Modulation of Thermal Insulation and Mechanical Property of Silica Aerogel Thermal Insulation Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SiO2 Aerogel Slurry and Thermal Insulation Coatings
2.1.1. Preparation of SiO2 Aerogel Slurry
2.1.2. Preparation of Thermal Insulation Coatings
2.2. Performance Tests
3. Results and Discussion
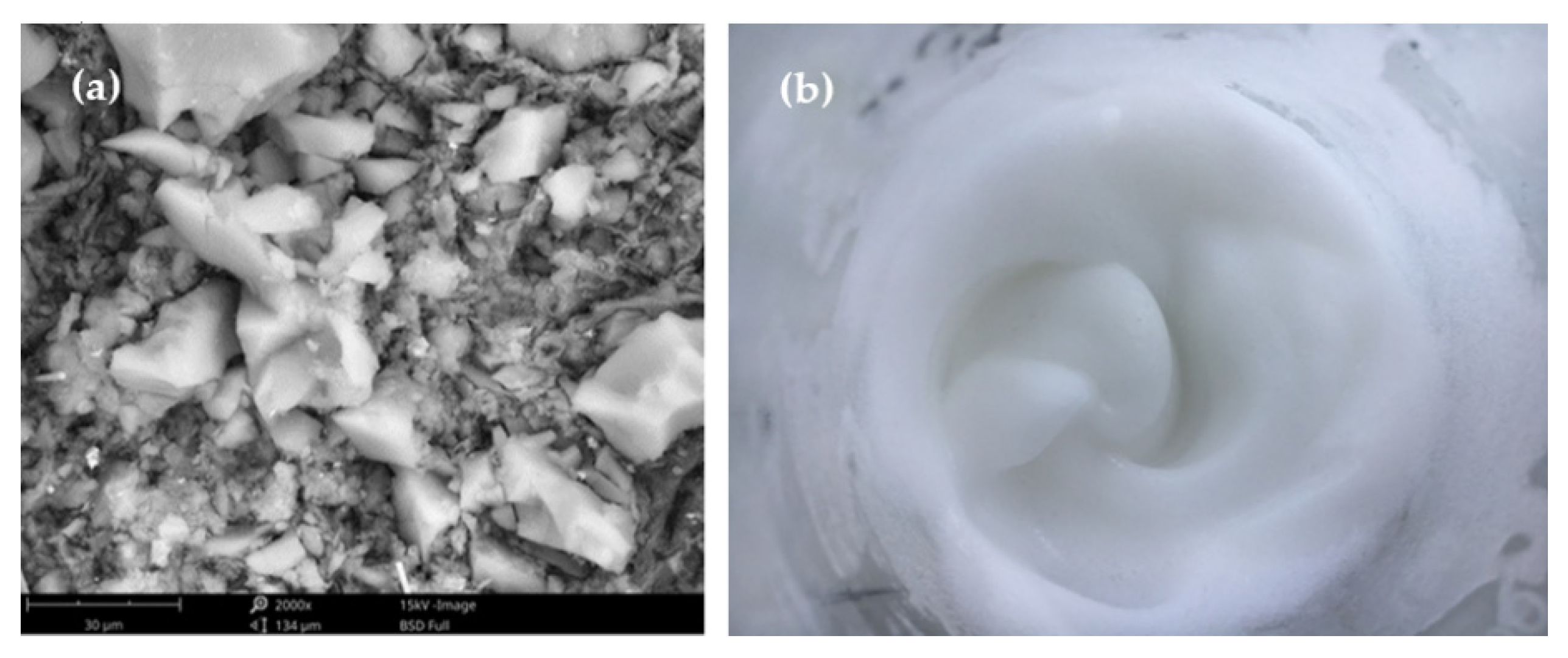
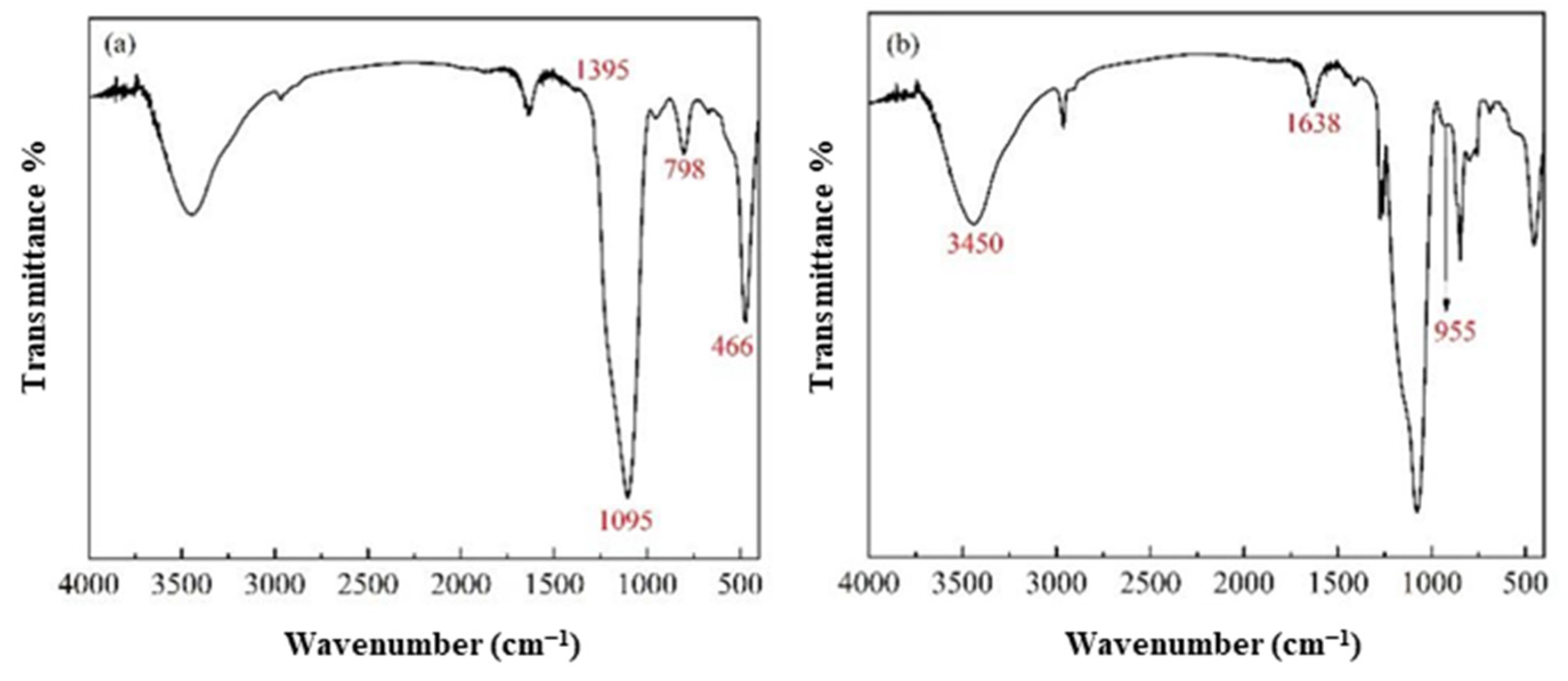
3.1. The SiO2 Aerogel Slurry
3.2. Modulation of Thermal Insulation and Mechanical Property
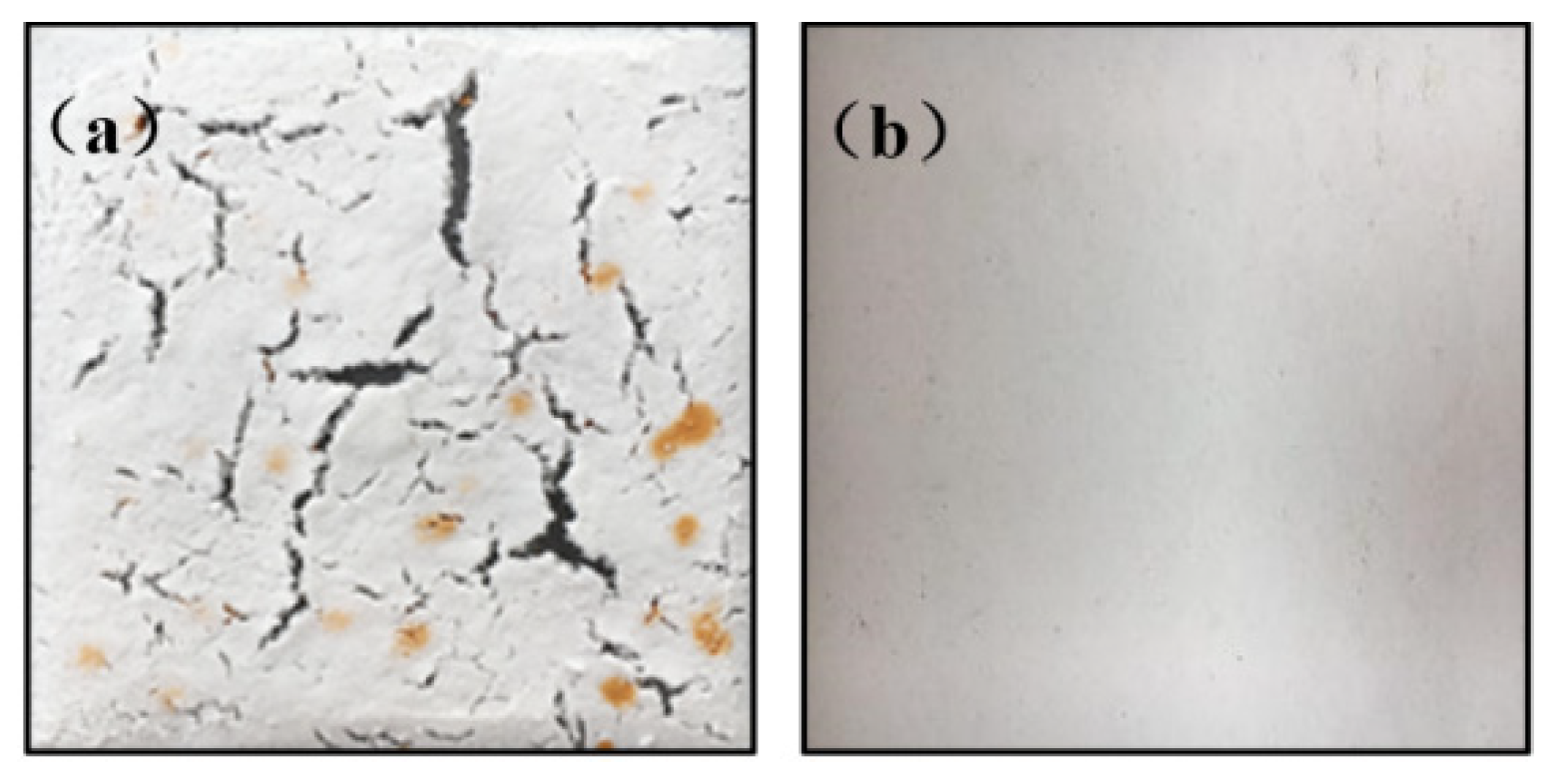
3.2.1. The Effects of Fibers on Coating Properties
3.2.2. The Effects of Mass Ratio of Silica Aerogel and Hollow Glass Microsphere on Coating Properties
3.2.3. The Effects of P/B ratio on Coating Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashrafi, O.; Navarri, P.; Hughes, R.; Lu, D. Heat recovery optimization in a Steam-Assisted Gravity Drainage (SAGD) plant. Energy 2016, 111, 981–990. [Google Scholar] [CrossRef]
- Gates, I.D.; Larter, S.R. Energy efficiency and emissions intensity of SAGD. Fuel 2014, 115, 706–713. [Google Scholar] [CrossRef]
- Lacey, D.D.; Natalia, R.G.; Nicholas, C.; Aayushi, B.; Malsha, U.; Liu, G.W.H.; Anita, S.B. A materials science perspective of midstream challenges in the utilization of heavy crude oil. ACS Omega 2022, 7, 1547–1574. [Google Scholar] [CrossRef]
- Yuan, J.Y.; McFarlane, R. Evaluation of steam circulation strategies for SAGD startup. J. Can. Pet. Technol. 2011, 50, 20–32. [Google Scholar] [CrossRef]
- Johnathan, H.; Quentin, F.; Khanh, V.; Seetha, R. Detection of corrosion under insulation on aerospace structures via pulsed eddy current thermography. Aerosp. Sci. Technol. 2022, 121, 107317. [Google Scholar] [CrossRef]
- Fu, H.Y.; Ding, Y.W.; Li, M.M.; Li, H.T.; Huang, X.L.; Wang, Z. Research on thermal performance and hygrothermal behavior of timber-framed walls with different external insulation layer: Insulation Cork Board and anti-corrosion pine plate. J. Build. Eng. 2021, 28, 101069. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Kish, J.; Alfantazi, A. Corrosion of new-generation steel in outer oil pipeline environments. J. Mater. Eng. Perform. 2017, 26, 214–220. [Google Scholar] [CrossRef]
- Melchers, R.E. The effect of corrosion on the structural reliability of steel offshore structures. Corros. Sci. 2005, 47, 2391–2410. [Google Scholar] [CrossRef]
- Kathleen, E.D.; Irene, A.D.; Heather, S.N.; Blake, W.S.; Bradley, S.S.; Pierre, J.S.; Joseph, M.S. Design features of offshore oil production platforms influence their susceptibility to biocorrosion. Appl. Microbiol. Biotechnol. 2017, 101, 6517–6529. [Google Scholar] [CrossRef]
- Khadim, M.A.; Sarbar, M.A. Role of asphaltene and resin in oil field emulsions. J. Pet. Sci. Eng. 1999, 23, 213–221. [Google Scholar] [CrossRef]
- Zach, J.; Hroudová, J.; Brožovský, J.; Krejza, Z.; Gailius, A. Development of thermal insulating materials on natural base for thermal insulation systems. Procedia Eng. 2013, 57, 1288–1294. [Google Scholar] [CrossRef]
- Lakatos, A. Comparison of the thermal properties of different insulating materials. Adv. Mater. Res. 2014, 899, 381–386. [Google Scholar] [CrossRef]
- Qi, Y.H.; Zhang, G.L.; Xia, J.Y. Progress in thermal insulation coatings. Paint Coat. Ind. 2019, 49, 80–87. [Google Scholar] [CrossRef]
- He, F.; Qi, Z.T.; Zhen, W.; Wu, J.Y.; Huang, Y.H.; Xiong, X.W.; Zhang, R.Z. Thermal conductivity of silica aerogel thermal insulation coatings. Int. J. Thermophys. 2019, 40, 92. [Google Scholar] [CrossRef]
- Søren, K. Quantitative analysis of silica aerogel-based thermal insulation coatings. Prog. Org. Coat. 2015, 89, 26–34. [Google Scholar] [CrossRef]
- Jin, L.; Li, P.Z.; Zhou, H.B.; Zhang, W.; Zhou, G.D.; Wang, C. Improving thermal insulation of TC4 using YSZ-based coating and SiO2 aerogel. Prog. Nat. Sci. Mater. Int. 2015, 25, 141–156. [Google Scholar] [CrossRef]
- Liu, Z.H.; Ding, Y.D.; Yang, H.B. Dispersion properties of aerogel slurry and application of its coatings in thermal insulation engineering. Rev. Téc. Ing. Univ. Zulia. 2016, 39, 153–161. [Google Scholar] [CrossRef]
- Liu, G.W.; Zhang, Y.; Thomas, M.P.; Ullah, A.; Pharr, M.; Guiton, B.S.; Banerjee, S. Negative thermal expansion HfV2O7 nanostructures for alleviation of thermal stress in nanocomposite coatings. ACS Appl. Mater. Interfaces 2021, 13, 44723–44732. [Google Scholar] [CrossRef]
- Li, J.T.; Han, B.Z. Development of silica aerogel and hollow glass microspheres based heat-insulating coatings. Paint Coat. Ind. 2013, 7, 24–28. [Google Scholar] [CrossRef]
- Wang, W.; Fei, Y.; Wang, T. Effects of infrared opacifiers on properties of silicate thermal insulating coatings. Bull. Chin. Ceram. Soc. 2017, 6, 2079–2082. [Google Scholar] [CrossRef]
- Wei, T.Y.; Lu, S.Y.; Chang, Y.C. Hydrophobic composite aerogels with high mechanical strength and low high-temperature thermal conductivities. J. Phys. Chem. B 2008, 112, 11881–11886. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Zhai, Q.G.; Li, X.L.; Yu, X.A. Preparation and studies on the interaction at the organic-inorganic interface of KH550 modified SiO2. Chem. Res. Appl. 2014, 26, 989–992. [Google Scholar] [CrossRef]
- Abha, M.; Pawan, K.T.; Singh, M.K.; Misra, D.S. FTIR studies of nitrogen doped carbon nanotubes. Diam. Relat. Mater. 2006, 15, 385–388. [Google Scholar] [CrossRef]
- Chen, W.; Wang, F.; Liang, J.C.; Tang, Q.G. Thermal insulation coatings containing sepiolite mineral fibers and their performance. Adv. Mater. Res. 2011, 178, 318–323. [Google Scholar] [CrossRef]
- Lian, Y.Y.; Wu, H.J.; You, X.H. Advances in effect of fiber species on improving mechanical and thermal insulation properties of silica aerogel. Bull. Chin. Ceram. Soc. 2017, 36, 1216–1222. [Google Scholar] [CrossRef]
- Yan, P.; Zhou, B.; Du, A.; Li, C.H. Mechanical property of silica aerogels reinforced with isocyanate. At. Energy Sci. Technol. 2014, 48, 1100–1105. [Google Scholar] [CrossRef]
- Yin, X.B.; Wang, X.C.; Zhang, J. Synthesis and characterization of SiO2 composite aerogels enhanced by the self growing nano-fibers. Chin. J. Inorg. Chem. 2014, 30, 603–608. [Google Scholar] [CrossRef]
- He, P.; Ruan, H.D.; Wang, C.Y.; Lu, H. Mechanical properties and thermal conductivity of thermal insulation board containing recycled thermosetting polyurethane and thermoplastic. Polymers 2021, 13, 4411. [Google Scholar] [CrossRef]
- Du, D.X.; Jiang, Y.G.; Feng, J.Z. Facile synthesis silica aerogel composites via ambient-pressure drying without surface modification or solvent exchange. Vacuum 2020, 173, 109117–109120. [Google Scholar] [CrossRef]
- Fei, Z.F.; Yang, Z.C.; Chen, G.B.; Li, K.F.; Zhao, S.; Su, G.H. Preparation and characterization of glass fiber/polyimide/sio2 composite aerogels with high specific surface area. J. Mater. Sci. 2012, 53, 12885–12893. [Google Scholar] [CrossRef]
- Sokolov, V.A.; Gasparyan, M.D.; Makhov, S.V. Corrosion resistance of chromium-containing refractories in molten alkali-free borosilicate glass E. Refract. Ind. Ceram. 2016, 57, 50–52. [Google Scholar] [CrossRef]
- Guo, J.Y.; Deng, X.H.; Wang, H.Z.; Zhou, L.Y. Modeling and simulation of vacuum low pressure carburizing process in gear steel. Coatings 2021, 11, 1003. [Google Scholar] [CrossRef]
- Ng, T.Y.; Yeo, J.J.; Liu, Z.S. A Molecular dynamics study of the thermal conductivity of nanoporous silica aerogel, obtained through negative pressure rupturing. J. Non-Cryst. Solids 2012, 358, 1350–1355. [Google Scholar] [CrossRef]
- Kalendová, A.; Veselý, D.; Kalenda, P. A study of the effects of pigments and fillers on the properties of anticorrosive paints. Pigm. Resin Technol. 2006, 35, 83–94. [Google Scholar] [CrossRef]
- Ibrahim, M.; Biwole, P.H.; Wurtz, E. A study on the thermal performance of exterior walls covered with a recently patented silica-aerogel-based insulating coating. Build. Environ. 2014, 81, 112–122. [Google Scholar] [CrossRef]
- Liu, T.W.; Wang, Z.; Li, G.Z.; Shi, G.P.; Zhao, X. Mechanical properties and thermal conductivity of lightweight thermal insulation composites. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Xi’an, China, 1–4 November 2018. [Google Scholar]
- Xing, Z.; Ke, H.; Wang, X.; Zheng, T.; Qiao, Y.; Chen, K.; Zhang, X.; Zhang, L.; Bai, C.; Li, A.Z. Investigation of the thermal conductivity of resin-based lightweight composites filled with hollow glass microspheres. Polymers 2020, 12, 518. [Google Scholar] [CrossRef] [PubMed]
- Bozsaky, D. Laboratory tests with liquid nano-ceramic thermal insulation coating. Procedia Eng. 2015, 123, 68–75. [Google Scholar] [CrossRef]

| Material | Type | Manufacturer |
|---|---|---|
| Hydrophobic SiO2 aerogel | AG-S 15 | Shanxi Yangzhong new material Co., Ltd., Taiyuan, China |
| Hollow glass bead | VS 5500 | 3M Co., Ltd., Saint Paul, MN, USA |
| Nano zirconia powder | EFUZR-D30D | Zhengzhou Cheng Ao chemical products Co., Ltd., Zhengzhou, China |
| Fiberglass | FG-11 | Yancheng Ailiwei fiber products Co., Ltd; diameter 11 μm, Yancheng, China |
| Ceramic fiber | 1430 | Zhejiang Jiuwei refractory material Co., Ltd.; granularity < 1 mm, Hangzhou, China |
| Wetting agent | APM 95 | Dow Chemical, Midland, MI, USA |
| Defoamer agent | BYK-025 | BYK-Chemie GmbH, Wesel, Germany |
| Dispersant | CTD-7101A | CNOOC Changzhou Paint & Coating Industry Research Institute Co., Ltd., Changzhou, China |
| Waterborne acrylic resin | CTD-6803 | CNOOC Changzhou Paint & Coating Industry Research Institute Co., Ltd., Changzhou, China |
| Film-forming agent | Texanol-12 | Eastman, Shanghai, China |
| Thickening thixotropic agent | F01-V | Lehmann & Voss & Co., Hamburg, Germany |
| Deionized water | - | made by the laboratory, 16.68 MΩ·cm |
| Carbon steel board | Q235 | bought on the market |
| Items | with Fibers | Without Fibers |
|---|---|---|
| λ/[W·(m·k)−1] | 0.051 | 0.069 |
| surface topography | no cracks | full of cracks |
| tensile strength/kPa | 1520 | 450 |
| Items | w (SiO2 Aerogel) : w (Hollow Glass Beads) | ||||
|---|---|---|---|---|---|
| 0:20 | 5:15 | 10:10 | 15:5 | 20:0 | |
| ΔT/℃ | 31 | 39 | 45 | 48 | 52 |
| λ/[W·(m·k)−1] | 0.082 | 0.065 | 0.050 | 0.043 | 0.036 |
| Adhesive strength/kPa | 1800 | 1530 | 1024 | 618 | 407 |
| Test Item | P/B Ratio | ||||
|---|---|---|---|---|---|
| 3:1 | 2:1 | 1:1 | 1:2 | 1:3 | |
| λ/[W·(m·k)−1] | 0.039 | 0.043 | 0.050 | 0.058 | 0.063 |
| Adhesive strength /kPa | 342 | 501 | 1024 | 1211 | 1460 |
| Tensile strength/kPa | 408 | 610 | 1580 | 2010 | 2600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, Z.; Ma, S.; Wang, H.; Guan, Z.; Lian, B.; Qiu, Y.; Jiang, Y. Modulation of Thermal Insulation and Mechanical Property of Silica Aerogel Thermal Insulation Coatings. Coatings 2022, 12, 1421. https://doi.org/10.3390/coatings12101421
Di Z, Ma S, Wang H, Guan Z, Lian B, Qiu Y, Jiang Y. Modulation of Thermal Insulation and Mechanical Property of Silica Aerogel Thermal Insulation Coatings. Coatings. 2022; 12(10):1421. https://doi.org/10.3390/coatings12101421
Chicago/Turabian StyleDi, Zhigang, Shengjun Ma, Huanhuan Wang, Zichao Guan, Bingjie Lian, Yunpeng Qiu, and Yiming Jiang. 2022. "Modulation of Thermal Insulation and Mechanical Property of Silica Aerogel Thermal Insulation Coatings" Coatings 12, no. 10: 1421. https://doi.org/10.3390/coatings12101421




