Microstructure and Corrosion Resistance of Two-Dimensional TiO2/MoS2 Hydrophobic Coating on AZ31B Magnesium Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2/MoS2 Films
2.3. Preparation of TiO2/MoS2 Films Modified by KH570
2.4. Analysis of Zeta Potential
2.5. Characteristics of Morphology, Chemical Composition, and Hydrophobic and Anticorrosion Properties
3. Results and Discussion
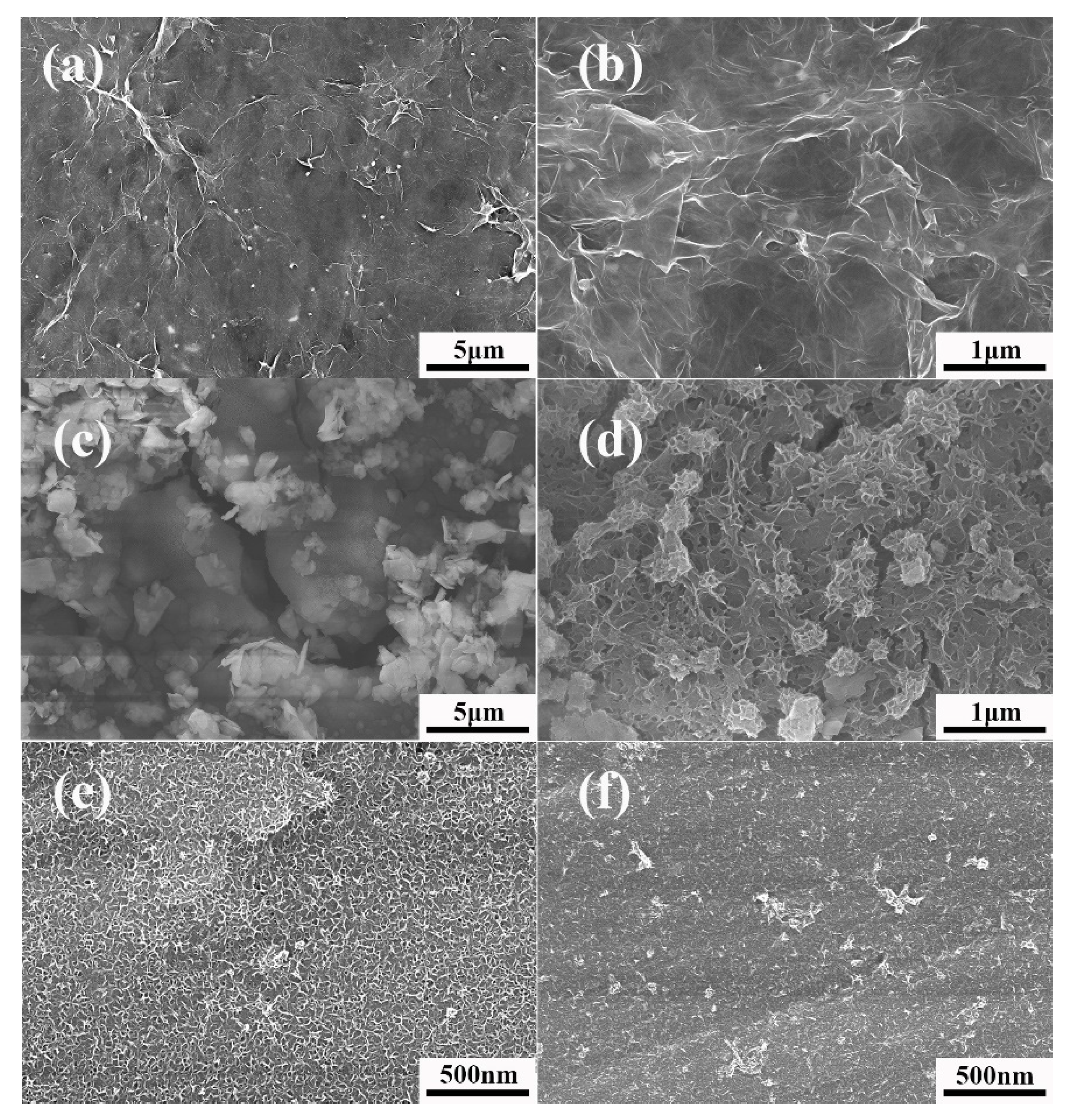
3.1. Morphology Analysis of TiO2/MoS2 Films
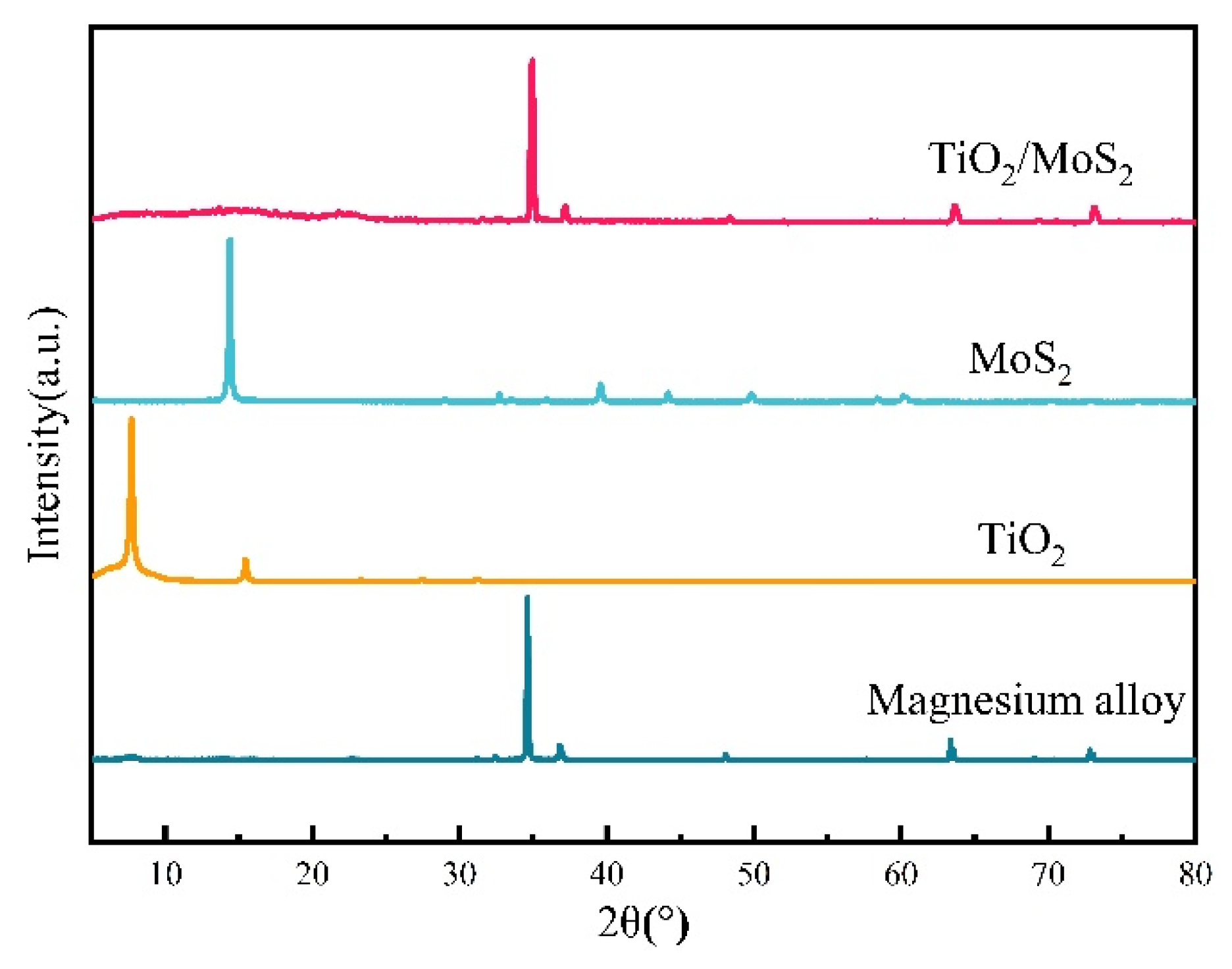
3.2. Compositional Analysis of TiO2/MoS2 Films
3.3. XPS of TiO2/MoS2 Coating
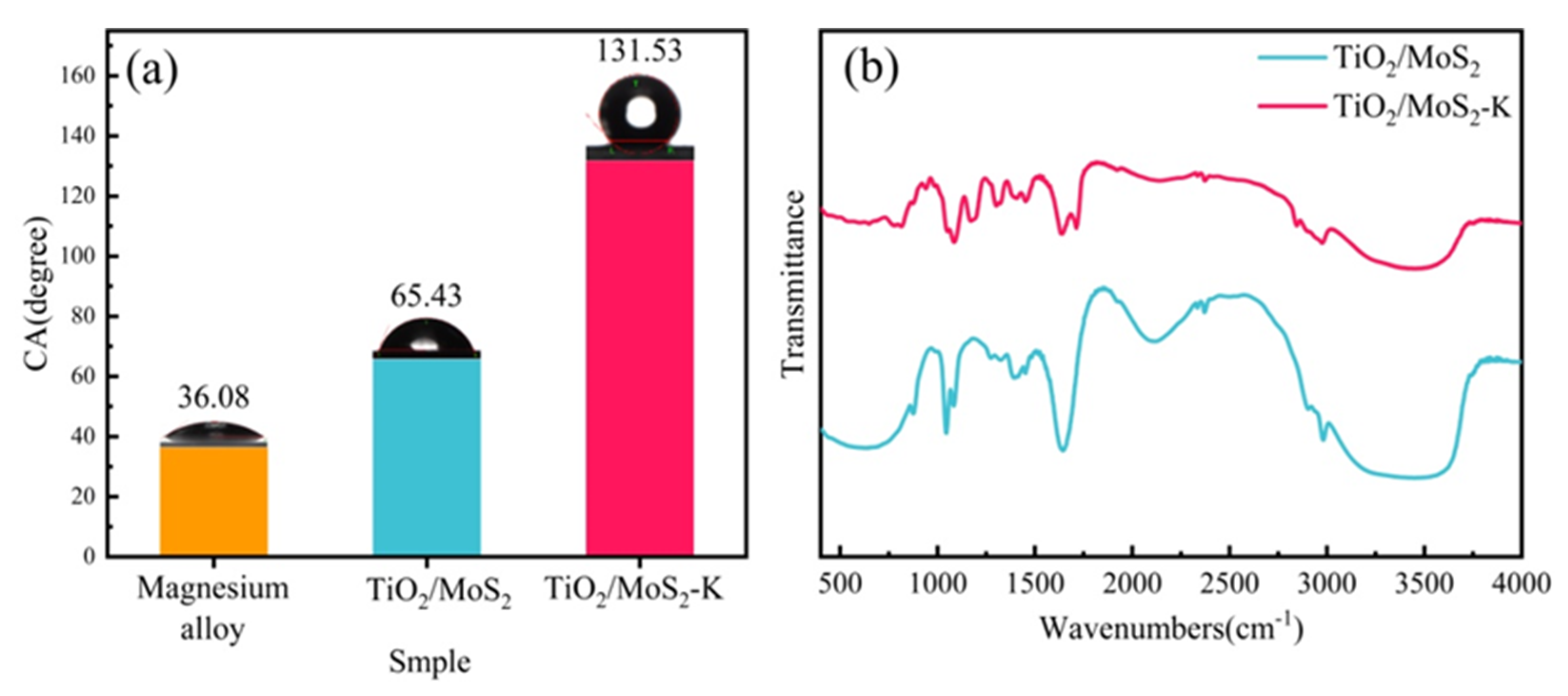
3.4. Hydrophilicity Test of TiO2/MoS2 Films
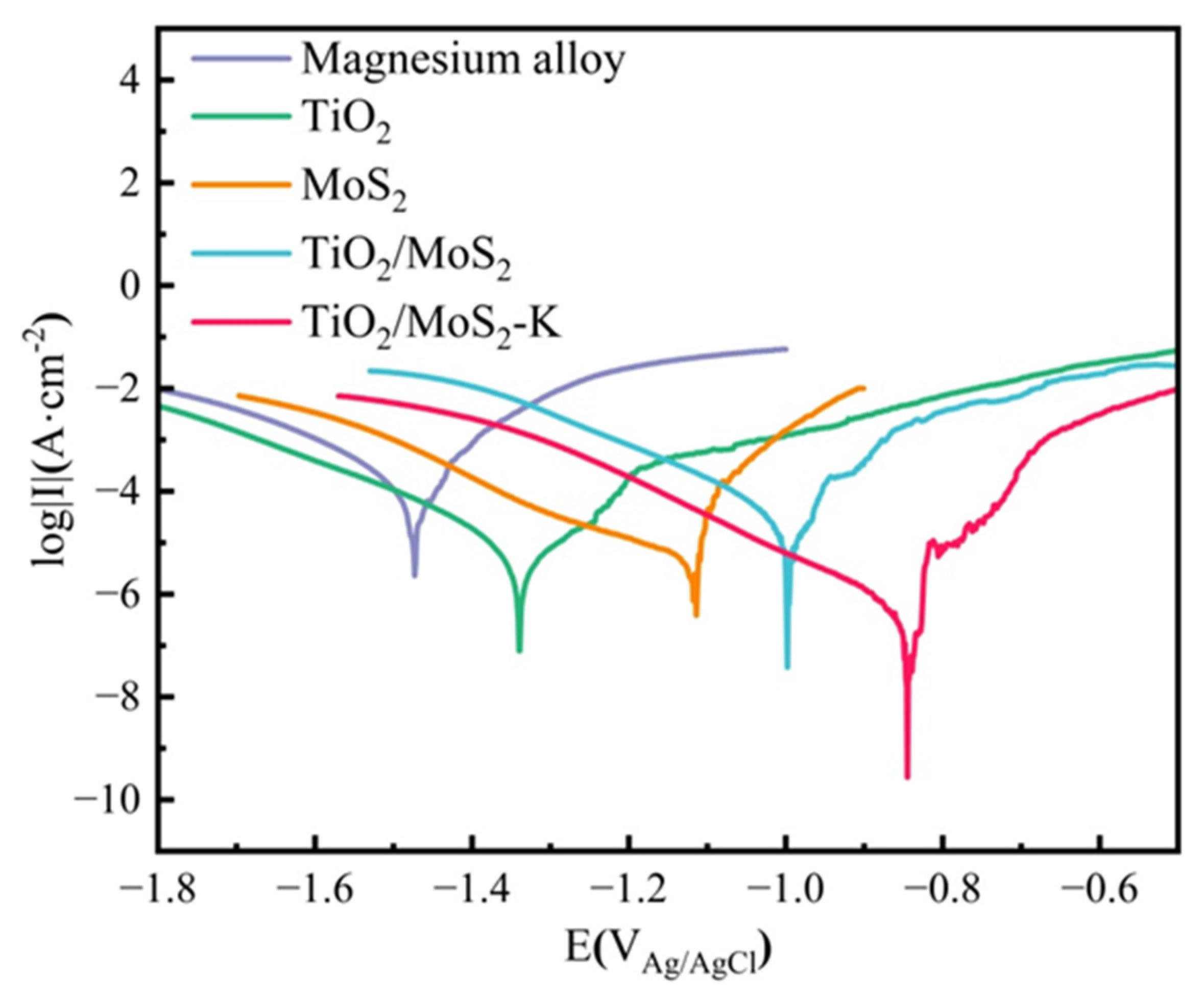
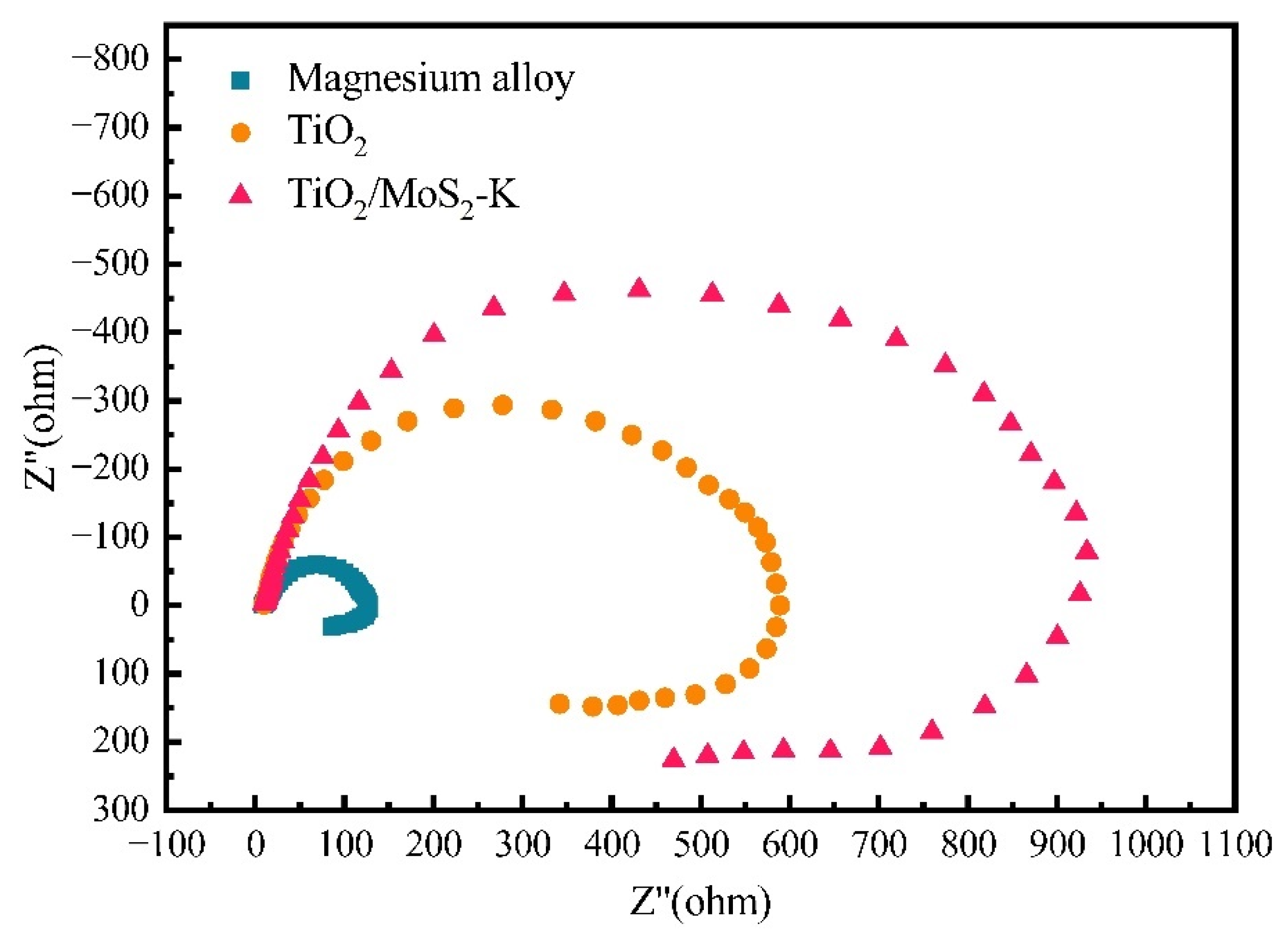
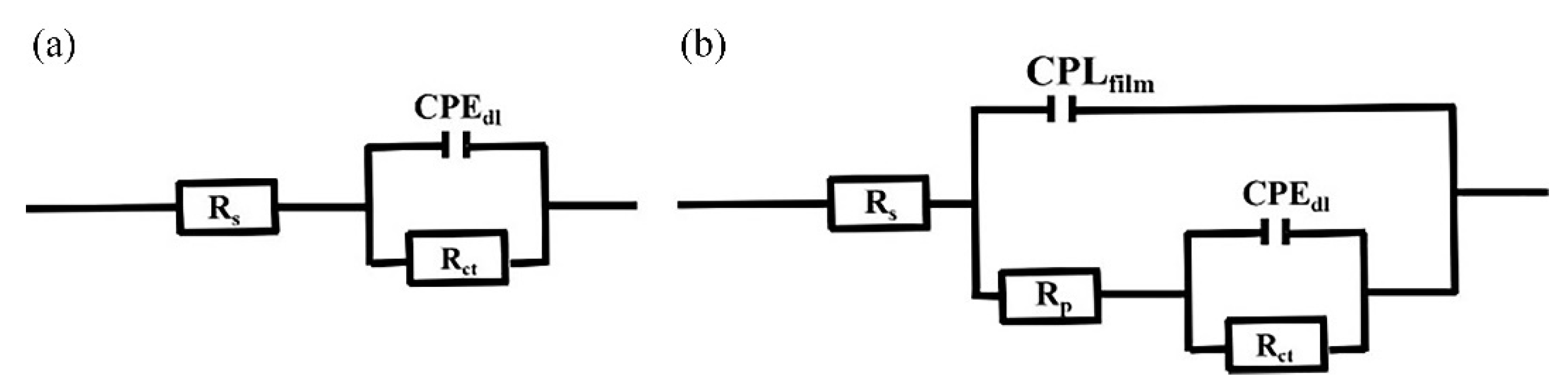
3.5. Electrochemical Test of TiO2/MoS2 Films
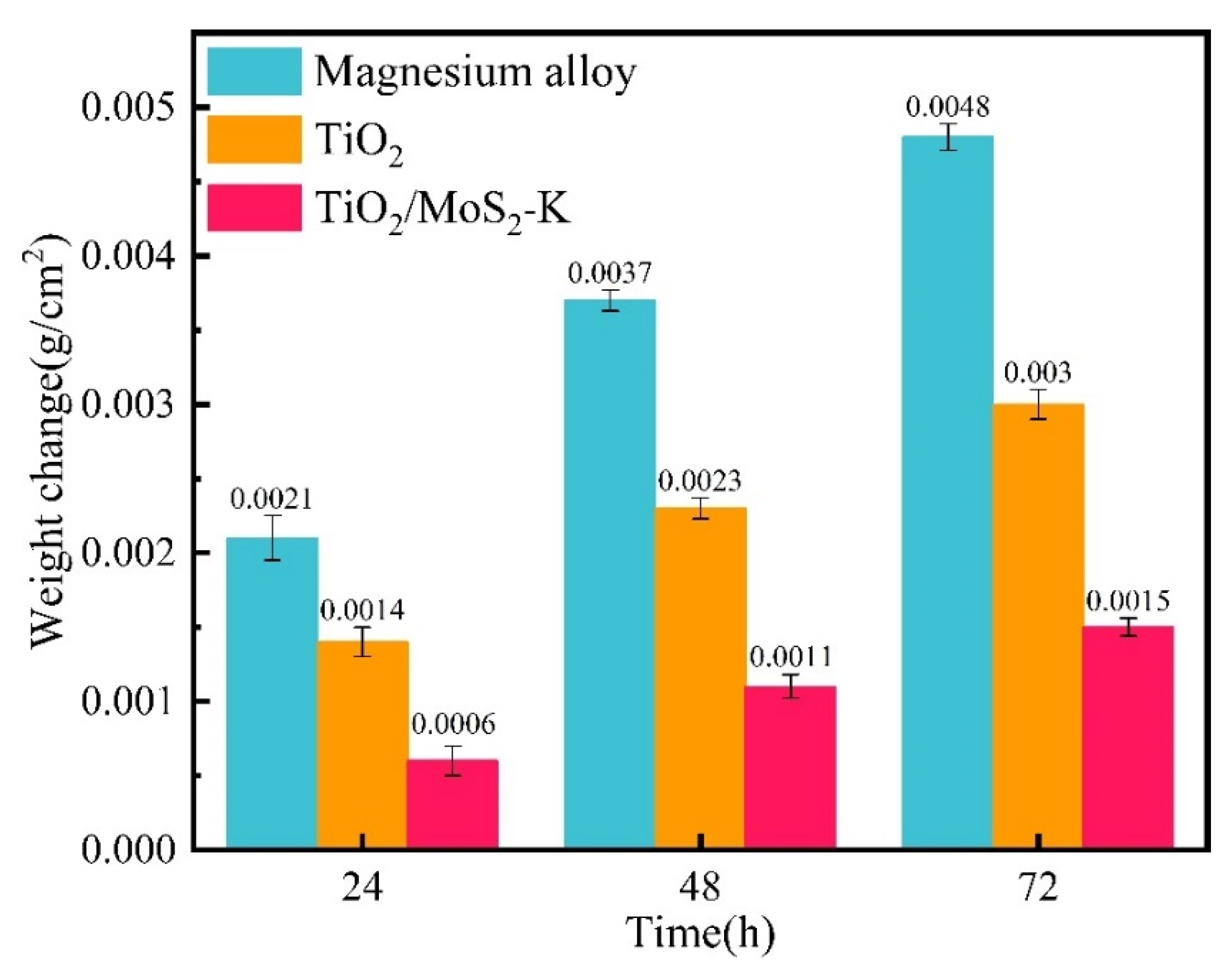
3.6. Salt Spray Corrosion Experiment of TiO2/MoS2 Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luo, K.; Zhang, L.; Wu, G.; Liu, W.; Ding, W. Effect of Y and Gd content on the microstructure and mechanical properties of Mg–Y–RE alloys. J. Magnes. Alloy. 2019, 7, 345–354. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy. 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Liu, H.; Tong, Z.; Yang, Y.; Zhou, W.; Chen, J.; Pan, X.; Ren, X. Preparation of phosphate conversion coating on laser surface textured surface to improve corrosion performance of magnesium alloy. J. Alloys Compd. 2021, 865, 158701. [Google Scholar] [CrossRef]
- Xianhua, C.; Yuxiao, G.; Fusheng, P. Research Progress in Magnesium Alloys as Functional Materials. Rare Met. Mater. Eng. 2016, 45, 2269–2274. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy. 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Siddique, S.; Bernussi, A.A.; Husain, S.W.; Yasir, M. Enhancing structural integrity, corrosion resistance and wear properties of Mg alloy by heat treated cold sprayed Al coating. Surf. Coat. Technol. 2020, 394, 125882. [Google Scholar] [CrossRef]
- Yeganeh, M.; Mohammadi, N. Superhydrophobic surface of Mg alloys: A review. J. Magnes. Alloy. 2018, 6, 59–70. [Google Scholar] [CrossRef]
- Chang, S.-H.; Niu, L.; Su, Y.; Wang, W.; Tong, X.; Li, G. Effect of the pretreatment of silicone penetrant on the performance of the chromium-free chemfilm coated on AZ91D magnesium alloys. Mater. Chem. Phys. 2016, 171, 312–317. [Google Scholar] [CrossRef]
- Ballam, L.R.; Arab, H.; Bestetti, M.; Franz, S.; Masi, G.; Sola, R.; Donati, L.; Martini, C. Improving the Corrosion Resistance of Wrought ZM21 Magnesium Alloys by Plasma Electrolytic Oxidation and Powder Coating. Materials 2021, 14, 2268. [Google Scholar] [CrossRef]
- Liu, C.; Liang, J.; Zhou, J.; Wang, L.; Li, Q. Effect of laser surface melting on microstructure and corrosion characteristics of AM60B magnesium alloy. Appl. Surf. Sci. 2015, 343, 133–140. [Google Scholar] [CrossRef]
- Lu, F.-F.; Ma, K.; Li, C.-X.; Yasir, M.; Luo, X.-T.; Li, C.-J. Enhanced corrosion resistance of cold-sprayed and shot-peened aluminum coatings on LA43M magnesium alloy. Surf. Coat. Technol. 2020, 394, 125865. [Google Scholar] [CrossRef]
- Yao, H.-L.; Yi, Z.-H.; Yao, C.; Zhang, M.-X.; Wang, H.-T.; Li, S.-B.; Bai, X.-B.; Chen, Q.-Y.; Ji, G.-C. Improved corrosion resistance of AZ91D magnesium alloy coated by novel cold-sprayed Zn-HA/Zn double-layer coatings. Ceram. Int. 2020, 46, 7687–7693. [Google Scholar] [CrossRef]
- Hosseini, M.R.; Ahangari, M.; Johar, M.H.; Allahkaram, S.R. Optimization of nano HA-SiC coating on AISI 316L medical grade stainless steel via electrophoretic deposition. Mater. Lett. 2021, 285, 129097. [Google Scholar] [CrossRef]
- Guan, S.; Hao, L.; Yoshida, H.; Itoi, T.; Cheng, Y.; Seki, S.; Nishina, Y.; Lu, Y. Enhanced photocatalytic activity and stability of TiO2/graphene oxide composites coatings by electrophoresis deposition. Mater. Lett. 2021, 286, 129258. [Google Scholar] [CrossRef]
- Sun, T.-Y.; Hao, Y.; Wu, Y.-H.; Zhao, W.-J.; Huang, L.-F. Corrosion Resistance of Ultrathin Two-Dimensional Coatings: First-Principles Calculations towards In-Depth Mechanism Understanding and Precise Material Design. Metals 2021, 11, 2011. [Google Scholar] [CrossRef]
- Mak, K.F.; Shan, J. Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides. Nat. Photonics 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Choudhary, N.; Jung, Y.; Thomas, J. Recent Advances in Two-Dimensional Nanomaterials for Supercapacitor Electrode Applications. ACS Energy Lett. 2018, 3, 482–495. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Bhandavat, R.; David, L.; Singh, G. Synthesis of Surface-Functionalized WS2 Nanosheets and Performance as Li-Ion Battery Anodes. J. Phys. Chem. Lett. 2012, 3, 1523–1530. [Google Scholar] [CrossRef]
- Ding, R.; Chen, S.; Lv, J.; Zhang, W.; Zhao, X.-d.; Liu, J.; Wang, X.; Gui, T.-J.; Li, B.-J.; Tang, Y.-Z.; et al. Study on graphene modified organic anti-corrosion coatings: A comprehensive review. J. Alloys Compd. 2019, 806, 611–635. [Google Scholar] [CrossRef]
- Seel, M.; Pandey, R. Proton and hydrogen transport through two-dimensional monolayers. 2D Mater. 2016, 3, 025004. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hou, T.; Wu, N.; Jiao, S.; Zhou, K.; Yin, J.; Suk, J.W.; Cui, X.; Zhang, M.; Li, S.; et al. Polycrystalline Few-Layer Graphene as a Durable Anticorrosion Film for Copper. Nano Lett. 2021, 21, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Bohm, S. Graphene against corrosion. Nat. Nanotechnol. 2014, 9, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Lim, A.T.O.; Huang, J. A cautionary note on graphene anti-corrosion coatings. Nat. Nanotechnol. 2017, 12, 834–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Xiao, X.; Wang, N.; Meng, G.; Gu, L. Graphene-like two-dimensional nanosheets-based anticorrosive coatings: A review. J. Mater. Sci. Technol. 2022, 129, 139–162. [Google Scholar] [CrossRef]
- Nurdiwijayanto, L.; Nishijima, H.; Miyake, Y.; Sakai, N.; Osada, M.; Sasaki, T.; Taniguchi, T. Solution-Processed Two-Dimensional Metal Oxide Anticorrosion Nanocoating. Nano Lett. 2021, 21, 7044–7049. [Google Scholar] [CrossRef]
- Shi, K.; Meng, X.; Xiao, S.; Chen, G.; Wu, H.; Zhou, C.; Jiang, S.; Chu, P.K. MXene Coatings: Novel Hydrogen Permeation Barriers for Pipe Steels. Nanomaterials 2021, 11, 2737. [Google Scholar] [CrossRef]
- Mujib, S.B.; Mukherjee, S.; Ren, Z.; Singh, G. Assessing corrosion resistance of two-dimensional nanomaterial-based coatings on stainless steel substrates. R. Soc. Open Sci. 2020, 7, 200214. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, W.; Wang, K.; Xu, J. GO-Ti3C2 two-dimensional heterojunction nanomaterial for anticorrosion enhancement of epoxy zinc-rich coatings. J. Hazard. Mater. 2021, 417, 126048. [Google Scholar] [CrossRef] [PubMed]
- Xi, K.; Wu, H.; Zhou, C.; Qi, Z.; Yang, K.; Fu, R.K.Y.; Xiao, S.; Wu, G.; Ding, K.; Chen, G.; et al. Improved corrosion and wear resistance of micro-arc oxidation coatings on the 2024 aluminum alloy by incorporation of quasi-two-dimensional sericite microplates. Appl. Surf. Sci. 2022, 585, 152693. [Google Scholar] [CrossRef]
- Wang, X. Preparation and Corrosion Resistance of AKT-Waterborne Polyurethane Coating. Int. J. Electrochem. Sci. 2020, 15, 1450–1464. [Google Scholar] [CrossRef]
- Kavimani, V.; Prakash, K.S.; Gunashri, R.; Sathish, P. Corrosion protection behaviour of r-GO/TiO2 hybrid composite coating on Magnesium substrate in 3.5 wt.% NaCl. Prog. Org. Coat. 2018, 125, 358–364. [Google Scholar] [CrossRef]
- Li, Z.; Ding, S.; Kong, L.; Wang, X.; Ashour, A.; Han, B.; Ou, J. Nano TiO2-engineered anti-corrosion concrete for sewage system. J. Clean. Prod. 2022, 337, 130508. [Google Scholar] [CrossRef]
- Kumar, A.M.; Khan, A.; Hussein, M.A.; Khan, M.Y.; Dafalla, H.; Suresh, B.; Ramakrishna, S. Hybrid nanocomposite coatings from PEDOT and BN-TiO2 nanosheets: Enhanced invitro corrosion resistance, wettability and biocompatibility for biomedical applications. Prog. Org. Coat. 2022, 170, 106946. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Lei, S.; Su, Y.; Yang, W.; Wang, J.; Qin, G.; Li, W. Formation and oxidation behavior of TiO2 modified Al2O3-Nb2O5/NbAl3 composite coating prepared by two-step methods. Surf. Coat. Technol. 2022, 433, 128081. [Google Scholar] [CrossRef]
- Devikala, S.; Kamaraj, P.; Arthanareeswari, M. Corrosion resistance behavior of PVA/TiO2 composite in 3.5% NaCl. Mater. Today Proc. 2018, 5, 8672–8677. [Google Scholar] [CrossRef]
- Rostami, S.; Mahdavi, S.; Alinezhadfar, M.; Mohseni, A. Tribological and corrosion behavior of electrochemically deposited Co/TiO2 micro/nano-composite coatings. Surf. Coat. Technol. 2021, 423, 127591. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y. Corrosion behaviour of Zn-Ni alloy and Zn-Ni-nano-TiO2 composite coatings electrodeposited from ammonium citrate baths. Process. Saf. Environ. Prot. 2020, 141, 366–379. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Fan, J.; Lotya, M.; O’Neill, A.; Fox, D.; Feng, Y.; Zhang, X.; Jiang, B.; Zhao, Q.; et al. Ultrafast saturable absorption of two-dimensional MoS2 nanosheets. ACS Nano 2013, 7, 9260–9267. [Google Scholar] [CrossRef]
- Asan, G.; Asan, A.; Çelikkan, H. The effect of 2D-MoS2 doped polypyrrole coatings on brass corrosion. J. Mol. Struct. 2020, 1203, 127318. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Chen, C.; Wu, Y.; Chen, J. MoS2 nanosheets modified SiO2 to enhance the anticorrosive and mechanical performance of epoxy coating. Prog. Org. Coat. 2019, 132, 316–327. [Google Scholar] [CrossRef]
- Hu, S.; Muhammad, M.; Wang, M.; Ma, R.; Du, A.; Fan, Y.; Cao, X.; Zhao, X. Corrosion resistance performance of nano-MoS2-containing zinc phosphate coating on Q235 steel. Mater. Lett. 2020, 265, 127256. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.; Xiao, G.; Xia, Y.; Li, H.; He, Z. Two-dimensional hybrid materials: MoS2-RGO nanocomposites enhanced the barrier properties of epoxy coating. Appl. Surf. Sci. 2018, 444, 511–521. [Google Scholar] [CrossRef]
- Sasaki, T.; Kooli, F.; Iida, M.; Michiue, Y.; Takenouchi, S.; Yajima, Y.; Izumi, F.; Chakoumakos, B.C.; Watanabe, M. A Mixed Alkali Metal Titanate with the Lepidocrocite-like Layered Structure. Preparation, Crystal Structure, Protonic Form, and Acid−Base Intercalation Properties. Chem. Mater. 1998, 10, 4123–4128. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Yuen, M.F.; Ng, T.-W.; Tang, Y.; Lee, C.-S.; Chen, X.; Zhang, W. Hierarchical composite structure of few-layers MoS2 nanosheets supported by vertical graphene on carbon cloth for high-performance hydrogen evolution reaction. Nano Energy 2015, 18, 196–204. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Bala, N. Corrosion behavior and characterization of HA/Fe3O4/CS composite coatings on AZ91 Mg alloy by electrophoretic deposition. Mater. Chem. Phys. 2019, 237, 121884. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.; Finklea, H.; Liu, X. A review of electrophoretic deposition of metal oxides and its application in solid oxide fuel cells. Adv. Colloid Interface Sci. 2020, 276, 102102. [Google Scholar] [CrossRef] [PubMed]
- Hoshide, T.; Zheng, Y.; Hou, J.; Wang, Z.; Li, Q.; Zhao, Z.; Ma, R.; Sasaki, T.; Geng, F. Flexible Lithium-Ion Fiber Battery by the Regular Stacking of Two-Dimensional Titanium Oxide Nanosheets Hybridized with Reduced Graphene Oxide. Nano Lett. 2017, 17, 3543–3549. [Google Scholar] [CrossRef]
- Maluangnont, T.; Matsuba, K.; Geng, F.; Ma, R.; Yamauchi, Y.; Sasaki, T. Osmotic Swelling of Layered Compounds as a Route to Producing High-Quality Two-Dimensional Materials. A Comparative Study of Tetramethylammonium versus Tetrabutylammonium Cation in a Lepidocrocite-type Titanate. Chem. Mater. 2013, 25, 3137–3146. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, D.; Liu, X.; Zhang, Y. Recent progress in superhydrophobic coating on Mg alloys: A general review. J. Magnes. Alloy. 2021, 9, 1471–1486. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, H.; Zeze, A.L.P.; Liu, X.; Tao, M. Coating performance, durability and anti-corrosion mechanism of organic modified geopolymer composite for marine concrete protection. Cem. Concr. Compos. 2022, 129, 104495. [Google Scholar] [CrossRef]
- Gong, C.; Jianzhong, L.; Cuicui, C.; Changfeng, L.; Liang, S. Study on silane impregnation for protection of high performance concrete. Procedia Eng. 2012, 27, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, Y.; Zou, Y.; Wu, Y.; Chen, G.; Ouyang, J.; Jia, D.; Zhou, Y. A self-adjusting PTFE/TiO2 hydrophobic double-layer coating for corrosion resistance and electrical insulation. Chem. Eng. J. 2020, 402, 126116. [Google Scholar] [CrossRef]
- Parichehr, R.; Dehghanian, C.; Nikbakht, A. Preparation of PEO/silane composite coating on AZ31 magnesium alloy and investigation of its properties. J. Alloys Compd. 2021, 876, 159995. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Q.; Chen, R.; Liu, J.; Zhang, H.; Li, R.; Takahashi, K.; Liu, P.; Wang, J. Fabrication of ZIF-8@SiO2 Micro/Nano Hierarchical Superhydrophobic Surface on AZ31 Magnesium Alloy with Impressive Corrosion Resistance and Abrasion Resistance. ACS Appl. Mater. Interfaces 2017, 9, 11106–11115. [Google Scholar] [CrossRef]
- Zhang, G.; Qin, S.; Yan, L.; Zhang, X. Simultaneous improvement of electromagnetic shielding effectiveness and corrosion resistance in magnesium alloys by electropulsing. Mater. Charact. 2021, 174, 111042. [Google Scholar] [CrossRef]
- Liu, H.; Tong, Z.; Zhou, W.; Yang, Y.; Jiao, J.; Ren, X. Improving electrochemical corrosion properties of AZ31 magnesium alloy via phosphate conversion with laser shock peening pretreatment. J. Alloys Compd. 2020, 846, 155837. [Google Scholar] [CrossRef]



| Sample | Zeta Potential/mV |
|---|---|
| TiO2 | −0.511 |
| MoS2 | −0.356 |
| Sample | Ecorr/(VAg/AgCl) | Icorr/(A·cm−2) |
|---|---|---|
| Magnesium alloy | −1.47 | 6.81 × 10−4 |
| TiO2 | −1.34 | 5.31 × 10−6 |
| MoS2 | −1.11 | 2.19 × 10−5 |
| TiO2/MoS2 | −1.00 | 3.69 × 10−7 |
| TiO2/MoS2-K | −0.85 | 6.73 × 10−8 |
| Sample | Rs (Ω·cm2) | CPEfilm (F/cm2) | Rp (Ω·cm2) | CPEdl-T (F/cm2) | CPEdl-P (F/cm2) | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|
| Magnesium alloy | 10.2 | - | - | 7.11 × 10−6 | - | 111.9 |
| TiO2 | 8.97 | 1.27 × 10−6 | 16.96 | 1.66 × 10−6 | 0.98 | 659.6 |
| TiO2/MoS2-K | 9.25 | 8.92 × 10−6 | 19.29 | 1.87 × 10−6 | 0.95 | 871.9 |
| Point | O | Mg | Mn | Ti | Na | Cl | Mo | S |
|---|---|---|---|---|---|---|---|---|
| A | 57.64 | 26.18 | 12.02 | 0 | 2.79 | 1.37 | 0 | 0 |
| B | 59.23 | 25.43 | 11.08 | 0 | 2.51 | 1.75 | 0 | 0 |
| C | 55.38 | 25.28 | 12.60 | 2.40 | 3.15 | 1.19 | 0 | 0 |
| D | 53.85 | 25.32 | 11.06 | 3.99 | 3.98 | 1.80 | 0 | 0 |
| E | 55.62 | 25.16 | 11.10 | 3.69 | 2.17 | 1.12 | 0.22 | 0.92 |
| F | 56.38 | 25.09 | 11.20 | 2.57 | 2.23 | 1.33 | 0.19 | 1.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, L.; Wu, H.; Mao, G.; Li, Z.; Zhang, L.; Liu, Q. Microstructure and Corrosion Resistance of Two-Dimensional TiO2/MoS2 Hydrophobic Coating on AZ31B Magnesium Alloy. Coatings 2022, 12, 1488. https://doi.org/10.3390/coatings12101488
Lai L, Wu H, Mao G, Li Z, Zhang L, Liu Q. Microstructure and Corrosion Resistance of Two-Dimensional TiO2/MoS2 Hydrophobic Coating on AZ31B Magnesium Alloy. Coatings. 2022; 12(10):1488. https://doi.org/10.3390/coatings12101488
Chicago/Turabian StyleLai, Longjie, Heng Wu, Guobing Mao, Zhengdao Li, Li Zhang, and Qi Liu. 2022. "Microstructure and Corrosion Resistance of Two-Dimensional TiO2/MoS2 Hydrophobic Coating on AZ31B Magnesium Alloy" Coatings 12, no. 10: 1488. https://doi.org/10.3390/coatings12101488






