1. Introduction
Great efforts are currently being made to use environmentally friendly coatings [
1,
2,
3,
4], but at the same time, there is a growing demand for antimicrobial coatings worldwide [
5,
6,
7], as evidenced by a large number of articles and patents [
8,
9,
10]. Combining these two requirements is not an easy task, as one of the biggest trends in the market for green coatings is the introduction of low or zero volatile organic solvent (VOC) technologies, leading to the use of waterborne or solvent-free coatings. Waterborne coatings are an excellent alternative to solvent-based coatings, as they are a universal, high-quality, and environmentally friendly choice that meets both European and US regulations that require VOCs to be below 350 g/L of water [
11,
12]. Acrylic latex paints are probably the most widely used commercially produced polymeric waterborne coatings [
12,
13], mainly due to their easy preparation and modification. In addition, they are characterized by low toxicity, high resistance to atmospheric conditions, fast drying at room temperature, and compatibility with a wide range of surfaces, such as metals, mineral substrates, or wood [
14,
15,
16]. An equally important advantage is good usability for the target customer, thanks to easy washing with water (before curing) and low odor [
17,
18,
19]. Due to these properties, they have become very popular despite their typical shortcomings, such as the formation of flash corrosion, and low resistance to high and low temperatures or solvents and water, which often limits their applicability [
20,
21,
22].
One way to improve the durability of latex coatings is to crosslink polymer chains with covalent or ionic bonds [
23,
24]. Researchers have developed a number of crosslinking mechanisms for latex films [
21,
25,
26], both two-pack and one-pack (self-crosslinking) compositions, with “two-pack in one pot” systems. The one-pack compositions are preferred, mainly for ease of use, as they do not require the addition of an external crosslinker, or the mixing of different latexes produced at different stages. In these systems, the crosslinking reaction is usually triggered either by a drastic decrease in pH or by evaporation of water during film drying [
27,
28]. A particularly effective crosslinking mechanism used in the “two-pack in one pot” latex compositions is based on a reaction between carbonyl groups in diacetone acrylamide repeat units (DAAM), which are part of the polymer chain, and hydrazide groups from adipic acid dihydrazide (ADH) dissolved in the aqueous phase (see
Figure 1) [
29,
30,
31,
32,
33]. The unique feature of this reaction is rapid curing at room temperature which further increases the barrier properties, mechanical properties, and water-repellent properties of coating films [
31].
A serious drawback of waterborne coatings is that they are sensitive to biodegradation by bacteria and fungi while in the can, or later as an applied coating film [
34,
35]. Various inorganic and organic materials have been used for this purpose in the past, but many of them are now banned due to their harmfulness to human health or the environment [
36,
37,
38] and thus non-compliance with relevant European legislation (the European Union specifically adopted Directive 98/8/ES). Current findings stimulate further reductions in the number of substances with antimicrobial effects [
35]. Therefore, new environmentally-friendly alternatives to antimicrobial protection should be sought [
39,
40], as microbial infectious diseases are a serious health and socio-economic problem which attracts the attention of the public around the world [
41].
Nanoparticles provide a new approach to the development of antimicrobial materials [
42,
43]. In particular, metal oxide (MeO) nanoparticles have the potential to be effective against a wide range of microorganisms (MO), such as aerobic bacteria, anaerobic bacteria, viruses, yeasts, and fungi [
5,
44,
45,
46,
47]. Metal cations are thought to destroy the enzymes of these MOs and are therefore unable to build up resistance [
48,
49,
50]. An example of a promising MeO with an antimicrobial effect is nanostructured La
2O
3 which has been found to inhibit the growth of bacteria, fungi, and yeast, and for this reason it was investigated in safety and biomedical applications [
51]. Lanthanum is a rare-earth element possessing unique physical and chemical properties such as high density, high melting point, high thermal conductance, and conductivity, which provides the potential for enhancing the useful properties of the coating [
52,
53].
In addition to antimicrobial properties, MeO nanoparticles can also have a positive effect on the structural properties of the coating [
54,
55]. If limited soluble MeO nanoparticles are used, the properties of acrylic latex coatings can also be improved by ionic (physical) crosslinking with subsequent complex formation, which occurs in the interfacial zones between adjacent latex particles, through ionic dipolar interactions [
24]. In the case of carboxy-functionalized latexes, ionic bonds are formed between carboxyl groups on the surface of latex particles in the presence of polyvalent metals or metal complexes [
56,
57,
58]. ZnO (often reported in the relevant literature) appears to be a suitable crosslinker, as it is a sparingly soluble metal oxide that does not dramatically affect latex stability [
59]. Ammonia, which is often used to ensure the stability of the latex system, reacts with zinc ions to form a zinc amine complex. Upon evaporation of ammonia and water in the film-forming process, zinc ions are released from the complex and react with the carboxyl groups on the surface of the latex particles at room temperature. The ionic crosslinking reaction is first formed between zinc ions and carboxyl groups and then transformed into a more thermodynamically stable coordinated complex (see
Figure 2). This effect is attributed to rearrangement caused by kinetic factors, during a process in which latex particles collect to form a tighter arrangement [
54]. Compared to conventional latexes, these compositions offer excellent thermal stability, hardness, resistance to water, weathering, etc. [
60,
61].
The problem of flash corrosion of latex coatings limits their use on metal substrates (especially steel), where the soluble iron salt is transferred to the coating film during drying [
62]. In practice, this problem is solved using often toxic flash corrosion inhibitors, such as sodium benzoate or sodium nitrite [
63,
64]. The corrosion potential of the metal and the nature of corrosion are mainly dependent on the concentration of hydrogen ions in the aqueous medium (pH). Pourbaix diagrams can be used to predict the corrosion behavior of a metal substrate [
65]. In the case of a steel substrate according to the Pourbaix diagram for iron, it can be expected that at a pH above 8.5 the steel passes into the passivity region and no iron oxidation occurs. Thus, the solution to this problem could be to alkalize the latex with an agent that will not leak during the drying process and will maintain a high pH (above 8.5) throughout the coating process. Nanostructured MgO appears to be a suitable candidate that dissociates into hydroxyl and magnesium ions in an aqueous medium, thus shifting the pH of the aqueous solution to the alkaline range [
55,
66,
67].
Because of concern for human health and environmental protection, nanoparticles have begun to be investigated regarding their cytotoxicity. In general, the toxicity depends on nanoparticle size and concentration [
68,
69]. When comparing the cytotoxicity data available in the literature for nanostructured ZnO [
70,
71], MgO [
72,
73,
74], and La
2O
3 [
75,
76,
77], ZnO, which has also been the most studied material, appears to be the most cytotoxic (with proven toxicity for aquatic organisms and human cells [
78,
79,
80,
81,
82,
83,
84,
85]), while MgO is considered the least harmful [
73] with the possible use as an anti-cancer treatment agent [
72].
The aim of this paper is to present and compare surface untreated MeO nanoparticles (specifically MgO, ZnO, La
2O
3, and combinations of MgO and ZnO) in the role of multi-functional additives for hygienic acrylic latex coating binders. This study follows, complements, and extends our previous research [
23,
56,
62,
66]. To the best of our knowledge, the applications of La
2O
3 nanoparticles, and combinations of nanostructured MgO and ZnO in latex coating compositions have not been presented so far. The effectiveness of the latexes was evaluated for biocidal effect with respect to the type of nanoparticles present and their concentration, both in the liquid binder (the in-can preservation stability) and their coating films. Several other benefits of using specific nanoparticles in the coating systems were highlighted.
2. Materials and Methods
2.1. Incoming Materials
The latexes were prepared from monomeric methyl methacrylate (MMA), n-butyl acrylate (BA), methacrylic acid (MAA) and diacetone acrylamide (DAAM) supplied by Sigma-Aldrich, Prague, Czech Republic. Adipic acid dihydrazide (ADH, active substance content > 98%; Sigma-Aldrich, Prague, Czech Republic) served as the crosslinking agent; Disponil FES 993 (anion-active surfactant based on sodium polyglycol ether sulfate; BASF, Prague, Czech Republic), as the emulsifier; ammonium persulfate (active substance content > 99.9%; Lach-Ner, Neratovice, Czech Republic), as the initiator; and nanostructured MeO with no surface treatment, as the antimicrobial and antifungal ingredient of the latex, namely (i) MgO nanoparticles with particle size < 200 nm (commercial name JR-NMg30, Xuancheng Jingrui New Materials Co., Xuancheng, China), (ii) ZnO nanoparticles with particle size < 100 nm (Sigma-Aldrich, Prague, Czech Republic) and (iii) La2O3 nanoparticles with particle size < 100 nm (Sigma-Aldrich, Prague, Czech Republic). All chemicals were used without further modifications such as purification (as supplied by the manufacturer).
2.2. Synthesis of Latexes
Using the technique of semi-continuous emulsion radical polymerization, four series of acrylic latexes were prepared, which differed in the type and content of inorganic nanoparticles. To allow interparticle crosslinking, DAAM providing ketone carbonyl functional groups for subsequent crosslinking with a hydrazide crosslinking agent was added to the polymer chain. DAAM and nanoparticles were delivered to the system only in the second phase of the monomer drip. The ratio composition of the monomers making up all the latexes was: 86 g MMA, 106 g BA, and 8 g MAA dosed in the first phase and 78 g MMA, 104 g BA, 8 g MAA, and 10 g DAAM dosed in the second phase. The ratio of acrylic monomers forming latex particles was chosen so that the calculated glass transition temperature (
Tg) of the latex polymer was around 10 °C (calculated according to Fox’s equation [
86]) to ensure sufficient film formation and non-stick coatings. The L
0 latex sample was a reference without the corresponding inorganic nanoparticles, while the L
ZnO samples contained ZnO nanoparticles, the L
MgO samples contained MgO nanoparticles and the L
La2O3 samples contained La
2O
3 nanoparticles, each with a concentration of 0.5, 1, and 1.5 wt.% (based on the total amount of monomers). The last series of L
MgO+ZnO acrylic latexes combined MgO and ZnO nanoparticles, with MgO nanoparticles each being supplied with a concentration of 1 wt.% and ZnO nanoparticles in a concentration of 0.25, 0.5 and 0.75 wt.% (based on the total amount of monomers).
The latexes were prepared in a glass reaction vessel under a nitrogen atmosphere at a polymerization temperature of 85 °C according to the procedure shown in
Table 1. The monomer emulsion was added dropwise to the stirred reactor (approximately 2 mL/min) in two phases; a 15 min pause was allowed between the two phases. When all the emulsion was added, the system was allowed to react for another 120 min to complete the polymerization process. (The temperature of the reaction vessel was maintained at 85 °C throughout the synthetic process).
To prepare a series of latexes with ZnO and La2O3 nanoparticles, respectively, the procedure involved the preparation of an aqueous suspension with nanoparticles. To prepare the nanoparticle suspension, the ZnO/La2O3 nanoparticles were mixed with water, which was intended to prepare a monomer emulsion for the second phase. To facilitate the fragmentation of the agglomerates, dispersion was performed using a T18 digital ULTRA-TURRAX disperser (IKA Works, Staufen, Germany) at 17,000 rpm for 15 min, followed by treatment in an ultrasonic ice bath KRAINTEK K 12.F (Kraintek s.r.o., Podhájská, Slovakia) for 1 h. The prepared aqueous nanoparticle suspension was then mixed with the monomers, emulsifier, and initiator to prepare a second phase monomer emulsion, dispersed at 3000 rpm for 3 min, and immediately metered into the reactor.
To prepare a series of latexes with MgO nanoparticles, the procedure involved the preparation of a monomer suspension with nanoparticles. First, the MgO nanopowder was mixed with the MMA and BA monomers, which were intended to prepare a monomer emulsion for the second phase. To facilitate the fragmentation of the agglomerates, dispersion was performed using a T18 digital ULTRA-TURRAX disperser (IKA Works, Staufen, Germany) at 14,000 rpm for 45 min while cooling in an ice bath followed by treatment in an ultrasonic ice bath KRAINTEK K-12.F (Kraintek s.r.o., Podhájská, Slovakia) for 45 min. The prepared monomer suspension with nanoparticles was then mixed with water, an emulsifier, initiator, and the rest of the monomers (MAA and DAAM) intended to prepare the second phase monomer emulsion, dispersed at 3000 rpm for 3 min and immediately metered into the reactor. In the case of a series of latexes with a combination of MgO and ZnO nanoparticles, the MgO nanoparticle monomer suspension and the ZnO nanoparticle aqueous suspension were prepared according to the procedures described above with the difference that half quantities of all individual components except the MeO nanoparticles were used for the preparation of both nanoparticle suspensions. In addition, the second phase drop was divided into two steps, the monomer emulsion with MgO nanoparticles was dropped in the first step followed by an immediate feeding of the monomer emulsion containing ZnO nanoparticles in the second step.
After synthesis, the solids were filtered to calculate the coagulate content, the pH of the cold latex was adjusted to 8.5 with 10% aqueous ammonia (for latexes having an initial pH below 8.5), and finally a 10% aqueous solution of ADH was added in an amount corresponding to a molar ratio of DAAM:ADH = 2:1, whereby the self-crosslinking latex binders were prepared.
All latexes were always synthesized in triplicate for each sample to ensure reliable results.
2.3. Characterization of Latexes
The coagulate and coarse impurity contents of the latexes were determined by sieve analysis according to CSN 64 9008; pH was measured with a Mettler Toledo FiveEasy FE20 pH-meter (Merck KGaA, Darmstadt, Germany) [
87]; the minimum film-forming temperature (MFFT) was determined by using a MFFT-60 instrument (Rhopoint Instruments, East Sussex, UK) according to ISO 2115; the storage stability of latexes was tested in two storing modes: (1) at 50 °C for 2 months; (2) at 25 °C for 2 years. The evaluation was performed using the zeta potential and the particle size by the dynamic light scattering (DLS) method on a Zetasizer Nano ZS (Malvern Panalytical, Malvern, UK) [
88]; the in-can antimicrobial efficiency of the liquid latexes was tested by using Preventol
® Dipslides (LANXESS Deutschland GmbH, Cologne, Germany). The in-can preservation test of antimicrobial efficiency consisted of submerging the agar part of the DipSlide into the latex for 10 s, followed by incubation at 30 °C for 120 h. The result was evaluated by using standards [
89].
The determination was made from each prepared latex (each latex was prepared three times) by means to ensure reliable results.
2.4. Preparation of Coating Films: Latex Application to the Substrate
The latexes were applied to glass panels with a size of 200 mm × 100 mm × 5 mm to test the hardness, chemical resistance, mechanical resistance (adhesion), gloss, transparency and water whitening of coating films. Mechanical (impact) resistance and corrosion resistance were tested on coating films applied to cold-rolled low-carbon steel panels (Q-Panel steel Class 11—ISO 3574 CR1, SAE 1008/1010; the chemical composition is 0.60% max Manganese, 0.15% max Carbon, 0.03% max Phosphorus and 0.035% max Sulfur) with a size of 152 mm × 102 mm × 0.8 mm. Both the glass and steel panels were cleaned thoroughly with chloroform before the tests [
90]. The liquid latexes were applied to the glass/steel substrates by using a film applicator coater (bird type applicator with a constant slot width, product of Zehntner GmbH, Schwerzenbach, Switzerland). The slot width was 150 µm for application on the glass panels and 250 µm for application on the steel panels. The coating films on the panels were allowed to dry for 10 days (except for the panels intended for flash corrosion examination) in an air-conditioned room.
For determination of the antimicrobial efficiency, the presence and location of inorganic nanoparticles and real nanoparticle content in the latex films, loose films about 1 mm thick were prepared by pouring the latexes into silicone molds. The loose films were then air-dried at RT for a month and then vacuum-dried at 30 °C to constant weight. Subsequently, for antimicrobial efficiency tests the samples were sterilized on both sides using UVR-Mi UV germicidal radiation for 20 min. For the antifungal efficiency tests, the latexes were applied also to sterile wooden squares with a size of 5 × 5 cm2. The latex was applied to the wooden panels with a brush in perpendicular directions in 4 layers, with a minimum drying time of 4 h between the layers; the tests themselves were performed after a drying period of 10 days.
Each test was performed in triplicate. All the coating films—on glass, steel, wood and in silicone moulds—were exposed to a temperature of 21 ± 2 °C and relative humidity of 55% in an air-conditioned room according to CSN EN 23270 prior to the tests.
2.5. Description of Embedded Nanoparticles: Characterization, Content and Location
Monitoring of the change in the chemical nature of the respective embedded metal oxide nanoparticles during the synthesis was performed by X-ray diffraction (XRD) on an Empyrean (PANAlytical, Almelo, The Netherlands) at 40 mA and 45 kV. The instrumental setup comprised a PIXcel3D-Medipix3 surface detector with a goniometer radius of 240 mm, a scan speed = 0.033453 °/s and Ni filter (to select the Cu K
α wavelength) on the diffracted beam. Using Topas software (version 4.2.1, 2010, Bruker Axs, Karlsruhe, Germany), the internal standard method was used, using 10 wt.% of internal standard α-Al
2O
3 (NIST SRM 676a) [
91]. The testing was performed on samples subjected to the simulation of polymerization carried out in the same way as in the synthesis of latexes (see
Table 1), only without the content of the relevant monomers, but also on the originally supplied nanoparticles of metal oxides. The aqueous suspension thus obtained was decanted to obtain an inorganic powder, which was dried at 80 °C to constant weight and analyzed.
Determination of real nanoparticle content in coating films of acrylic latexes was performed by using of an inductively coupled plasma optical emission spectrometry (ICP-OES) using an Integra 6000 (GBC Scientific Equipment, Braeside, Australia) [
92]. From the values obtained, the nanoparticle content was calculated using the simplified assumption that all nanoparticles are in the coating film only in the form of the respective metal oxide. (except for La
2O
3 based, where La
2O
2CO
3 was considered according to XRD analysis results).
The presence and location of inorganic nanoparticles on the surface of the latex coating films were evaluated employing an atomic force microscopy (AFM). The measurement was done applying AFM Dimension Icon (Bruker, Billerica, MA, USA) in PeakForce quantitative nanoscale mechanical mode using ScanAsyst-Air tips (k = 0.4 N·m
−1). The topography and mechanical behavior of the film surface were simultaneously monitored at a scanning frequency of 0.5 Hz with a resolution of 512 × 512 pixels at the area 5 × 5 μm
2 in a similar way as in [
55]. The presence and distribution of inorganic nanoparticles inside the latex coatings was observed by means of scanning electron microscopy (SEM) using a LYRA 3 scanning electron microscope (Tescan, Brno, Czech Republic). The cryo-fractures of the polymer films were covered with a 20 nm carbon layer and measured at an accelerating voltage of 5 kV.
The change in the morphology of the nanoparticles before and after their exposure to the polymerization conditions was also monitored by SEM, by the same method of preparation as for XRD. The nanoparticles were gilded with 18 nm Au layer and measured at an accelerated voltage of 10 kV.
The determination was made from each prepared latex (each latex was prepared three times) to ensure reliable results.
2.6. Coating Film Property Assessment
The degree of crosslinking of acrylic latexes was evaluated based on gel content and crosslink density. The gel content was determined according to CSN EN ISO 6427 by extraction in a Soxhlet extractor with tetrahydrofuran for 24 h. Crosslinking density was performed by swelling loose films of acrylic latexes in toluene at 35 °C for 7 days. The sample was then withdrawn, quickly dried with gauze and weighed to obtain the weight of the swollen sample. Equations (1)–(4), employing the theory of Flory and Rehner [
93], were used to calculate the crosslink density (expressed as moles of crosslinks per cm
3 of polymer network), as given in the following:
where the abbreviations indicate:
Mc—average molecular weight between crosslinking;
V1—molar volume of toluene (106.3 cm/mol);
ρp—density of polymer that was calculated to be 1.11 g/cm
3 for the BA/MMA/MAA (53/43/4 by weight) copolymer from 1.06, 1.18 and 1.015 g/cm
3 for poly(BA), poly(MMA) and poly(MAA), respectively;
φ—volume fraction of the gel polymer in the swollen gel;
Wp and
Ws are the weight fractions of the gel polymer and solvent (toluene) in the swollen gel, respectively;
ρs—density of solvent (0.8669 g/cm
3);
χ—polymer and solvent interaction parameter;
δ1—solubility parameter of polymer that was calculated to be 9.16 (cal/cm
3)
1/2 for the BA/MMA/MAA (53/43/4 by weight) copolymer from 9.0, 9.3 and 9.8 (cal/cm
3)
1/2 for poly(BA), poly(MMA) and poly(MAA), respectively; and
δ2—solubility parameter of toluene, 8.9 (cal/cm
3)
1/2 [
94,
95].
The optical properties of coating films were evaluated with respect to gloss, transparency and water whitening. The gloss was evaluated according to CSN EN ISO 2813 (using a gloss-measuring geometry at 20°). Since these are transparent coatings, the glass panels on which the measurement was performed were sprayed with black matte paint (RAL 9005) and the measurement was determined by a micro TRI-gloss μ instrument (BYK-Gardner, Wesel, Germany). The transparency and water whitening of coating films were evaluated by light transmission (transmittance measurement at a wavelength of 500 nm) using a ColorQuest XE Spectrometer (Hunterlab, Reston, VA, USA). Initially, transparency measurements were performed and then the coating films were exposed to distilled water for 48 h at room temperature, then measurements were immediately performed on the exposed areas of the coating film. The degree of water whitening—W in % was calculated by W = 100(T0 − Tt)/T0, where the abbreviations indicate: T0—the transmittance of sample before distilled water exposure and Tt—the transmittance of sample after performing the immersion test.
The hardness of the coating films was evaluated according to CSN EN ISO 1522—Paints and varnishes—Pendulum damping test; Persoz type pendulum (3034M001 pendulum, Elcometer Instruments GmbH, Aalen, Germany). The mechanical properties (mechanical resistance) of the coating films were assessed according to CSN EN ISO 6272-2—Paints and varnishes—Rapid-deformation (impact resistance) test using an Elcometer 1615 variable impact tester (Elcometer Instruments GmbH, Aalen, Germany) with a steel falling weight of 1000 g with 20 ± 1 mm hemispherical end. Tests according to CSN ISO 2409, the cross-cut test, were also made. An Elcometer cross-cut system (Elcometer Instruments GmbH, Aalen, Germany) with 6 parallel knives 1 mm apart was used. After making the grid, the damage was visually assessed according to a classification scale from 0 to 5, where 0 indicates the best result (
Table S1, see Supplementary Materials). Chemical resistance of the coating films was assessed according to ASTM D-4752-10—rubbing test with methyl ethyl ketone (MEK).
Flash corrosion resistance of the coating films was evaluated after the coating was applied to the steel substrate and assessed through a laboratory test to identify any flash corrosion [
64]. The laboratory test was made on coating films deposited on steel panels which, after drying at 21 ± 2 °C, RH 50 ± 5% for 2 h, were stored in a refrigerator at 5 °C for 16 h. After removal from the refrigerator, the entire coating film was uniformly covered with a filter paper that was pre-wetted with distilled water, and the system was covered with a heavy glass plate to achieve close contact of the coating film with the water. The water was allowed to act for 2 h at room temperature. Then the filter paper was removed, the coating film was dried, and corrosion phenomena were scored as per ASTM D 610-85; coloration was scored using Gardner’s iodometric scale (see
Table S2) [
63].
All coating film evaluation tests were always performed in triplicate for each sample to ensure reliable results.
2.7. Testing of Antimicrobial Efficiency of Coating Films
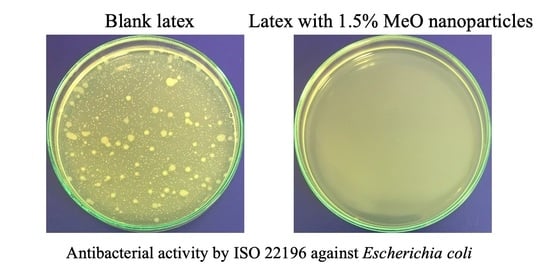
The antimicrobial efficiency of the coating films was evaluated using a modified method ISO 22196 [
96] to determine the antibacterial activity. Based on this modified method, the most effective concentration of the given nanoparticles was selected for each series and was subsequently tested using the ISO 22196 method. For these selected samples, their antifungal efficiency of coating films on a wooden substrate was further determined using a modified method ASTM D5590 [
97]. Four bacterial strains were used for antimicrobial tests, namely
Staphylococcus aureus (
S. aureus, CCM 4516),
Escherichia coli (
E. coli, CCM 4517),
Enterococcus faecalis (
E. faecalis, CCM 3956) and
Klebsiella pneumoniae (
K. pneumoniae, CCM 4425) and two fungal strains, namely
Penicillium chrysogenum (
P. chrysogenum, CCM 8034) and
Aspergillus brasiliensis (
A. brasiliensis, CCM 8222). All the microbial strains were provided by the Czech Collection of Microorganisms (Masaryk University, Brno, Czech Republic).
The testing of antibacterial activity according to ISO 22196 method and the modified ISO 22196 method was performed on sterile loose films with dimensions of 25 × 25 mm
2 which were inoculated with 0.1 mL of a specific standardized bacterial suspension (24 h culture), namely
S. aureus (7.4 × 10
6 cfu/mL),
E. coli (6.9 × 10
6 cfu/mL),
E. faecalis (6.1 × 10
6 cfu/mL) and
K. pneumoniae (5.8 × 10
6 cfu/mL). The inoculated samples were then covered with a sterile polypropylene film (sterilized with 70% ethanol) with a size of 20 × 20 mm
2. Then the samples were incubated at 35 °C for 24 h at 95% RH. In the case of the modified ISO 22196, the samples were imprinted on a Plate Count Agar (HIMEDIA, Mumbai, India) after the removal of the polypropylene film. Each sample was imprinted on three different agar areas (see
Figure S1) and incubated at 35 °C for 24 h. The results were then evaluated from 0 to 5, with 0 representing the best antibacterial activity, i.e., without the growth of bacterial colonies.
If the ISO 22196 method was followed, after removing the polypropylene film, the samples were rinsed with 2.5 mL of SCDLP flushing solution (Lach-ner a.s., Neratovic, Czech Republic) and a dilution series from 0 to 10
−7 was prepared from the thus prepared microbial suspension. From the dilution series prepared in this way, 1 mL was always taken, which was placed in a Petri dish and filled with 45 °C warm MPA nutrient agar No. 2 (HIMEDIA, India), and the whole system was mixed and incubated at 35 °C. After a 24 h incubation, bacterial contamination readings were performed (for dilutions with 30–300 bacterial colonies). The arithmetic mean was calculated from the obtained values and a conversion was carried out according to the Equations (5) and (6):
where N is the number of viable bacteria per cm
2 of test sample; R is antimicrobial activity; C is the average number of bacteria; D is the dilution value; V is the volume of rinsing solution added to the sample; A is the surface area of the cover foil in mm
2; U
t is the average value of the logarithm of viable bacteria in units of cells/cm
2 of untreated test samples after 24 h of incubation; and A
t is the average value of the logarithm of viable bacteria in units of cells/cm
2 of test specimens after 24 h of incubation.
Testing of antifungal activity was performed on sterile 40 × 40 mm2 wooden panels treated with the coating film. The 5-day cultures were suspended in physiological saline solution with 0.005% (w/v) of TWEEN® 20 (Sigma-Aldrich, Prague, Czech Republic). Specifically standardized fungal suspensions were used, namely P. chrysogenum (2.4 × 106 spores/mL) and A. brasiliensis (3.1 × 106 spores/mL). Sterile samples placed in the center of a Petri dish with MALT agar (HIMEDIA, Mumbai, India) were inoculated with 0.1 mL of the appropriate fungal suspension, which was evenly distributed (with an L-shaped hockey stick) over the entire surface. The samples thus prepared were incubated at 25 ± 2 °C and 90% RH for 28 days. The results were then evaluated by standard specification from 0 to 4, with 0 representing the best antifungal activity, i.e., without the growth of mould.
All antimicrobial tests were always performed in triplicate for each sample to ensure reliable results of the antimicrobial efficacy.
4. Conclusions
This work was devoted to the development of hygienic acrylic latexes which are expected to provide antimicrobial activity both in the liquid state and subsequently as a coating film on various substrates without the use of commercial (often toxic) biocidal additives. Various types of surface untreated MeO nanoparticles have been used as functional antimicrobial additives, namely MgO, ZnO, La2O3, and combinations of MgO and ZnO. To overcome the typical shortcomings of acrylic latexes, keto-hydrazide interfacial crosslinking has been introduced into the latexes. The inorganic nanoparticles were inserted into the latex during semi-continuous emulsion polymerization and latexes prepared in this way showed long-term stability. It was found that all used MeO nanoparticles provided antimicrobial properties and at the highest concentration used (theoretically 1.5 wt.%, practically about 1.3 wt.% in a dry coating film, and about 0.5 wt.% in liquid latex) ensured in-can antimicrobial stability of liquid latexes and bactericidal activity of coatings against three of four tested bacteria (S. aureus, E. coli, and K. pneumaniae). Only the coatings with the highest concentration of inserted MgO nanoparticles (1.31 wt.%) provided bactericidal activity against E. faecalis, probably due to the high alkalinity of the coatings caused by the hydration of MgO. In contrast, only latex coatings containing ZnO nanoparticles showed fungicidal activity, which is probably related to the highest concentration of reactive oxygen species formed in the case of ZnO nanoparticles. In addition to antimicrobial activity, the incorporation of MeO nanoparticles into acrylic latex was shown to provide additional advantages. All types of inorganic nanoparticles were found to act as effective ionic crosslinkers, providing excellent resistance to MEK and water whitening without compromising the gloss and transparency of coatings. Furthermore, it was found that the incorporation of MgO nanoparticles into latex caused a significant decrease in MFFT due to hydroplasticization of the carboxy-functionalized emulsion copolymer, which was more pronounced with increasing concentration of MgO nanoparticles (converted predominantly to Mg(OH)2). The insertion of MgO nanoparticles in latex also increased resistance to flash corrosion of steel substrates, including the number of microscopic corrosion centers, where at the highest concentration of nanoparticles, no corrosion center formation was evident, probably due to latex alkalinity. It can be concluded that environmentally-friendly one-component self-crosslinking latexes using MeO nanoparticles as antimicrobial additives and self-crosslinking agents were developed. These materials could be perfect binders for interior hygienic paints or clear coats providing durable protection for various materials including steel. Moreover, these latexes could also find application in exterior coatings which are particularly threatened by water and moisture, including microbial colonization.