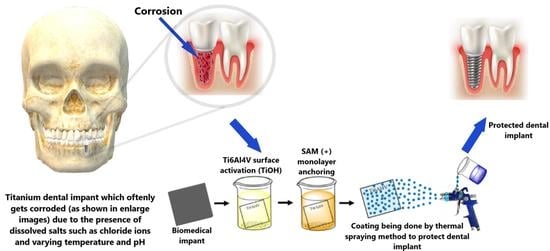
Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants
Abstract
1. Introduction
2. Commonly Utilized Biomedical Alloys and Their Corrosion Mitigation
2.1. Stainless Steel
2.2. Co–Cr Alloys
2.3. Ti Alloys
3. Surface Modification Techniques for Biomedical Metals and Alloys
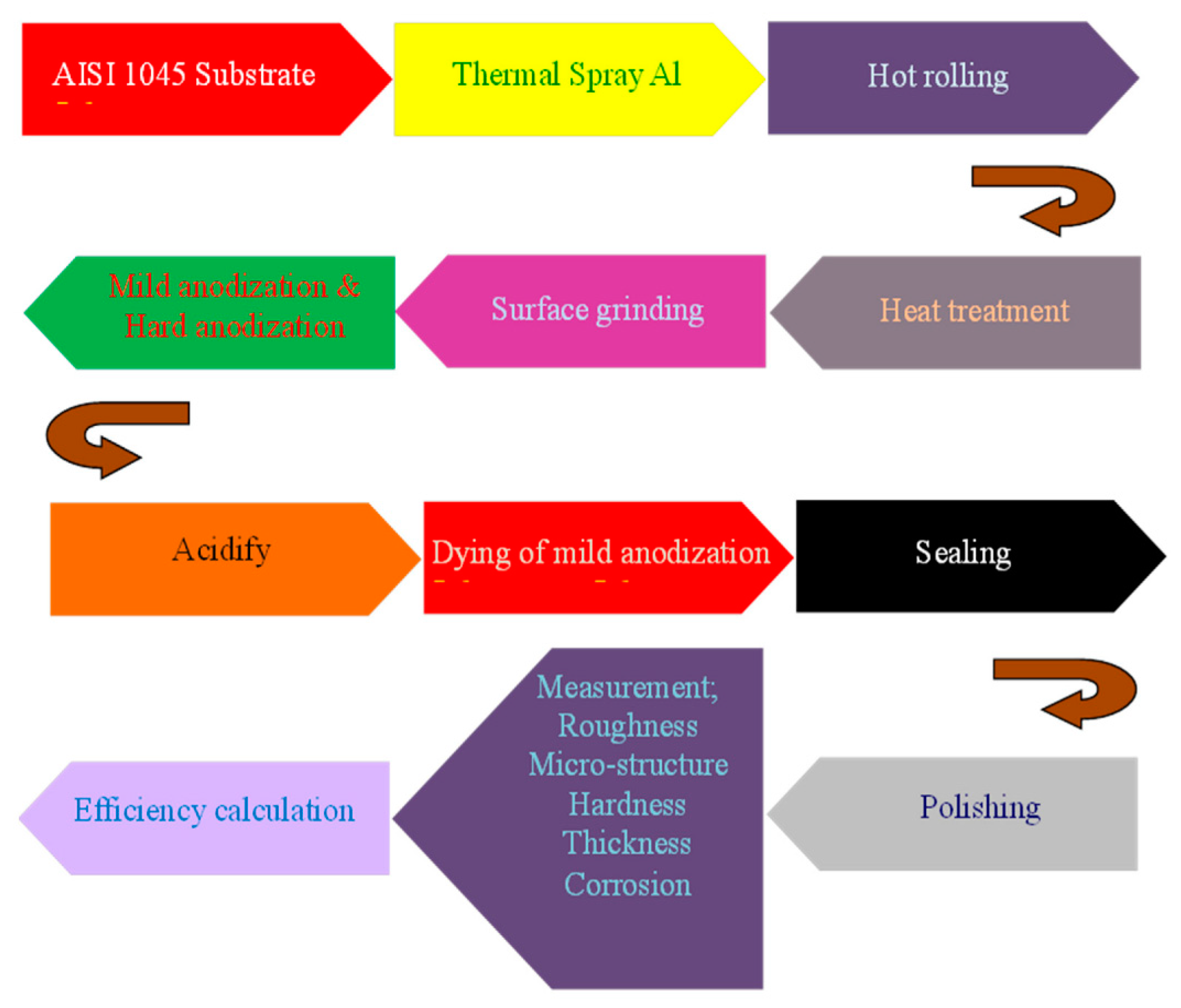
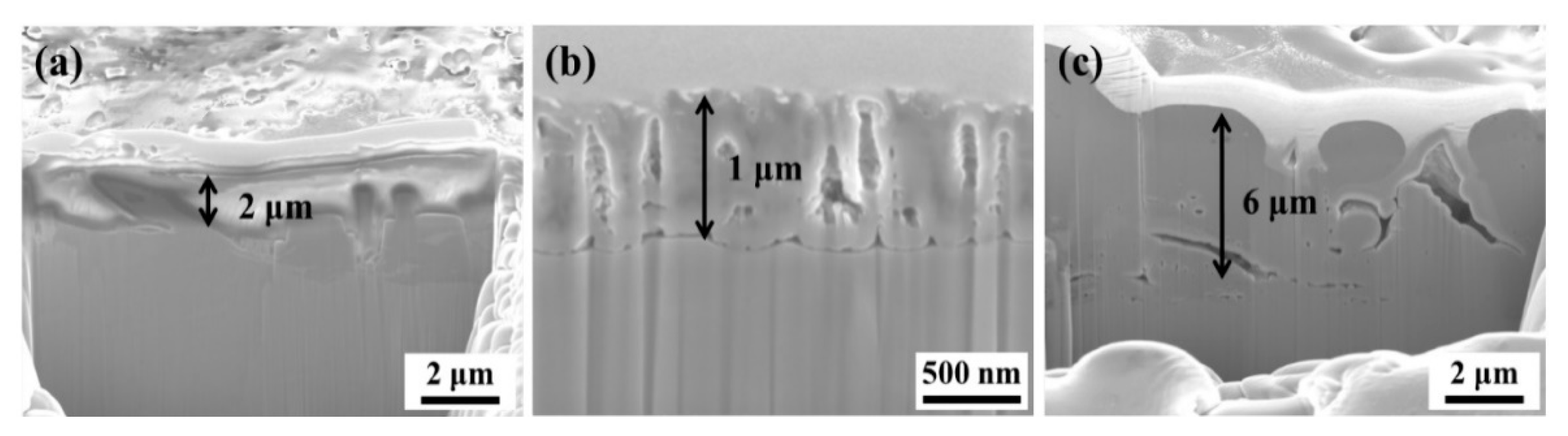
3.1. Thermal Spraying
3.2. Glow Discharge Plasma
3.3. Ion Implantation
3.4. Ultrasonic Nanocrystal Surface Modification Techniques (UNSM)
3.5. Physical Vapor Deposition
3.6. Plasma-Assisted Surface Treatments
3.7. Photon Irradiation
3.8. Ion-beam Modification
4. Surface Modification Coatings for Biomedical Metals and Alloys
4.1. Nanostructure Coatings
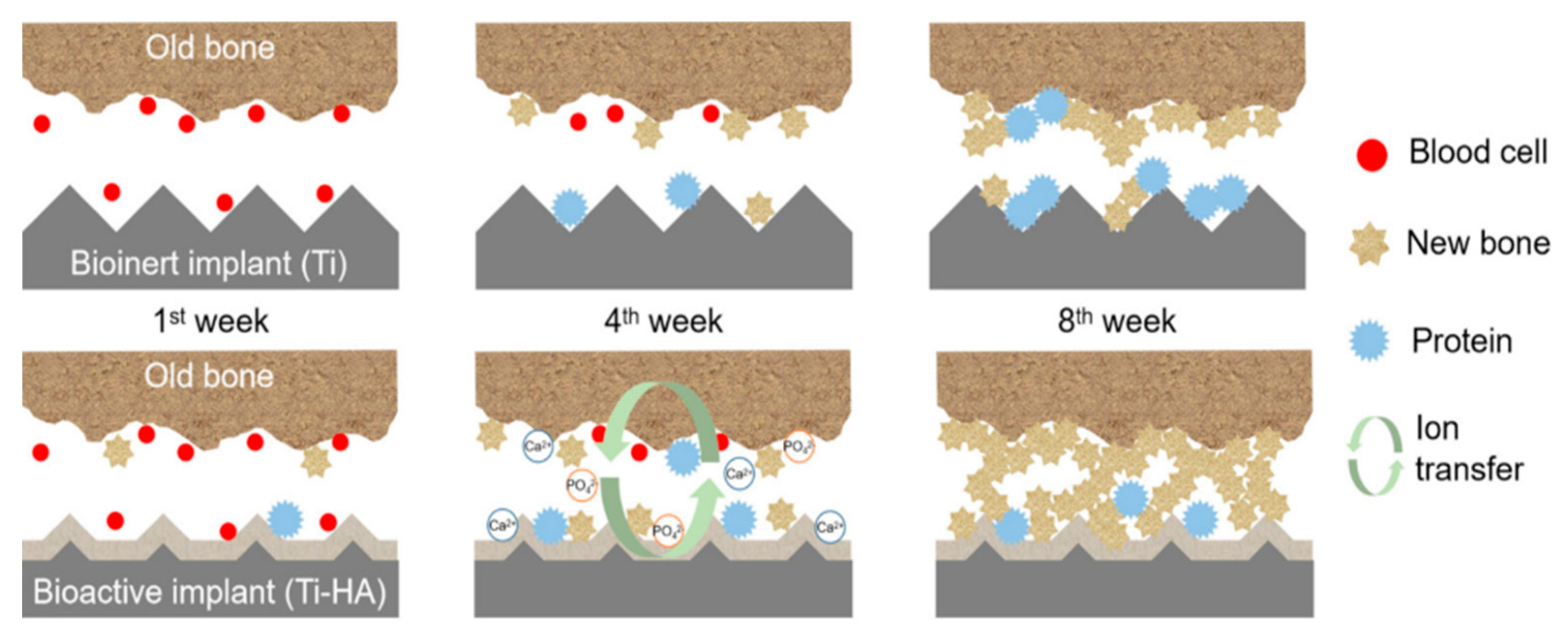
4.2. Bio-inert Material Coatings
4.3. Bioactive Glass Coatings
4.4. Hydroxyapatite (HA) Coatings
4.5. Electrophoretic Deposition (EPD)
4.6. Bio-mimetic Coating (Smart Biomaterials)
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molino, G.; Bari, A.; Baino, F.; Fiorilli, S.; Vitale-Brovarone, C. Electrophoretic Deposition of Spray-Dried Sr-Containing Mesoporous Bioactive Glass Spheres on Glass–Ceramic Scaffolds for Bone Tissue Regeneration. J. Mater. Sci. 2017, 52, 9103–9114. [Google Scholar] [CrossRef]
- Barfeie, A.; Wilson, J.; Rees, J. Implant Surface Characteristics and Their Effect on Osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiorilli, S.; Vitale-Brovarone, C. Composite Biomaterials Based on Sol-Gel Mesoporous Silicate Glasses: A Review. Bioengineering 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Kačiulis, S.; Mattogno, G.; Pandolfi, L.; Cavalli, M.; Gnappi, G.; Montenero, A. XPS Study of Apatite-Based Coatings Prepared by Sol-Gel Technique. Appl. Surf. Sci. 1999, 151, 1–5. [Google Scholar] [CrossRef]
- Sreekanth, D.; Rameshbabu, N. Development and Characterization of MgO/Hydroxyapatite Composite Coating on AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation Coupled with Electrophoretic Deposition. Mater. Lett. 2012, 68, 439–442. [Google Scholar] [CrossRef]
- Schindhelm, K.; Milthorpe, B.K. An Overview of Biomaterials. Australas. Phys. Eng. Sci. Med. 2003, 9, 29–32. [Google Scholar]
- Dos Santos, G.A. The Importance of Metallic Materials as Biomaterials. Adv. Tissue Eng. Regen. Med. Open Access 2017, 3, 300–302. [Google Scholar] [CrossRef]
- Yadav, S.; Gangwar, S. An Overview on Recent Progresses and Future Perspective of Biomaterials. IOP Conf. Ser. Mater. Sci. Eng. 2018, 404, 012013. [Google Scholar] [CrossRef]
- Martinez-Boubeta, C.; Simeonidis, K.; Oró, J.; Makridis, A.; Serantes, D.; Balcells, L. Finding the Limits of Magnetic Hyperthermia on Core-Shell Nanoparticles Fabricated by Physical Vapor Methods. Magnetochemistry 2021, 7, 49. [Google Scholar] [CrossRef]
- Chiang, P.C.; Chen, C.W.; Tsai, F.T.; Lin, C.K.; Chen, C.C. Hard Anodization Film on Carbon Steel Surface by Thermal Spray and Anodization Methods. Materials 2021, 14, 3580. [Google Scholar] [CrossRef]
- Liu, R.; Yuan, S.; Lin, N.; Zeng, Q.; Wang, Z.; Wu, Y. Application of Ultrasonic Nanocrystal Surface Modification (UNSM) Technique for Surface Strengthening of Titanium and Titanium Alloys: A Mini Review. J. Mater. Res. Technol. 2021, 11, 351–377. [Google Scholar] [CrossRef]
- Bano, S.; Rincon Romero, A.; Islam, M.T.; Grant, D.M.; Ahmed, I.; Hussain, T. Development and Characterization of Phosphate-Based Glass Coatings via Suspension High-Velocity Oxy-Fuel (SHVOF) Thermal Spray Process. J. Therm. Spray Technol. 2021, 30, 1862–1874. [Google Scholar] [CrossRef]
- Blum, M.; Sayed, M.; Mahmoud, E.M.; Killinger, A.; Gadow, R.; Naga, S.M. In Vitro Evaluation of Biologically Derived Hydroxyapatite Coatings Manufactured by High Velocity Suspension Spraying. J. Therm. Spray Technol. 2021, 30, 1891–1904. [Google Scholar] [CrossRef]
- Sharma, S.; Ganjoo, R.; Saha, S.K.; Kang, N.; Thakur, A.; Assad, H.; Kumar, A. Investigation of Inhibitive Performance of Betahistine Dihydrochloride on Mild Steel in 1M HCl Solution. J. Mol. Liq. 2021, 347, 118383. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A. Encapsulated Nanoparticles in Organic Polymers for Corrosion Inhibition; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128193594. [Google Scholar]
- Sandilya, S.; Thakur, A.; Suresh Singh, S.; Kumar, A. Recent Advances, Synthesis and Characterization of Bio-Based Based Polymers from Natural Sources. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 70–93. [Google Scholar]
- Sharma, S.; Ganjoo, R.; Saha, S.K.; Kang, N.; Thakur, A.; Assad, H.; Sharma, V.; Kumar, A. Experimental and Theoretical Analysis of Baclofen as a Potential Corrosion Inhibitor for Mild Steel Surface in HCl Medium. J. Adhes. Sci. Technol. 2021, 36, 2067–2092. [Google Scholar] [CrossRef]
- Kaur, M.; Thakur, A.; Sharma, V.; Kumar, A. Tricine as An Effective Corrosion Inhibitor For Aluminium In Acidic Medium. Think India J. 2019, 22, 3200–3219. [Google Scholar]
- Parveen, G.; Bashir, S.; Thakur, A.; Saha, S.K.; Banerjee, P.; Kumar, A. Experimental and Computational Studies of Imidazolium Based Ionic Liquid 1-Methyl- 3-Propylimidazolium Iodide on Mild Steel Corrosion in Acidic Solution Experimental and Computational Studies of Imidazolium Based Ionic Liquid 1-Methyl- 3-Propylimidazolium. Mater. Res. Express 2020, 7, 016510. [Google Scholar] [CrossRef]
- Bashir, S.; Lgaz, H.; Chung, I.M.; Kumar, A. Effective Green Corrosion Inhibition of Aluminium Using Analgin in Acidic Medium: An Experimental and Theoretical Study. Chem. Eng. Commun. 2020, 8, 1121–1130. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Sustainable Inhibitors for Corrosion Mitigation in Aggressive Corrosive Media: A Comprehensive Study. J. Bio-Tribo-Corros. 2021, 7, 67. [Google Scholar] [CrossRef]
- Benčina, M.; Resnik, M.; Starič, P.; Junkar, I. Use of Plasma Technologies for Antibacterial Surface Properties of Metals. Molecules 2021, 26, 1418. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, R.; Boparai, K.S. Development and Surface Improvement of FDM Pattern Based Investment Casting of Biomedical Implants: A State of Art Review. J. Manuf. Process. 2018, 31, 80–95. [Google Scholar] [CrossRef]
- Khor, K.A.; Gu, Y.W.; Pan, D.; Cheang, P. Microstructure and Mechanical Properties of Plasma Sprayed HA/YSZ/ Ti-6Al-4V Composite Coatings. Biomaterials 2004, 25, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cao, W.; Li, H. Biomedical Alloys and Physical Surface Modifications: A Mini-Review. Materials 2022, 15, 66. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J. Indus. Eng. Chem. 2019, 76, 91–115. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y.; Wang, L. Surface Modification of Titanium and Titanium Alloys: Technologies, Developments, and Future Interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Huang, S.; Liang, N.; Hu, Y.; Zhou, X.; Abidi, N. Polydopamine-Assisted Surface Modification for Bone Biosubstitutes. Biomed Res. Int. 2016, 2016, 2389895. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bao, C.; Wismeijer, D.; Wu, G. The Physicochemical/Biological Properties of Porous Tantalum and the Potential Surface Modification Techniques to Improve Its Clinical Application in Dental Implantology. Mater. Sci. Eng. C 2015, 49, 323–329. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Supriyanto, E.; Murugesan, S.; Balaji, A.; Asokan, M.K. Biomaterials in Cardiovascular Research: Applications and Clinical Implications. Biomed Res. Int. 2014, 2014, 459465. [Google Scholar] [CrossRef]
- Izman, S.; Rafiq, M.; Anwar, M.; Nazim, E.M.; Rosliza, R.; Shah, A.; Hass, M.A. Surface Modification Techniques for Biomedical Grade of Titanium Alloys: Oxidation, Carburization and Ion Implantation Processes. Titan. Alloy. Towar. Achiev. Enhanc. Prop. Divers. Appl. 2012, 42, 201–228. [Google Scholar] [CrossRef][Green Version]
- Thakur, A.; Kaya, S.; Abousalem, A.S.; Sharma, S.; Ganjoo, R.; Assad, H.; Kumar, A. Computational and Experimental Studies on the Corrosion Inhibition Performance of an Aerial Extract of Cnicus Benedictus Weed on the Acidic Corrosion of Mild Steel. Process Saf. Environ. Prot. 2022, 161, 801–818. [Google Scholar] [CrossRef]
- Schäfer, E. Effect of Physical Vapor Deposition on Cutting Efficiency of Nickel-Titanium Files. J. Endod. 2002, 28, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.J.; Bai, Y.; Ma, W.; Chen, W. dong Suspension Plasma-Sprayed Fluoridated Hydroxyapatite/Calcium Silicate Composite Coatings for Biomedical Applications. J. Therm. Spray Technol. 2019, 28, 1025–1038. [Google Scholar] [CrossRef]
- Hacking, S.A.; Zuraw, M.; Harvey, E.J.; Tanzer, M.; Krygier, J.J.; Bobyn, J.D. A Physical Vapor Deposition Method for Controlled Evaluation of Biological Response to Biomaterial Chemistry and Topography. J. Biomed. Mater. Res. Part A 2007, 82, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, X.; Ding, M.H.; Zheng, H.; Zhang, H.S.; Zhang, B.; Li, X.Q.; Wu, G.Y. Surface Modification of Biomedical AISI 316L Stainless Steel with Zirconium Carbonitride Coatings. Appl. Surf. Sci. 2015, 340, 113–119. [Google Scholar] [CrossRef]
- Yang, S.; Dillon, O.W.; Puleo, D.A.; Jawahir, I.S. Enhancement of Wear Resistance for Improved Functional Performance of Co-Cr-Mo Hip Implants through Cryogenic Surface Treatment: A Case Study. Mach. Sci. Technol. 2021, 25, 455–476. [Google Scholar] [CrossRef]
- Bagherifard, S.; Slawik, S.; Fernández-Pariente, I.; Pauly, C.; Mücklich, F.; Guagliano, M. Nanoscale Surface Modification of AISI 316L Stainless Steel by Severe Shot Peening. Mater. Des. 2016, 102, 68–77. [Google Scholar] [CrossRef]
- Chikarakara, E.; Naher, S.; Brabazon, D. Process Mapping of Laser Surface Modification of AISI 316L Stainless Steel for Biomedical Applications. Appl. Phys. A Mater. Sci. Process. 2010, 101, 367–371. [Google Scholar] [CrossRef]
- Ou, K.L.; Chou, H.H.; Liu, C.M.; Peng, P.W. Surface Modification of Austenitic Stainless Steel with Plasma Nitriding for Biomedical Applications. Surf. Coat. Technol. 2011, 206, 1142–1145. [Google Scholar] [CrossRef]
- Demirci, S.; Dalmış, R.; Dikici, T.; Tünçay, M.M.; Kaya, N.; Güllüoğlu, A.N. Effect of Surface Modifications of Additively Manufactured Ti-6Al-4V Alloys on Apatite Formation Ability for Biomedical Applications. J. Alloys Compd. 2021, 887, 161445. [Google Scholar] [CrossRef]
- González, J.E.; de Armas, G.; Negrin, J.; Beltrán, A.M.; Trueba, P.; Gotor, F.J.; Peón, E.; Torres, Y. Influence of Successive Chemical and Thermochemical Treatments on Surface Features of Ti6Al4V Samples Manufactured by SLM. Metals 2021, 11, 313. [Google Scholar] [CrossRef]
- Sánchez-Bodón, J.; Del Olmo, J.A.; Alonso, J.M.; Moreno-Benítez, I.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2022, 14, 165. [Google Scholar] [CrossRef]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired Surface Modification of Orthopedic Implants for Bone Tissue Engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef]
- Fox, K.E.; Tran, N.L.; Nguyen, T.A.; Nguyen, T.T.; Tran, P.A. Surface Modification of Medical Devices at Nanoscale-Recent Development and Translational Perspectives; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128134771. [Google Scholar]
- Hasan, A.; Saxena, V.; Pandey, L.M. Surface Functionalization of Ti6Al4V via Self-Assembled Monolayers for Improved Protein Adsorption and Fibroblast Adhesion. Langmuir 2018, 34, 3494–3506. [Google Scholar] [CrossRef]
- Lin, M.; Zhao, Y.; Wang, S.Q.; Liu, M.; Duan, Z.F.; Chen, Y.M.; Li, F.; Xu, F.; Lu, T.J. Recent Advances in Synthesis and Surface Modification of Lanthanide-Doped Upconversion Nanoparticles for Biomedical Applications. Biotechnol. Adv. 2012, 30, 1551–1561. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium-Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Martin, M.; Dahotre, N.B. Influence of Laser Surface Modification on Corrosion Behavior of Stainless Steel 316L and Ti-6Al-4V in Simulated Biofluid. Surf. Eng. 2005, 21, 297–306. [Google Scholar] [CrossRef]
- Salahinejad, E.; Hadianfard, M.J.; Macdonald, D.D.; Sharifi, S.; Mozafari, M.; Walker, K.J.; Tahmasbi Rad, A.; Madihally, S.V.; Vashaee, D.; Tayebi, L. Surface Modification of Stainless Steel Orthopedic Implants by Sol-Gel ZrTiO4 and ZrTiO4-PMMA Coatings. J. Biomed. Nanotechnol. 2013, 9, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Golenji, R.B.; Alipour, F.; Hadavi, M.M.; Mobasherpour, I. Hydroxyapatite/Hydroxyapatite-Magnesium Double-Layer Coatings as Potential Candidates for Surface Modification of 316 LVM Stainless Steel Implants. Ceram. Int. 2020, 46, 25374–25381. [Google Scholar] [CrossRef]
- Lu, T.; Qiao, Y.; Liu, X. Surface Modification of Biomaterials Using Plasma Immersion Ion Implantation and Deposition. Interface Focus 2012, 2, 325–336. [Google Scholar] [CrossRef]
- Noqta, O.A.; Aziz, A.A.; Usman, I.A.; Bououdina, M. Recent Advances in Iron Oxide Nanoparticles (IONPs): Synthesis and Surface Modification for Biomedical Applications. J. Supercond. Nov. Magn. 2019, 32, 779–795. [Google Scholar] [CrossRef]
- Lee, J.Y.; Aguilar, L.E.; Park, C.H.; Kim, C.S. UV Light Assisted Coating Method of Polyphenol Caffeic Acid and Mediated Immobilization of Metallic Silver Particles for Antibacterial Implant Surface Modification. Polymers 2019, 11, 1200. [Google Scholar] [CrossRef]
- Moxon, K.A.; Kalkhoran, N.M.; Markert, M.; Sambito, M.A.; McKenzie, J.L.; Webster, J.T. Nanostructured Surface Modification of Ceramic-Based Microelectrodes to Enhance Biocompatibility for a Direct Brain-Machine Interface. IEEE Trans. Biomed. Eng. 2004, 51, 881–889. [Google Scholar] [CrossRef]
- Variola, F.; Brunski, J.; Orsini, G.; De Oliveira, P.T.; Nanci, A. Nanoscale Surface Modifications of Medically-Relevant Metals: State-of-the Art and Perspectives. Nanoscale 2012, 3, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Amanov, A. A Promising Post-Additive Manufacturing Surface Modification for Tailoring Gradient Nanostructure and Harmonic Structure in Co–Cr–Mo Alloy. Vacuum 2020, 182, 109702. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Hu, X.; Dong, H. Surface Modification of a Medical Grade Co-Cr-Mo Alloy by Low-Temperature Plasma Surface Alloying with Nitrogen and Carbon. Surf. Coatings Technol. 2013, 232, 906–911. [Google Scholar] [CrossRef]
- Valkov, S.; Parshorov, S.; Andreeva, A.; Nikolova, M.; Petrov, P. Surface Modification of Co-Cr-Mo Alloys by Electron-Beam Treatment. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1056, 012008. [Google Scholar] [CrossRef]
- Amanov, A. Effect of Post-Additive Manufacturing Surface Modification Temperature on the Tribological and Tribocorrosion Properties of Co-Cr-Mo Alloy for Biomedical Applications. Surf. Coatings Technol. 2021, 421, 127378. [Google Scholar] [CrossRef]
- Mahapatro, A. Bio-Functional Nano-Coatings on Metallic Biomaterials. Mater. Sci. Eng. C 2015, 55, 227–251. [Google Scholar] [CrossRef]
- Chauhan, S.; Upadhyay, L.S.B. Biosynthesis of Iron Oxide Nanoparticles Using Plant Derivatives of Lawsonia Inermis (Henna) and Its Surface Modification for Biomedical Application. Nanotechnol. Environ. Eng. 2019, 4, 8. [Google Scholar] [CrossRef]
- Song, C.; Sun, W.; Xiao, Y.; Shi, X. Ultrasmall Iron Oxide Nanoparticles: Synthesis, Surface Modification, Assembly, and Biomedical Applications. Drug Discov. Today 2019, 24, 835–844. [Google Scholar] [CrossRef]
- Prodana, M.; Stoian, A.B.; Burnei, C.; Ionita, D. Innovative Coatings of Metallic Alloys Used as Bioactive Surfaces in Implantology: A Review. Coatings 2021, 11, 649. [Google Scholar] [CrossRef]
- Xue, T.; Attarilar, S.; Liu, S.; Liu, J.; Song, X.; Li, L.; Zhao, B.; Tang, Y. Surface Modification Techniques of Titanium and Its Alloys to Functionally Optimize Their Biomedical Properties: Thematic Review. Front. Bioeng. Biotechnol. 2020, 8, 603072. [Google Scholar] [CrossRef]
- Florea, D.A.; Albuleț, D.; Grumezescu, A.M.; Andronescu, E. Surface Modification—A Step Forward to Overcome the Current Challenges in Orthopedic Industry and to Obtain an Improved Osseointegration and Antimicrobial Properties. Mater. Chem. Phys. 2020, 243, 122579. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Hafezi, M.; Hadipour, M.; Nadernezhad, A.; Aghaie, E.; Behnamian, Y.; Abu Osman, N.A. Effect of Tricalcium Magnesium Silicate Coating on the Electrochemical and Biological Behavior of Ti-6Al-4V Alloys. PLoS ONE 2015, 10, e0138454. [Google Scholar] [CrossRef]
- Celesti, C.; Gervasi, T.; Cicero, N.; Giofrè, S.V.; Espro, C.; Piperopoulos, E.; Gabriele, B.; Mancuso, R.; Lo Vecchio, G.; Iannazzo, D. Titanium Surface Modification for Implantable Medical Devices with Anti-Bacterial Adhesion Properties. Materials 2022, 15, 3283. [Google Scholar] [CrossRef]
- Schweitzer, L.; Cunha, A.; Pereira, T.; Mika, K.; Do Rego, A.M.B.; Ferraria, A.M.; Kieburg, H.; Geissler, S.; Uhlmann, E.; Schoon, J. Preclinical in Vitro Assessment of Submicron-Scale Laser Surface Texturing on Ti6al4v. Materials 2020, 13, 5342. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. [Google Scholar] [CrossRef]
- Tortorella, S.; Buratti, V.V.; Maturi, M.; Sambri, L.; Franchini, M.C.; Locatelli, E. Surface-Modified Nanocellulose for Application in Biomedical Engineering and Nanomedicine: A Review. Int. J. Nanomedicine 2020, 15, 9909–9937. [Google Scholar] [CrossRef]
- Okazaki, Y.; Katsuda, S.I. Biological Safety Evaluation and Surface Modification of Biocompatible Ti–15Zr–4Nb Alloy. Materials 2021, 14, 731. [Google Scholar] [CrossRef]
- Delfi, M.; Ghomi, M.; Zarrabi, A.; Mohammadinejad, R.; Taraghdari, Z.B.; Ashrafizadeh, M.; Zare, E.N.; Agarwal, T.; Padil, V.V.T.; Mokhtari, B.; et al. Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis 2020, 2, 117–139. [Google Scholar] [CrossRef]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Applications of Conducting Polymers and Their Issues in Biomedical Engineering. J. R. Soc. Interface 2010, 7, S559–S579. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface Modification of Polymers by Plasma Treatments for the Enhancement of Biocompatibility and Controlled Drug Release. Surf. Coatings Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Govindarajan, T.; Shandas, R. A Survey of Surface Modification Techniques for Next-Generation Shape Memory Polymer Stent Devices. Polymers 2014, 6, 2309–2331. [Google Scholar] [CrossRef]
- Raval, N.; Kalyane, D.; Maheshwari, R.; Tekade, R.K. Surface Modifications of Biomaterials and Their Implication on Biocompatibility; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128144282. [Google Scholar]
- Kyzioł, K.; Kaczmarek, Ł.; Brzezinka, G.; Kyzioł, A. Structure, Characterization and Cytotoxicity Study on Plasma Surface Modified Ti-6Al-4V and γ-TiAl Alloys. Chem. Eng. J. 2014, 240, 516–526. [Google Scholar] [CrossRef]
- Shrivastava, V.P.; Subedi, D.P. Surface Modification of Polymeric Biomaterials by Coating Herbal Extracts. J. Nano Res. Adv. Mater. Polym. Sci. 2020, 1, 1–10. [Google Scholar]
- Xu, J.; Zhang, J.; Shi, Y.; Tang, J.; Huang, D.; Yan, M.; Dargusch, M.S. Surface Modification of Biomedical Ti and Ti Alloys: A Review on Current Advances. Materials 2022, 15, 1749. [Google Scholar] [CrossRef]
- Bouazizi, N.; Vieillard, J.; Samir, B.; Derf, F. Le Advances in Amine-Surface Functionalization of Inorganic Adsorbents for Water Treatment and Antimicrobial Activities: A Review. Polymers 2022, 14, 378. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (Peo) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Abazari, S.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Daroonparvar, M.; Berto, F. A Comprehensive Review on Surface Modifications of Biodegradable Magnesium-Based Implant Alloy: Polymer Coatings Opportunities and Challenges. Coatings 2021, 11, 747. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Jang, T.S.; Kim, D.; Yoon, C.B.; Han, G.; Kim, H.E.; Jung, H. Do Characterization of Titanium Surface Modification Strategies for Osseointegration Enhancement. Metals 2021, 11, 618. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Z. Biomedical Applications of Carbon Nanomaterials. In Biomedical Applications and Toxicology of Carbon Nanomaterials; Wiley-VCH: Hoboken, NJ, USA, 2016; pp. 131–162. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants-A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface Modification of Stainless Steel for Biomedical Applications: Revisiting a Century-Old Material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Witkowska, J.; Tarnowski, M.; Choińska, E.; Kulpa, M.; Szade, J.; Raugh, G.; Święszkowski, W.; Wierzchoń, T. Plasma Modification of Carbon Coating Produced by Rf Cvd on Oxidized Niti Shape Memory Alloy under Glow-Discharge Conditions. Materials 2021, 14, 4842. [Google Scholar] [CrossRef] [PubMed]
- Devgan, S.; Sidhu, S.S. Evolution of Surface Modification Trends in Bone Related Biomaterials: A Review. Mater. Chem. Phys. 2019, 233, 68–78. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-Surface Modification of Biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Muthukumarasamy, N.; Flores, M.; Rajesh, G.; Paraskevopoulos, K.M.; Pouroutzidou, G.K.; Theodorou, G.S.; Ioannidou, K.; Lusvarghi, L.; Velauthapillai, D.; et al. Incorporation of Nanosized Carbon over Hydroxyapatite (HAp) Surface Using DC Glow Discharge Plasma for Biomedical Application. Vacuum 2021, 190, 110300. [Google Scholar] [CrossRef]
- Ponnusamy, S.; Ali, H.H.; Dutt, F.; Rahman, S.U.; Salah, A.A.; Pipalia, M.; Baier, R.E.; Arany, P.R. Redox Signaling Induces Laminin Receptor Ribosomal Protein-SA Expression to Improve Cell Adhesion Following Radiofrequency Glow Discharge Treatments. Sci. Rep. 2022, 12, 7742. [Google Scholar] [CrossRef]
- Huang, R.; Liu, L.; Li, B.; Qin, L.; Huang, L.; Yeung, K.W.K.; Han, Y. Nanograins on Ti-25Nb-3Mo-2Sn-3Zr Alloy Facilitate Fabricating Biological Surface through Dual-Ion Implantation to Concurrently Modulate the Osteogenic Functions of Mesenchymal Stem Cells and Kill Bacteria. J. Mater. Sci. Technol. 2021, 73, 31–44. [Google Scholar] [CrossRef]
- Akpek, A. Analysis of Surface Properties of Ag and Ti Ion-Treated Medical Textiles by Metal Vapor Vacuum Arc Ion Implantation. Coatings 2021, 11, 102. [Google Scholar] [CrossRef]
- Tel, G. Ion Implantation of 109Ag Stable Isotope as a Tracer in SS316L Biomedical Implant for Failure Detection. Failure Detection. In Introduction to Distributed Algorithms; Cambridge University Press: Cambridge, UK, 2012; pp. 505–519. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Wang, J.; Xu, C.; He, J.; Bai, X. Improving Surface Properties of Fe-Based Laser Cladding Coating Deposited on a Carbon Steel by Heat Assisted Ultrasonic Burnishing. J. Mater. Res. Technol. 2021, 12, 100–116. [Google Scholar] [CrossRef]
- Dang, J.; An, Q.; Lian, G.; Zuo, Z.; Li, Y.; Wang, H.; Chen, M. Surface Modification and Its Effect on the Tensile and Fatigue Properties of 300M Steel Subjected to Ultrasonic Surface Rolling Process. Surf. Coat. Technol. 2021, 422, 127566. [Google Scholar] [CrossRef]
- de Oliveira, A.; Placias, F.G.; Sobrinho, A.S.d.S.; Leite, D.M.G.; Miyakawa, W.; Neto, J.J.; Koh, I.H.J.; Liberatore, A.M.A.; dos Santos, M.A.; Matieli, J.E.; et al. Secondary Ion Mass Spectrometry and Atomic Force Microscopy Analysis of Silver-Doped Diamond-like Carbon Films on Titanium Alloy (Ti6Al4V) for Possible Biomedical Application. Thin Solid Films 2021, 719, 138487. [Google Scholar] [CrossRef]
- Kandasamy, K.; Surendhiran, S.; Syed Khadar, Y.A.; Rajasingh, P. Ultrasound-Assisted Microwave Synthesis of CdS/MWCNTs QDs: A Material for Photocatalytic and Corrosion Inhibition Activity. Mater. Today Proc. 2020, 47, 757–762. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Du, Q.; Li, Z.S.; Yang, Q.Z. Mechanisms for the Direct Electron Transfer of Cytochrome c Induced by Multi-Walled Carbon Nanotubes. Sensors 2012, 12, 10450–10462. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S. Analytical Characterisation and Corrosion Behaviour of Bis-Aminosilane Coatings Modified with Carbon Nanotubes Activated with Rare-Earth Salts Applied on AZ31 Magnesium Alloy. Surf. Coat. Technol. 2008, 202, 4766–4774. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Zhu, G.; Lu, Z.; Zhang, Y.; Zhao, X.; Hou, B. Developing Multi-Wall Carbon Nanotubes/Fusion-Bonded Epoxy Powder Nanocomposite Coatings with Superior Anti-Corrosion and Mechanical Properties. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 628, 127309. [Google Scholar] [CrossRef]
- Prasannakumar, R.S.; Chukwuike, V.I.; Bhakyaraj, K.; Mohan, S.; Barik, R.C. Electrochemical and Hydrodynamic Flow Characterization of Corrosion Protection Persistence of Nickel/Multiwalled Carbon Nanotubes Composite Coating. Appl. Surf. Sci. 2020, 507, 145073. [Google Scholar] [CrossRef]
- Dalla, P.T.; Tragazikis, I.K.; Exarchos, D.A.; Dassios, K.G.; Barkoula, N.M.; Matikas, T.E. Effect of Carbon Nanotubes on Chloride Penetration in Cement Mortars. Appl. Sci. 2019, 9, 1032. [Google Scholar] [CrossRef]
- Pak, A.; Masoudi, M.; Elmkhah, H. Effect of Ultrasonic Peening on the Surface Properties of Nano-Layered CrN/CrAlN Coating Deposited by CAPVD Method on D3 Tool Steel. Surf. Interfaces 2022, 28, 101618. [Google Scholar] [CrossRef]
- Zare, E.N.; Lakouraj, M.M.; Moosavi, E. Poly (3-Aminobenzoic Acid) @ MWCNTs Hybrid Conducting Nanocomposite: Preparation, Characterization, and Application as a Coating for Copper Corrosion Protection. Compos. Interfaces 2016, 23, 571–583. [Google Scholar] [CrossRef]
- Yin, Y.; Lü, Y.; Wu, P.; Cai, C. Direct Electrochemistry of Redox Proteins and Enzymes Promoted by Carbon Nanotubes. Sensors 2005, 5, 220–234. [Google Scholar] [CrossRef]
- Hong, J.; Zhao, Y.X.; Xiao, B.L.; Moosavi-Movahedi, A.A.; Ghourchian, H.; Sheibani, N. Direct Electrochemistry of Hemoglobin Immobilized on a Functionalized Multi-Walled Carbon Nanotubes and Gold Nanoparticles Nanocomplex-Modified Glassy Carbon Electrode. Sensors 2013, 13, 8595–8611. [Google Scholar] [CrossRef]
- Nur-e-alam, M.; Basher, M.K.; Vasiliev, M.; Das, N. Physical Vapor-deposited Silver (Ag)-based Metal-dielectric Nanocomposites for Thin-film and Coating Applications. Appl. Sci. 2021, 11, 6746. [Google Scholar] [CrossRef]
- Vignesh, R.; Sakthinathan, G.; Velusamy, R.; Ramakrishna, S. An In-Vitro Evaluation Study on the Effects of Surface Modification via Physical Vapor Deposition on the Degradation Rates of Magnesium-Based Biomaterials. Surf. Coatings Technol. 2021, 411, 126972. [Google Scholar] [CrossRef]
- Arora, B.; Attri, P. Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. J. Compos. Sci. 2020, 4, 135. [Google Scholar] [CrossRef]
- Pinyou, P.; Blay, V.; Chansaenpak, K.; Lisnund, S. Paracetamol Sensing with a Pencil Lead Electrode Modified with Carbon Nanotubes and Polyvinylpyrrolidone. Chemosensors 2020, 8, 133. [Google Scholar] [CrossRef]
- Long, D.; Wu, G.; Zhu, G. Noncovalently Modified Carbon Nanotubes with Carboxymethylated Chitosan: A Controllable Donor-Acceptor Nanohybrid. Int. J. Mol. Sci. 2008, 9, 120–130. [Google Scholar] [CrossRef]
- Li, S.; Zhou, J.; Noroozifar, M.; Kerman, K. Gold-Platinum Core-Shell Nanoparticles with Thiolated Polyaniline and Multi-Walled Carbon Nanotubes for the Simultaneous Voltammetric Determination of Six Drug Molecules. Chemosensors 2021, 9, 24. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, C.; Zehri, A.; Ye, L.; Wang, N.; Zhmud, B.; Lu, H.; Liu, J. Surface Modification of Graphene for Use as a Structural Fortifier in Water-Borne Epoxy Coatings. Coatings 2019, 9, 754. [Google Scholar] [CrossRef]
- Siddiki, M.K.; Venkatesan, S.; Qiao, Q. Nb 2O 5 as a New Electron Transport Layer for Double Junction Polymer Solar Cells. Phys. Chem. Chem. Phys. 2012, 14, 4682–4686. [Google Scholar] [CrossRef] [PubMed]
- Shypylenko, A.; Pshyk, A.V.; Grześkowiak, B.; Medjanik, K.; Peplinska, B.; Oyoshi, K.; Pogrebnjak, A.; Jurga, S.; Coy, E. Effect of Ion Implantation on the Physical and Mechanical Properties of Ti-Si-N Multifunctional Coatings for Biomedical Applications. Mater. Des. 2016, 110, 821–829. [Google Scholar] [CrossRef]
- Pashkuleva, I.; Marques, A.P.; Vaz, F.; Reis, R.L. Surface Modification of Starch Based Biomaterials by Oxygen Plasma or UV-Irradiation. J. Mater. Sci. Mater. Med. 2010, 21, 21–32. [Google Scholar] [CrossRef]
- Amiri, H.; Mohammadi, I.; Afshar, A. Electrophoretic Deposition of Nano-Zirconia Coating on AZ91D Magnesium Alloy for Bio-Corrosion Control Purposes. Surf. Coatings Technol. 2017, 311, 182–190. [Google Scholar] [CrossRef]
- Pishbin, F.; Cordero-Arias, L.; Cabanas-Polo, S.; Boccaccini, A.R. Bioactive Polymer-Calcium Phosphate Composite Coatings by Electrophoretic Deposition; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9781782423164. [Google Scholar]
- Meng, X.; Kwon, T.Y.; Yang, Y.; Ong, J.L.; Kim, K.H. Effects of Applied Voltages on Hydroxyapatite Coating of Titanium by Electrophoretic Deposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 78, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Singh, I.; Boccaccini, A.R. Multi-Walled Carbon Nanotube-Reinforced Hydroxyapatite Layers on Ti6AI4V Medical Implants by Electrophoretic Deposition (EPD). Adv. Eng. Mater. 2008, 10, 131–138. [Google Scholar] [CrossRef]
- Drevet, R.; Ben Jaber, N.; Fauré, J.; Tara, A.; Ben Cheikh Larbi, A.; Benhayoune, H. Electrophoretic Deposition (EPD) of Nano-Hydroxyapatite Coatings with Improved Mechanical Properties on Prosthetic Ti6Al4V Substrates. Surf. Coat. Technol. 2016, 301, 94–99. [Google Scholar] [CrossRef]
- Šugár, P.; Ludrovcová, B.; Kalbáčová, M.H.; Šugárová, J.; Sahul, M.; Kováčik, J. Laser Surface Modification of Powder Metallurgy-Processed Ti-Graphite Composite Which Can Enhance Cells’ Osteo-Differentiation. Materials 2021, 14, 6067. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface Modified Techniques and Emerging Functional Coating of Dental Implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Raghavan, R.; RavindranP, A.; Purushothaman, P. Surface Treatments of Implant: A Review. Int. J. Sci. Healthc. Res. 2020, 5, 128. [Google Scholar]
- Koopaie, M.; Bordbar-Khiabani, A.; Kolahdooz, S.; Darbandsari, A.K.; Mozafari, M. Advanced Surface Treatment Techniques Counteract Biofilm-Associated Infections on Dental Implants. Mater. Res. Express 2020, 7, 015417. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Cinca, N.; Garcia-Giralt, N.; Dosta, S.; Cano, I.G.; Nogués, X.; Guilemany, J.M. In-Vitro Comparison of Hydroxyapatite Coatings Obtained by Cold Spray and Conventional Thermal Spray Technologies. Mater. Sci. Eng. C 2020, 107, 110306. [Google Scholar] [CrossRef]
- Pantaroto, H.N.; Cordeiro, J.M.; Pereira, L.T.; de Almeida, A.B.; Nociti Junior, F.H.; Rangel, E.C.; Azevedo Neto, N.F.; da Silva, J.H.D.; Barão, V.A.R. Sputtered Crystalline TiO2 Film Drives Improved Surface Properties of Titanium-Based Biomedical Implants. Mater. Sci. Eng. C 2021, 119, 111638. [Google Scholar] [CrossRef] [PubMed]
- Polo, T.O.B.; da Silva, W.P.; Momesso, G.A.C.; Lima-Neto, T.J.; Barbosa, S.; Cordeiro, J.M.; Hassumi, J.S.; da Cruz, N.C.; Okamoto, R.; Barão, V.A.R.; et al. Plasma Electrolytic Oxidation as a Feasible Surface Treatment for Biomedical Applications: An in Vivo Study. Sci. Rep. 2020, 10, 10000. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, N.C.; Chung, S.; Webster, T.J. New Nanoscale Surface Modifications of Metallic Biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Woodhead Publishing: Sawston, UK, 2015; pp. 249–273. [Google Scholar] [CrossRef]
- Liu, J.X.; Yang, D.Z.; Shi, F.; Cai, Y.J. Sol-Gel Deposited TiO2 Film on NiTi Surgical Alloy for Biocompatibility Improvement. Thin Solid Films 2003, 429, 225–230. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Cho, J.; Subhani, T.; Kaya, C.; Kaya, F. Electrophoretic Deposition of Carbon Nanotube-Ceramic Nanocomposites. J. Eur. Ceram. Soc. 2010, 30, 1115–1129. [Google Scholar] [CrossRef]
- Pishbin, F.; Simchi, A.; Ryan, M.P.; Boccaccini, A.R. A Study of the Electrophoretic Deposition of Bioglass® Suspensions Using the Taguchi Experimental Design Approach. J. Eur. Ceram. Soc. 2010, 30, 2963–2970. [Google Scholar] [CrossRef]
- Esteban, J.; Vallet-Regí, M.; Aguilera-Correa, J.J. Antibiotics-and Heavy Metals-Based Titanium Alloy Surface Modifications for Local Prosthetic Joint Infections. Antibiotics 2021, 10, 1270. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, H.; Kim, H.E.; Lee, E.J.; Jang, T.S.; Jung, H. Do Nano-topographical Control of Ti-nb-zr Alloy Surfaces for Enhanced Osteoblastic Response. Nanomaterials 2021, 11, 1507. [Google Scholar] [CrossRef] [PubMed]
- Chico, B.; Pérez-Maceda, B.T.; José, S.S.; Escudero, M.L.; García-Alonso, M.C.; Lozano, R.M. Corrosion Behaviour and J774a.1 Macrophage Response to Hyaluronic Acid Functionalization of Electrochemically Reduced Graphene Oxide on Biomedical Grade Cocr. Metals 2021, 11, 1078. [Google Scholar] [CrossRef]
- Rojas, O.; Prudent, M.; López, M.E.; Vargas, F.; Ageorges, H. Influence of Atmospheric Plasma Spraying Parameters on Porosity Formation in Coatings Manufactured from 45S5 Bioglass® Powder. J. Therm. Spray Technol. 2020, 29, 185–198. [Google Scholar] [CrossRef]
- Zhang, M.; Pu, X.; Chen, X.; Yin, G. In-Vivo Performance of Plasma-Sprayed CaO–MgO–SiO2-Based Bioactive Glass-Ceramic Coating on Ti–6Al–4V Alloy for Bone Regeneration. Heliyon 2019, 5, e02824. [Google Scholar] [CrossRef]
- Dudek, K.; Dulski, M.; Losiewicz, B. Functionalization of the NiTi Shape Memory Alloy Surface by HAp/SiO2/Ag Hybrid Coatings Formed on SiO2-TiO2 Glass Interlayer. Materials 2020, 13, 1648. [Google Scholar] [CrossRef] [PubMed]
- Cheang, P.; Khor, K.A. Addressing Processing Problems Associated with Plasma Spraying of Hydroxyapatite Coatings. Biomaterials 1996, 17, 537–544. [Google Scholar] [CrossRef]
- Mavis, B.; Taş, A.C. Dip Coating of Calcium Hydroxyapatite on Ti-6Al-4V Substrates. J. Am. Ceram. Soc. 2000, 83, 989–991. [Google Scholar] [CrossRef]
- Al-Amin, M.; Abdul-Rani, A.M.; Danish, M.; Rubaiee, S.; Bin Mahfouz, A.; Thompson, H.M.; Ali, S.; Unune, D.R.; Sulaiman, M.H. Investigation of Coatings, Corrosion and Wear Characteristics of Machined Biomaterials through Hydroxyapatite Mixed-Edm Process: A Review. Materials 2021, 14, 3597. [Google Scholar] [CrossRef] [PubMed]
- Maleki-Ghaleh, H.; Khalil-Allafi, J. Characterization, Mechanical and in Vitro Biological Behavior of Hydroxyapatite-titanium-carbon Nanotube Composite Coatings Deposited on NiTi Alloy by Electrophoretic Deposition. Surf. Coatings Technol. 2019, 363, 179–190. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Khalil-Allafi, J.; Horandghadim, N.; Keikhosravani, P.; Hosseini, M.G. Structural Characterization, Mechanical, and Electrochemical Studies of Hydroxyapatite-Titanium Composite Coating Fabricated Using Electrophoretic Deposition and Reaction Bonding Process. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Khalili, V.; Khalil-Allafi, J.; Maleki-Ghaleh, H.; Paulsen, A.; Frenzel, J.; Eggeler, G. The Influence of Si as Reactive Bonding Agent in the Electrophoretic Coatings of HA–Si–MWCNTs on NiTi Alloys. J. Mater. Eng. Perform. 2016, 25, 390–400. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Khalil-Allafi, J. Effect of Hydroxyapatite-Titanium-MWCNTs Composite Coating Fabricated by Electrophoretic Deposition on Corrosion and Cellular Behavior of NiTi Alloy. Mater. Corros. 2019, 70, 2128–2138. [Google Scholar] [CrossRef]

























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, A.; Kumar, A.; Kaya, S.; Marzouki, R.; Zhang, F.; Guo, L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12, 1459. https://doi.org/10.3390/coatings12101459
Thakur A, Kumar A, Kaya S, Marzouki R, Zhang F, Guo L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings. 2022; 12(10):1459. https://doi.org/10.3390/coatings12101459
Chicago/Turabian StyleThakur, Abhinay, Ashish Kumar, Savaş Kaya, Riadh Marzouki, Fan Zhang, and Lei Guo. 2022. "Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants" Coatings 12, no. 10: 1459. https://doi.org/10.3390/coatings12101459
APA StyleThakur, A., Kumar, A., Kaya, S., Marzouki, R., Zhang, F., & Guo, L. (2022). Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings, 12(10), 1459. https://doi.org/10.3390/coatings12101459