Abstract
An in situ Mg-Al hydrotalcite (LDH) film was prepared using a one-step hydrothermal method on the surface of a medical magnesium alloy. The importance and influence of the reaction parameters on the corrosion resistance of the LDH coatings were optimized and investigated through an orthogonal array and range analysis. The reaction parameters included the temperature, reaction time, pH, and concentration of the aluminum source. The relationship between the parameters and corrosion resistance performance of each coating was compared with the chemical composition, electrochemical corrosion current, and hydrogen evolution rate. Suitable reaction parameters were obtained. The morphology, element distribution, adhesion strength, and electrochemical properties of the preferred coatings were further analyzed and evaluated to optimize the treatment process. The results showed that temperature had the most significant impact on the quality of the LDH coating; a suitably high temperature, a longer reaction time, a higher aluminum source concentration, and a high pH were conducive to forming high-quality LDH coatings. There was an inverse relationship between the corrosion resistance and the LDH-to-Mg(OH)2 content ratio of the coatings. The optimal reaction parameters for this Mg-Al LDH coating on the substrate were 130 °C for 8 h at a pH of 13 using a 10 mM Al3+ solution.
1. Introduction
Magnesium alloys have attracted significant attention for bone repair due to their good biocompatibility, biodegradability, and mechanical properties, which are similar to those of bones [1]. Mg2+ from magnesium alloy degradation has been shown to promote the adsorption and growth of bone cells [2]. Biodegradable magnesium alloy implants have been beneficial in enabling patients with a bone injury to avoid a second operation after orthopedic implantation. Magnesium alloys are considered potential materials for biomedical development [3]. Due to their rapid degradation in the human body and inevitable corrosion, the wide application of magnesium alloys in the clinical orthopedic field has been limited [4,5].
Recently, various treatment methods, including alloying and processing deformation, have been widely applied to improve the corrosion resistance of magnesium alloy [6]. Many researchers have reported that the use of a suitable surface treatment can effectively enhance the corrosion resistance and reduce the corrosion rate of magnesium alloys; this is another common approach for modifying the corrosion resistance of alloys [5]. Many surface-modification methods have been used to improve the corrosion resistance of magnesium alloys; these include electrochemical deposition [7], chemical conversion coating [8], biodegradable polymer coating [9], and micro-arc oxidation (MAO) [10]. In addition to the corrosion resistance requirements, implants of magnesium alloys in living bodies are often required to provide a certain level of functionality after the clinical implantation to promote the healing process of the damaged physiological tissues of the body [11]. Studies have focused on coatings as carriers for biomedical agents to satisfy specific clinical needs; this has been a leading research direction for magnesium alloy surface treatments.
A layered double hydroxide is a compound composed of positively charged metal hydroxide layers filled with negatively charged anions. Due to its good stability and positive charge characteristics, LDH is a suitable carrier for use in the medical field [12,13]. Hydrotalcite, as a type of surface-coating material, provides good corrosion resistance for magnesium alloys; this has excellent potential, especially for substrate protection [14,15]. Some methods for the preparation of Mg-Al hydrotalcite (Mg-Al LDH) have been developed on magnesium alloy surfaces. The in situ growth approach has been used and does not destroy the alloy substrate [16,17]. By using a metal matrix to supply Mg2+ as the reactant magnesium source, the interfacial bonding force between the matrix and coating layer can be effectively enhanced.
The preparation of Mg-Al hydrotalcite on medical magnesium alloys by the hydrothermal method for in situ growth coating is often complicated and requires a longer reaction time [10,16,18]. The parameters of the reactions, such as the reaction time, pH of the reaction solution, and reaction temperature, have a significant direct impact on the properties; these include the morphology, composition, and corrosion resistance of the obtained coating. Pan et al. [19] investigated the effect of the reaction temperatures on an Mg-Al LDH film on the surface of an AZ31 magnesium alloy that was pretreated with micro-arc oxidation. They found that the reaction temperature influenced the corrosion resistance of the coating by affecting the crystallinity and densification of the LDH. They also showed that the variation in the pH of the reacting solution influenced the crystallinity and the density of the lamellar structure in the LDH layer [20]. The corrosion resistance of the coating was changed by varying the pH.
Song et al. [18] developed an Mg-Al hydrotalcite film on the surface of an AZ31 magnesium alloy using a two-step method. Their results showed that the temperature increase reduced the coating thickness and the occurrence of a crack layer in the precursor film during pretreatment. The transformation of the hydrotalcite film in the second step was mainly controlled by the pH; the optimum preparation conditions for this LDH coating were determined. Changing the preparation parameters caused evident variations in the morphology and composition of the coating layers; this led to differences in the corrosion resistance and degradation rate of the matrix-related properties. The importance of these reaction parameters on coating performance is crucial and needs to be investigated. Previous studies tended to focus on the influence of a single reaction parameter on the coating properties; this approach is insufficient for the full optimization of the corrosion resistance of a coating.
Each type of double hydroxide has its own process to obtain the optimum property [21,22]. For this study, the Mg-Al LDH coatings were prepared on an aluminum-free medical magnesium alloy using an in situ growth approach. The impact and importance of the reaction parameters on the morphology, composition, and corrosion resistance of the final LDH films were systematically investigated; the reaction parameters included the Al source concentration, pH, temperature, and reaction time. The selection of the process parameter order for studying the in situ hydrothermal synthetic LDH coatings was accomplished by comparing the impact significance; this allowed the determination of the optimal preparation process for the LDH film on the Mg-Zn-Zr-Sr alloy.
2. Experimental Materials and Methods
2.1. Preparation of the Substrate Material
The Mg-Zn-Zr-Sr alloy substrates were prepared according to our previous study [23]. Samples with a size of Φ8 mm × 2 mm were polished with 320#, 800#, and 1500# SiC sandpapers; the sample was washed with distilled water and acetone to degrease the surface and then stored in dry conditions.
2.2. Preparation of the LDH Coating
The factor levels of the orthogonal experiments are listed in detail in Table 1. To prepare the LDH coating, a 2 M NaOH solution was added to an Al(NO3)3 solution (100 mL) to adjust the pH. The samples were placed into a hydrothermal reaction kettle and immersed in the mixture solution. After treatment under the preset conditions, as presented in Table 2, the samples were cooled, washed with deionized water, and then dried. The entire experimental process is shown in Figure 1.

Table 1.
Factor level in orthogonal experiments.

Figure 1.
Schematic illustration of the experiment.
2.3. Microstructure Characterization
The chemical composition and crystal phase of the LDH coating were characterized by X-ray diffraction (XRD, RigakuD/max/2500PCRigaku, Tokyo, Japan) using Cu Kα radiation (40 kV, 40 mA) with a glancing angle at a scanning rate of 3°/min and a scanning angle from 5° to 80°. The surface microstructure and the elemental distribution of the coatings were characterized using a field-emission scanning electron microscope (FESEM, Quanta FEG 250, FEI, Ann Arbor, MI, USA) and energy-dispersive X-ray spectroscopy (EDS).
2.4. Corrosion Behavior
Electrochemical polarization and impedance experiments were carried out with a Zennium electrochemical workstation (Zahner, Frankfurt, Germany ) in simulated body fluid (SBF) solution at 37 °C using a three-electrode test. The sample was utilized as a reaction electrode; a saturated calomel electrode and a graphite electrode were used as reference electrodes and auxiliary electrodes, respectively. Before testing, each exposed surface (testing area Φ8 mm) was immersed in SBF solution for 30 min to stabilize the specimen’s open-circuit potential (OCP) in a relatively stable range. The electrochemical impedance spectroscopy (EIS) test was performed with frequencies between 0.01 Hz and 100 kHz. A potentiodynamic polarization curve test was performed at a scanning rate of 1 mV/s in the frequency range of 10−2–105 Hz. The cathode Tafel slope (βc), anode Tafel slope (βa), and corrosion current density (Icorr) were calculated using the results of the potentiodynamic polarization experiment. The polarization resistance (Rp) was calculated using Equation (1) [24,25].
The hydrogen evolution rate (HER) test was conducted as described in our previous study [26]. Briefly, the sample was immersed in the SBF at 37 °C, with an immersion ratio of 35.5 mL/cm2; the hydrogen produced during the soaking process was then collected by the drainage method. The volume of hydrogen produced was recorded and three parallel samples were used for each sample. The hydrogen evolution rate, VH (mL·cm−2), was calculated according to the volume of hydrogen that evolved.

Table 2.
Design and results of the orthogonal array with, factor 5 and level 4 in Table 1.
Table 2.
Design and results of the orthogonal array with, factor 5 and level 4 in Table 1.
| Run | Control Factors | Results | |||||
|---|---|---|---|---|---|---|---|
| A: Temperature (°C) | B: pH Value | C: Time (h) | D: Concentration of Al(NO3)3 (mM) | Relative Content of LDH (%) | Icorr × 10−7 (A/cm2) | H2 Volume after 51 h Immersion (mL/cm2) | |
| 1 | 95 | 9 | 8 | 0.01 | 1.875 | 2.635 | 0.104 |
| 2 | 95 | 10 | 10 | 0.1 | 1.725 | 2.839 | 0.111 |
| 3 | 95 | 11 | 12 | 1 | 0.423 | 1.185 | 0.0757 |
| 4 | 95 | 12 | 14 | 10 | 2.932 | 1.725 | 0.106 |
| 5 | 95 | 13 | 16 | 100 | 0.154 | 30.391 | 1.843 |
| 6 | 110 | 9 | 10 | 1 | 1.549 | 3.253 | 0.154 |
| 7 | 110 | 10 | 12 | 10 | 2.667 | 1.873 | 0.0934 |
| 8 | 110 | 11 | 14 | 100 | 0.705 | 7.033 | 0.285 |
| 9 | 110 | 12 | 16 | 0.01 | 0.732 | 6.843 | 0.275 |
| 10 | 110 | 13 | 8 | 0.1 | 3.512 | 1.424 | 0.102 |
| 11 | 120 | 9 | 12 | 100 | 2.067 | 2.419 | 0.109 |
| 12 | 120 | 10 | 14 | 0.01 | 3.800 | 13.836 | 0.244 |
| 13 | 120 | 11 | 16 | 0.1 | 0.449 | 11.462 | 1.024 |
| 14 | 120 | 12 | 8 | 1 | 0.406 | 12.302 | 1.006 |
| 15 | 120 | 13 | 10 | 10 | 0.254 | 21.638 | 1.255 |
| 16 | 130 | 9 | 14 | 0.1 | 0.368 | 18.546 | 1.438 |
| 17 | 130 | 10 | 16 | 1 | 1.959 | 2.552 | 0.113 |
| 18 | 130 | 11 | 8 | 10 | 2.971 | 1.635 | 0.107 |
| 19 | 130 | 12 | 10 | 100 | 1.471 | 9.987 | 1.118 |
| 20 | 130 | 13 | 12 | 0.01 | 3.818 | 1.302 | 0.104 |
| 21 | 150 | 9 | 16 | 10 | 0.700 | 7.218 | 0.947 |
| 22 | 150 | 10 | 8 | 100 | 0.923 | 5.521 | 0.247 |
| 23 | 150 | 11 | 10 | 0.01 | 0.058 | 86.565 | 6.546 |
| 24 | 150 | 12 | 12 | 0.1 | 0.229 | 22.357 | 1.105 |
| 25 | 150 | 13 | 14 | 1 | 0.196 | 23.659 | 1.113 |
| Blank | - | - | - | - | 256.770 | 2.871 | |
2.5. Bonding Strength Test
The bonding strengths between the coatings and the metal substrate were tested using an automatic scratch instrument. An indenter with a 0.2 mm tip radius was used to perform the test under an applied load of 30 N with a scratch length of 3 mm. The critical load was the force corresponding to the first signal peak (N). Each sample was tested three times to obtain an average.
3. Results
3.1. Analysis of the Range of the Orthogonal Test Results
The XRD patterns of the orthogonal experimental samples are shown in Figure 2. The diffraction peaks, located at 11.4° and 18.7°, corresponded to Mg-Al LDH and Mg(OH)2, respectively, in the coating. This confirmed that the obtained coatings were a mixture of in situ hydrotalcite and magnesium hydroxide [16]. It has been reported that the proportion of hydrotalcite in the coating is significantly related to its reaction conditions [27,28]. The crystal plane diffraction peak (003) that was assigned to LDH at approximately 2θ = 11.5° was observed in Figure 2 [29]. The ratio of the crystal plane strength between LDH (003) and Mg(OH)2 (001) in the XRD pattern [28], namely, ILDH (003)/IMg(OH)2 (001), was estimated for all specimens. The ILDH (003)/IMg(OH)2 (001), the hydrogen evolution rate (HER), and electrochemical self-etching current density (Icorr) are listed in Table 2. The variations of this data are shown in Figure 3. The increase in the LDH content in the coating resulted in the decline of the corresponding Icorr and HER. Both have similar curves, due to the ion exchange from LDH; this decelerated the corrosion rate of the substrate [8,18]. Using the XRD results, Mg-Al LDH was fabricated under the preset conditions in the orthogonal tests. The preparation conditions for the hydrothermal reactions were optimized to obtain high-quality LDH coatings that provided more effective protection for the alloy matrix.
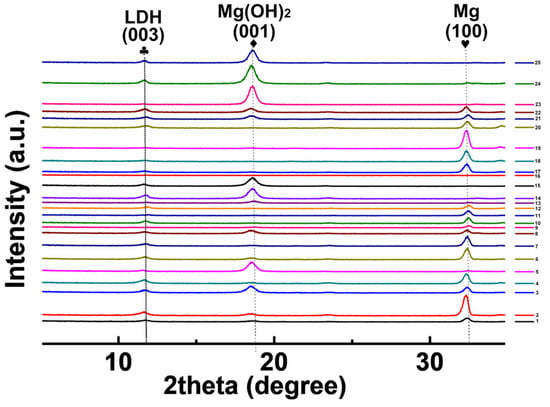
Figure 2.
The XRD patterns of the orthogonal array samples.
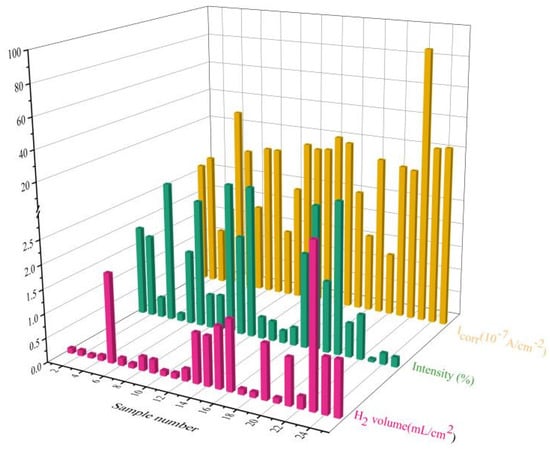
Figure 3.
Variations of all samples in relation to the ILDH (003)/IMg(OH)2 (001) (Intensity), HER (H2 volume), and Icorr (Icorr).
Range analysis was utilized to calculate the range of testing values of the design factors at each level in an orthogonal array; this was beneficial for the acquisition of the sequence to determine the important target-designed factors and the optimization of the preparation conditions. For orthogonal tests with multiple factors, a range analysis was first performed for each factor to optimize the treatment process. Then, the optimal conditions were obtained through other characterizations or property performances. In Table 3, the symbols Hi (i = 1, 2, 3, 4, 5) and hi (i = 1, 2, 3, 4, 5), and the R values correspond to the summations, average values, and difference between the maximum and minimum average values of the experimental results at different levels of the same factor, respectively. A higher value of R indicates a more significant effect of the factor on alloy properties [30]. Since the variation of Icorr and the HER of the samples were similar, ILDH(003)/IMg(OH)2(001) and Icorr were used to evaluate the corrosion rate of the coating in series alloys under different treatment conditions. The results are listed in Table 2 and Table 3.

Table 3.
Analysis of a range of orthogonal experiments.
Among all the tested factors, A (temperature) had the most prominent impact on the ILDH(003)/IMg(OH)2(001) with the highest R-value, while D (concentration of Al(NO3)3) had the weakest impact on the ILDH(003)/IMg(OH)2(001) with the lowest R-value. The influence of the preparation factors in Table 1 on the comparative content of LDH in the coatings was in the following decreasing order: A > B > C > D; this showed that the optimal condition for the LDH content was A4B5C1D4. The same analysis process was used for the Icorr values. The effects of the corresponding factors in decreasing order were A > C > B > D; this differed from that of ILDH(003)/IMg(OH)2(001). The optimal combination of treatment conditions was determined to be A4C4B5D4. The influence of pH and treatment time on ILDH(003)/IMg(OH)2(001) and Icorr were different; the optimal levels of treatment time in these two combinations were also different. Further analyses, including surface morphology, in vitro degradation behavior, and coating binding strength under both conditions (condition 1 = A4B5C1D4 and condition 2 = A4C4B5D4) were conducted to determine the optimal reaction parameters showing prominent corrosion resistance.
3.2. Characteristics of the Optimizing Coatings
The surface morphologies obtained by SEM are shown in Figure 4, their electrochemical parameters of the coatings are shown in the Table 4. As observed, there were prominent differences between conditions 1 and 2. Specifically, the condition-1 coating was denser and uniform, with no apparent microcracks on its surface. Under a high resolution (Figure 4b), the flaky hexagonal structures of LDH with a 20–80 nm thickness intersected one another; the integrated and compact surface indicated the better crystalline integrity of the LDH sheets [19]. Holes can sometimes appear as hydrogen-releasing channels; no apparent holes were observed on the chemical conversion coatings of the magnesium alloy. In Figure 4c, some microcracks appeared on the condition-2 coating, and some flocculent particles were also observed. In the high-resolution microstructure, changes in the flaky structure were observed. Their thicknesses decreased compared to the samples in Figure 4b; this indicated that the integrity of the LDH was changed or destroyed. EDS analysis showed that the weight element content of Al in condition 1 was lower than that in condition 2. This was probably due to the increase in the reaction time, which was favorable for increasing the platelet size [18]. The LDH growth was combined with the continuous formation of Mg(OH)2 during this process; the Mg(OH)2 formation often occurred before that of LDH [10]. Although the surface of condition 2 had a higher Al content, the surface morphology of condition 1 was more integrated and compact; this indicated the potential for the better corrosion resistance performance of this coating.
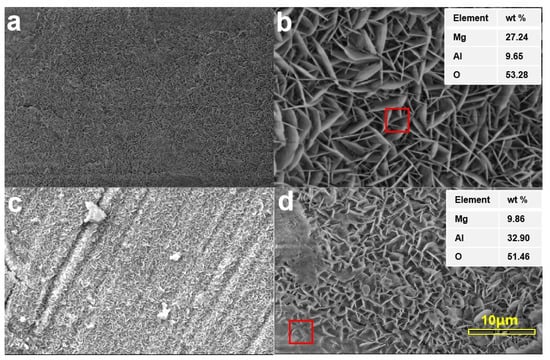
Figure 4.
Surface morphologies and EDS spectra of the coatings in (a,b) condition 1 and (c,d) condition 2.

Table 4.
Electrochemical parameters of the coatings in conditions 1 and 2.
Electrochemical analysis of the optimized samples was performed in the SBF solution in Figure 5. The potential of the LDH-coated alloy substrate in condition 1 and condition 2 was approximately −1.47 and −1.42 V, respectively. The corresponding Icorr of the samples were 1.18 and 1.21 μA/cm2, respectively; this indicated an improved anticorrosive performance of the condition-1 coating. The higher value of Rp further confirmed the improved corrosive protection of the condition-1 sample. The impedance spectra of the coated samples are shown in Figure 6. The capacitive loop was related to the charge transfer process, due to the presence of coatings. The Nyquist plots clearly showed a larger diameter of the impedance arc of the condition-1 coating; this indicated that it was more resistant to corrosion [31,32]. The |Z| value of the condition-2 coating was 3.78 × 103 Ω·cm2 (Figure 6b); this was lower than that of the condition-1 coating (1.07 × 104 Ω·cm2). This further confirmed the better corrosion resistance of the condition-1 coating. Figure 6c shows the Bode plots of the phase angle versus the frequency of the samples. The phase angle of the condition-1 coating in the intermediate frequency range was relatively flatter and indicated that the coating was denser and more uniform [32]. The equivalent fitted circuits of the coatings are shown in Figure 6d, and the corresponding fitting data are listed in Table 5. R1, Rs, Rct, and RL are the resistance of the coating, the solution resistance, the charge transfer resistance, and the resistance at a low frequency with an inductance (L), respectively. CPE1 and CPE2 represent the constant phase elements. CPE1 and R1 are related to the coating resistance. The values of R1 and Rct for the condition-1 coating were higher than those for the condition-2 coating. This indicated a better corrosion resistance of the condition-1 coating. RL and L were related to the peeling away of the coating and the formation of corrosion products.
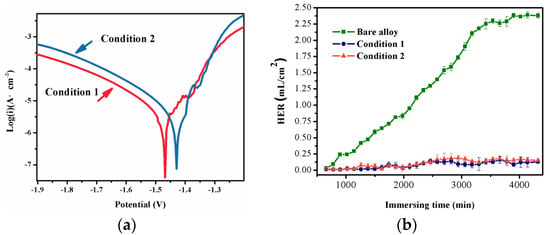
Figure 5.
Polarization curves (a) and the hydrogen evolution rate (HER) (b) for the coatings in conditions 1 and 2. The HER curve shows the bare alloy for comparison.
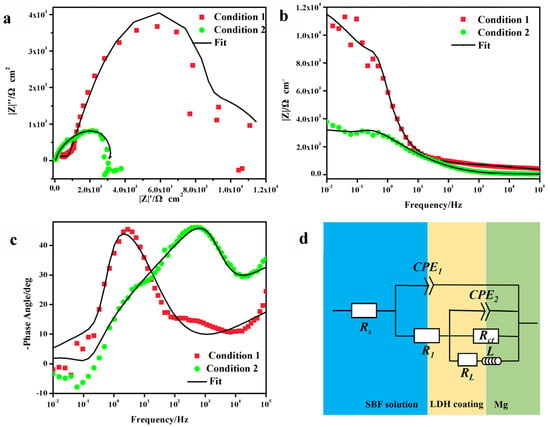
Figure 6.
Impedance spectra of the coatings in conditions 1 and 2: (a) Nyquist images. (b) Bode plots of |Z| vs. frequency. (c) Bode plots of phase angle vs. frequency. (d) Fitting circuit image.

Table 5.
Electrochemical data for the coatings in conditions 1 and 2, obtained via equivalent circuit fitting of EIS curves.
The hydrogen evolution rate (HER) test in Figure 5 shows that the bare alloy corroded more rapidly, and the HER was significantly increased. For the entire testing process, the final HER of the bare alloy, the condition-1 alloy, and the condition-2 alloy were 2.4, 0.13, and 0.15 mL/cm2, respectively. This implied that the corrosion of the metal matrix was effectively restrained. There was no noticeable increase in the HER of the coated specimens in the immersion experiment; this indicated significant corrosion resistance and the protection of the LDH coatings. The difference in the HER results between the condition-1 and -2 coatings was not significant.
The bonding strengths of the coatings before and after the HER test were analyzed using a scratch tester. The critical loadings, LC1, of the condition-1 and 2 coatings before the HER test corresponded to the location of the peak of the acoustic emission signals and were 23.6 ± 0.46 N and 19.6 ± 0.72 N in Table 6, respectively. Two other signals were observed (Figure 7). The signal loading, LC2, corresponded to a further extension of the scratch on the coating; the final loading signal, LC3, was attributed to the destruction of the LDH coating on the metal substrate. These results implied that the condition-1 coating had a better bonding force. After the HER test, the binding strength of the LDH coating and alloy substrate decreased significantly; the condition-1 coating still had a comparatively higher loading after 51 h of soaking. The coating in the solution was gradually destroyed by its interaction with the environment, microcracks, or defects; hydrotalcite dissolution inevitably occurred during this process [15]. Galvanic corrosion between the substrate and coating occurred; this led to the accumulation of Mg(OH)2 with relatively looser corrosion products on the surface. The binding strength of the coating with the inferior quality was significantly decreased and easily cracked after soaking. A denser and integrated coating surface was required to effectively protect the substrate from corrosion; this further demonstrated the better performance of the condition-1 coating.

Table 6.
Loads at critical failure for the coatings in conditions 1 and 2, before and after an HER test for 51 h.
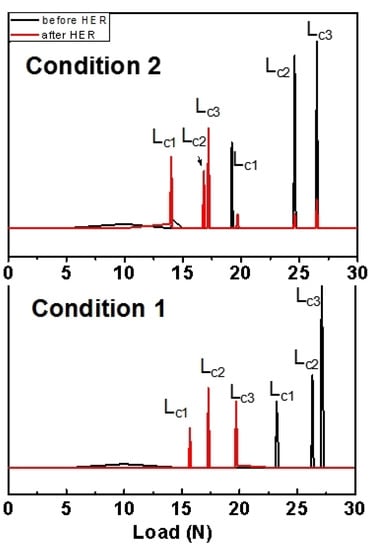
Figure 7.
Acoustic emission signal curves of the coatings in conditions 1 and 2 before (black line) and after (red line) the HER test for 51 h.
4. Discussions
The quality of the LDH coating that was obtained via the in situ hydrothermal approach directly affected the corrosion resistance of the Mg alloys. The formation process of the Mg-Al LDH film without the addition of a magnesium source in a hydrothermal solution was very complicated [16,19]; the chemical composition and surface morphology of the coated film exhibited a series of component conversions by varying the process parameters. The growth mechanism of the Mg-Al LDH coatings in conditions 1 and 2 are shown in Figure 8. Since the magnesium source was provided by the substrate, the in situ coating thus formed was mainly composed of Mg(OH)2 and LDH [28,29]; the replacement phenomenon occurred during the formation procedure. After immersion in the solution, the substrate gradually dissolved, released Mg2+, and formed Mg(OH)2. In an alkaline environment, the Al3+ from Al(NO3)3 combined with OH- enriched in the solution to form a complex ion of [Al13(OH)32(H2O)]7+; this was followed by adsorption onto Mg(OH)2. Some of the Mg2+ in Mg (OH)2 were replaced by Al3+ and then combined with NO3- in the solution; the original LDH granules were gradually formed [19,33]. The cations in the LDH structure formed octahedral coordination with hydroxyl groups by ordinary bridge groups; these were closely arranged together [17,34]. Therefore, the structure of LDH was more stable in the coating, compared to Mg(OH)2.
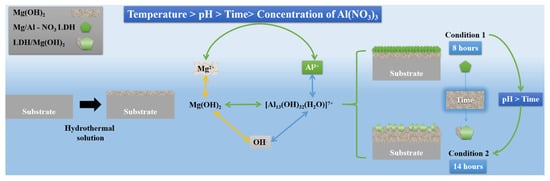
Figure 8.
Growth mechanism of the Mg-Al LDH coatings in conditions 1 and 2.
The reaction temperature for the hydrothermal process influenced the growth orientation and density of the double-layered hydroxide layer in the coating, due to the relationship between the temperature and the formation and growth of the LDH crystal nucleus [35,36]. Increasing the temperature promoted the dissociation of Mg(OH)2 in the coating to provide more Mg2+ for replacement [19]. It was advantageous to promote the diffusion and exchange of each ion in the reacting solution. As the temperature increased, the Mg(OH)2 layer formed a deposition membrane that gradually thickened and hindered the electron and ion transfer for chemical reactions on the surface and the substrate. The concentrations of Mg2+ and OH− in the surface layer were reduced and the dissolution of the alloy substrate reached equilibrium. The overall chemical reaction of the coating slowed down. Regarding the condition-1 and -2 treatment results, the SEM morphology and relative LDH content in the coating suggested that a suitably high temperature (130 °C in this study) favored a high-quality LDH coating and improved the corrosion resistance. The nucleation speed of LDH was positively correlated with the reaction temperature [36], and a higher temperature was associated with a faster nucleation velocity [37,38]. When the temperature was too high, large numbers of crystal nuclei formed rapidly; this increased the defects in the densification process of the LDH layer. The corrosion resistance of the film deteriorated with increasing temperature.
The pH affected the chemical composition of the reaction solution, and an appropriate pH was crucial to maintain the Mg2+ and OH− concentrations and control the LDH formation. The composition of LDH was significantly related to the OH− concentration [39]. An unsuitable pH level led to the inhibition of the conversion of Mg substrate to Mg2+ ions or the decline in Mg2+ concentration through OH−; this limited the composition uniformity and compactness of the LDH coatings [39]. This was possibly why the pH preceded the reaction time, as shown in Table 3.
The variation of the reaction time affected the corrosion resistance of the LDH coating [18]. Specifically, the formed LDH crystal nucleus gradually grew after a suitable time; the nanosheet size, component transition, and densification of LDH were improved during this process. Extending the reaction time produced more Mg(OH)2 on the surface of the matrix and reduced the relative content of LDH in the coating [33]. This led to a worsened corrosion resistance of the condition-2 coating than that of the condition-1 coating. The ion concentration in the solution and the crystal nucleus provided the capability for growth of the sheet size, component transition, or densification of LDH. This caused the greater significance of temperature and pH in enhancing the corrosion resistance of coatings.
5. Conclusions
The effect of the preparation parameters on the corrosion resistance of in situ Mg-Al hydrotalcite coatings on biomedical magnesium alloys was investigated using the orthogonal test method. The optimized preparation conditions for the LDH coating were obtained. The results are summarized as follows:
(1) The optimized preparation conditions for the LDH coating with integrated properties of surface morphology, composition, corrosion resistance, and bonding strength were a temperature of 130 °C, a reaction time of 8 h, a pH of 13, and an aluminum nitrate concentration of 10 mM.
(2) The temperature had the most significant impact on the quality of the LDH coating; a suitably high temperature, longer reaction time, higher aluminum source concentration, and high pH were conducive to the formation of a high-quality in situ LDH coating.
(3) The corrosion resistance of the coating was related to the LDH content in the final coating. An increase in the hydrotalcite content was correlated to a lower corrosion current density of the coating.
Author Contributions
Conceptualization, A.T.; Data curation, W.W., Y.C. and W.L. (Wen Liu); Formal analysis, Y.Z. and A.T.; Funding acquisition, Y.Z. and M.C.; Investigation, A.T.; Project administration, M.C.; Resources, W.W., Y.C. and W.L. (Wen Liu); Supervision, W.L. (Wei Li) and M.C.; Validation, W.L. (Wei Li) and W.L. (Wen Liu); Writing—original draft, Y.Z.; Writing—review & editing, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support for this work from the National Natural Science Foundation of China (52171241, 51801137 and 51871166) and the Joint Foundation of the National Natural Science Foundation of China (U1764254).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloy. 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Kazakova, G.; Safronova, T.; Golubchikov, D.; Shevtsova, O.; Rau, J.V. Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair. Materials 2021, 14, 4857. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, J.; Li, Y.; Wang, L.; Jing, L.; Li, M.; Yu, S.; Cheng, Y.; Zheng, Y.J.B.S. Based on the synergistic effect of Mg2+ and antibacterial peptides to improve the corrosion resistance, antibacterial ability and osteogenic activity of magnesium-based degradable metals. J. Biomater. Sci. 2021, 9, 807–825. [Google Scholar] [CrossRef]
- Seitz, J.-M.; Eifler, R.; Bach, F.-W.; Maier, H.J. Magnesium degradation products: Effects on tissue and human metabolism. J. Biomed. Mater. Res. Part A 2013, 102, 3744–3753. [Google Scholar] [CrossRef]
- Heimann, R.B. Magnesium alloys for biomedical application: Advanced corrosion control through surface coating. Surf. Coat. Technol. 2020, 405, 126521. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.; Sun, M.; Ding, W. Recent developments and applications on high-performance cast magnesium rare-earth alloys. J. Magnes. Alloy. 2020, 9, 1–20. [Google Scholar] [CrossRef]
- Wu, F.; Liang, J.; Peng, Z.; Liu, B. Electrochemical deposition and characterization of Zn-Al layered double hydroxides (LDHs) films on magnesium alloy. Appl. Surf. Sci. 2014, 313, 834–840. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.-G.; Zeng, R.-C.; Li, S.-Q.; Cui, H.-Z.; Song, L.; Han, E.-H. Corrosion resistance of Mg–Al-LDH coating on magnesium alloy AZ31. Surf. Coat. Technol. 2014, 258, 1152–1158. [Google Scholar] [CrossRef]
- Wong, H.M.; Yeung, K.W.K.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.K.; Cheung, K.M.C. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar] [CrossRef]
- Li, C.-Y.; Gao, L.; Fan, X.-L.; Zeng, R.-C.; Chen, D.-C.; Zhi, K.-Q. In vitro degradation and cytocompatibility of a low temperature in-situ grown self-healing Mg-Al LDH coating on MAO-coated magnesium alloy AZ31. Bioact. Mater. 2020, 5, 364–376. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Z.; Zang, S.; Yu, X.; Yang, H.; Chang, S. Research Progress on Surface Treatments of Biodegradable Mg Alloys: A Review. ACS Omega 2020, 5, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Z.P.; Lu, J.; Tang, Z.Y.; Zhao, H.J.; Good, D.A.; Wei, M.Q. Potential for Layered Double Hydroxides-Based, Innovative Drug Delivery Systems. Int. J. Mol. Sci. 2014, 15, 7409–7428. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, D.; Li, M.; Liu, X.; Zhang, Y.; Qian, S.; Peng, F. Osteogenesis, angiogenesis and immune response of Mg-Al layered double hydroxide coating on pure Mg. Bioact. Mater. 2020, 6, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.; Balan, P.; Birbilis, N. Advances in LDH coatings on Mg alloys for biomedical applications: A corrosion perspective. Appl. Clay Sci. 2020, 202, 105948. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. In situ growth of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros. Sci. 2011, 53, 3281–3288. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. Study of the in situ growth mechanism of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros. Sci. 2012, 63, 148–158. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. In Situ Growth Process of Mg–Al Hydrotalcite Conversion Film on AZ31 Mg Alloy. J. Mater. Sci. Technol. 2015, 31, 384–390. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Z.C.; Pan, F.S.; Tang, A.T.; Zhang, G.; Liu, L. Influence of Reaction Temperature on the Controlled Growth of Mg-Al LDH Film. Int. J. Electrochem. Sci. 2017, 12, 6352–6364. [Google Scholar] [CrossRef]
- Wu, L.; Pan, F.; Liu, Y.; Zhang, G.; Tang, A.; Atrens, A. Influence of pH on the growth behaviour of Mg–Al LDH films. Surf. Eng. 2017, 34, 674–681. [Google Scholar] [CrossRef]
- Reichle, W. Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ionics 1986, 22, 135–141. [Google Scholar] [CrossRef]
- Costantino, U.; Marmottini, F.; Nocchetti, M.; Vivani, R. New synthetic routes to hydrotalcite-like compounds—Characterisation and properties of the obtained materials. Eur. J. Inorg. Chem. 1998, 1998, 1439–1446. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Chen, M.; Zhao, Y.; You, C.; Li, Y.; Chen, G. In Vitro and in Vivo Degradation Behavior and Biocompatibility Evaluation of Microarc Oxidation-Fluoridated Hydroxyapatite-Coated Mg–Zn–Zr–Sr Alloy for Bone Application. ACS Biomater. Sci. Eng. 2019, 5, 2858–2876. [Google Scholar] [CrossRef]
- Koga, G.Y.; Albert, B.; Roche, V.; Nogueira, R.P. A comparative study of mild steel passivation embedded in Belite-Ye’elimite-Ferrite and Porland cement mortars. Electrochim. Acta 2018, 261, 66–77. [Google Scholar] [CrossRef]
- Zhao, Y.-B.; Liu, H.-P.; Li, C.-Y.; Chen, Y.; Li, S.-Q.; Zeng, R.-C.; Wang, Z.-L. Corrosion resistance and adhesion strength of a spin-assisted layer-by-layer assembled coating on AZ31 magnesium alloy. Appl. Surf. Sci. 2018, 434, 787–795. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, Z.; Tang, C.; Li, W.; You, C.; Chen, M. The mechanical and corrosion resistance of Mg-Zn-Ca-Ag alloys: The influence of Ag content. J. Mater. Res. Technol. 2020, 9, 10863–10875. [Google Scholar] [CrossRef]
- Ishizaki, T.; Kamiyama, N.; Watanabe, K.; Serizawa, A. Corrosion resistance of Mg(OH)2/Mg-Al layered double hydroxide composite film formed directly on combustion-resistant magnesium alloy AMCa602 by steam coating. Corros. Sci. 2015, 92, 76–84. [Google Scholar] [CrossRef]
- Nakamura, K.; Tsunakawa, M.; Shimada, Y.; Serizawa, A.; Ishizaki, T. Formation mechanism of Mg-Al layered double hydroxide-containing magnesium hydroxide films prepared on Ca-added flame-resistant magnesium alloy by steam coating. Surf. Coat. Technol. 2017, 328, 436–443. [Google Scholar] [CrossRef]
- Ishizaki, T.; Chiba, S.; Suzuki, H. In Situ Formation of Anticorrosive Mg-Al Layered Double Hydroxide-Containing Magnesium Hydroxide Film on Magnesium Alloy by Steam Coating. ECS Electrochem. Lett. 2013, 2, C15–C17. [Google Scholar] [CrossRef]
- Zhang, X.H.; Su, G.C.; Han, Y.Y.; Ai, X.H.; Yan, W.L. A study on the composition optimization and mechanical properties of Al-Mg-Si cast alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Processing 2010, 527, 3852–3856. [Google Scholar] [CrossRef]
- Cui, X.-J.; Lin, X.-Z.; Liu, C.-H.; Yang, R.-S.; Zheng, X.-W.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Zou, Y.-H.; Wang, J.; Cui, L.-Y.; Zeng, R.-C.; Wang, Q.-Z.; Han, Q.-X.; Qiu, J.; Chen, X.-B.; Chen, D.-C.; Guan, S.-K.; et al. Corrosion resistance and antibacterial activity of zinc-loaded montmorillonite coatings on biodegradable magnesium alloy AZ31. Acta Biomater. 2019, 98, 196–214. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Wang, W.; Zhao, Y.; Chen, M. Microstructure, corrosion resistance, and antibacterial properties of an Ag/Mg-Al layered double hydroxide coating synthesized in situ on biomedical Mg-Zn-Ca alloy. Ceram. Int. 2021, 48, 4172–4187. [Google Scholar] [CrossRef]
- Gou, G.J.; Ma, P.H.; Chu, M.X. Kinetics of synthetic of Cl- type hydrotalcitelike with coprecipitation reaction. Acta Phys. -Chim. Sin. 2004, 20, 1357–1363. [Google Scholar]
- Eliseev, A.; Lukashin, A.; Vertegel, A.; Tarasov, V.; Tret’yakov, Y.D. A Study of Crystallization of Mg–Al Double Hydroxides. In Doklady Chemistry; Kluwer Academic Publishers-Plenum Publishers: Drive Norwell, MA, USA, 2002; pp. 339–343. [Google Scholar]
- Heng-xu, X.; Jun-li, S.; Si-yuan, L.; Li-feng, H.; Hua-yun, D.; Bao-sheng, L.; Ying-hui, W. Effect of hydrothermal reaction temperature on structure and corrosion resistance of CaAl-LDH film on AZ31 magnesium alloy. Chin. J. Nonferrous Met. 2021, 31, 298–309. [Google Scholar]
- Zhiqiang, L.; Libin, Y.; Zuoliang, S.; Yanfei, W.; Liang, Z.; Xiaoyu, Z. Experimental research on growth and crystal size control of potassium chloride in batch cooling crystallization. Inorg. Chem. Ind. 2019, 51, 33–36. [Google Scholar]
- Zhang, G.; Wu, L.; Tang, A.T.; Chen, X.B.; Ma, Y.L.; Long, Y.; Peng, P.; Ding, X.X.; Pan, H.L.; Pan, F.S. Growth behavior of MgAl-layered double hydroxide films by conversion of anodic films on magnesium alloy AZ31 and their corrosion protection. Appl. Surf. Sci. 2018, 456, 419–429. [Google Scholar] [CrossRef]
- Chen, J.; Feng, J.; Yan, L.; Li, H.; Xiong, C.; Ma, S. In situ growth process of Mg–Fe layered double hydroxide conversion film on MgCa alloy. J. Magnes. Alloy. 2020, 9, 1019–1027. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).








