Broadband Spectrum Light-Driven PANI/Au/Beta-Cyclodextrin Nanocomposite and Its Light-Triggered Interfacial Carrier Transfer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Loading of Aniline in The Hydrophobic Cavities of Beta-Cyclodextrin
2.3. Synthesis of Au/Polyaniline Nanocomposite Functionalized with Beta-Cyclodextrin
2.4. Morphology Observations with SEM
2.5. Morphology Observation with TEM
2.6. Measurement of UV–Vis Spectrum
2.7. Measurement of FTIR Spectra
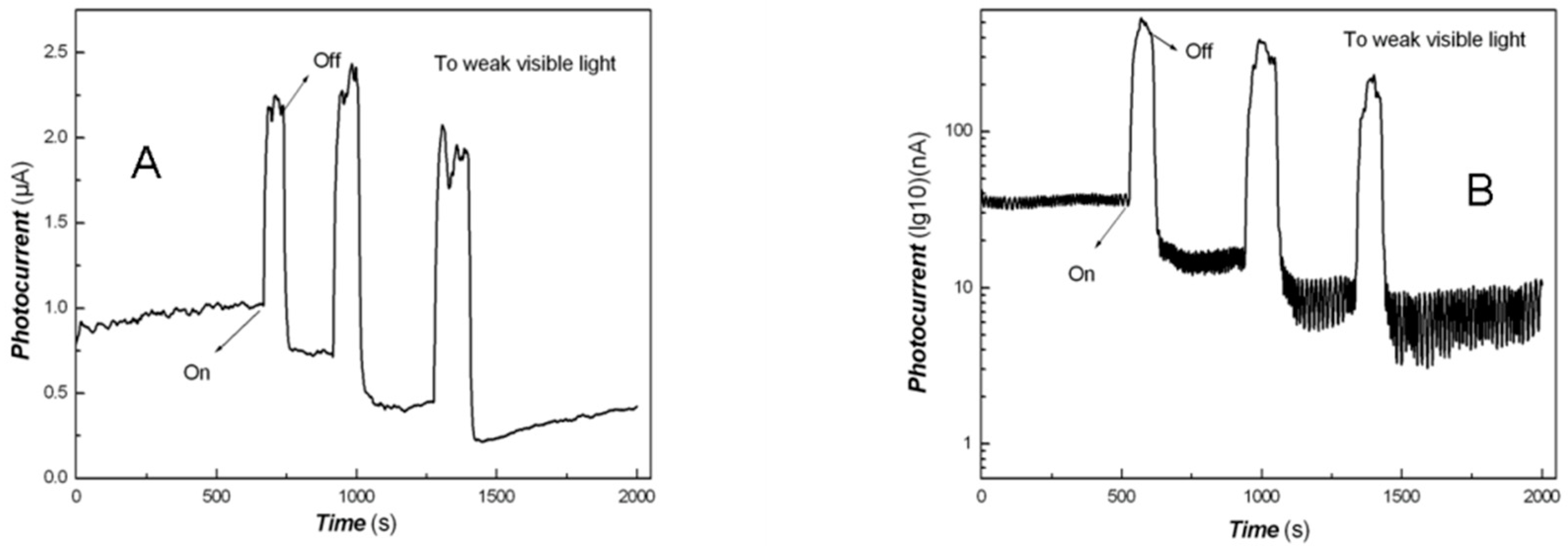
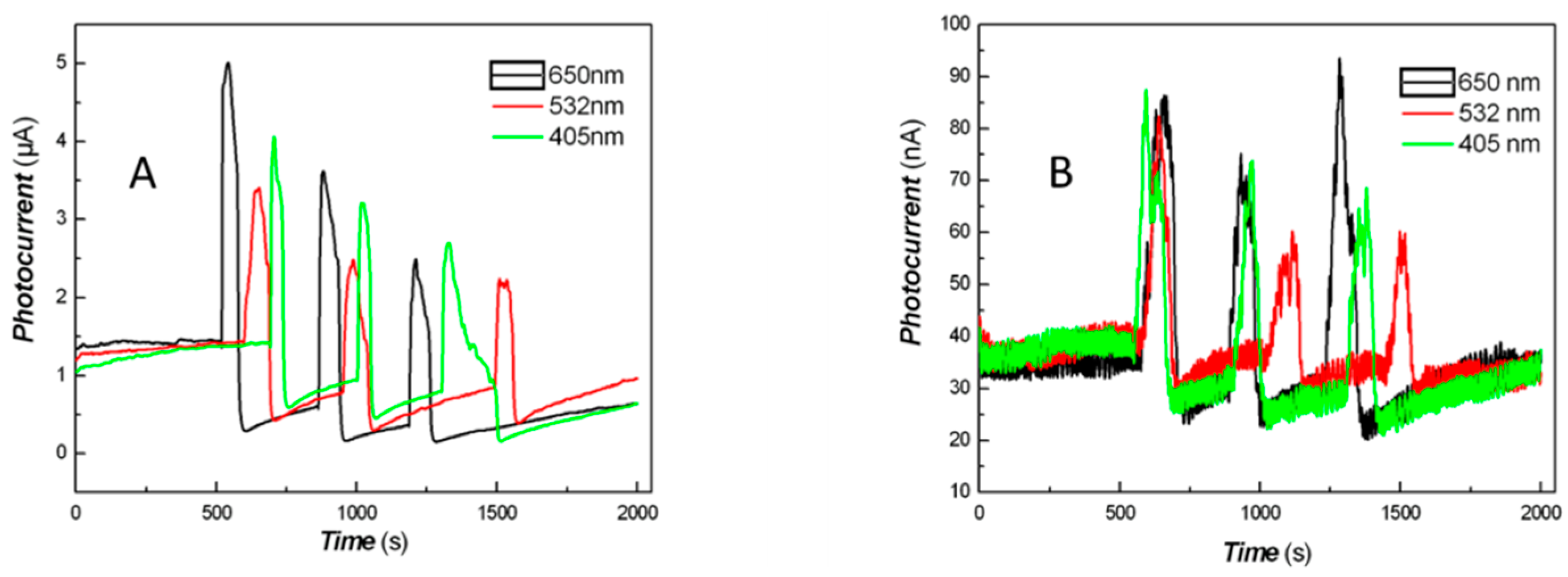
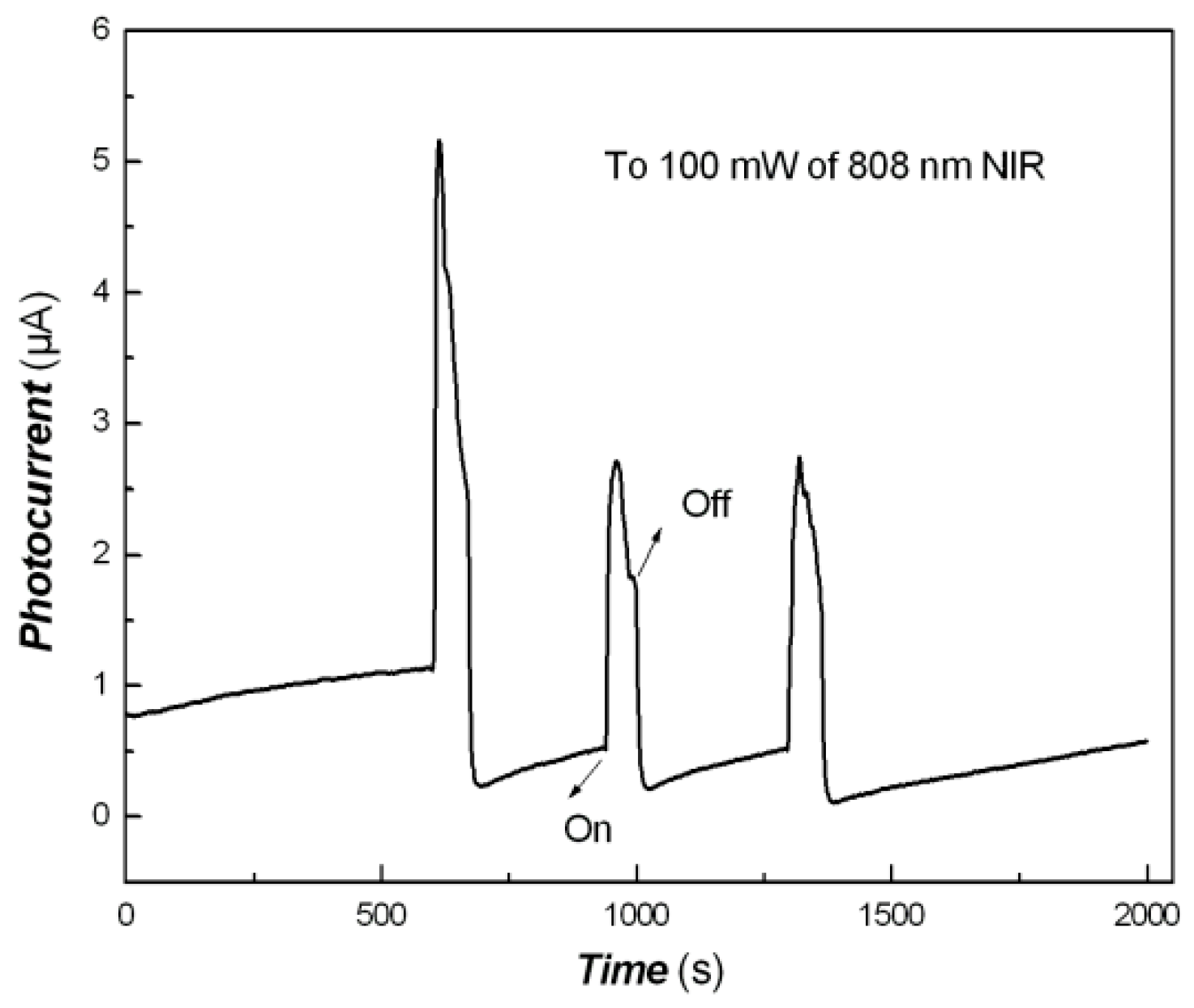
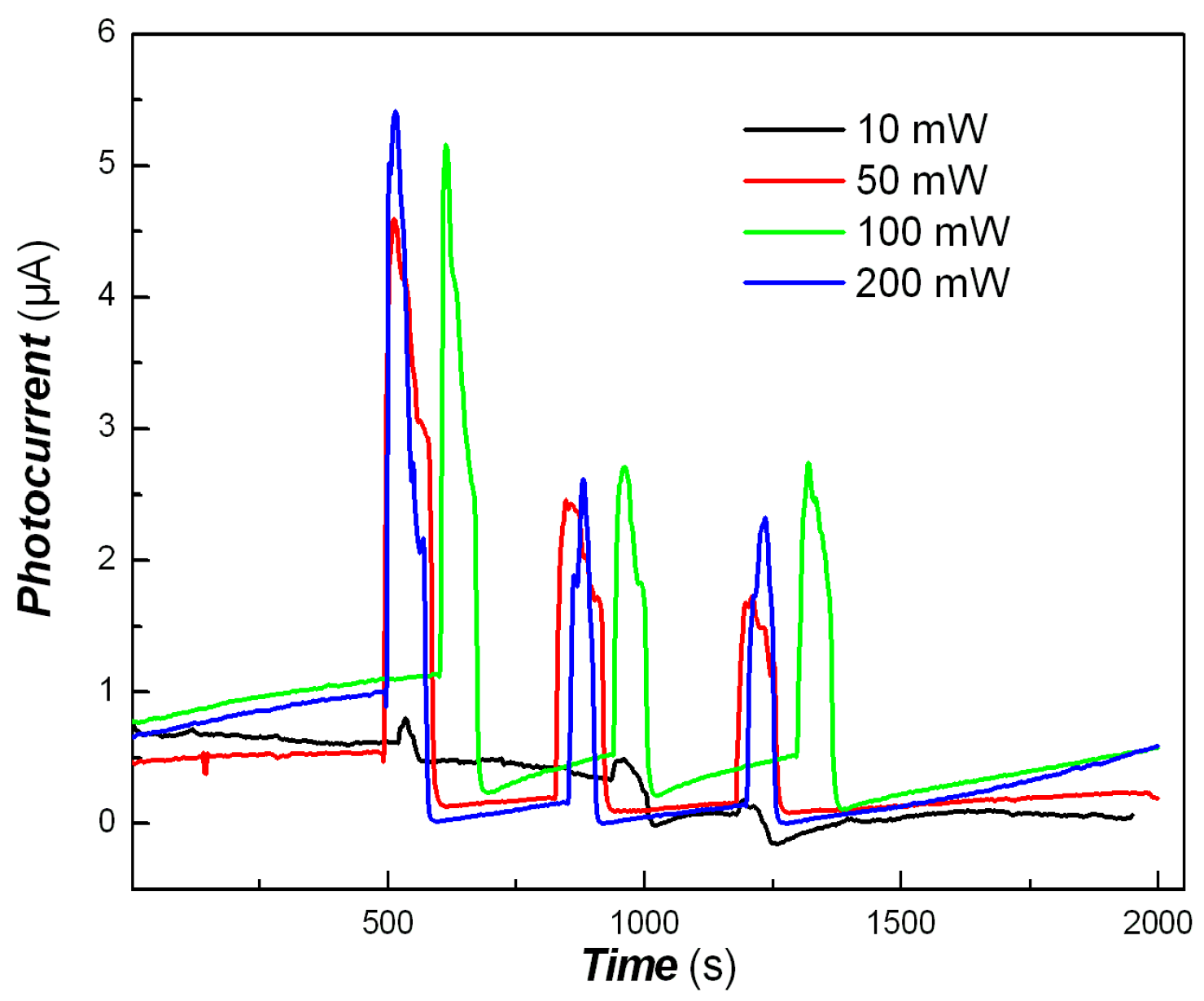
2.8. Photo-Responses of Nanocomposite to Visible Light and NIR
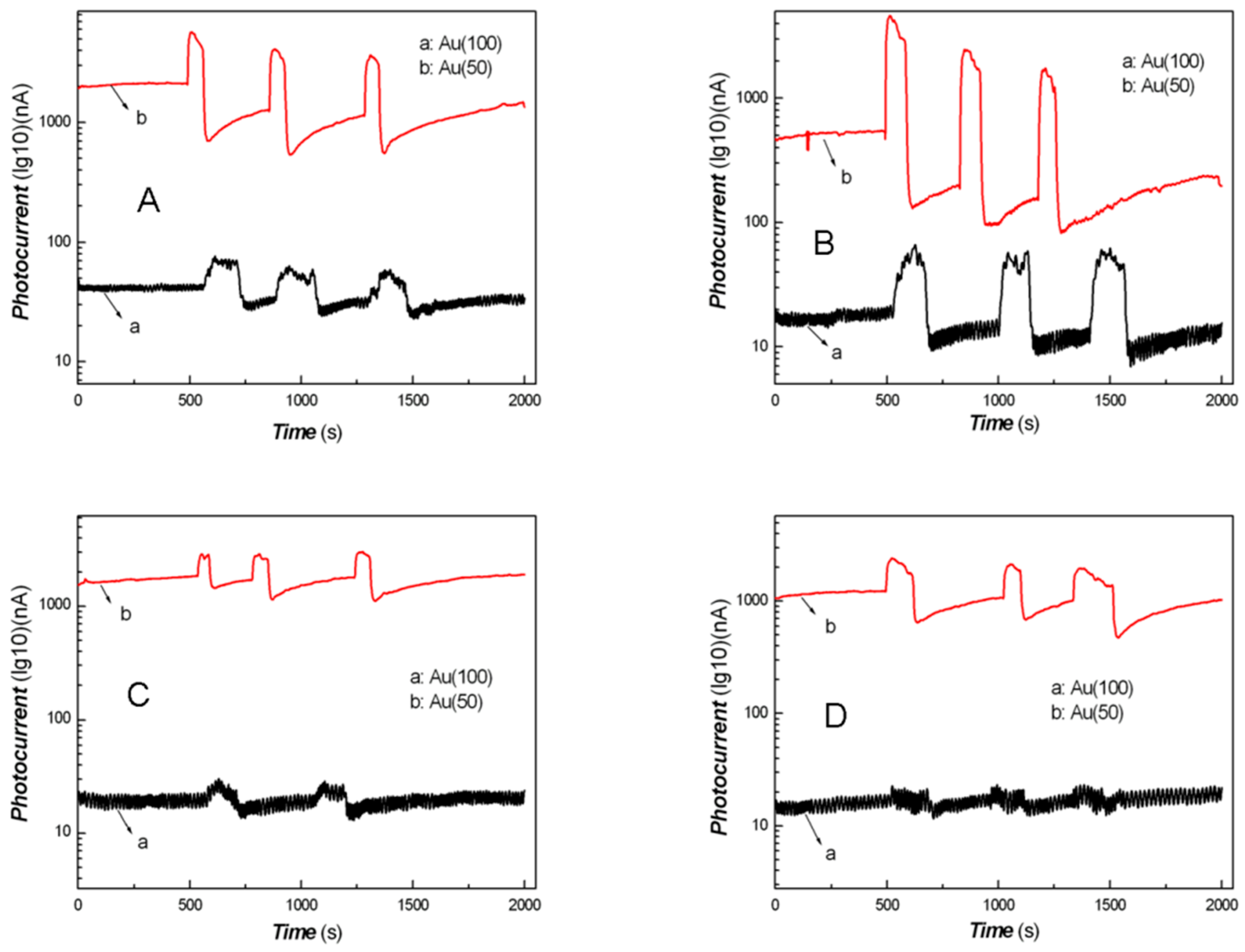
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alkuam, E.; Mohammed, M.; Chen, T. Fabrication of CdS nanorods and nanoparticles with PANI for (DSSCs) dye-sensitized solar cells. Sol. Energy 2017, 150, 317–324. [Google Scholar] [CrossRef]
- He, B.; Tang, Q.; Wang, M.; Chen, H.; Yuan, S. Robust Polyaniline−Graphene Complex Counter Electrodes for Efficient Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 8230–8236. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Yan, K.; Lin, Z.; Loo, J.S.C.; Pan, L.; Zhang, Q.; Zhang, J.; Zhu, J. Inkjet-printed porous polyaniline gel as an efficient anode for microbial fuel cells. J. Mater. Chem. A 2016, 4, 14555–14559. [Google Scholar] [CrossRef]
- Wu, R.; Tsai, M.; Ho, K.; Wei, T.; Hsieh, T.; Han, Y.; Kuo, C.; Tseng, P.; Wang, Y. Sulfonated polyaniline nanofiber as Pt-catalyst conducting support for proton exchange membrane fuel cell. Polymer 2014, 55, 2035–2043. [Google Scholar] [CrossRef]
- Hursa′n, D.; Korma′nyos, A.; Rajeshwar, K.; Jana′ky, C. Polyaniline films photoelectrochemically reduce CO2 to alcohols. Chem. Commun. 2016, 52, 8858–8861. [Google Scholar] [CrossRef]
- Jeon, J.; Kwon, S.R.; Lutkenhaus, J.L. Polyaniline nanofiber/electrochemically reduced graphene oxide layer-by-layer electrodes for electrochemical energy storage. J. Mater. Chem. A 2015, 3, 3757–3767. [Google Scholar] [CrossRef]
- Ge, D.; Yang, L.; Honglawan, A.; Li, J.; Yang, S. In Situ Synthesis of Hybrid Aerogels from Single-Walled Carbon Nanotubes and Polyaniline Nanoribbons as Free-Standing, Flexible Energy Storage Electrodes. Chem. Mater. 2014, 26, 1678–1685. [Google Scholar] [CrossRef]
- Ramphal, I.A.; Hagerman, M.E. Water-Processable Laponite/Polyaniline/Graphene Oxide Nanocomposites for Energy Applications. Langmuir 2015, 31, 1505–1515. [Google Scholar] [CrossRef]
- Wang, G.; Peng, J.; Zhang, L.; Zhang, J.; Dai, B.; Zhu, M.; Xia, L.; Yu, F. Two-dimensional SnS2@PANI nanoplates with high capacity and excellent stability for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 3659–3666. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, Z.; Wang, Z.; Liu, Y.; Wang, X.; Qiu, J. Dually Fixed SnO2 Nanoparticles on Graphene Nanosheets by Polyaniline Coating for Superior Lithium Storage. ACS Appl. Mater. Interfaces 2015, 7, 2444–2451. [Google Scholar] [CrossRef]
- Lin, N.; Zhou, J.; Wang, L.; Zhu, Y.; Qian, Y. Polyaniline-Assisted Synthesis of Si@C/RGO as Anode Material for Rechargeable Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Htut, K.Z.; Kim, M.; Lee, E.; Lee, G.; Baeck, S.H.; Shim, S.E. Biodegradable polymer-modified graphene/polyaniline electrodes for supercapacitors. Synth. Met. 2017, 227, 61–70. [Google Scholar] [CrossRef]
- Gu, D.; Ding, C.; Qin, Y.; Jiang, H.; Wang, L.; Shen, L. Behavior of electrical charge storage/release in polyaniline electrodes of symmetric supercapacitor. Electrochim. Acta 2017, 245, 146–155. [Google Scholar] [CrossRef]
- Chang, C.; Hu, Z.; Lee, T.; Huang, Y.; Ji, W.; Liu, W.; Yeh, J.; Wei, Y. Biotemplated hierarchical polyaniline composite electrodes with high performance for flexible supercapacitors. J. Mater. Chem. A 2016, 4, 9133–9145. [Google Scholar]
- Liu, Q.; Jing, S.; Wang, S.; Zhuo, H.; Zhong, L.; Peng, X.; Sun, R. Flexible nanocomposites with ultrahigh specific areal capacitance and tunable properties based on a cellulose derived nanofiber-carbon sheet framework coated with polyaniline. J. Mater. Chem. A 2016, 4, 13352–13362. [Google Scholar] [CrossRef]
- Chao, J.; Deng, J.; Zhou, W.; Liu, J.; Hu, R.; Yang, L.; Zhu, M.; Schmidt, O.G. Hierarchical nanoflowers assembled from MoS2/polyaniline sandwiched nanosheets for high-performance supercapacitors. Electrochim. Acta 2017, 243, 98–104. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Meng, Y.; Wei, Z. Conducting Polymer Nanowire Arrays for High Performance Supercapacitors. Small 2014, 10, 14–31. [Google Scholar] [CrossRef]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and Polypyrrole Pseudocapacitor Electrodes with Excellent Cycling Stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef]
- Tian, Y.; Cong, S.; Su, W.; Chen, H.; Li, Q.; Geng, F.; Zhao, Z. Synergy of W18O49 and Polyaniline for Smart Supercapacitor Electrode Integrated with Energy Level Indicating Functionality. Nano Lett. 2014, 14, 2150–2156. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.; Deng, J.; Zhang, Y.; Sun, X.; Chen, P.; Fang, X.; Zhang, Z.; Guan, G.; Peng, H. Electrochromic Fiber-Shaped Supercapacitors. Adv. Mater. 2014, 26, 8126–8132. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, J.; Lin, T.; LÜ, Q.; Chen, G. Nanocomposites of Sulfonic Polyaniline Nanoarrays on Graphene Nanosheets with an Improved Supercapacitor Performance. Chem. Eur. J. 2015, 21, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yu, D.; Zhang, Q.; Goh, K.; Wei, L.; Yong, Y.; Jiang, R.; Wei, J.; Chen, Y. Ternary Hybrids of Amorphous Nickel Hydroxide–Carbon Nanotube-Conducting Polymer for Supercapacitors with High Energy Density, Excellent Rate Capability, and Long Cycle Life. Adv. Funct. Mater. 2015, 25, 1063–1073. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Lei, H.; Li, B. An organic–inorganic broadband photodetector based on a single polyaniline nanowire doped with quantum dots. Nanoscale 2016, 8, 15529–15537. [Google Scholar] [CrossRef]
- Barman, T.; Pal, A.R. Plasmonic Photosensitization of Polyaniline Prepared by a Novel Process for High-Performance Flexible Photodetector. ACS Appl. Mater. Interfaces 2015, 7, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Lee, B.; Nguyen, V.N.; Dao, V. Novel photocatalytic activity of vanadium-doped tantalum nitride sensitized/protected by polyaniline for efficient visible light water splitting. J. Catal. 2017, 352, 13–21. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X. Two dimensional conjugated polymers with enhanced optical absorption and charge separation for photocatalytic hydrogen evolution. Energy Environ. Sci. 2014, 7, 1902–1906. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Z.; Li, H.; Sun, X.; Peng, H. Conjugated polymer composite artificial muscle with solvent-induced anisotropic mechanical actuation. J. Mater. Chem. A 2014, 2, 17272–17280. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, C.; Peng, L.; Yu, G. Conductive “Smart” Hybrid Hydrogels with PNIPAM and Nanostructured Conductive Polymers. Adv. Funct. Mater. 2015, 25, 1219–1225. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, X.; Dou, S.; Zhang, L.; Zhang, H.; Lv, H.; Wang, L.; Zhao, J.; Li, Y. A comprehensive study of electrochromic device with variable infrared emissivity based on polyaniline conducting polymer. Sol. Energy Mater. Sol. Cells 2017, 170, 120–126. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, J.; Wang, C. Acid and base dual-controlled cargo molecule release from polyaniline gated-hollow mesoporous silica nanoparticles. Polym. Chem. 2016, 7, 6467–6474. [Google Scholar] [CrossRef]
- Gong, X.; Fei, G.; Fu, W.; Fang, M.; Gao, X.; Zhong, B.; Zhang, L. Flexible strain sensor with high performance based on PANI/PDMS films. Org. Electron. 2017, 47, 51–56. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, J.; Kahng, Y.H.; Kim, N.; Kim, Y.J.; Lee, J.; Lee, T.; Lee, K. Graphene-Conducting Polymer Hybrid Transparent Electrodes for Efficient Organic Optoelectronic Devices. Adv. Funct. Mater. 2014, 24, 1847–1856. [Google Scholar] [CrossRef]
- Meng, D.; Yang, S.; Guo, L.; Li, G.; Ge, J.; Huang, Y.; Bielawski, C.W.; Geng, J. The enhanced photothermal effect of graphene/conjugated polymer composites: Photoinduced energy transfer and applications in photocontrolled switches. Chem. Commun. 2014, 50, 14345–14348. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, L.; Zhang, D.; Yang, D.; Guo, J.; Wei, J. Fabrication of polyaniline/silver nanoparticles/multi-walled carbonnanotubes composites for flexible microelectronic circuits. Synth. Met. 2014, 192, 15–22. [Google Scholar] [CrossRef]
- Jiang, N.; Shao, L.; Wang, J. (Gold Nanorod Core)/(Polyaniline Shell) Plasmonic Switches with Large Plasmon Shifts and Modulation Depths. Adv. Mater. 2014, 26, 3282–3289. [Google Scholar] [CrossRef]
- Ramana, G.V.; Srikanth, V.V.S.S.; Padya, B.; Jain, P.K. Carbon nanotube–polyaniline nanotube core–shell structures for electrochemical applications. Eur. Polym. J. 2014, 57, 137–142. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Chen, F.; Sun, X.; Li, X.; Yu, Z.; Wan, P.; Chen, X. Hierarchical graphene–polyaniline nanocomposite films for high-performance flexible electronic gas sensors. Nanoscale 2016, 8, 12073–12080. [Google Scholar] [CrossRef]
- Rao, H.; Chen, M.; Ge, H.; Lu, Z.; Liu, X.; Zou, P.; Wang, X.; He, H.; Zeng, X.; Wang, Y. A novel electrochemical sensor based on Au@PANI composites film modified glassy carbon electrode binding molecular imprinting technique for the determination of melamine. Biosens. Bioelectron. 2017, 87, 1029–1035. [Google Scholar] [CrossRef]
- Wang, W.; Xu, G.; Cui, X.T.; Sheng, G.; Luo, X. Enhanced catalytic and dopamine sensing properties of electrochemically reduced conducting polymer nanocomposite doped with pure graphene oxide. Biosens. Bioelectron. 2014, 58, 153–156. [Google Scholar] [CrossRef]
- Rana, U.; Paul, N.D.; Mondal, S.; Chakraborty, C.; Malik, S. Water soluble polyaniline coated electrode: A simple and nimbleelectrochemical approach for ascorbic acid detection. Synth. Met. 2014, 192, 43–49. [Google Scholar] [CrossRef]
- Yang, T.; Yang, R.; Chen, H.; Nan, F.; Ge, T.; Jiao, K. Electrocatalytic Activity of Molybdenum Disulfide Nanosheets Enhanced by Self-Doped Polyaniline for Highly Sensitive and Synergistic Determination of Adenine and Guanine. ACS Appl. Mater. Interfaces 2015, 7, 2867–2872. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, E.A.; Komkova, M.A.; Nikitina, V.N.; Zaryanov, N.V.; Voronin, O.G.; Karyakina, E.E.; Yatsimirsky, A.K.; Karyakin, A.A. Reagentless Polyol Detection by Conductivity Increase in the Course of Self-Doping of Boronate-Substituted Polyaniline. Anal. Chem. 2014, 86, 11690–11695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, C.; Luo, J.; Long, Y.; Wang, C.; Yu, T.; Xiao, D. A polyaniline microtube platform for direct electron transfer of glucose oxidase and biosensing applications. J. Mater. Chem. B 2015, 3, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Radoiˇci´c, M.; Ciri´c-Marjanovi´c, G.; Spasojevi´c, V.; Ahrenkiel, P.; Mitri´ca, M.; Novakovi´cd, T.; Saponji´ca, Z. Superior photocatalytic properties of carbonized PANI/TiO2 nanocomposites. Appl. Catal. B Environ. 2017, 213, 155–166. [Google Scholar] [CrossRef]
- Liu, Z.; Miao, Y.; Liu, M.; Ding, Q.; Tjiu, W.W.; Cui, X.; Liu, T. Flexible polyaniline-coated TiO2/SiO2 nanofiber membranes with enhanced visible-light photocatalytic degradation performance. J. Colloid Interface Sci. 2014, 424, 49–55. [Google Scholar] [CrossRef]
- Bogdanović, U.; Vodnik, V.; Mitrić, M.; Dimitrijević, S.; Škapin, S.D.; Žunič;, V.; Budimir, M.; Stoiljković, M. Nanomaterial with High Antimicrobial Efficacy of Copper/Polyaniline Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 1955–1966. [Google Scholar] [CrossRef]
- Poyraz, S.; Cerkez, I.; Huang, T.S.; Liu, Z.; Kang, L.; Luo, J.; Zhang, X. One-Step Synthesis and Characterization of Polyaniline Nanofiber/Silver Nanoparticle Composite Networks as Antibacterial Agents. ACS Appl. Mater. Interfaces 2014, 6, 20025–20034. [Google Scholar] [CrossRef]
- Zhang, X.; Goux, W.J.; Manohar, S.K. Synthesis of polyaniline nanofibers by “Nanofiber Seeding”. J. Am. Chem. Soc. 2004, 126, 4502–4503. [Google Scholar] [CrossRef]
- Huang, J.; Kaner, R.B. Nanofiber formation in the chemical polymerization of aniline: A mechanistic study. Angew. Chem. Int. Ed. 2004, 43, 5817–5821. [Google Scholar] [CrossRef]
- Li, C.; Bai, H.; Shi, G. Conducting polymer nanomaterials: Electro-synthesis and applications. Chem Soc. Rev. 2009, 38, 2397–2409. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Zhang, G.; He, H. Polyaniline nanowires on Si surfaces fabricated with DNA templates. J. Am. Chem. Soc. 2004, 126, 7097–7101. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Virji, S.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber gas sensors: Examination of response mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Nanostructure polyaniline sensors. Chem. Eur. J. 2004, 10, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, G.; Wang, M.; Cheng, Y.; Bai, R.; Chen, H. Preparation of Nano-wire Structured Polyaniline Composite and its Gas-sensitivity Studies. Chem. A Eur. J. 2006, 12, 3254–3260. [Google Scholar] [CrossRef]
- Ma, X.; Wang, M.; Li, G.; Chen, H.; Bai, R. Preparation of Polyaniline-TiO2 Composite Film with in-situ Polymerization Approach and Its Gas-sensitivity at Room Temperature. Mater. Chem. Phys. 2006, 98, 241–247. [Google Scholar] [CrossRef]
- Ma, X.; Gao, M.; He, X.; Li, G. Morphology Tailoring of Nano/Micro-structured Conductive Polymers, Composites and Their Applications in Chemical Sensors. Recent Pat. Nanotechnol. 2010, 4, 150–163. [Google Scholar] [CrossRef]
- Ma, X.; Wang, M.; Chen, H.; Li, G.; Sun, J.; Bai, R. Preparation of Water Soluble Poly (aniline) and its Gas-sensitivity. Green Chem. 2005, 7, 507–513. [Google Scholar] [CrossRef]
- Ma, X.; Li, G.; Wang, M.; Bai, R.; Yang, F.; Chen, H. Unusual Electrical Response of Poly(aniline) Composite Film on Exposure to the Atmosphere of Base and its Applications on Sensors. Green Chem. 2006, 8, 63–69. [Google Scholar] [CrossRef]
- Xu, S.; Gao, T.; Feng, X.; Fan, X.; Liu, G.; Mao, Y.; Yu, X.; Lin, J.; Luo, X. Near infrared fluorescent dual ligand functionalized Au NCs based multidimensional sensor array for pattern recognition of multiple proteins and serum discrimination. Biosens. Bioelectron. 2017, 97, 203–207. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Zhu, J.; Tong, Q. Pd-Au@carbon dots nanocomposite: Facile synthesis and application as an ultrasensitive electrochemical biosensor for determination of colitoxin DNA in human serum. Biosens. Bioelectron. 2017, 94, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Han, Y.; Yu, Y.; Xu, M.; Zhang, X.; Huang, L.; Dong, S. RGO/Au NPs/N-doped CNTs supported on nickel foam as an anode for enzymatic biofuel cells. Biosens. Bioelectron. 2017, 97, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Amoli-Diva, M.; Sadighi-Bonabi, R.; Pourghazi, K. Switchable on/off drug release from gold nanoparticles-grafted dual light- and temperature-responsive hydrogel for controlled drug delivery. Mater. Sci. Eng. C 2017, 76, 242–248. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Sun, X.; Ma, H.; Zhang, Y.; Wei, Q. Dual-responsive electrochemical immunosensor for prostate specific antigen detection based on Au-CoS/graphene and CeO2/ionic liquids doped with carboxymethyl chitosan complex. Biosens. Bioelectron. 2017, 94, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tai, H.; Zhang, P.; Ye, Z.; Su, Y.; Jiang, Y. Enhanced ammonia-sensing properties of PANI-TiO2-Au ternaryself-assembly nanocomposite thin film at room temperature. Sens. Actuators B 2017, 246, 85–95. [Google Scholar] [CrossRef]
- Schmidt, I.; Gad, A.; Scholz, G.; Boht, H.; Martens, M.; Schilling, M.; Wasisto, H.S.; Waag, A.; Schröder, U. Gold-modified indium tin oxide as a transparent window in optoelectronic diagnostics of electrochemically active biofilms. Biosens. Bioelectron. 2017, 94, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Liu, Y.; Zhang, L.; Wang, F.; Lu, Y. High performance surface-enhanced Raman scattering sensing basedon Au nanoparticle-monolayer graphene-Ag nanostar array hybrid system. Sens. Actuators B 2017, 247, 850–857. [Google Scholar] [CrossRef]
- Gole, A.; Murphy, C.J. Seed-Mediated Synthesis of Gold Nanorods: Role of the Size and Nature of the Seed. Chem. Mater. 2004, 16, 3633–3640. [Google Scholar] [CrossRef]
- Placido, T.; Comparelli, R.; Giannici, F.; Cozzoli, P.D.; Capitani, G.; Striccoli, M.; Agostiano, A.; Curri, M.L. Photochemical Synthesis of Water-Soluble Gold Nanorods: The Role of Silver in Assisting Anisotropic Growth. Chem. Mater. 2009, 21, 4192–4202. [Google Scholar] [CrossRef]
- Park, K.; Drummy, L.F.; Wadams, R.C.; Koerner, H.; Nepal, D.; Fabris, L.; Vaia, R.A. Growth Mechanism of Gold Nanorods. Chem. Mater. 2013, 25, 555–563. [Google Scholar] [CrossRef]
- Fazal, S.; Jayasree, A.; Sasidharan, S.; Koyakutty, M.; Nair, S.V.; Menon, D. Green Synthesis of Anisotropic Gold Nanoparticles for Photothermal Therapy of Cancer. ACS Appl. Mater. Interfaces 2014, 6, 8080–8089. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, S.; Xi, C.; Chen, Y.; Li, X.; Zhang, L.; Wang, G.; Chen, Y.; Chen, Z. A novel magnetic β-cyclodextrin modified graphene oxide adsorbent with high recognition capability for 5 plant growth regulators. Food Chem. 2018, 239, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A. Facile and efficient electrochemical enantiomer recognition of phenylalanine using β-Cyclodextrin immobilized on reduced grapheme oxide. Biosens. Bioelectron. 2017, 94, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Liu, Z.; Dong, K.; Ju, E.; Ren, J.; Du, Y.; Li, Z.; Qu, X. A Smart “Sense-Act-Treat” System: Combining a Ratiometric pH Sensor with a Near Infrared Therapeutic Gold Nanocage. Adv. Mater. 2014, 26, 6635–6641. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, S.; Shimpi, N.G. A β-cyclodextrin based binary dopant for polyaniline: Structural, thermal, electrical, and sensing performance. Mater. Sci. Eng. B 2017, 220, 13–21. [Google Scholar] [CrossRef]
- Ma, M.; Zhe, T.; Song, W.; Guo, P.; Wang, J.; Wang, J. A comparative study on the glucose sensors modified by two different β-cyclodextrin functionalized reduced graphene oxide based Au nanocomposites synthesized through developed post immobilization and in situ growth technologies. Sens. Actuators B 2017, 253, 818–829. [Google Scholar] [CrossRef]
- Chen, J.; He, P.; Bai, H.; He, S.; Zhang, T.; Zhang, X.; Dong, F. Poly (β-cyclodextrin)/carbon quantum dots modified glassy carbon electrode: Preparation, characterization and simultaneous electrochemical determination of dopamine, uric acid and tryptophan. Sens. Actuators B 2017, 252, 9–16. [Google Scholar] [CrossRef]
- Li, C.; Wu, Z.; Yang, H.; Deng, L.; Chen, X. Reduced graphene oxide-cyclodextrin-chitosan electrochemical sensor: Effective and simultaneous determination of o- and p-nitrophenols. Sens. Actuators B 2017, 251, 446–454. [Google Scholar] [CrossRef]
- Luo, M.; Hua, Y.; Liang, Y.; Han, J.; Liu, D.; Zhao, W.; Wang, P. Synthesis of novel β-cyclodextrin functionalized S, N co-doped carbon dots for selective detection of testosterone. Biosens. Bioelectron. 2017, 98, 195–201. [Google Scholar] [CrossRef]
- Sedghi, R.; Heidari, B.; Yassari, M. Novel molecularly imprinted polymer based on β-cyclodextrin@graphene oxide: Synthesis and application for selective diphenylamine determination. J. Colloid Interface Sci. 2017, 503, 47–56. [Google Scholar] [CrossRef]
- Peng, L.; Ye, L.; Cao, J.; Chang, Y.; Li, Q.; An, M.; Tan, Z.; Xu, J. Cyclodextrin-based miniaturized solid phase extraction for biopesticides analysis in water and vegetable juices samples analyzed by ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Food Chem. 2017, 226, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, M.; Wang, M.; Tong, P.; Lu, Q.; Zhang, L. Magnetic porous β-cyclodextrin polymer for magnetic solid-phase extraction of microcystins from environmental water samples. J. Chromatogr. A 2017, 1503, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ma, J.; Feng, W.; Jia, Q. Specific enrichment of glycoproteins with polymer monolith functionalized with glycocluster grafted β-cyclodextrin. J. Chromatogr. A 2017, 1512, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Skorb, E.V.; Möhwald, H. “Smart” Surface Capsules for Delivery Devices. Adv. Mater. Interfaces 2014, 1, 1400237. [Google Scholar] [CrossRef]
- Hu, Q.; Tang, G.; Chu, P.K. Cyclodextrin-Based Host−Guest Supramolecular Nanoparticles for Delivery: From Design to Applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef]
- Kettel, M.J.; Schaefer, K.; Groll, J.; Moeller, M. Nanogels with High Active β-Cyclodextrin Content as Physical Coating System with Sustained Release Properties. ACS Appl. Mater. Interfaces 2014, 6, 2300–2311. [Google Scholar] [CrossRef]
- Constantin, M.; Bucatariu, S.; Ascenzi, P.; Simionescu, B.C. Gheorghe Fundueanu, Poly (NIPAAm-co-β-cyclodextrin) microgels with drug hosting and temperature-dependent delivery properties. React. Funct. Polym. 2014, 84, 1–9. [Google Scholar] [CrossRef]
- Li, R.Q.; Niu, Y.L.; Zhao, N.N.; Yu, B.R.; Mao, C.; Xu, F.J. Series of New β-Cyclodextrin-Cored Star-like Carriers for Gene Delivery. ACS Appl. Mater. Interfaces 2014, 6, 3969–3978. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, F.; Xing, P.; Chu, X.; Hao, A. A binary solvent gel as drug delivery carrier. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 577–584. [Google Scholar] [CrossRef]
- Xiong, Q.; Cui, M.; Bai, Y.; Liu, Y.; Liu, D.; Song, T. A supramolecular nanoparticle system based on β-cyclodextrin-conjugated poly-l-lysine and hyaluronic acid for co-delivery of gene and chemotherapy agent targeting hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2017, 155, 93–103. [Google Scholar] [CrossRef]
- Varan, G.; Varan, C.; Erdoğar, N.; Hıncal, A.A.; Bilensoy, E. Amphiphilic cyclodextrin nanoparticles. Int. J. Pharm. 2017, 531, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Li, L.; Zhang, Y.; Chen, K.; Wang, H.; Gong, R. Carboxymethyl-β-cyclodextrin grafted chitosan nanoparticles as oral delivery carrier of protein drugs. React. Funct. Polym. 2017, 117, 10–15. [Google Scholar] [CrossRef]
- Krzak, A.; Swiech, O.; Majdecki, M.; Bilewicz, R. Complexing daunorubicin with β -cyclodextrin derivative increases drug intercalation into DNA. Electrochim. Acta 2017, 247, 139–148. [Google Scholar] [CrossRef]
- Adeoye, O.; Cabral-Marques, H. Cyclodextrin nanosystems in oral drug delivery: A mini review. Int. J. Pharm. 2017, 531, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, T.; Lachmanski, L.; Manoli, F.; Menendez-Miranda, M.; Manet, I.; Guo, Z.; Wu, L.; Zhang, J.; Gref, R. Cyclodextrin-based metal-organic frameworks particles as efficient carriers for lansoprazole: Study of morphology and chemical composition of individual particles. Int. J. Pharm. 2017, 531, 424–432. [Google Scholar] [CrossRef]
- Salzano, G.; Wankar, J.; Ottani, S.; Villemagne, B.; Baulard, A.R.; Willand, N.; Brodin, P.; Manet, I.; Gref, R. Cyclodextrin-based nanocarriers containing a synergic drug combination: A potential formulation for pulmonary administration of antitubercular drugs. Int. J. Pharm. 2017, 531, 577–587. [Google Scholar] [CrossRef]
- Coviello, V.; Sartini, S.; Quattrini, L.; Baraldi, C.; Gamberini, M.C.; Motta, C.L. Cyclodextrin-based nanosponges for the targeted delivery of the anti-restenotic agent DB103: A novel opportunity for the local therapy of vessels wall subjected to percutaneous intervention. Eur. J. Pharm. Biopharm. 2017, 117, 276–285. [Google Scholar] [CrossRef]
- Stjern, L.; Voittonen, S.; Weldemichel, R.; Thuresson, S.; Agnes, M.; Benkovics, G.; Fenyvesi, É.; Malanga, M.; Yannakopoulou, K.; Feiler, A.; et al. Cyclodextrin-mesoporous silica particle composites for controlled antibiotic release. A proof of concept toward colon targeting. Int. J. Pharm. 2017, 531, 595–605. [Google Scholar] [CrossRef]
- Cutrone, G.; Casas-Solvas, J.M.; Vargas-Berenguel, A. Cyclodextrin-Modified Inorganic Materials for the Construction of Nanocarriers. Int. J. Pharm. 2017, 531, 621–639. [Google Scholar] [CrossRef]
- d’Angelo, I.; Fraix, A.; Ungaro, F.; Quaglia, F.; Miro, A. Poly(ethylene oxide)/hydroxypropyl- β -cyclodextrin films for oromucosal delivery of hydrophilic drugs. Int. J. Pharm. 2017, 531, 606–613. [Google Scholar] [CrossRef]
- Elezaby, R.S.; Gad, H.A.; Metwally, A.A.; Geneidi, A.S.; Awad, G.A. Self-assembled amphiphilic core-shell nanocarriers in line with the modern strategies for brain delivery. J. Control. Release 2017, 261, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, G.; Zhou, Z.; Gao, L.; Tao, Q. Sensitive complex micelles based on host-guest recognition from chitosan-graft-β-cyclodextrin for drug release. Int. J. Biol. Macromol. 2017, 105, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Wang, Y.; He, P.; Zhan, Y.; Sun, Z.; Li, Y.; Zhang, Y. Study on β-cyclodextrin-complexed nanogels with improved thermal response for anticancer drug delivery. Mater. Sci. Eng. C 2017, 78, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Cyclodextrin-functionalized mesostructured silica nanoparticles for removal of polycyclic aromatic hydrocarbons. J. Colloid Interface Sci. 2017, 497, 233–241. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Sadegh, H.; Ali, G.A.M.; Bharti, A.K.; Makhlouf, A.S.H. Facile route synthesis of novel graphene oxide-β-cyclodextrin nanocomposite and its application as adsorbent for removal of toxic bisphenol A from the aqueous phase. J. Mol. Liq. 2017, 237, 466–472. [Google Scholar] [CrossRef]
- Tan, P.; Hu, Y. Improved synthesis of graphene/β-cyclodextrin composite for highly efficient dye adsorption and removal. J. Mol. Liq. 2017, 242, 181–189. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Xu, D.; Huang, X.; Xu, X.; Zheng, S.; Zhang, Y.; Lin, H. Metal–organic framework preparation using magnetic graphene oxide–β- cyclodextrin for neonicotinoid pesticide adsorption and removal. Carbohydr. Polym. 2017, 175, 584–591. [Google Scholar] [CrossRef]
- Ragavan, K.V.; Rastogi, N.K. β-Cyclodextrin capped graphene-magnetite nanocomposite for selective adsorption of Bisphenol-A. Carbohydr. Polym. 2017, 168, 129–137. [Google Scholar] [CrossRef]
- Parsamanesh, M.; Tehrani, A.D.; Mansourpanah, Y. Supramolecular hydrogel based on cyclodextrin modified GO as a potent natural organic matter absorbent. Eur. Polym. J. 2017, 92, 126–136. [Google Scholar] [CrossRef]
- Tang, C.; Shiri, M.; Zhang, H.; Ayinla, R.T.; Wang, K. Light-Driven Charge Transport and Optical Sensing in Molecular Junctions. Nanomaterials 2022, 12, 698. [Google Scholar] [CrossRef] [PubMed]
- Sendler, T.; Luka-Guth, K.; Wieser, M.; Wolf, J.; Helm, M.; Gemming, S.; Kerbusch, J.; Huhn, T.; Erbe, A. Light-Induced Switching of Tunable Single-Molecule Junctions. Adv. Sci. 2015, 2, 1500017. [Google Scholar] [CrossRef]
- van der Molen, S.J.; Liljeroth, P. Charge transport through molecular switches. J. Phys. Condens. Matter. 2010, 22, 133001. [Google Scholar] [CrossRef] [PubMed]
- Taherinia, D.; Frisbie, C.D. Photoswitchable Hopping Transport in Molecular Wires 4 nm in Length. J. Phys. Chem. C 2016, 120, 6442–6449. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Y.; Su, J.; Radjenovic, P.M.; Wang, Y.; Zheng, J.; Teng, B.; Shao, Y.; Zhou, X.; Li, J. Probing Interfacial Electronic Effects on Single-Molecule Adsorption Geometry and Electron Transport at Atomically Flat Surfaces. Angew. Chem. Int. Ed. 2021, 60, 15452–15458. [Google Scholar] [CrossRef]
- Jia, C.; Wang, J.; Yao, C.; Cao, Y.; Zhong, Y.; Liu, Z.; Liu, Z.; Guo, X. Conductance Switching and Mechanisms in Single-Molecule Junctions. Angew. Chem. Int. Ed. 2013, 52, 8666–8670. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, T. Recent progress in the development of molecular-scale electronics based on photoswitchable molecules. J. Mater. Chem. C 2020, 8, 821–848. [Google Scholar] [CrossRef]
- Schwartz, T.; Hutchison, J.A.; Genet, C.; Ebbesen, T.W. Reversible Switching of Ultrastrong Light-Molecule Coupling. Phys. Rev. Lett. 2011, 106, 196405. [Google Scholar] [CrossRef]
- Zhuang, M.; Ernzerhof, M. Mechanism of a molecular electronic photoswitch. Phys. Rev. B 2005, 72, 073104. [Google Scholar] [CrossRef]
- Browne, W.R.; Feringa, B.L. Light Switching of Molecules on Surfaces. Annu. Rev. Phys. Chem. 2009, 60, 407–428. [Google Scholar] [CrossRef]
- Choi, S.H.; Frisbie, C.D. Enhanced Hopping Conductivity in Low Band Gap Donor-Acceptor Molecular Wires Up to 20 nm in Length. J. Am. Chem. Soc. 2010, 132, 16191–16201. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Risko, C.; Delgado, M.C.R.; Kim, B.S.; Bre´das, J.; Frisbie, C.D. Transition from Tunneling to Hopping Transport in Long, Conjugated Oligo-imine Wires Connected to Metals. J. Am. Chem. Soc. 2010, 132, 4358–4368. [Google Scholar] [CrossRef]
- Trindade, E.C.A.; Antônio, R.V.; Brandes, R.; de Souza, L.; Neto, G.; Vargas, V.M.M.; Carminatti, C.A.; Recouvreux, D.d.S. Carbon fiber-embedded bacterial cellulose/polyaniline nanocomposite with tailored for microbial fuel cells electrode. J. Appl. Polym. Sci. 2020, 137, 49036. [Google Scholar] [CrossRef]
- Krishna, M.; Ghosh, A.; Muthuraj, D.; Das, S.; Mitra, S. Electrocatalytic Activity of Polyaniline in Magnesium−Sulfur Batteries. J. Phys. Chem. Lett. 2022, 13, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Almtiri, M.; Dowell, T.J.; Giri, H.; Wipf, D.O.; Scott, C.N. Electrochemically Stable Carbazole-Derived Polyaniline for Pseudocapacitors. ACS Appl. Polym. Mater. 2022, 4, 3088–3097. [Google Scholar] [CrossRef]
- Çıplak, Z.; Yıldız, A.; Yıldız, N. Green preparation of ternary reduced graphene oxide-au@polyaniline nanocomposite for supercapacitor application. J. Energy Storage 2020, 32, 101846. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, W.; Lan, B.; Wen, J.; Zhang, S.; Luo, P.; Zhang, R.; Hu, S.; Liu, Q. Polyaniline Nanorod Arrays as a Cathode Material for High-Rate Zinc-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 12360–12367. [Google Scholar] [CrossRef]
- Dianat, N.; Rahmanifar, M.S.; Noori, A.; El-Kady, M.F.; Chang, X.; Kaner, R.B.; Mousavi, M.F. Polyaniline-Lignin Interpenetrating Network for Supercapacitive Energy Storage. Nano Lett. 2021, 21, 9485–9493. [Google Scholar] [CrossRef]
- Kuchena, S.F.; Wang, Y. Superior Polyaniline Cathode Material with Enhanced Capacity for Ammonium Ion Storage. ACS Appl. Energy Mater. 2020, 3, 11690–11698. [Google Scholar] [CrossRef]
- Adhikari, A.D.; Shauloff, N.; Turkulets, Y.; Shalish, I.; Jelinek, R. Tungsten-Disulfide/Polyaniline High Frequency Supercapacitors. Adv. Electron. Mater. 2021, 7, 2100025. [Google Scholar] [CrossRef]
- Yavuz, A.; Erdogan, P.Y.; Zengin, H. The use of polyaniline films on flexible tape for supercapacitor applications. Int. J. Energy Res. 2020, 44, 1–15. [Google Scholar] [CrossRef]
- Korent, A.; Trafela, S.; Soderˇznik, K.Z.; Samardˇzija, Z.; Sturm, S.; Roˇzman, K.Z. Au-decorated electrochemically synthesised polyaniline-based sensory platform for amperometric detection of aqueous ammonia in biological fluids. Electrochim. Acta 2022, 430, 141034. [Google Scholar] [CrossRef]
- Farooqi, B.A.; Ashraf, A.; Farooq, U.; Ayub, K. Comparative study on sensing abilities of polyaniline and graphene polyaniline composite sensors toward methylamine and ammonia. Polym Adv. Technol. 2020, 31, 3351–3360. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Sridevi, V. Conducting, crystalline and electroactive polyaniline-Au nanocomposites through combined acid and oxidative doping pathways for biosensing applications: Detection of dopamine. Mater. Chem. Phys. 2019, 235, 121728. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, J. Non-enzymatic electrochemical sensor for nitrite based on a graphene oxide–polyaniline–Au nanoparticles nanocomposite. Microchem. J. 2021, 164, 106034. [Google Scholar] [CrossRef]
- Chaudhary, V.; Chavali, M. Novel methyl-orange assisted core-shell polyaniline-silver nanosheets for highly sensitive ammonia chemiresistors. J. Appl. Polym. Sci. 2021, 138, 51288. [Google Scholar] [CrossRef]
- Lin, L.; Miao, C.; Weng, S.; Ying, S.; Chen, F.; Ye, C.; Zhuang, H.; You, D. Ternary Pt–Au–FeOOH-decorated polyaniline nanocomposite for sensitive dopamine electrochemical detection. J. Electroanal. Chem. 2020, 877, 114519. [Google Scholar] [CrossRef]
- Verma, A.; Chaudhary, P.; Singh, A.; Tripathi, R.K.; Yadav, B.C. ZnS Nanosheets in a Polyaniline Matrix as Metallopolymer Nanohybrids for Flexible and Biofriendly Photodetectors. ACS Appl. Nano Mater. 2022, 5, 4860–4874. [Google Scholar] [CrossRef]
- Hua, M.; Hwang, G.; Chuang, Y.; Chen, S.; Tsai, R. Soluble n-Doped Polyaniline: Synthesis and Characterization. Macromolecules 2000, 33, 6235–6238. [Google Scholar] [CrossRef]
- Han, Z.; Liu, Q.; Zhao, H.; Shi, Y.; Liu, Y.; Chen, J. Preparation and properties of polyaniline-silver composite by glucose reduction. Polym. Compos. 2022, 43, 1121–1127. [Google Scholar] [CrossRef]
- Miyashita, R.; Goto, H. Electro-Magneto-Optically Active Polyaniline/Hydroxypropyl Cellulose Composite. ACS Appl. Polym. Mater. 2022, 4, 796–805. [Google Scholar] [CrossRef]
- Lin, C.; Xue, S.; Ji, C.; Huang, S.; Tung, V.; Kaner, R.B. Conducting Polyaniline for Antifouling Ultrafiltration Membranes: Solutions and Challenges. Nano Lett. 2021, 21, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Lin, C.; Hasan, M.M.F.; Kaner, R.; Sant, G.N. Highly Permeable Polyaniline−Graphene Oxide Nanocomposite Membranes for CO2 Separations. ACS Appl. Polym. Mater. 2019, 1, 3233–3241. [Google Scholar] [CrossRef]
- Mondal, S.; Rana, U.; Das, P.; Malik, S. Network of Polyaniline Nanotubes for Wastewater Treatment and Oil/Water Separation. ACS Appl. Polym. Mater. 2019, 1, 1624–1633. [Google Scholar] [CrossRef]
- Perea-López, N.; Elías, A.L.; Berkdemir, A.; Castro-Beltran, A.s.; Gutiérrez, H.R.; Feng, S.; Lv, R.; Hayashi, T.; López-Urías, F.; Ghosh, S.; et al. Photosensor Device Based on Few-Layered WS2 Films. Adv. Funct. Mater. 2013, 23, 5511–5517. [Google Scholar] [CrossRef]
- Lv, S.; Bian, L.; Qiu, J.; Zhang, X.; Gao, M.; He, X.; Ma, X.; Li, G. Surface Modification of Graphene Oxide with Pyrene Derivatives and their Photo-Switching Behaviors. Mater. Sci. Forum 2017, 898, 1739–1748. [Google Scholar] [CrossRef]
- Gao, M.; Guo, B.; Ma, L.; Zhang, B.; He, X.; Bian, L.; Ma, X.; Li, G. NIR (Near-Infrared) Driven Carbon Nanotube Modified with Dendrimers. Mater. Sci. Forum 2016, 848, 551–556. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, B.; Cong, Q.; He, X.; Gao, M.; Li, G. Organic-inorganic nanocomposites of ZnO-CuO-chitosan with improved properties. Mater. Chem. Phys. 2016, 178, 88–97. [Google Scholar] [CrossRef]

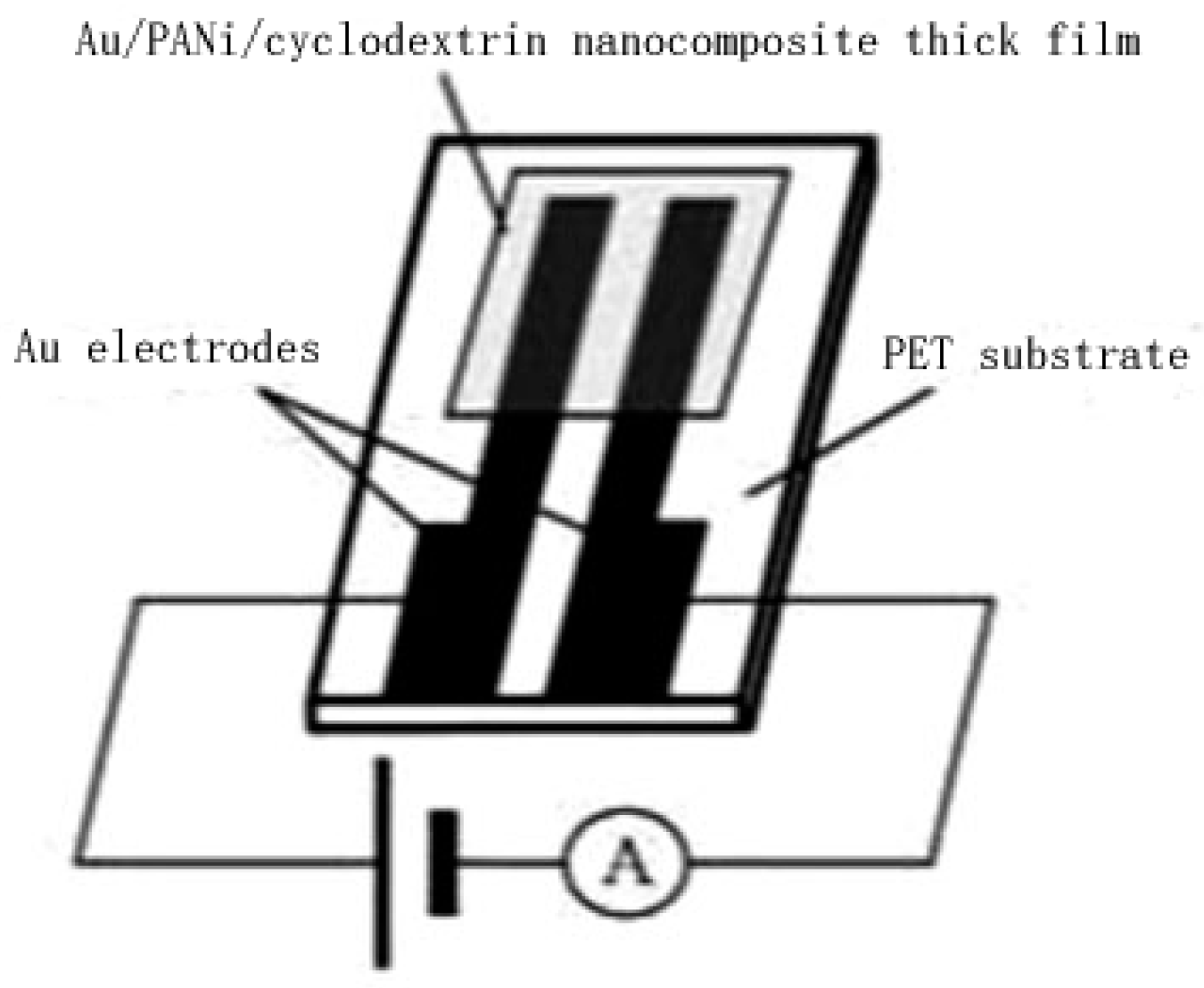
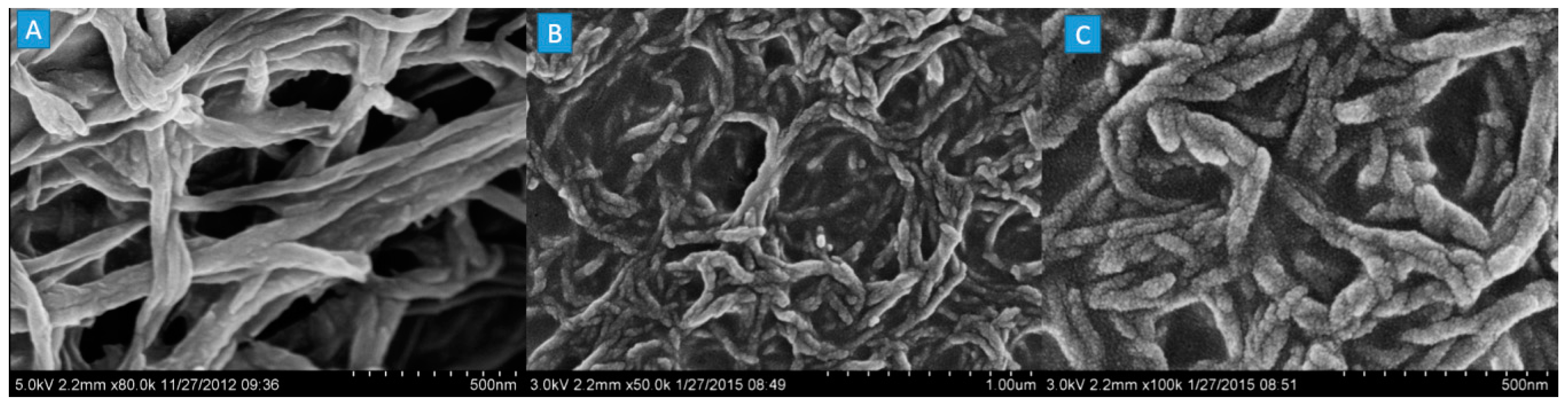
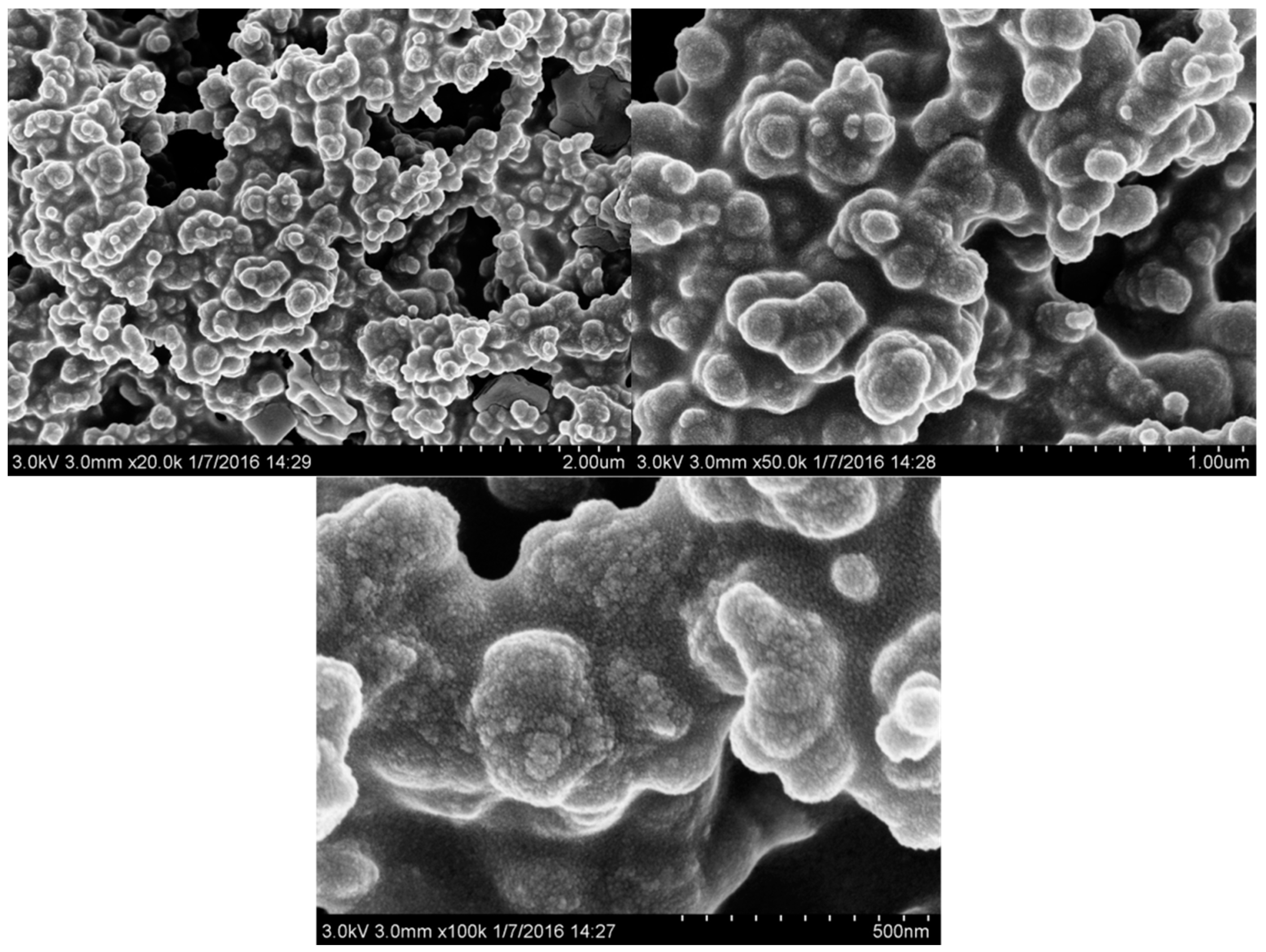
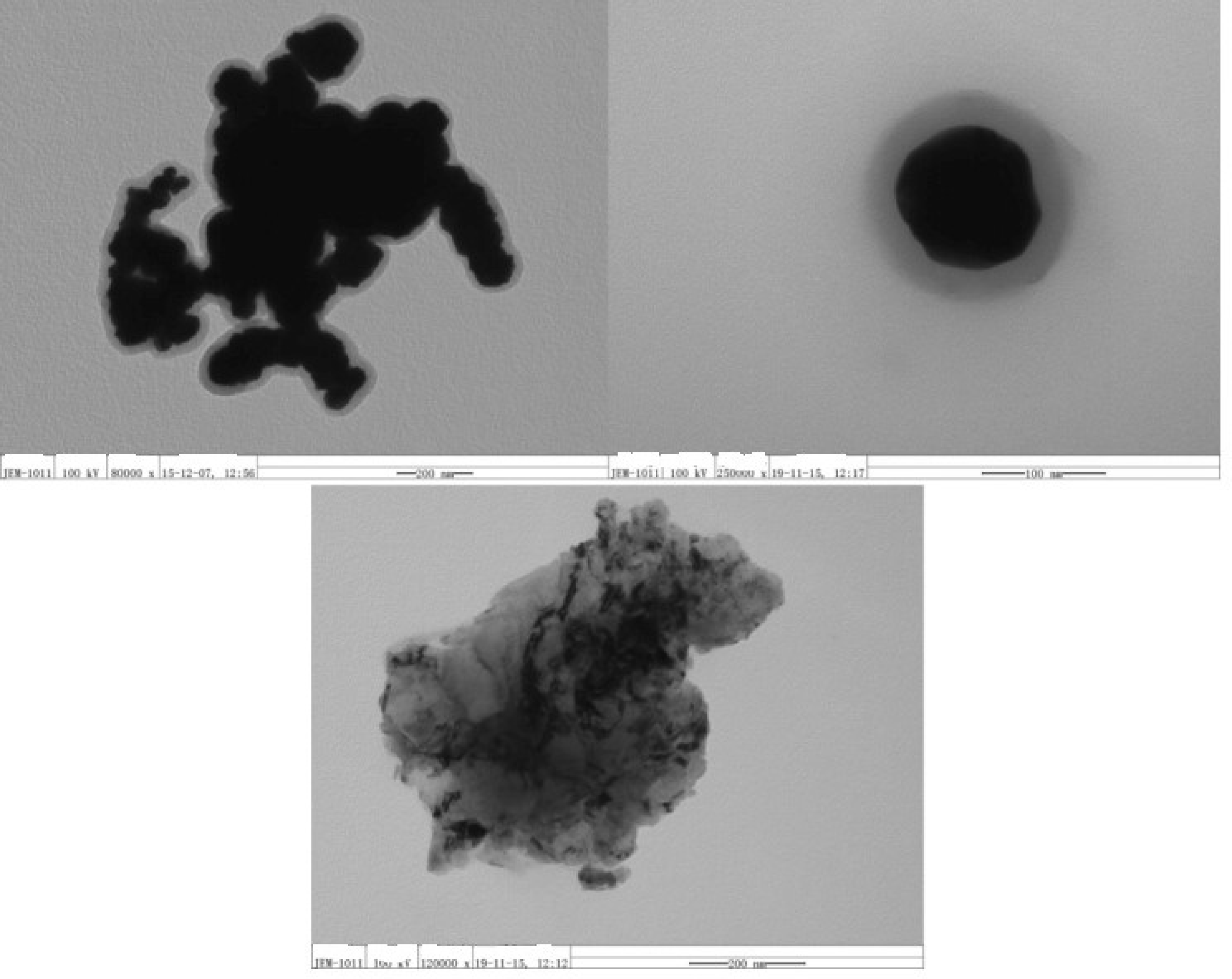
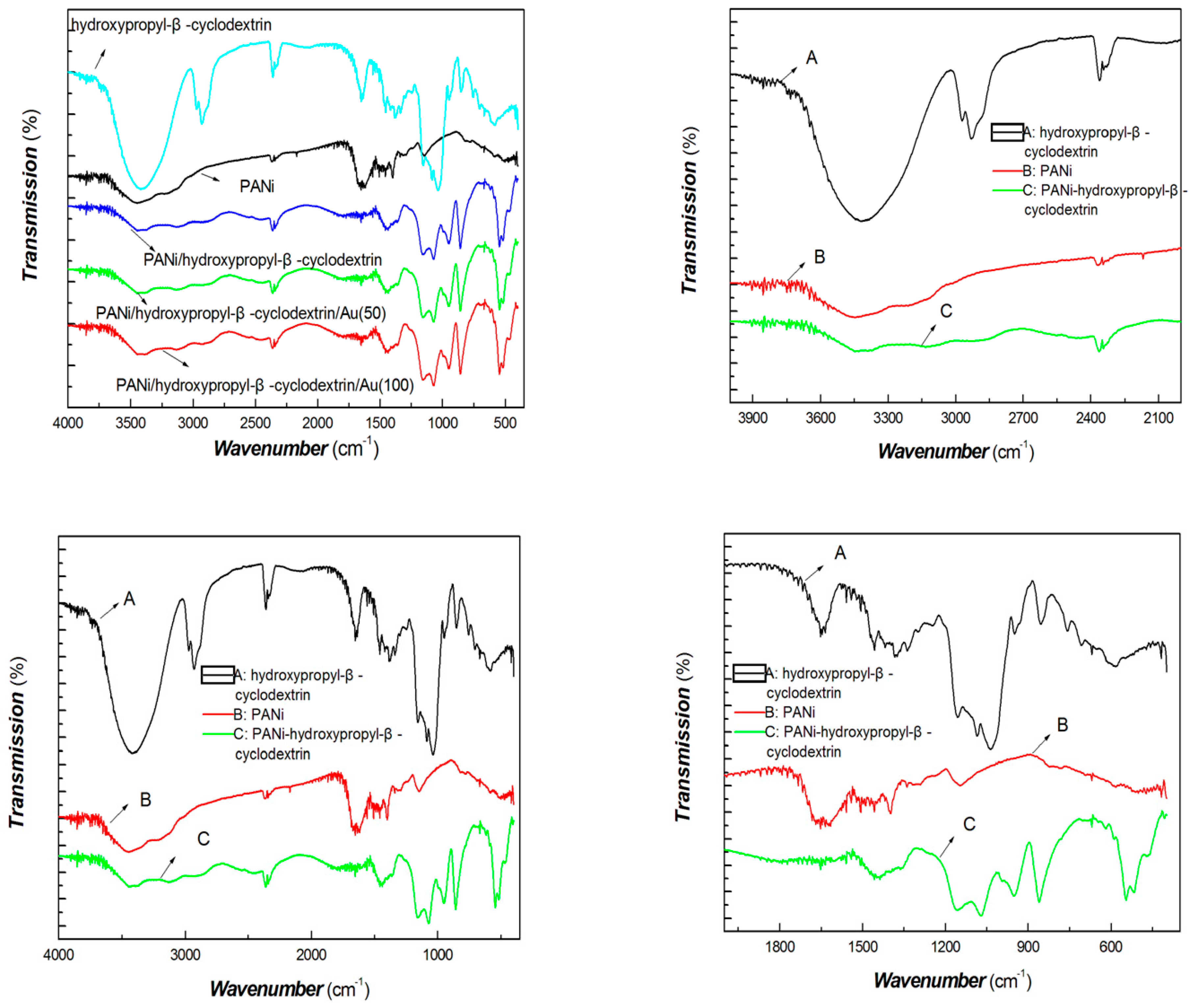
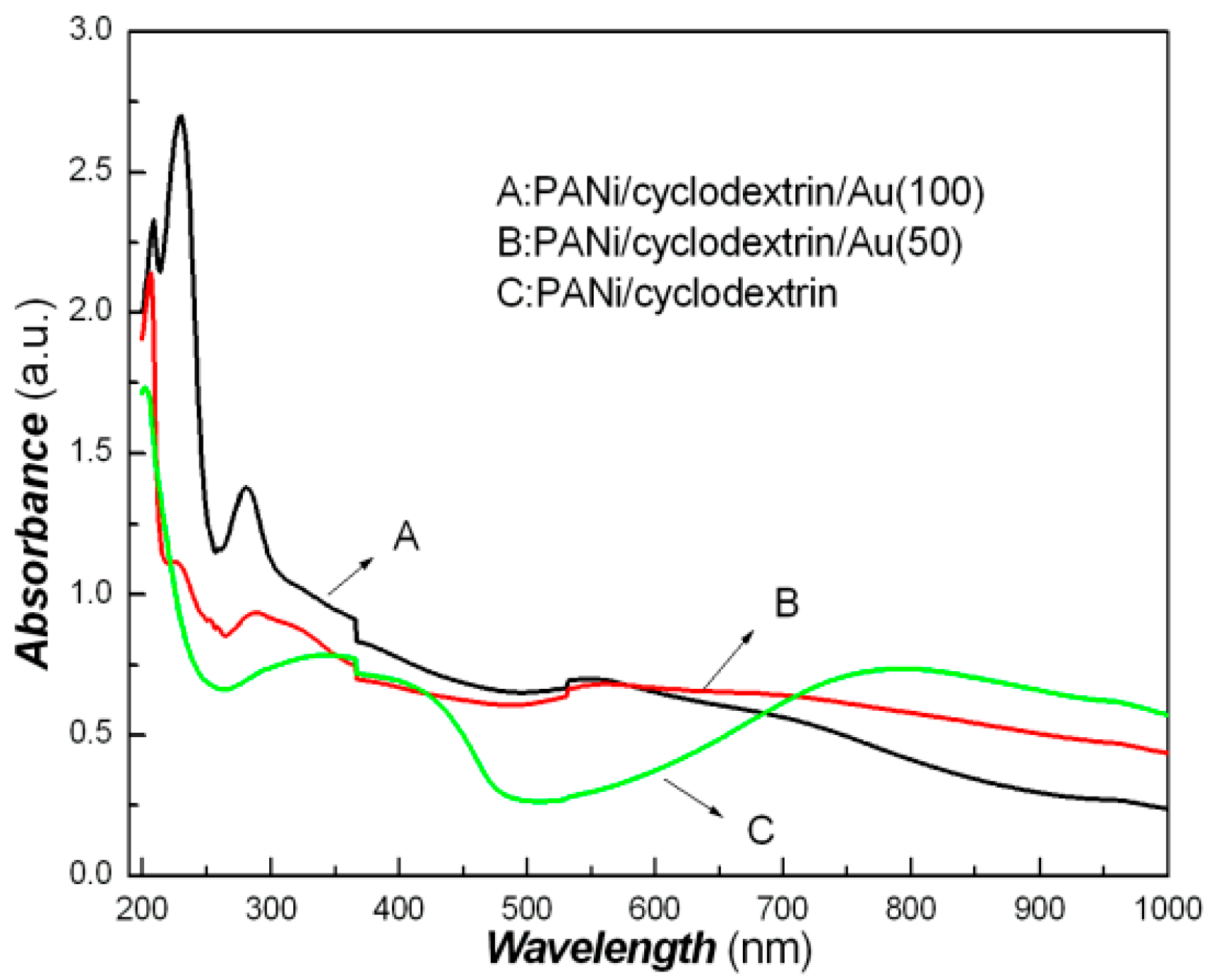
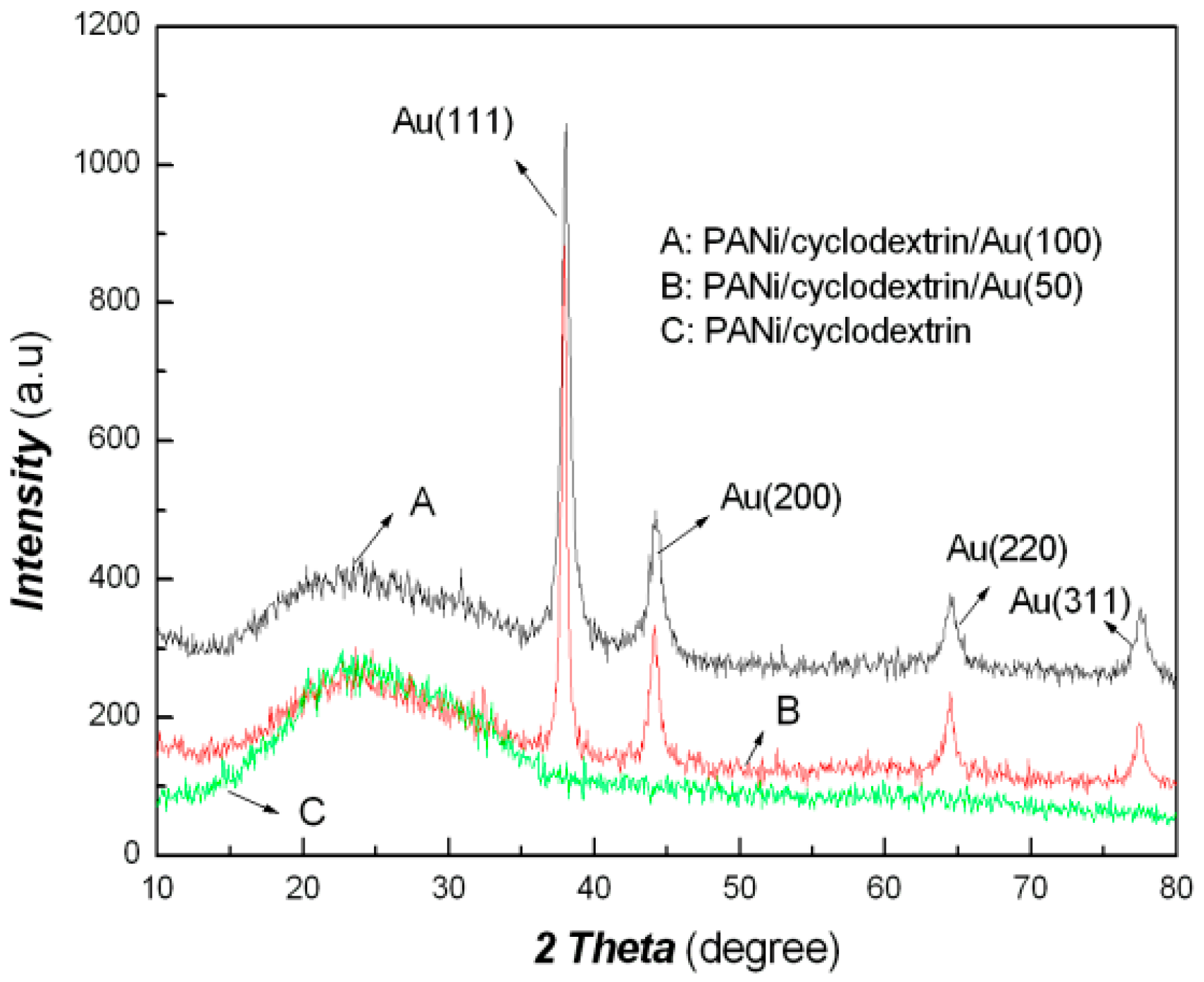
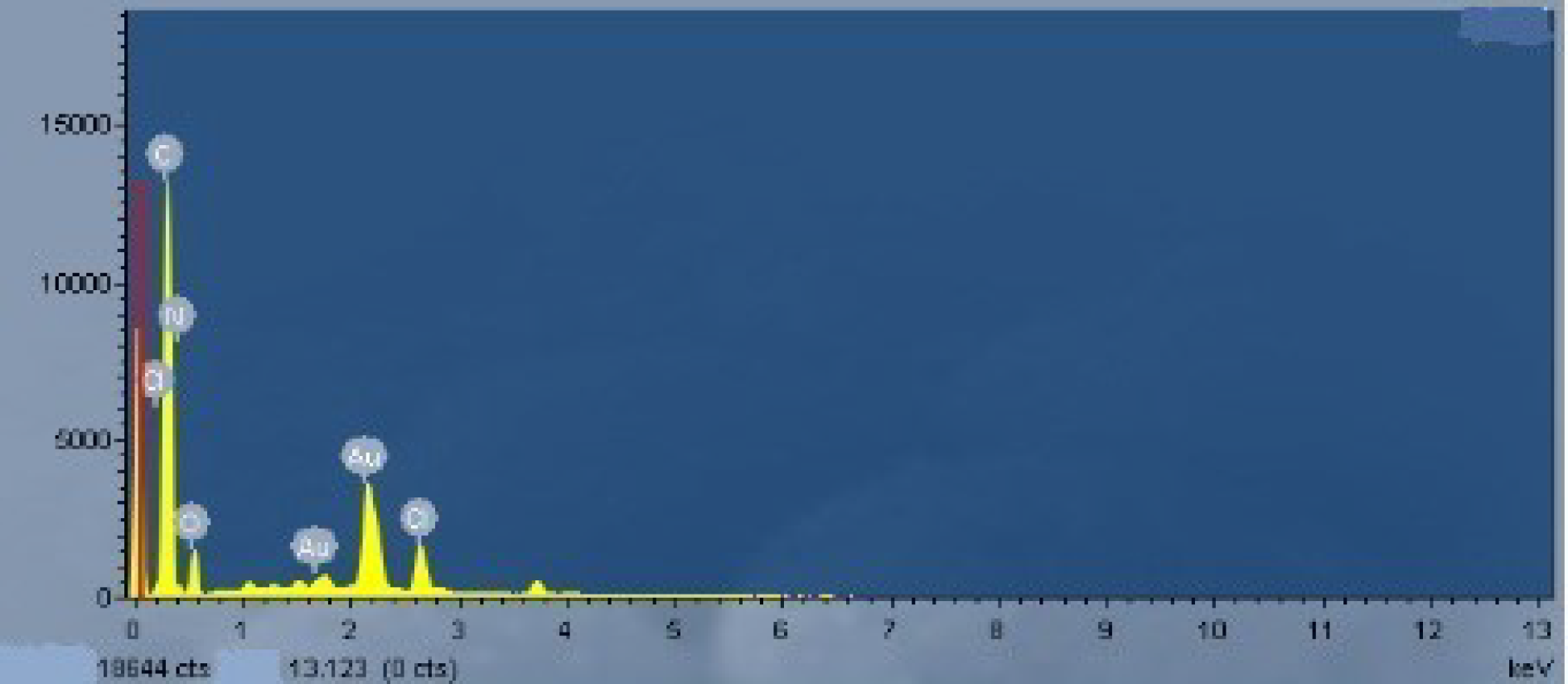
| Sample No. | Loading Aniline Solution | Loading Chloroauric Acid (0.02 M) | Hydroxypropyl-β-cyclodextrin (about 10mg/mL) | Loading Persulfate Solution (0.02 M) |
|---|---|---|---|---|
| 1 | 50 mL(0.036 M) | - | 0.5 g | 50 mL |
| 2 | 50 mL(0.036 M) | 50 mL | 0.5 g | - |
| 3 | 100 mL(0.036 M) | 100 mL | 1.0 g | - |
| 4 (for comparison) | 50 mL(0.02 M) | - | - | 50 mL |
| Element | C | N | O | Au | Cl, etc. | Total |
|---|---|---|---|---|---|---|
| Mass percent (%) | 62.61 | 7.47 | 9.96 | 15.09 | 4.87 | 100 |
| Atomic percent (%) | 79.11 | 8.09 | 9.45 | 1.16 | 2.19 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Li, C.; Zhang, X.; Gao, M.; Li, G. Broadband Spectrum Light-Driven PANI/Au/Beta-Cyclodextrin Nanocomposite and Its Light-Triggered Interfacial Carrier Transfer. Coatings 2022, 12, 1401. https://doi.org/10.3390/coatings12101401
Ma X, Li C, Zhang X, Gao M, Li G. Broadband Spectrum Light-Driven PANI/Au/Beta-Cyclodextrin Nanocomposite and Its Light-Triggered Interfacial Carrier Transfer. Coatings. 2022; 12(10):1401. https://doi.org/10.3390/coatings12101401
Chicago/Turabian StyleMa, Xingfa, Caiwei Li, Xintao Zhang, Mingjun Gao, and Guang Li. 2022. "Broadband Spectrum Light-Driven PANI/Au/Beta-Cyclodextrin Nanocomposite and Its Light-Triggered Interfacial Carrier Transfer" Coatings 12, no. 10: 1401. https://doi.org/10.3390/coatings12101401
APA StyleMa, X., Li, C., Zhang, X., Gao, M., & Li, G. (2022). Broadband Spectrum Light-Driven PANI/Au/Beta-Cyclodextrin Nanocomposite and Its Light-Triggered Interfacial Carrier Transfer. Coatings, 12(10), 1401. https://doi.org/10.3390/coatings12101401



_Ma.jpg)



