai, J.; Zu, Z.K.; Li, C.; Lyu, P.; Guan, Q.F.; Li, Y.X. Hot corrosion behavior of arc ion plating NiCoCrAlYSiHf coating via high--current pulsed electron beam. Oxid. Met. 2020, 94, 569–586. [Google Scholar] [CrossRef]
- Zhang, F.G. Tungsten carbide phase transformation under non-equilibrium solidification of high intensity pulsed ion and electron beams. Vacuum 2019, 159, 254–260. [Google Scholar]
- Golovin, Y.I.; Finkel, V.M.; Sletkov, A.A. Effects of current pulses on crack propagation kinetics in silicon iron. Strength Mater. 1977, 9, 204–210. [Google Scholar] [CrossRef]
- Finkel, V.M.; Ivanov, V.M.; Golovin, Y.I. Crack healing in metals by crossed electric and magnetic fields. Strength Mater. 1983, 15, 501–506. [Google Scholar] [CrossRef]
- Fu, Y.M.; Bai, X.Z.; Qiao, G.Y.; Hu, Y.D. Technique for producing crack arrest by electromagnetic heating. Mater. Sci. Technol.-Lond. 2001, 17, 1653–1656. [Google Scholar] [CrossRef]
- Fu, Y.M.; Bai, X.Z.; Zheng, L.J. Numerical simulation and experimental research on crack arresting using electromagnetic heat effects. Int. J. Nonlin. Sci. Num. 2001, 2, 375–378. [Google Scholar] [CrossRef]
- Murray, J.W.; Clare, A.T. Repair of EDM induced surface cracks by pulsed electron beam irradiation. J. Mater. Process. Technol. 2012, 212, 2642–2651. [Google Scholar] [CrossRef]
- Li, Q.L.; Xia, T.D.; Lan, Y.F.; Zhao, W.J.; Fan, L.; Li, P.F. Effect of rare earth cerium addition on the microstructure and tensile properties of hypereutectic Al–20%Si alloy. J. Alloys Compd. 2013, 562, 25–32. [Google Scholar] [CrossRef]
- Huang, Z.L.; Wang, K.; Zhang, Z.M.; Li, B.; Xue, H.S.; Yang, D.Z. Effects of Mg content on primary Mg2Si phase in hypereutectic Al–Si alloys. Trans. Nonferr. Metal. Soc. 2015, 25, 3197–3203. [Google Scholar] [CrossRef]
- Ma, P.; Wei, Z.J.; Jia, Y.D.; Zou, C.M.; Scudino, S.; Prashanth, K.G.; Yu, Z.S.; Yang, S.L.; Li, C.G.; Eckert, J. Effect of high pressure solidification on tensile properties and strengthening mechanisms of Al–20Si. J. Alloys Compd. 2016, 688, 88–93. [Google Scholar] [CrossRef]
- Shi, W.X.; Gao, B.; Tu, G.F.; Li, S.W. Effect of Nd on microstructure and wear resistance of hypereutectic Al–20Si alloy. J. Alloys Compd. 2010, 508, 480–485. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Mg-Si (Aluminum-Magnesium-Silicon). J. Phase Equilib. Diff. 2007, 28, 189–191. [Google Scholar] [CrossRef]
- Akrami, A.; Nasiri, N.; Kulish, V. Fractal dimension analysis of Mg2Si particles of Al-15% Mg2Si composite and its relationships to mechanical properties. Results Mater. 2020, 7, 100118–100123. [Google Scholar] [CrossRef]
- Ganiger, B.; Chandrashekharaiah, T.M.; Prasad, T.B.; Kabadi, V.R. Studies on relationship between wear behaviour and microstructure of a hypereutectic Al–Si alloy. Mater. Today Proc. 2018, 5, 25165–25173. [Google Scholar] [CrossRef]
- Li, Q.L.; Xia, T.D.; Lan, Y.F.; Li, P.F.; Fan, L. Effects of rare earth Er addition on microstructure and mechanical properties of hypereutectic Al–20% Si alloy. Mater. Sci. Eng. A 2013, 588, 97–102. [Google Scholar] [CrossRef]
- Hao, Y.; Gao, B.; Tu, G.F.; Li, S.W.; Dong, C.; Zhang, Z.G. Improved wear resistance of Al–15Si alloy with a high current pulsed electron beam treatment. Nucl. Instrum. Meth. B 2011, 269, 1499–1505. [Google Scholar] [CrossRef]
- Hao, Y.; Gao, B.; Tu, G.F.; Li, S.W.; Hao, S.Z.; Dong, C. Surface modification of Al–20Si alloy by high current pulsed electron beam. Appl. Surf. Sci. 2011, 257, 3913–3919. [Google Scholar] [CrossRef]
- Gao, B.; Hao, Y.; Zhuang, W.F.; Tu, G.F.; Shi, W.X.; Li, S.W.; Hao, S.Z.; Dong, C.; Li, M.C. Study on continuous solid solution of Al and Si elements of a high current pulsed electron beam treated hypereutectic Al17.5Si alloy. Phys. Procedia 2011, 18, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Gao, B.; Lv, J.K.; Sun, S.C.; Hao, Y.; Tu, G.F. Halo evolution of hypereutectic Al–17.5Si alloy treated with high-current pulsed electron beam. J. Nanomater. 2015, 2015, 247. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Hu, L.; Li, S.W.; Hao, Y.; Zhang, Y.D.; Tu, G.F.; Grosdidier, T. Study on the nanostructure formation mechanism of hypereutectic Al–17.5Si alloy induced by high current pulsed electron beam. Appl. Surf. Sci. 2015, 346, 147–157. [Google Scholar] [CrossRef]
- Gao, B.; Li, K.; Xing, P.F. Formation of a double-layer ultrafine crystal structure for high-current pulsed electron beam-treated Al–20Si–5Mg alloy. Coatings 2019, 9, 413. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Gao, B.; Xu, N.; Sun, Y.; Denisov, V.V.; Hu, L. The Influence of neodymium element on the crater structure formed on Al–17.5Si alloy surface processed by high-current pulsed electron beam. Coatings 2020, 10, 922. [Google Scholar] [CrossRef]


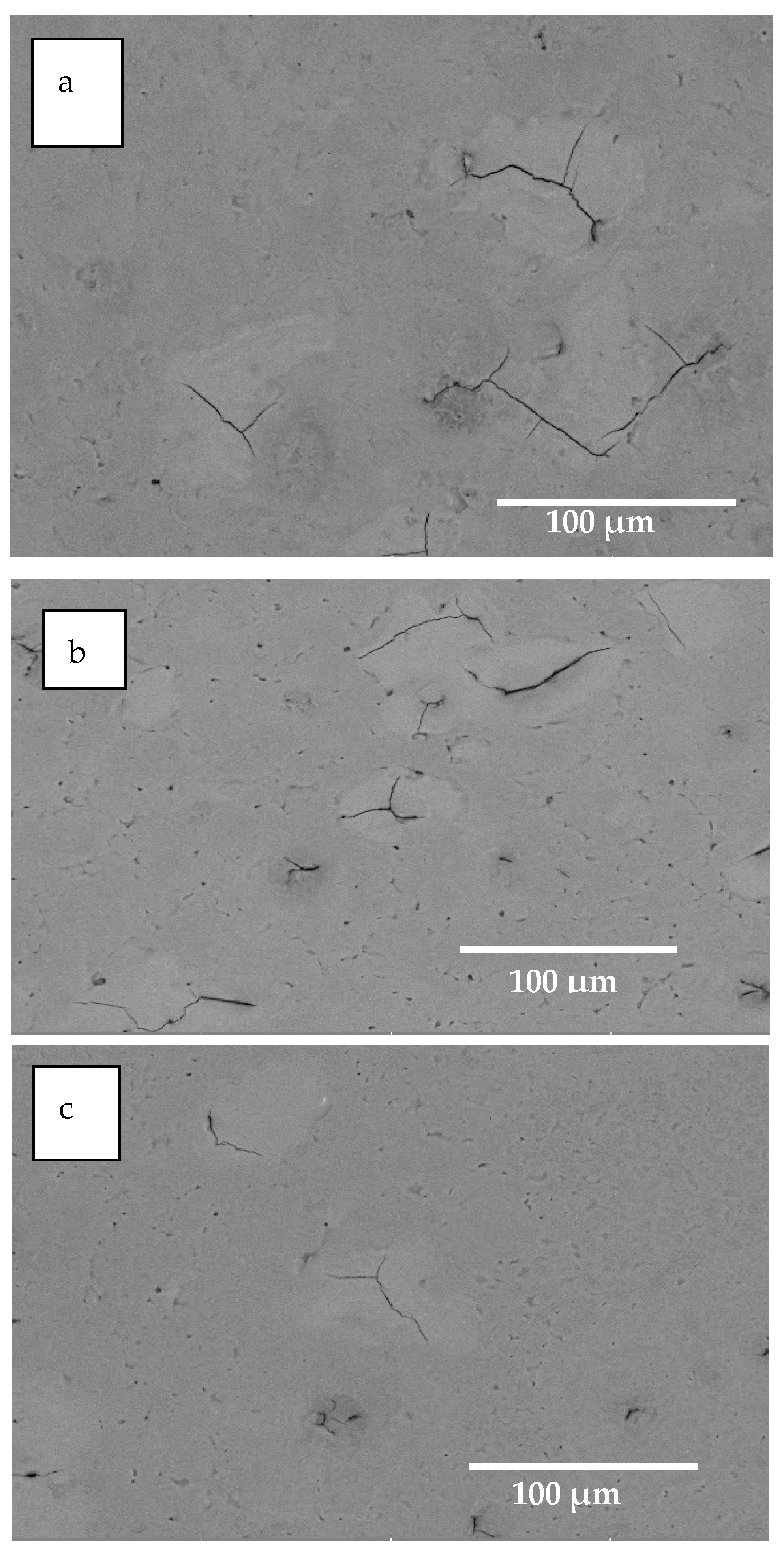

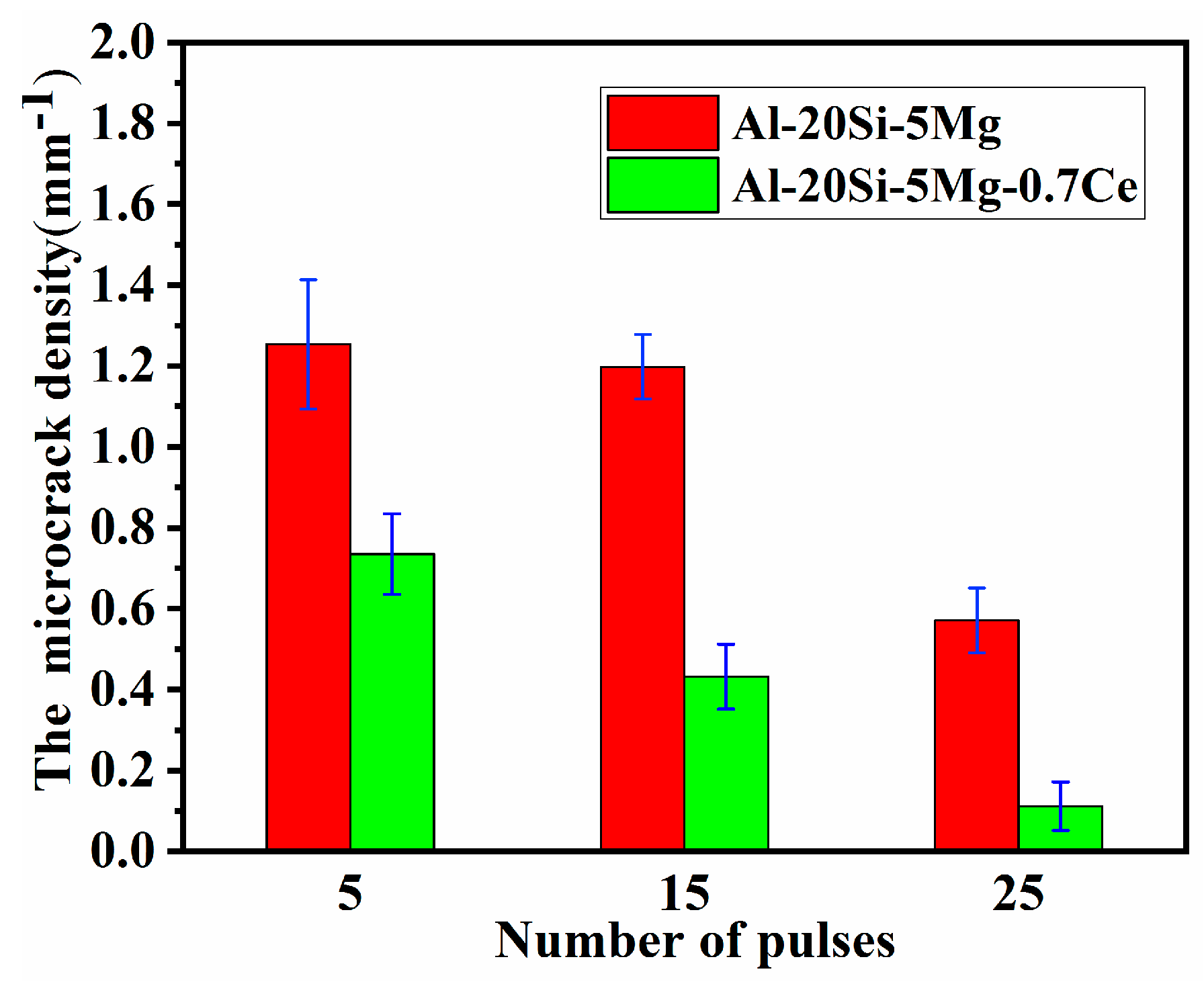
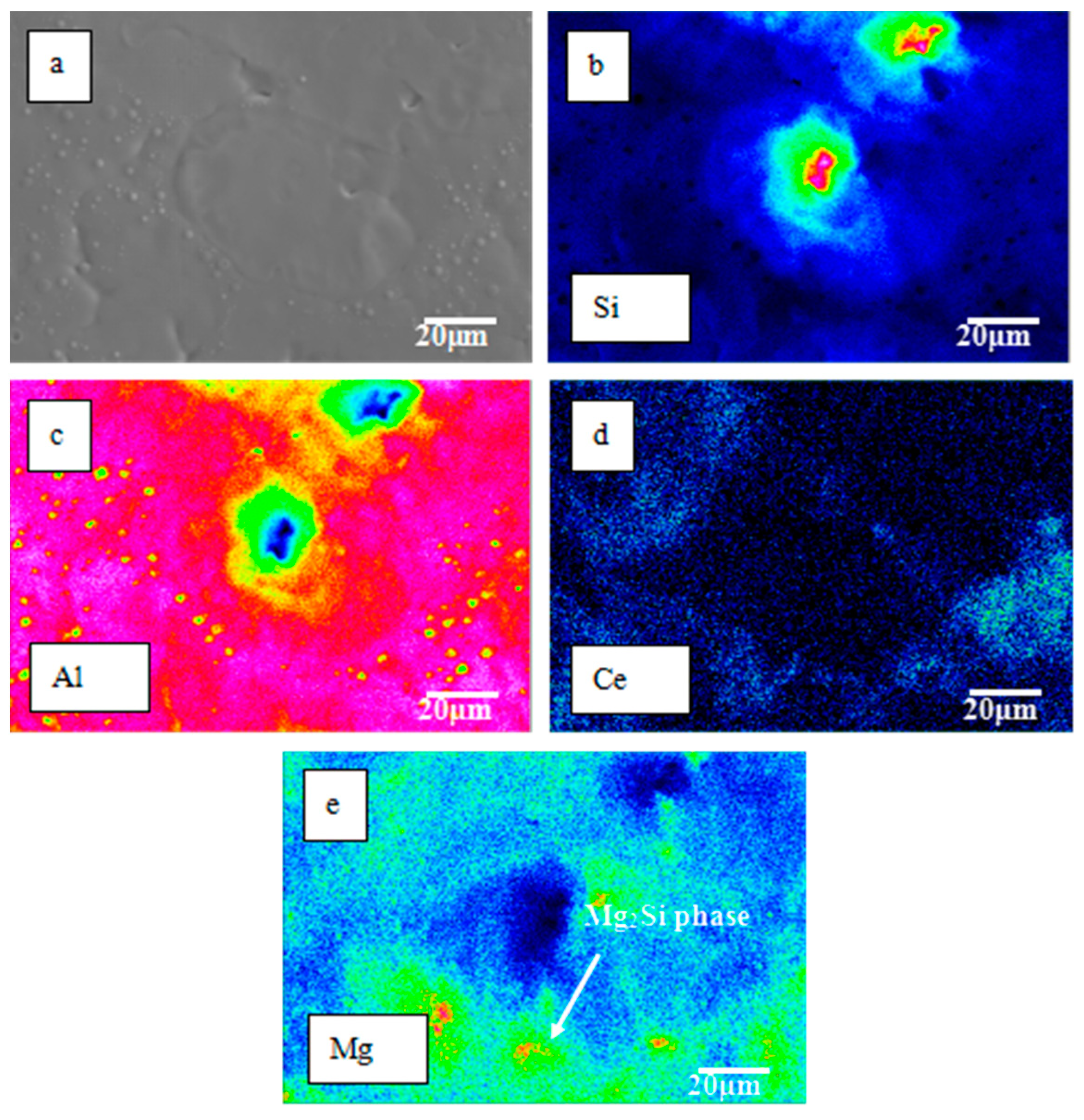
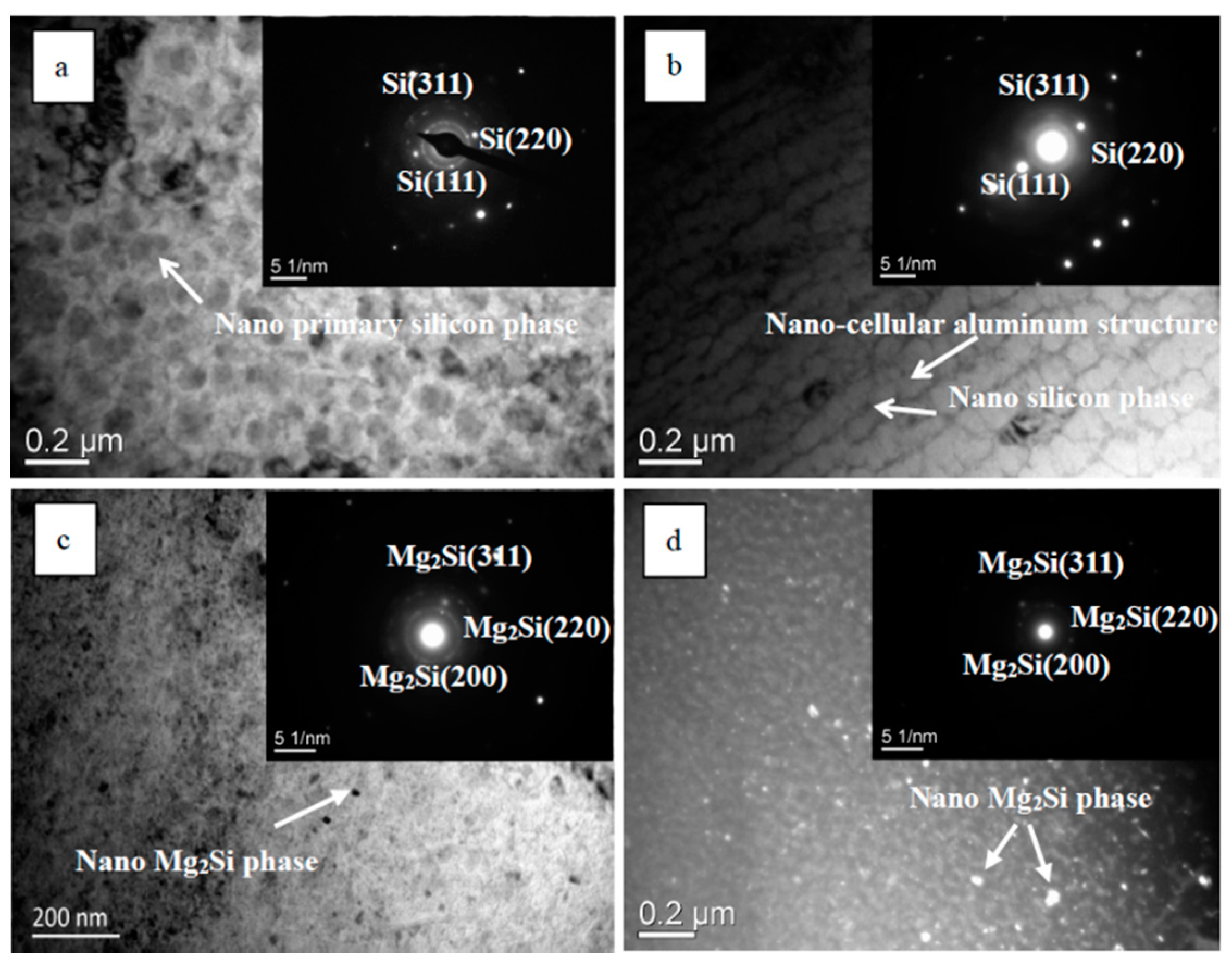
| Alloys | Al | Si | Mg | Ce | Impurity |
|---|---|---|---|---|---|
| Al–20Si–5Mg alloy | 75.06 | 20.05 | 4.79 | – | ≤0.1 |
| Al–20Si–5Mg–0.7Ce alloy | 75.01 | 19.54 | 4.69 | 0.66 | ≤0.1 |
| Alloy Sample | HCPEB Pulses | Ecoor (mV) | Icorr (μA/cm2) |
|---|---|---|---|
| Al–20Si–5Mg alloy | 0 | −972.8 | 52.88 |
| 5 | −875.0 | 7.745 | |
| 15 | −726.5 | 4.756 | |
| 25 | −710.3 | 3.965 | |
| Al–20Si–5Mg–0.7Ce alloy | 0 | −847.2 | 15.40 |
| 5 | −758.4 | 2.798 | |
| 15 | −727.9 | 2.459 | |
| 25 | −707.7 | 0.01021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Gao, B.; Xu, N.; Sun, Y.; Zhang, Y.; Xing, P. Effect of Cerium and Magnesium on Surface Microcracks of Al–20Si Alloys Induced by High-Current Pulsed Electron Beam. Coatings 2022, 12, 61. https://doi.org/10.3390/coatings12010061
Hu L, Gao B, Xu N, Sun Y, Zhang Y, Xing P. Effect of Cerium and Magnesium on Surface Microcracks of Al–20Si Alloys Induced by High-Current Pulsed Electron Beam. Coatings. 2022; 12(1):61. https://doi.org/10.3390/coatings12010061
Chicago/Turabian StyleHu, Liang, Bo Gao, Ning Xu, Yue Sun, Ying Zhang, and Pengfei Xing. 2022. "Effect of Cerium and Magnesium on Surface Microcracks of Al–20Si Alloys Induced by High-Current Pulsed Electron Beam" Coatings 12, no. 1: 61. https://doi.org/10.3390/coatings12010061






