Epitaxial Growth and Optical Properties of Laser Deposited CdS Thin Films
Abstract
:1. Introduction
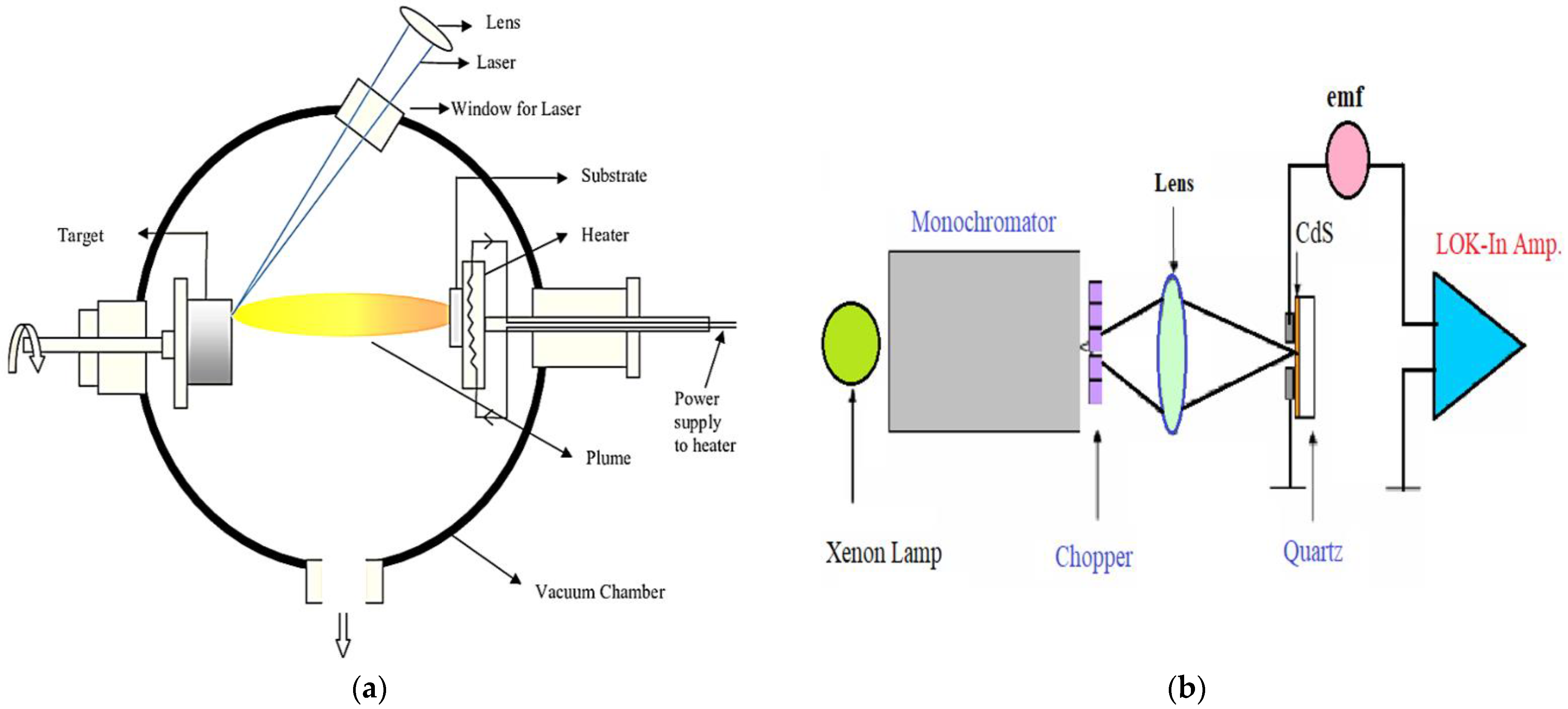
2. Experimental Details
2.1. Preparation CdS Thin Films
2.2. Characterization Techniques
3. Results and Discussion
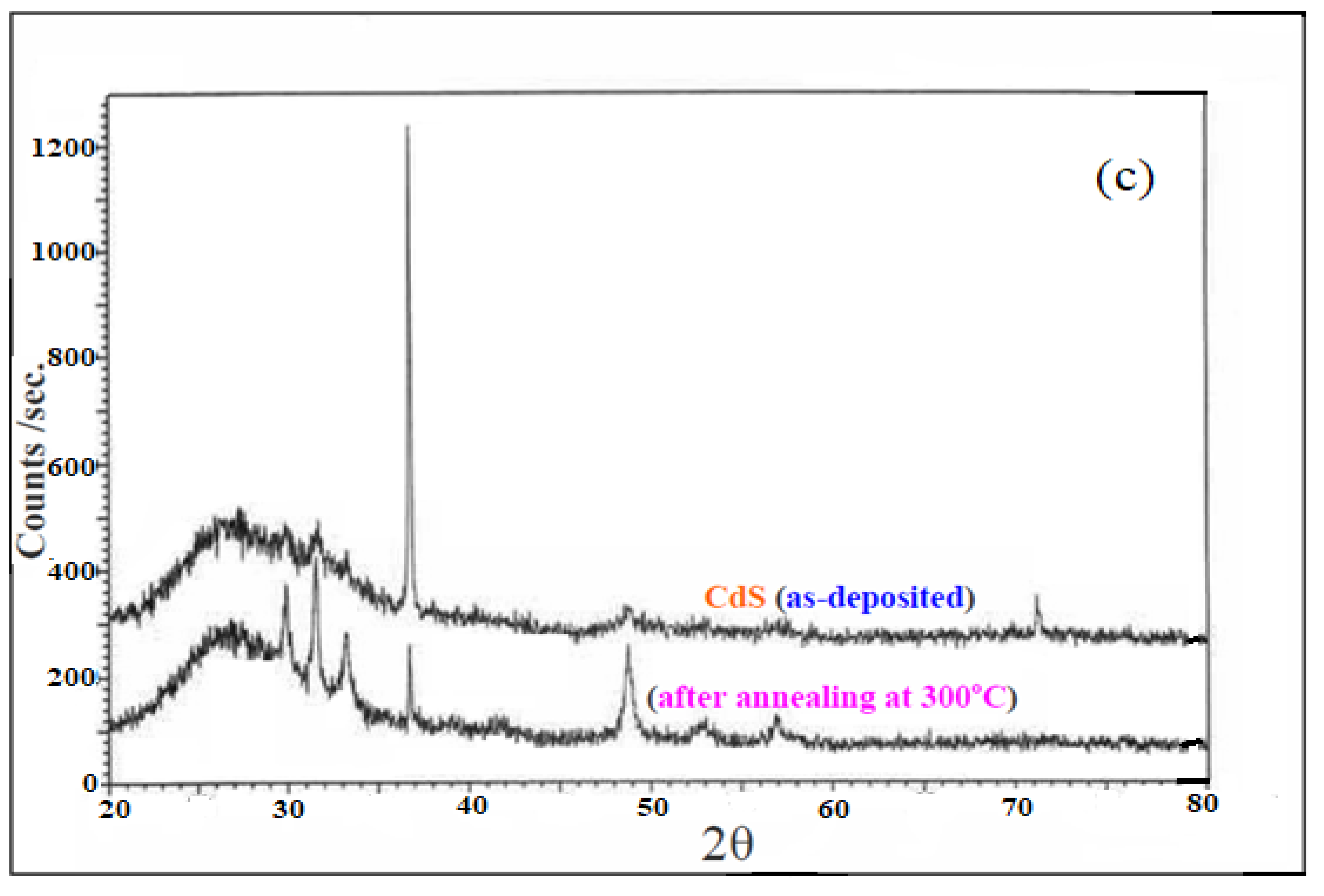
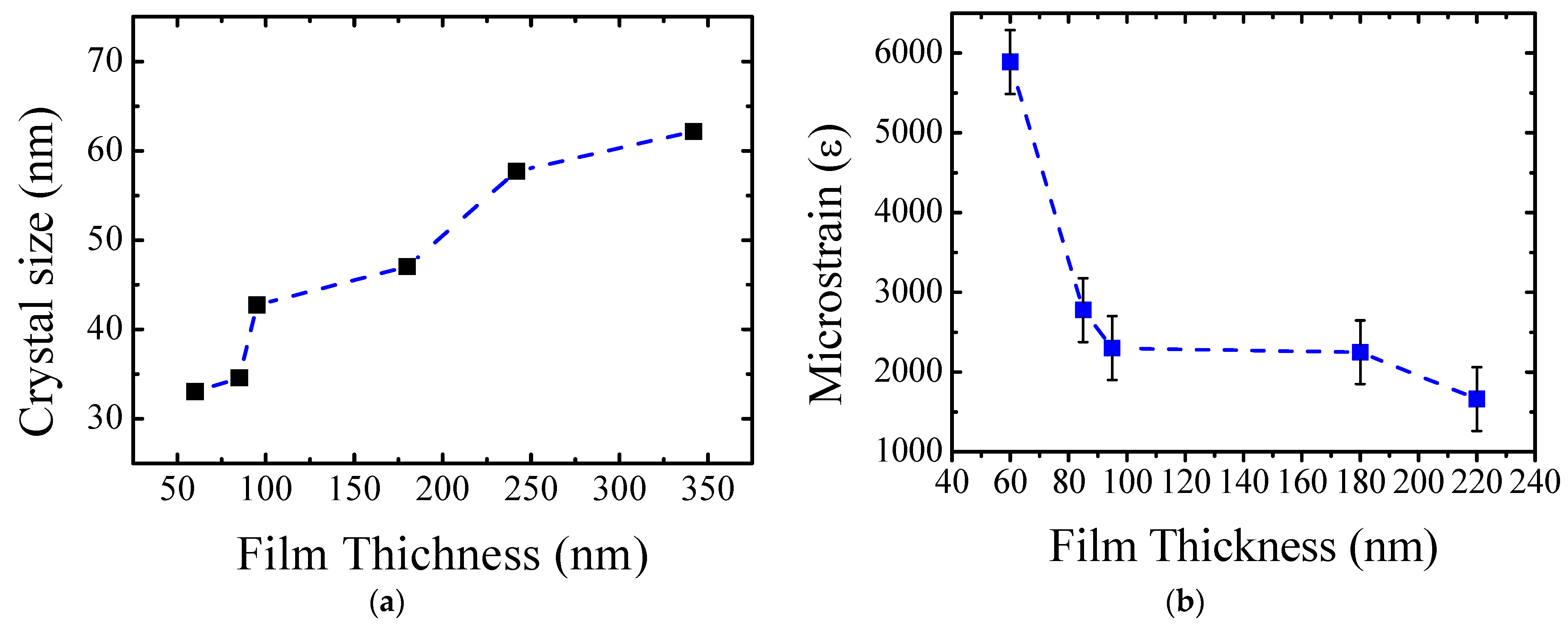
3.1. Structural Analysis
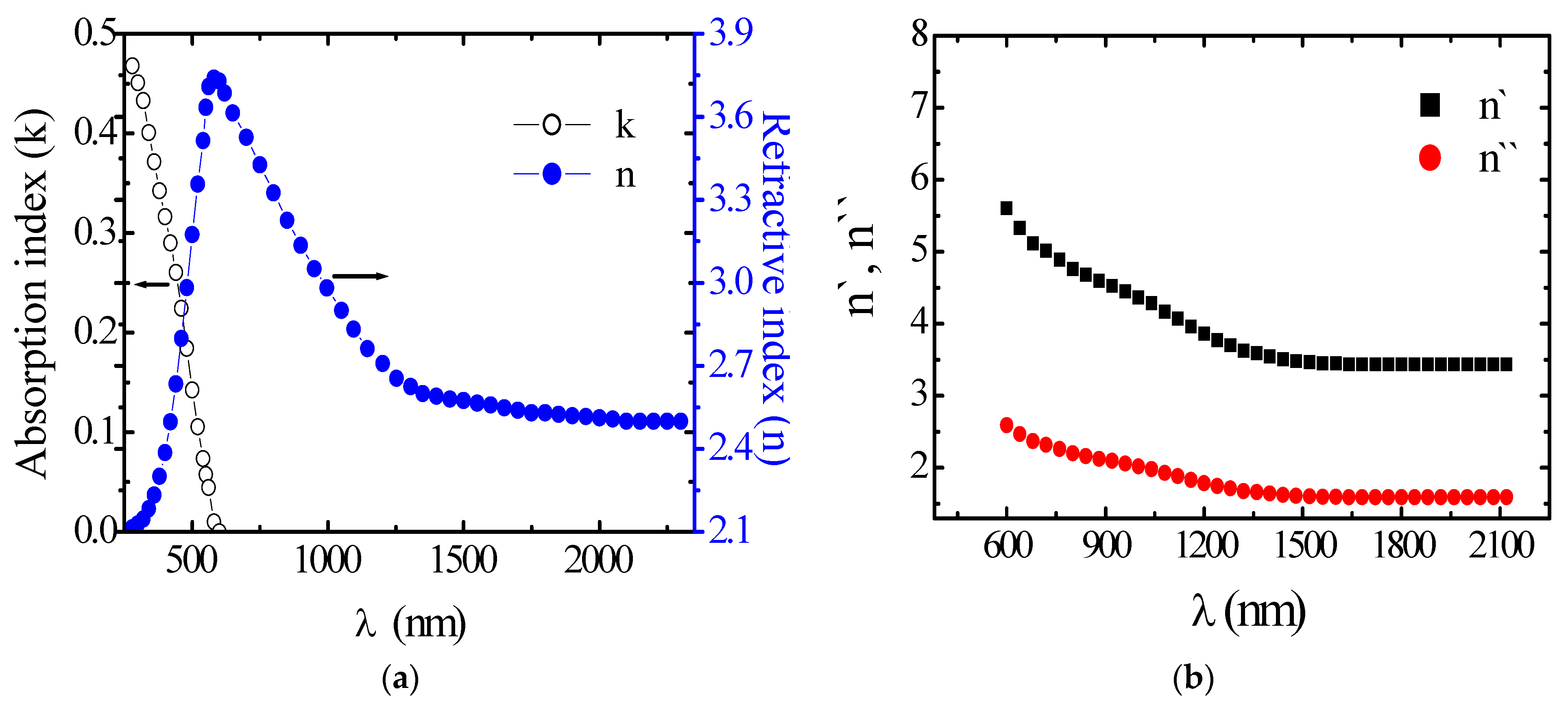
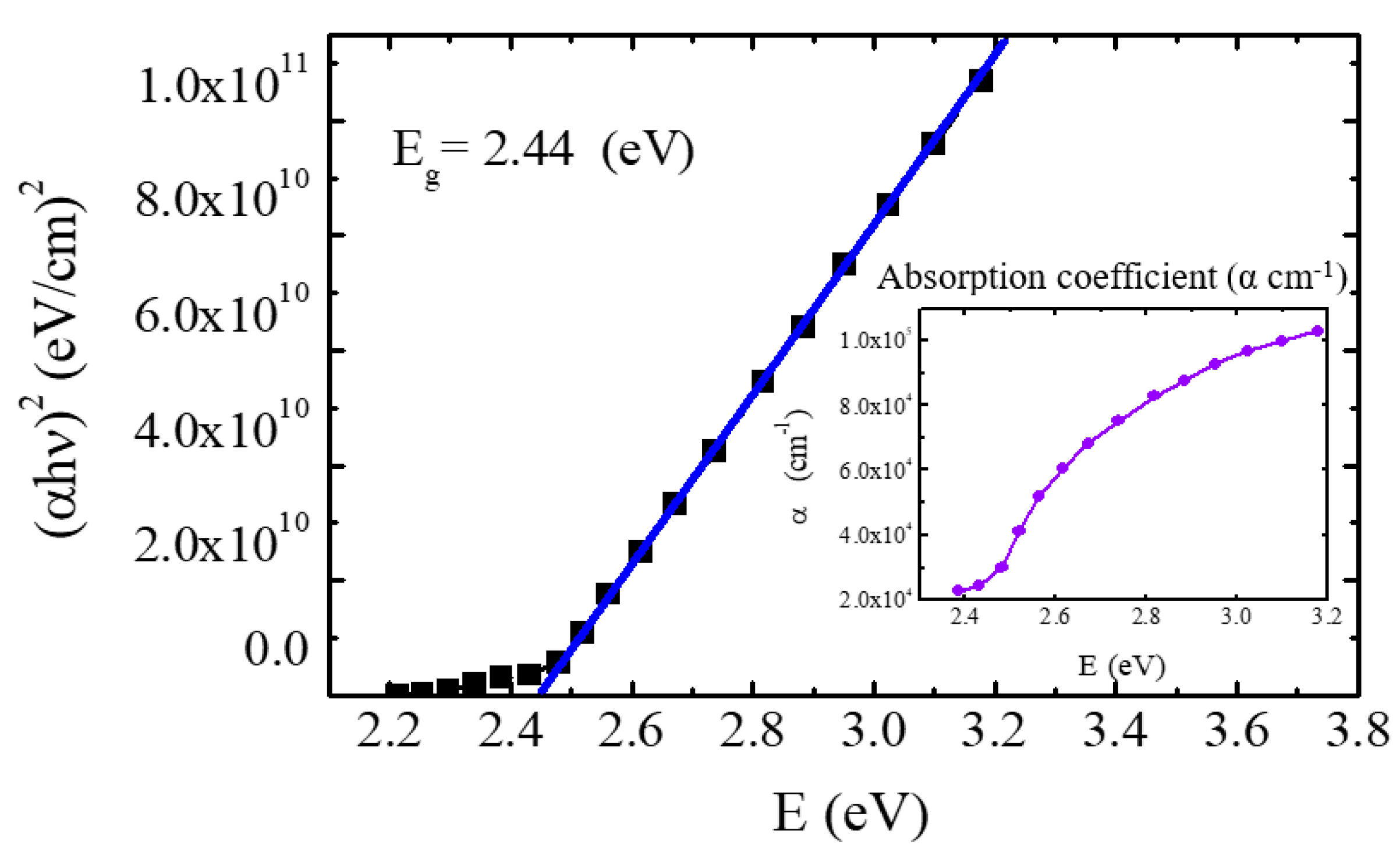
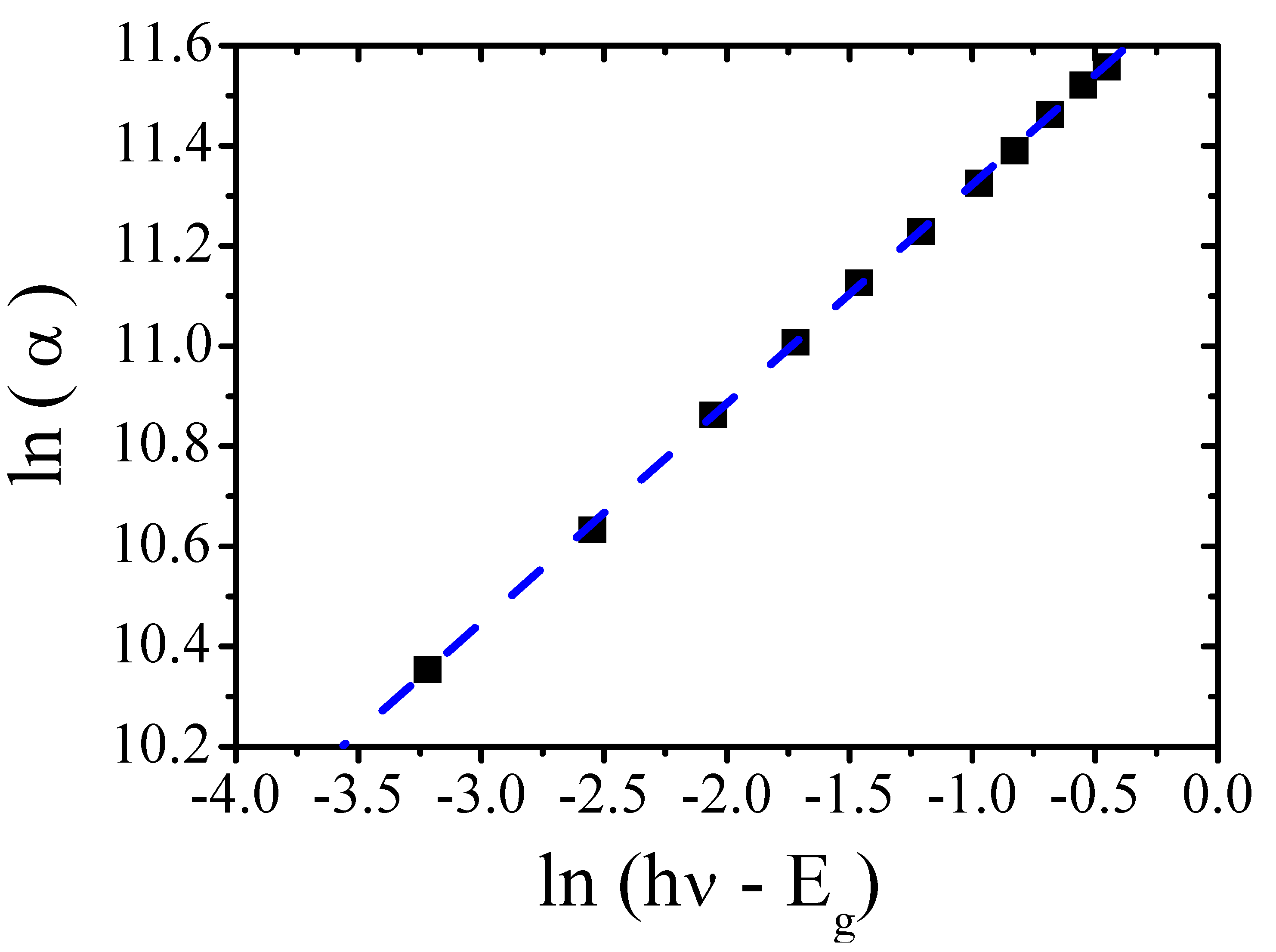
3.2. Optical Properties
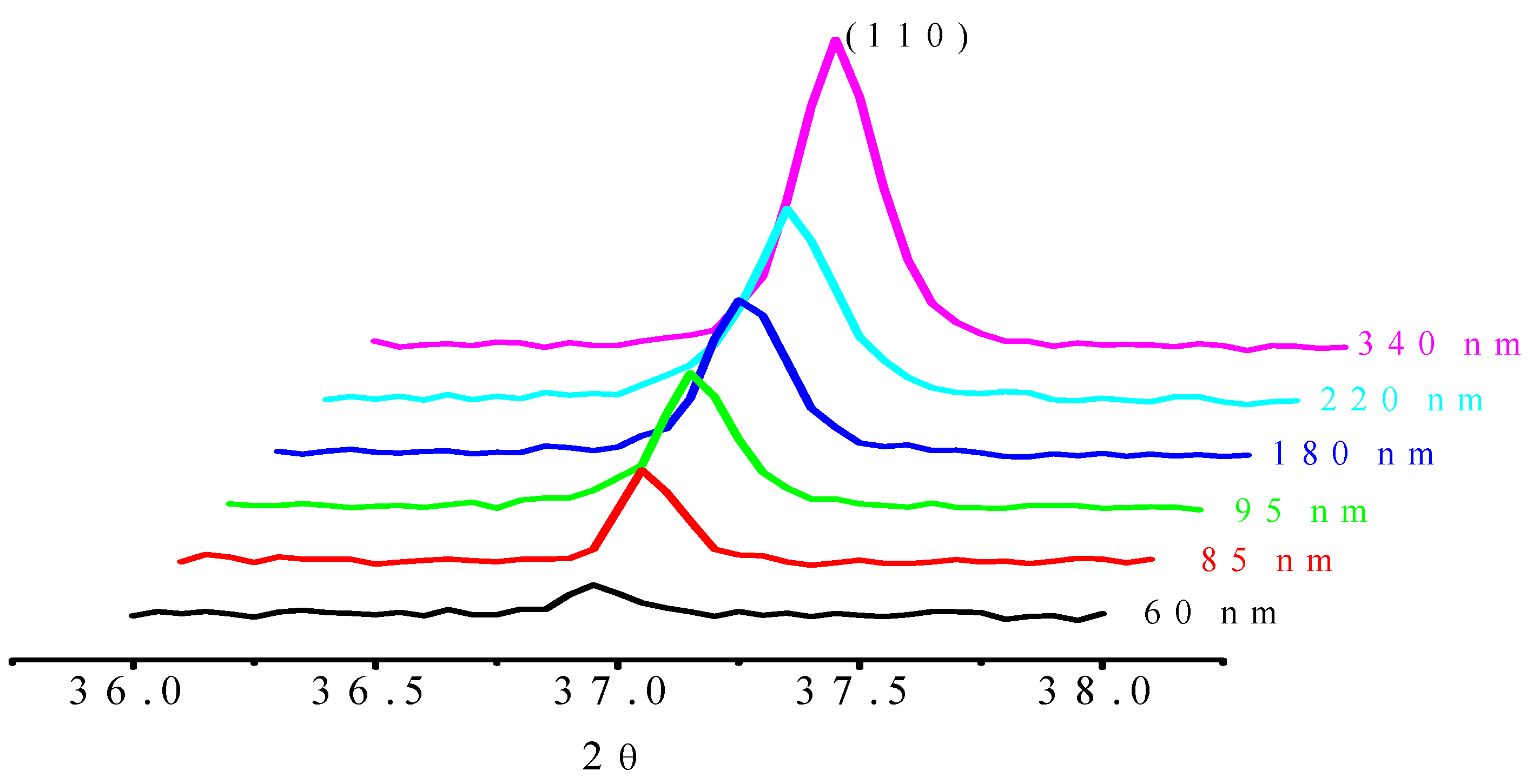
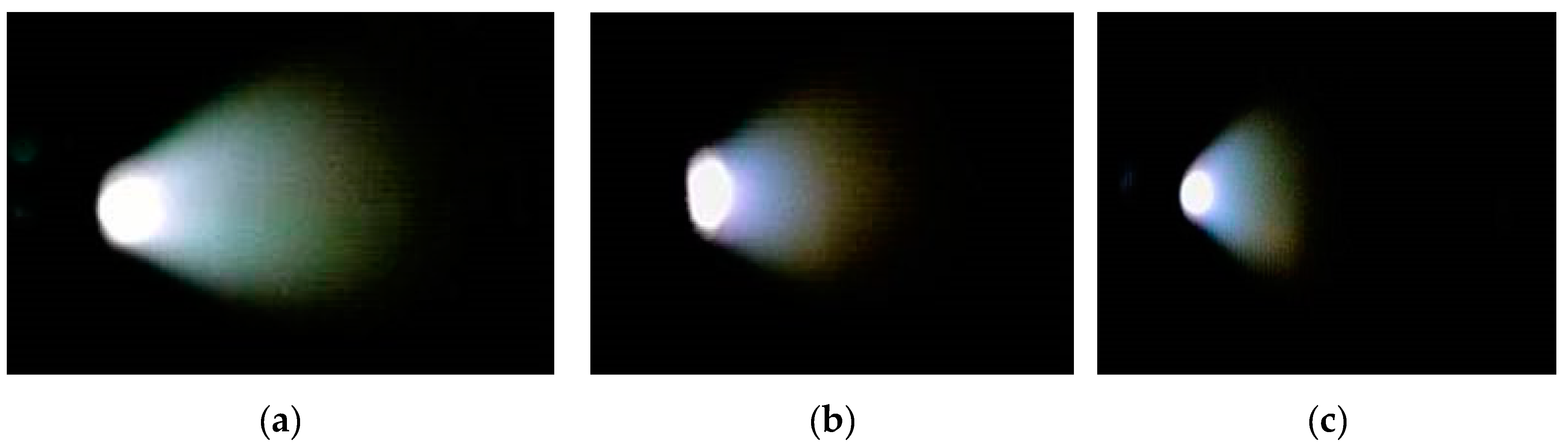
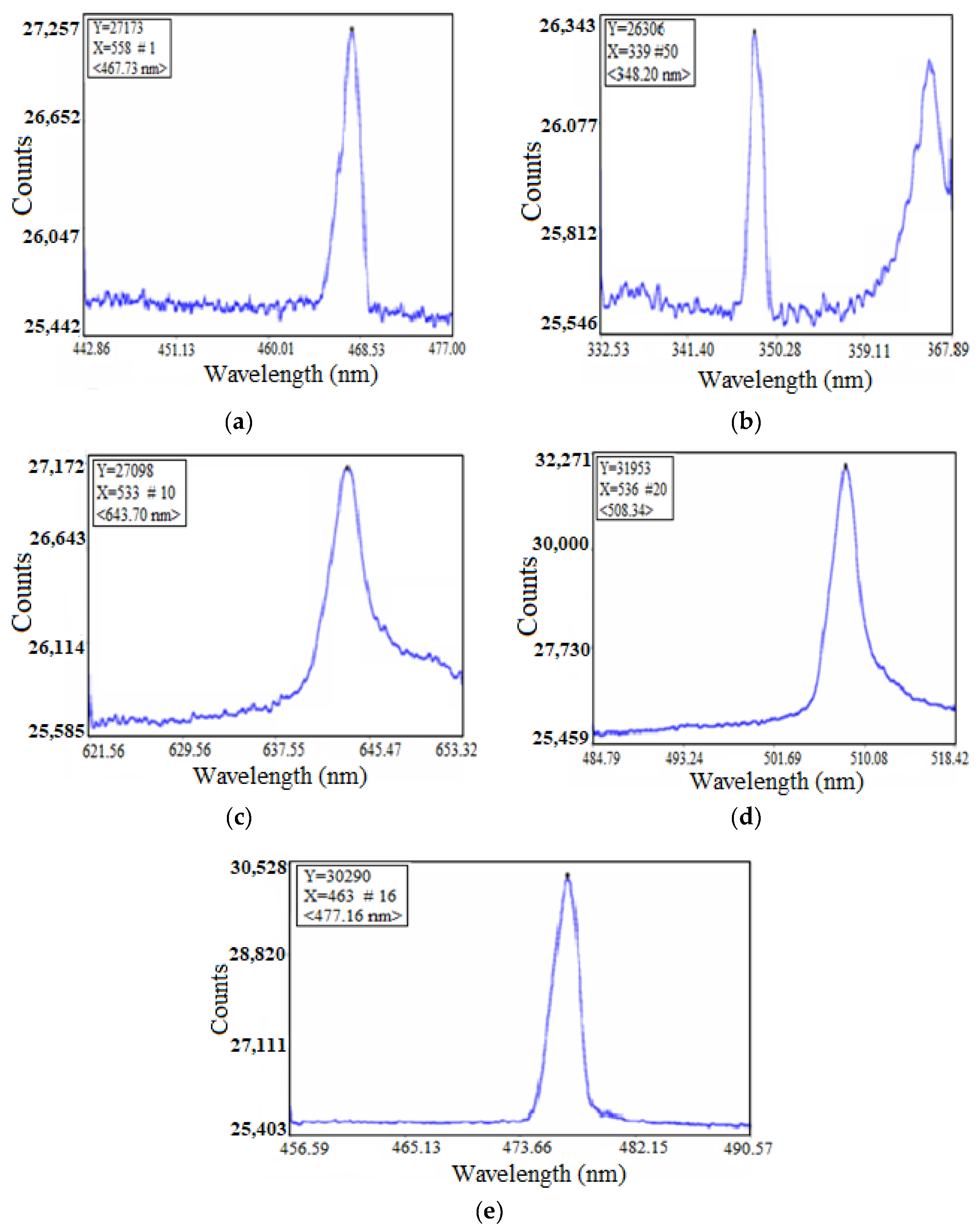
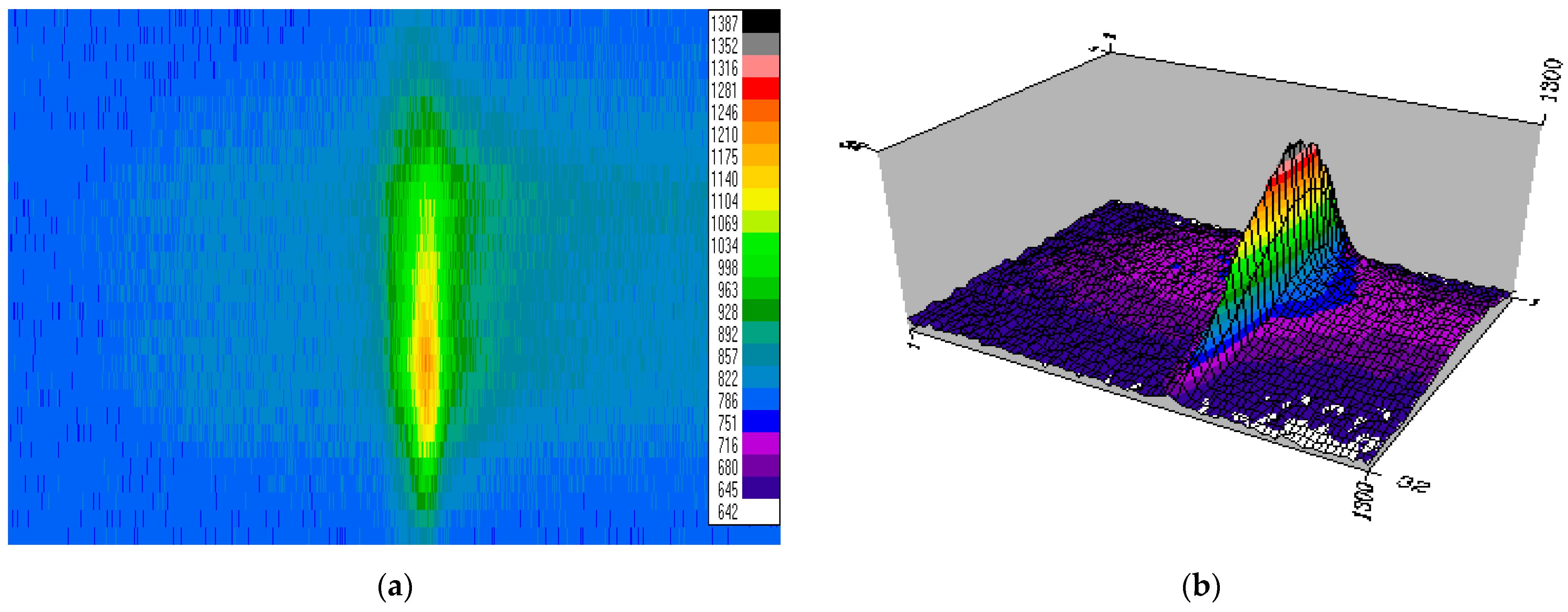
3.3. Plasma Plume and Spectral Profiles
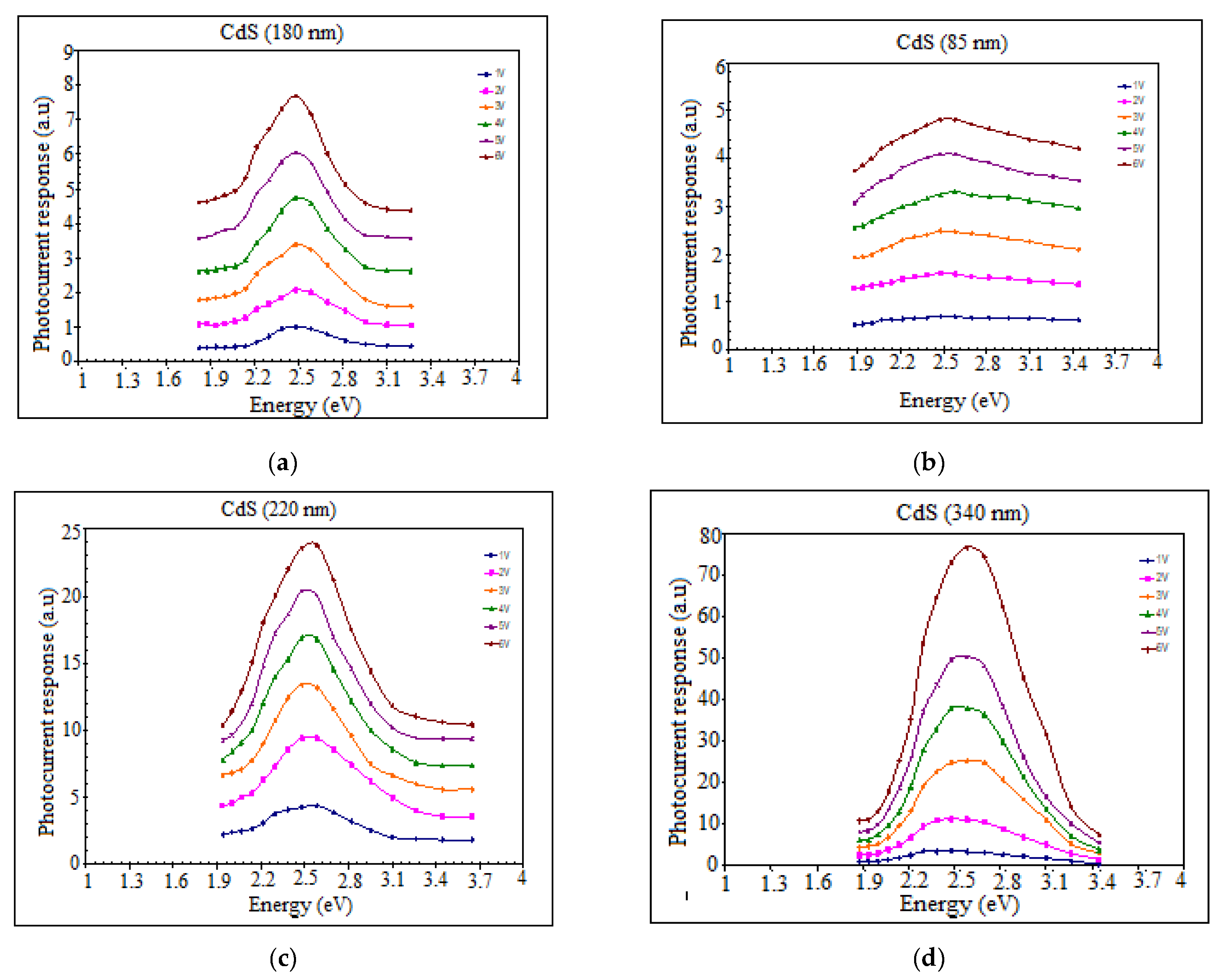
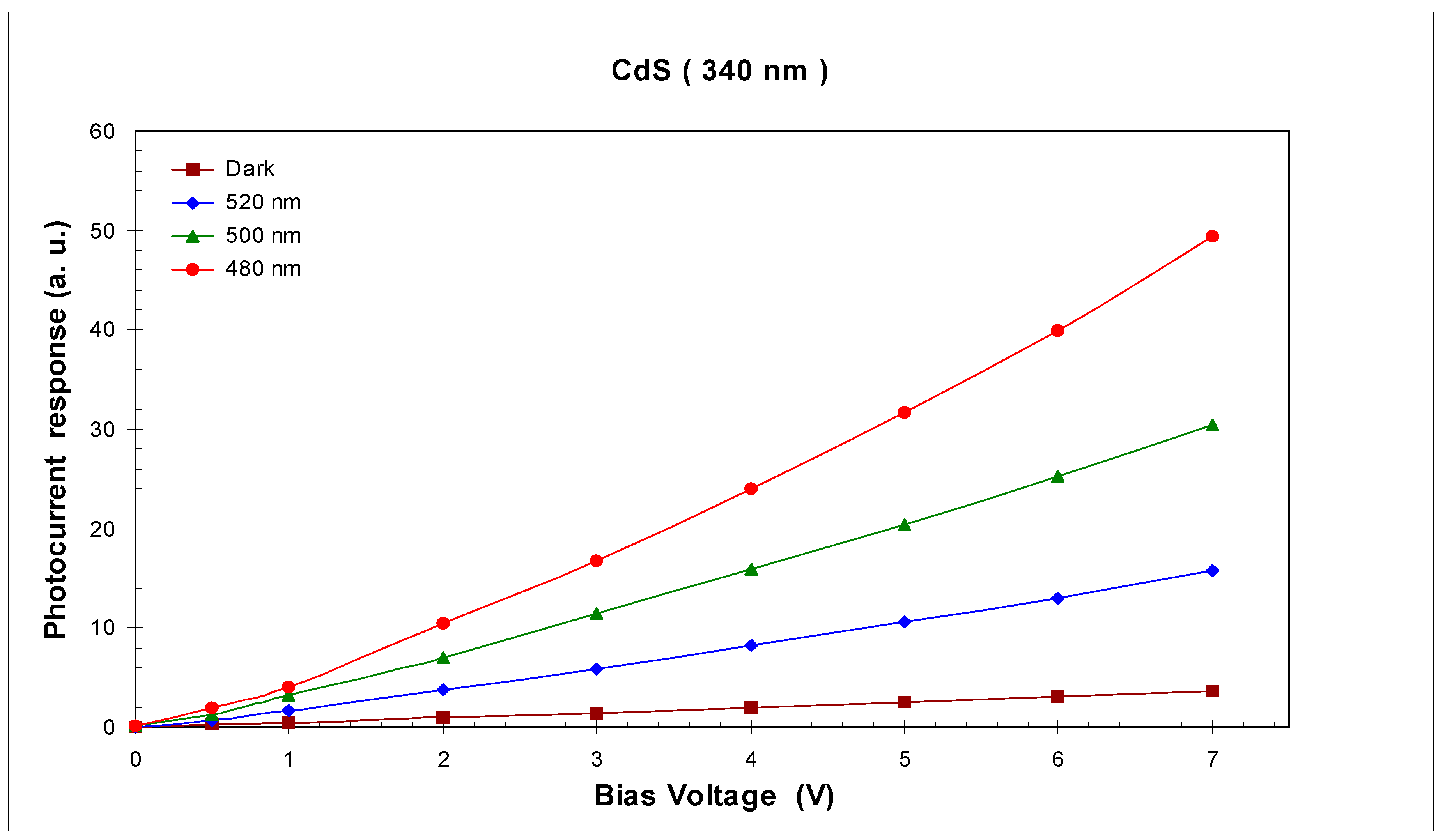
3.4. Photoconductivity Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, K.; Ahamed, M.B. Surface modification of cadmium sulfide thin film honeycomb nanostructures: Effect of in situ tin doping using chemical bath deposition. Appl. Surf. Sci. 2016, 361, 277–282. [Google Scholar] [CrossRef]
- Perlikowski, I.; Zielony, E.; Özdal, T.; Kavak, H. Structural properties and electrical characteristics of p-n junctions based on kesterite cu2znsns4 layers for thin-film solar cells. Energies 2021, 14, 5182. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Wu, D.; Li, R.; Wang, X.; Wang, Y.; Zhu, W.; Wei, Q.; Du, B. A generalized in situ electrodeposition of Zn doped CdS-based photoelectrochemical strategy for the detection of two metal ions on the same sensing platform. Biosens. Bioelectron. 2016, 77, 936. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, S.; Atasoy, Y.; Tomakin, M.; Bacaksız, E. Comparative studies of CdS, CdS: Al, CdS: Na and CdS:(Al–Na) thin films prepared by spray pyrolysis. Superlattices Microstruct. 2015, 88, 299. [Google Scholar] [CrossRef]
- Kim, W.; Baek, M.; Yong, K. Fabrication of ZnO/CdS, ZnO/CdO core/shell nanorod arrays and investigation of their ethanol gas sensing properties. Sens. Actuators B Chem. 2016, 223, 599–605. [Google Scholar] [CrossRef]
- Özdal, T.; Chtouki, T.; Kavak, H.; Figa, V.; Guichaoua, D.; Erguig, H.; Mysliwiec, J.; Sahraoui, B. Effect of annealing temperature on morphology and optoelectronics properties of spin coated CZTS thin films. J. Inorg. Organomet. Polym. Mater. 2021, 31, 89. [Google Scholar] [CrossRef]
- Trajic, J.; Gilic, M.; Romcevic, N.; Romcevic, M.; Stanisic, G.; Hadzic, B.; Petrovic, M.; Yahia, Y. Raman spectroscopy of optical properties in CdS thin films. Sci. Sinter. 2015, 47, 145. [Google Scholar] [CrossRef]
- Croitoru, N.; Jakobson, S. Properties of CdS films and Cu2S-CdS junctions prepared by chemical printing. Thin Solid Films 1979, 56, L5–L7. [Google Scholar] [CrossRef]
- Canevari, V.; Romeo, N.; Sberveglieri, G.; Azzi, S.; Tosi, A.; Curti, M.; Zanotti, L. Low resistivity CdS thin films grown by flashǦ evaporation at low substrate temperature (150–200 °C). J. Vac. Sci. Technol. A 1984, 2, 9–10. [Google Scholar] [CrossRef]
- Piel, A.; Murray, H. Etude de la conduction Poole-Frenkel dissymétrique dans des structures Al-CdS-Au. Thin Solid Films 1977, 44, 65–73. [Google Scholar] [CrossRef]
- Shay, J.L.; Wagner, S.; Bettini, M.; Bachmann, K.J.; Buehler, E. InP—CdS solar cells. IEEE Trans. Electron Devices 1977, 24, 483–486. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Tite, T.; Loir, A.; Maddi, C.; Donnet, C.; Garrelie, F. Review of graphene growth from a solid carbon source by pulsed laser deposition (PLD). Front. Chem. 2018, 6, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhmaidi, Z.O.; Abd-Lefdil, M.; El Khakani, M.A. Photoconversion optimization of pulsed-laser-deposited p-CZTS/n-Si-nanowires heterojunction-based photovoltaic devices. Nanomaterials 2020, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Kossowsky, R.; Jelinek, M.; Novak, J.; Jelínek, M.; Trtík, V.; Jastrabík, L. Pulsed laser deposition of thin films. In Physics and Materials Science of High Temperature Superconductors, IV; Kossowsky, R., Jelinek, M., Novák, J., Eds.; Springer: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Ogugua, S.N.; Ntwaeaborwa, O.M.; Swart, H.C. Latest development on pulsed laser deposited thin films for advanced luminescence applications. Coatings 2020, 10, 1078. [Google Scholar] [CrossRef]
- Martínez-Landeros, V.H.; Hernandez-Como, N.; Gutierrez-Heredia, G.; Quevedo-Lopez, M.A.; Aguirre-Tostado, F.S. Structural, chemical and electrical properties of CdS thin films fabricated by pulsed laser deposition using varying background gas pressure. Thin Solid Films 2019, 682, 24–28. [Google Scholar] [CrossRef]
- Abd, J.A.; Mohammed, W.M.; Al-Nafiey, A. CdS nanostructured thin films synthesized by pulsed laser deposition for solar cell technology. Key Eng. Mater. 2021, 882, 155–164. [Google Scholar] [CrossRef]
- Hussein, W.S.; Ahmed, A.F.; Aadim, K.A. Influence of laser energy and annealing on structural and optical properties of CdS films prepared by laser induced plasma. Iraqi J. Sci. 2020, 61, 1307–1312. [Google Scholar] [CrossRef]
- Hernandez-Como, N.; Martinez-Landeros, V.; Mejia, I.; Aguirre-Tostado, F.S.; Nascimento, C.D.; de Azevedo, G.M.; Krugc, C.; Quevedo-Lopez, M.A. Defect control in room temperature deposited cadmium sulfide thin films by pulsed laser deposition. Thin Solid Films 2014, 550, 665–668. [Google Scholar] [CrossRef]
- Moore, J.H.; Davis, C.C.; Coplan, M.A. Building Scientific Apparatus: A practical Guide to Design and Construction; Preseus Books: New York, NY, USA, 1991. [Google Scholar]
- Mittra, J.; Abraham, G.J.; Kesaria, M.; Bahl, S.; Gupta, A.; Shivaprasad, S.M.; Viswanadham, C.S.; Kulkarni, U.D.; Dey, G.K. Role of substrate temperature in the pulsed laser deposition of zirconium oxide thin film. Mater. Sci. Forum 2012, 710, 757–761. [Google Scholar] [CrossRef]
- Tolansky, S. Multiple-Beam Interference Microscopy of Metals; Academic Press: London, UK, 1970. [Google Scholar]
- Tolansky, S. Multiple-Beam Interferometry of Surface and Films; Oxford University: London, UK, 1978. [Google Scholar]
- Yakovlev, V.V.; Lazarov, V.; Reynolds, J.; Josifovska, M.G. Laser-induced phase transformations in semiconductor quantum dots. Appl. Phys. Lett. 2000, 76, 2050. [Google Scholar] [CrossRef]
- Amith, A. Thickness dependence of structural and electrical properties of Cds films for solar cells. J. Vac. Sci. Technol. 1978, 15, 353. [Google Scholar] [CrossRef]
- Wilson, J.B.; Woods, J. The electrical properties of evaporated films of cadmium sulphide. J. Phys. Chem. Sol. 1973, 34, 171. [Google Scholar] [CrossRef]
- Mahalingam, T.; John, V.S. Microstructural characterization of electrosynthesized ZnTe thin films. Cryst. Res. Technol. 2002, 37, 329. [Google Scholar] [CrossRef]
- Ioffe, A.F. Physics of Semiconductor, 491; Info Search Limited: London, UK, 1957. [Google Scholar]
- Heavens, O.S. Optical Properties of Thin Solid Films; IOP Publishing: London, UK, 1955. [Google Scholar]
- Atwee, T.; Kunze, H.-J. Spectroscopic investigation of two equal colliding plasma plumes of boron nitride. J. Phys. 2002, 35, 524. [Google Scholar]
- Morrow, T.; Sakeek, H.F.; El-Astal, A.; Walmsley, D.G.J. Absorption and emission spectra of the YBCO laser plume. J. Supercond. Nov. Magn. 1994, 7, 823. [Google Scholar] [CrossRef]
- Wang, X.T.; Man, B.Y.; Hu, X.Y. Optical spectroscopy of plasma produced by laser ablation of Ti alloy in air. J. Appl. Phys. 1996, 80, 1783. [Google Scholar] [CrossRef]
- Brackmann, C.; Nygren, J.; Bai, X.; Li, Z.; Bladh, H.; Axelsson, B.; Denbratt, I.; Koopmans, L. Per-Erik Bengtsson Marcus Aldén Laser-induced fluorescence of formaldehyde in combustion using third harmonic Nd. YAG Laser Excit. 2003, 59, 33347. [Google Scholar]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD. 2020 NIST Atomic Spectra Database (Ver.5.8) [online September 2021, Spectrmmeter] National Institute of Standards and Technology Gaithrs. Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 26 September 2021).
- Dutta, J.; Bhattacharyya, D.; Chdhuri, A.S.; Pal, A.K. Cadmium sulfide thickness effect on the photoresponse of the CdS(A1) spray/poly(3-methylthiophene) solid state junction. Sol. Energy Mater. Sol. Cells 1993, 29, 209. [Google Scholar]
- Heijne, L. Physical Principles of photoconductivity. Philips Tech. Rev. 1963, 25, 120. [Google Scholar]
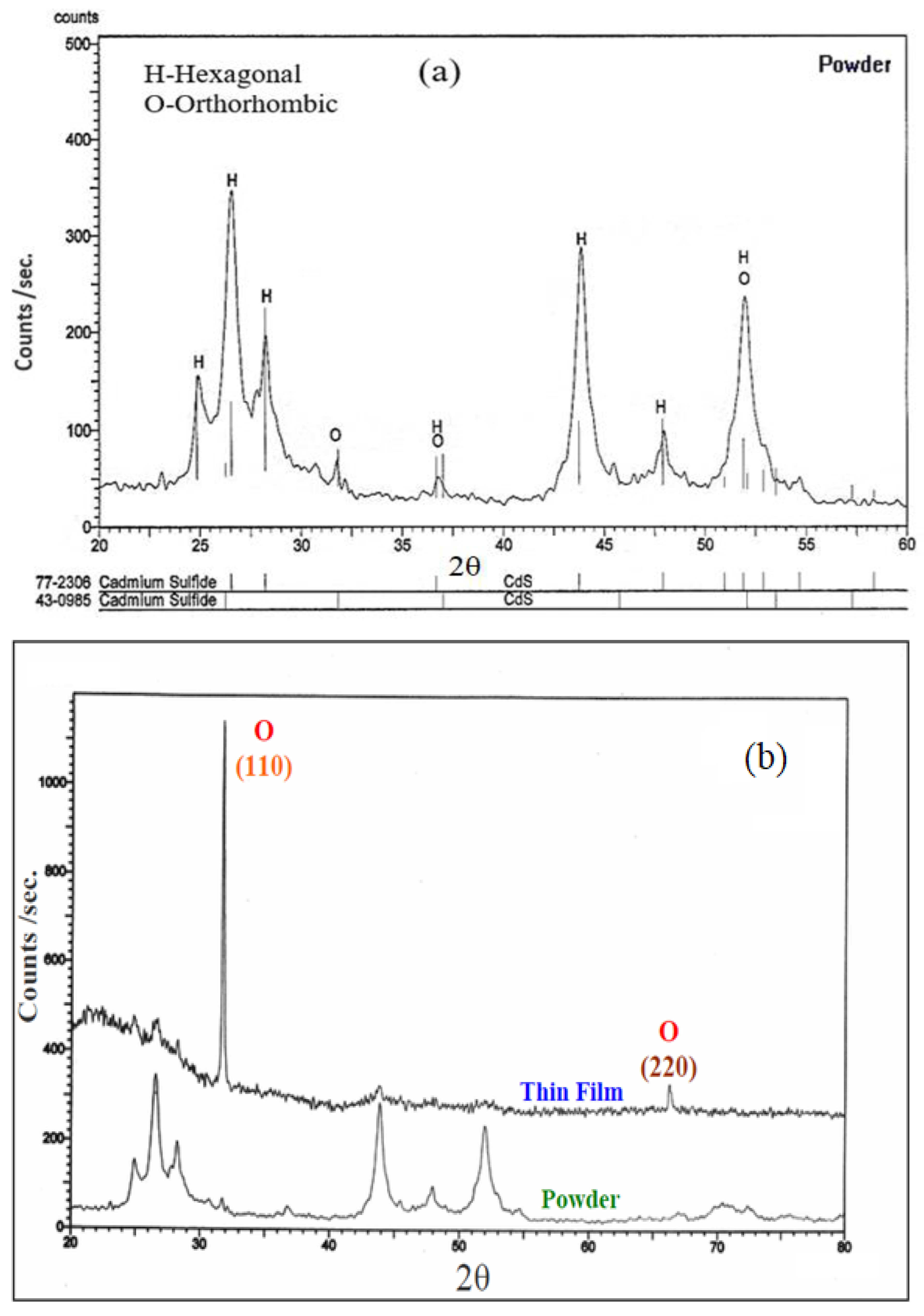


| CdS in the Powder Form. | CdS Thin Film of Thickness 340 nm. | CdS Thin Film of Thickness 340 nm after Annealing at 300 °C. | ||||||||||||||
| dExp | I/Io | ICDD77-2306 | dEXp | I/Io | ICDD 43-0985 | dExp | I/Io | ICDD 77-2306 | ||||||||
| d Å | I/Io | hkl | d Å | I/Io | hkl | d Å | I/Io | hkl | ||||||||
| 3.58 | 38 | 3.581 | 63 | 100 | 3.35 | 8 | 3.395 | 30 | 001 | 3.57 | 74 | 3.581 | 63 | 100 | ||
| 3.35 | 100 | 3.356 | 45 | 002 | 2.81 | 100 | 2.812 | 80 | 110 | 3.36 | 100 | 3.356 | 45 | 002 | ||
| 3.16 | 48 | 3.160 | 100 | 101 | 2.10 | 2 | 2.170 | 80 | 111 | 3.16 | 60 | 3.160 | 100 | 101 | ||
| 2.44 | 7 | 2.449 | 25 | 102 | 1.41 | 6 | 1.410 | 30 | 220 | 2.45 | 5 | 2.449 | 25 | 102 | ||
| 2.10 | 87 | 2.068 | 43 | 110 | 2.06 | 77 | 2.068 | 43 | 110 | |||||||
| 1.90 | 20 | 1.867 | 41 | 103 | 1.89 | 13 | 1.867 | 41 | 103 | |||||||
| 1.76 | 68 | 1.760 | 31 | 112 | 1.76 | 18 | 1.760 | 31 | 112 | |||||||
| 1.68 | 6 | 1.678 | 02 | 004 | 1.25 | 5 | 1.257 | 79 | 105 | |||||||
| 1.39 | 4 | 1.398 | 12 | 203 | 1.19 | 2 | 1.193 | 04 | 300 | |||||||
| 1.30 | 4 | 1.303 | 04 | 114 | 1.12 | 1 | 1.124 | 04 | 302 | |||||||
| 1.26 | 2 | 1.257 | 79 | 105 | ||||||||||||
| 1.19 | 11 | 1.193 | 04 | 300 | ||||||||||||
| 1.15 | 3 | 1.158 | 10 | 213 | ||||||||||||
| 1.2 | 7 | 1.124 | 04 | 302 | ||||||||||||
| T | ||||
|---|---|---|---|---|
| 60 | 5888 | −8987 | 8.08 × 107 | 3668.927 |
| 85 | 2778 | −12,097 | 1.46 × 108 | 4938.58 |
| 95 | 2300 | −12,575 | 1.58 × 108 | 5133.722 |
| 180 | 2248 | −12,627 | 1.59 × 108 | 5154.951 |
| 220 | 1661 | −13,214 | 1.75 × 108 | 5394.593 |
| Obtained Spectral Data (nm) | 348.20 | 467.73 | 508.34 | 643.70 |
| Reference Spectral Data (nm) [34] | 349.90 | 467.80 | 508.58 | 643.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadalla, A.S.; Al-shamiri, H.A.S.; Alshahrani, S.M.; Khalil, H.F.; El Nahas, M.M.; Khedr, M.A. Epitaxial Growth and Optical Properties of Laser Deposited CdS Thin Films. Coatings 2022, 12, 87. https://doi.org/10.3390/coatings12010087
Gadalla AS, Al-shamiri HAS, Alshahrani SM, Khalil HF, El Nahas MM, Khedr MA. Epitaxial Growth and Optical Properties of Laser Deposited CdS Thin Films. Coatings. 2022; 12(1):87. https://doi.org/10.3390/coatings12010087
Chicago/Turabian StyleGadalla, Atef S., Hamdan A. S. Al-shamiri, Saad Melhi Alshahrani, Huda F. Khalil, Mahmoud M. El Nahas, and Mohamed A. Khedr. 2022. "Epitaxial Growth and Optical Properties of Laser Deposited CdS Thin Films" Coatings 12, no. 1: 87. https://doi.org/10.3390/coatings12010087
APA StyleGadalla, A. S., Al-shamiri, H. A. S., Alshahrani, S. M., Khalil, H. F., El Nahas, M. M., & Khedr, M. A. (2022). Epitaxial Growth and Optical Properties of Laser Deposited CdS Thin Films. Coatings, 12(1), 87. https://doi.org/10.3390/coatings12010087







