Abstract
This work considers the ability of carbon-nanotube (CNT)-coated plasma to inactivate bioaerosols. Escherichia coli (E. coli) and λ virus phage were chosen as the challenge bioaerosols. A test chamber was used to simulate an indoor environment and to control the air exchange rate (ACH). The results demonstrated that CNT-coated plasma had a significant antimicrobial effect on both bacterial and viral bioaerosols. The experimental results revealed that CNT-coated plasma under an operating voltage of 6.0 kV and an ACH of 0.5 h−1 had significantly higher bioaerosol inactivating ability (kCNT, 0.24 and 0.23 min−1 for E. coli and λ virus phage, respectively) compared to the results without using CNT-coated plasma (kn, 0.09 and 0.08 min−1 for E. coli and λ virus phage, respectively). Under the higher ACH condition, the system demonstrated higher bioaerosol removal efficiency due to the mechanical effect of clean air exchange. Increasing flow rate and operating voltage could raise the inactivating ability of the CNT-coated plasma system. A CNT-coated plasma air-cleaning device was tested in a real indoor medical environment and yielded bacterial bioaerosol removal efficiency in the range from 70% to 80% within 6 h of operation (7.5 Lpm and 6.0 kV), which suggests that CNT-coated plasma treatment has the potential for further application in improving indoor air quality.
1. Introduction
Bioaerosols, as the major indoor contamination sources, can cause lots of respiratory system problems, like allergic syndromes, toxic reactions, and infectious diseases, and obviously affect indoor air quality in modern society [1]. Among these bioaerosols, bacteria, and viruses, as the major pathogenic components, cause serious health damage and cost consumption in health-care facilities. For example, the SARS-CoV-2 nosocomial infection, has been discovered in numerous health-care facilities on a global scale [2,3,4]. In hospital investigations, Escherichia coli (E. coli) was frequently isolated as a potentially antibiotic-resistant infectious agent [5,6,7]. For protecting patients and workers in health-care facilities, plenty of physical and chemical air-cleaning techniques such as negative ion releasing, electrostatic precipitation, particle filtration, ozone generation, nanoparticle, photocatalytic oxidation, ultraviolet germicidal irradiation, and disinfectant spraying, have been developed and evaluated to inactivate indoor bioaerosols contamination [8].
In the experiment of Li and Wang [9], they demonstrated a bactericidal effect against bacteria and fungal bioaerosols that began to become significant while the ozone concentration was increased to 10 ppm in the air, which is higher than the upper limit of 0.1 ppm recommended by the U.S. National Institute of Occupational Safety and Health. Regarding the effectiveness of titanium dioxide photocatalytic filters in controlling bioaerosols, Lin and Li [10] found that they were weak in removing bioaerosols at wavelengths under 365 nm ultraviolet light. When Lin and Li [11] used ultraviolet germicidal irradiation to inactive indoor bacterial and fungal bioaerosols, they found that it had the best removal efficiency for E. coli bioaerosols and the least-satisfactory performance for controlling fungal spores of Penicillium citrinum. Although ultraviolet germicidal irradiation significantly decreases the concentration of indoor bioaerosols greatly after use in an indoor environment for 10 min, it may also produce a secondary pollutant (ozone and nitrogen oxides) and potentially cause health risk to users. Gosh et al. [12] utilized an Ag-nanoparticle capped graphene oxide (GO) nano-sheet based supramolecular hydrogel to inactivate E. coli and Bacillus subtilis in broth. The results show that GO dispersed supramolecular hydrogel capped silver nanoparticles are extremely potent for inducing lethality in pathogenic bacteria. Furthermore, supramolecular hydrogel capped nano systems have been shown to produce a variety of bioactivities related to microorganism inactivation [13,14]. Chuang et al. [15] reported that spraying electrolyzed water containing 100–200 ppm free available chlorine achieves significant airborne bacterial contamination reducing effects. However, this liquid-disinfectant intervening technique is not suitable for peroneal-occupied space because of a potential visual-annoying problem. Hsu et al. [16] used the chitosan-coated antimicrobial filter to inactivate E. coli and Bacillus subtilis bioaerosols in the environmental-controlled chamber. The results revealed that 2.5% (w/w) of chitosan-coated antimicrobial filters have significantly more additional bioaerosol inactivating ability than conventional non-chemical coated air filters. Nevertheless, research by Hsu et al. also indicated indoor air parameters including face velocity and relative humidity, which are difficult to carefully control but have a significant influence on bioaerosol survival rates.
In recent years, non-thermal plasma systems have attracted attention and have been widely applied in environmental engineering to inactivate various pollutants. In particular, the capability of nonthermal plasma to remove gas phase pollutants has been extensively examined [17,18]. Non-thermal plasma has also been proven to be useful in inactivating many microorganisms such as viruses, Gram-negative, and Gram-positive bacteria on surfaces and aqueous solutions. [19,20,21,22,23,24].
Recently, non-thermal plasma has been applied to inactivate indoor bioaerosols. Gallagher et al. [25] have used it to achieve a 5-log reduction of indoor E. coli bioaerosol during a 2 min testing time. The most common ways to induce plasma inactivation mechanisms for eliminating microorganisms are UV-C and VUV (vacuum ultraviolet) irradiation in a wavelength range below 300 nm, which produces diffusion of oxidative species (O, O3, and O2*) or oxygen-containing radicals (e.g., OH and NO), a bombardment of the cell wall by charged particles (electrons and ions), and localized, periodic, and short-term heating of microorganisms. All the various plasma active components can synergistically interact. Furthermore, this work identified the OH radical as the main component of the inactivation mechanism during microbial inhibition.
Liang et al. [26] identified that dielectric barrier discharge (DBD) nonthermal plasma could inactivate bioaerosols. The result showed that less than 2% of B. subtilis bioaerosols survived the plasma treatment for 0.12 s, while none of the P. fluorescens bioaerosols survived. This work demonstrated that bacterial bioaerosols suffered significant viability loss and membrane and DNA damage. Helbich et al. [27] used non-thermal plasma to inactivate bacterial bioaerosols in a downstream airflow in a biological process. The plasma system was able to remove approximately 1–4 log units. Bisag et al. [28] applied cold atmospheric plasma devices to inactivate SARS-CoV-2 bioaerosols effectively. Cold atmospheric plasma can generate bioactive components including reactive oxygen and nitrogen species, electric fields, and UV-rays.
Traditionally, non-thermal plasma systems require high operating voltage [26] and have high ozone generation [21]. In 1991, the authors of [29] utilized a carbon-nanotube as the discharging electrode under plasma processing. In addition, according to the previous study, the carbon nanotube has excellent field electron emission abilities [30]. Thus, using the carbon nanotube as the charging electrode in a plasma system could enable a decrease in the threshold operating voltage and ozone concentration.
Previously, we used a discharging electrode coated with carbon nanotube for plasma processing under continuous flow bioaerosol inactivation [31]. This study went on to develop a novel self-fabricated indoor air purifying apparatus using carbon-nanotube coated plasma and install it in an environmental-controlled testing chamber to better understand its performance for indoor cleaning applications.
Therefore, this work aimed to investigate the inactivating performance of the carbon-nanotube coated plasma air-cleaning apparatus (CNT-coated plasma apparatus), characterized by its overall purifying performance in inactivating bacterial and viral bioaerosols.
The inactivating ability of this apparatus was determined by the survival of E. coli and lambda phage (λ virus, non-infectious virus) bioaerosols in the testing chamber under various operating conditions. E. coli, as mentioned above, is usually found in healthcare facilities and should be highly regulated due to its antibiotic-resistance risk [5,6,7]. λ virus, is similar to SARS-CoV-2 in size and was utilized as the surrogate for studying airborne dispersion of SARS-CoV-2 in a previous study [32]. Three essential operating factors of the CNT plasma apparatus that majorly affect inactivating performance: air exchange rate (ACH), operating voltage, and pumping flowrate, were included and explored in the study.
2. Theory
Bioaerosol is suspended particles in the air, and its viable concentration might be affected by physical (gravity deposition, wall loss, air mixing, ventilating dilution) and biological (desiccation, external stress during sampling) factors. For consistently determining the inactivating performance of CNT plasma apparatus, the following equations were used in this experiment to analyze the bioaerosol concentration decay, which included physical interfering factors:
where C is the bioaerosol concentration (CFU/m3 for E. coli and PFU/ for λ virus); C0 and Ct are the initial concentration of target bioaerosols and the concentration at time t, respectively; t is the residence time (min); C0, Ct, and t were all measured in each experimental set. Then, k is the first-order decaying coefficient of bioaerosol concentration (min−1) as a regression coefficient in an exponential regression analysis specified by Equation (2); and kn and kCNT are the first-order decaying coefficients of bioaerosol concentration associated with natural decay and using CNT coated plasma apparatus, respectively.
dC/dt = −kC
Ct = C0 exp(−kt), k = kn or kCNT
3. Materials and Methods
3.1. Carbon-Nanotube Coated Plasma Air-Cleaning Apparatus
This study fabricated a CNT-coated plasma apparatus for inactivating bioaerosols. The carbon-nanotube coated plasma apparatus included an exhaust fan, two carbon nanotube discharge electrodes, and a DC power supply. These components were set in a 22 × 20 × 16 cm3 stainless steel mixing column to format an indoor air treating apparatus for bacterial and viral bioaerosol inactivation in testing chambers and field medical environments.
For the carbon-nanotube electrode coating procedure, this study used silver glue adhesion over the stainless steel electrode and discharge voltage released by a power supply as the carbon-nanotube powder. As for the carbon-nanotube, an arc-discharge produced multi-wall carbon nanotube (MWCNT, purchased from TECO Nanotech Inc., Taipei, Taiwan) product was utilized. The scanning and transmission electron microscope observed structure and dimensions of MWCNT (provided by TECO Nanotech Inc., Taipei, Taiwan) are shown in Figure 1. MWCNT has an outside and inside diameter range of 15~20 nm and 3~5 nm, respectively. The length of MWCNT is ≤5 nm.
Figure 1.
(a) Scanning electron microscope image of multi-wall carbon nanotube and (b) transmission electron microscope image of multi-wall carbon nanotube (purchased from TECO Nanotech Inc., Taipei, Taiwan). Outside diameter: 15~20 nm, inside diameter: 3~5 nm, and length ≤5 nm.
Two carbon-nanotube coated discharge electrodes were connected to the positive and negative electrodes of the power supply, respectively. The power supply connected carbon-nanotube coated discharge electrodes were subsequently set on the wall of the mixing column aligned with each other (shown in Figure 2). The distance between the two electrodes was 1.0 cm, and the operating voltages of the plasma apparatus were set at 4.5, 6.0, and 7.5 kV.
Figure 2.
Schematic diagram of the CNT plasma apparatus.
This CNT plasma apparatus was operated by setting three different flow rates, 7.5, 15.1, and 22.6 Lpm (the face velocities through the cross-sectional area were equal to 0.1, 0.2, and 0.3 m/s), to understand the effects of different flow rates on bioaerosol inactivating ability.
3.2. Testing Bioaerosols
For the indoor bioaerosol inactivating tests, Escherichia coli (E. coli, Bioresource Collection and Research Center in Hsinchu, Taiwan, BCRC 10675) and λ virus phage (BCRC 70193) were chosen as the challenge bioaerosols. Both bioaerosols were purchased and activated as described in our previous study [7,8].
First, three E. coli colonies from agar plate cultures were extracted into a conical flask, and a loop was used containing 30 mL of tryptic soy broth (TSB, Bacto™, Franklin Lakes, NJ, USA). Then, the TSB culture was shaken at 85 rpm for 16–24 h at a temperature of 37 °C. Following incubation, the TSB culture was centrifuged at 2500 rpm for 5 min. After removing the supernatant, 30 mL of PBS solution (phosphate buffered saline, pH 7.2) was added and the E. coli sediments were resuspended. Then, a PBS buffer solution was used to minimize the osmotic pressure between the microbial cellular fluids and the buffer solution. The TSB medium was removed after repeating the above steps (except for incubation). The final PBS solution (E. coli stock) was used for bioaerosol generation. Colony-forming units (CFUs) were counted on the agar plates to evaluate the concentration of viable E. coli in the PBS solution.
The λ virus phage (BCRC 70193) was selected as the model strain of viral bioaerosol in this study. As a harmless virus, it has an isometric head of approximately 0.05 μm in diameter, a thin flexible tail of approximately 0.15 μm in length, and a diameter of approximately 7 nm. The experiment chose E. coli K12S (BCRC 14894) as the host of the λ virus phage and the phage was assessed as a prospective surrogate for water-borne and food-borne viruses. Each TSA plate contained 1 mL of a 16 h E. coli K12S (host cell) TSB culture for 2 h (each experimental set required six plates). Then, 100 μL of viral stock was added to each plate after removing the redundant E. coli K12S TSB culture from the surface of the TSA plate. The host culture and λ virus phage stock were spread uniformly over the surface of the agar plates using a sterile bent glass rod, and then all plates were incubated for 8–16 h at a temperature of 37 °C. Following incubation, 6 mL of sterilized distilled water was added to each plate, then shaken at 50–60 rpm for 5 min. To remove the host cells and generate the bioaerosol, the supernatant was centrifuged at 10,000 rpm for 10 min and filtered with a 0.22 μm Millex GS filter unit (Millipore Corporation, Carrigtwohill, Co., Country Cork, Ireland). Finally, the same method was used to count the plaque-forming units (PFU) (the double layer agar method) to determine the stock’s infective λ virus phage concentration.
3.3. Experimental Setup
Figure 3 shows the experimental setup for the aerosol inactivation experiment. In this experiment, the setup required an aerosol nebulizer, charge neutralizer, makeup air device, carbon-nanotube coated plasma system, and bioaerosol impactor. First, using a three-jet collision nebulizer (BGI Inc., Waltham, MA, USA), the model bacterial strains from suspension into the chamber were aerosolized at a 2.5 L/min flow rate. After drying the bioaerosol with the diffusion dryer, the dried bioaerosol was passed through a Kr-85 radioactive source (model 3077, TSI Inc., Shoreview, MN, USA) that neutralized it to the Boltzmann charge equilibrium. Then the bioaerosol was delivered into the stainless steel test chamber (80 × 80 × 80 cm3). As in an indoor environment, it is easy to dilute and remove the bacterial aerosols in the test chamber by increasing the fresh air intake, which decays the airborne concentration. To make sure that natural ventilation decay is affected by the fresh air intake rather than plasma intervention, some mechanical ventilation experimental parameters of the testing chamber were controlled in the study.
Figure 3.
Schematic diagram of the experimental setup.
Figure 3 shows all the mechanical components comprising the environmental-controlled test chamber, including a mechanical supply fan, a return fan, a pump, and a makeup air section. The testing chamber was 80 × 80 × 80 cm3 and fabricated with stainless steel. The testing chamber was viewed as well stabilized for 6 h to meet the demanded conditions before the experiment started. As mentioned before, in the real indoor environment, the bioaerosols might be diluted by outdoor air ventilation (such as open windows, open doors, using an air-conditioner). This ventilation dilution may interfere with our performance investigation of the CNT plasma apparatus. Therefore, in this testing chamber, the number of air exchange rates and airflow will be controlled and maintained by two fans and a pump to simulate a real indoor environment. That means the ACH in this chamber setup is equal to the total airflow per volume of the tested chamber. The filtrated clean air delivery in this experimental setup was employed as makeup air, which was changed to evaluate its effect on the decaying effects of bioaerosols at a fixed total mechanical delivered airflow rate.
In the first experimental part of this study, two total ACH parameters were set, 0.5 and 1.0 h−1. The relative humidity inside the chamber was monitored with Q-trak (Model 8550, TSI Inc., Shoreview, MN, USA), and the ratio of the flow rate of a dry gas stream to that of a humidified gas stream generated by a water vapor saturator was set at 30%. In the test chamber, E. coli bioaerosols were collected following the Taiwan Environmental Analysis Laboratory guideline (NIEA E301.11C). The bacterial aerosol samples were collected by the BioStage single-stage viable cascade impactor (SKC Inc., Eighty Four, PA, USA), which was loaded with tryptic soya agar (BactoTM TSA, BD, Franklin Lakes, NJ, USA) and operated at a flow rate of 28.3 L/min for 30 s. For each sample, three replicates were performed. The TSA plate samples were incubated at a temperature of 30 ± 1 °C for 48 ± 2 h. After incubation, the sample colonies formed on the plate were manually counted and converted to airborne E. coli bioaerosol concentration in CFU/m3.
For the λ virus bioaerosols sampling, an AGI-30 sampler (Model 7540, ACE GLASS Inc., Vineland, NJ, USA) and a sampling pump (All Field Tech, Taipei, Taiwan) were used. The efficiency for aerosolized viruses of this sampler has been shown to be better than others. Its collection efficiency is a function of the particle diameter and the sampling flow rate [33], and the sampling flow rate and sampling time were 12.5 LPM and 6–8 min. Sterilized distilled water was used for sampling the aerosolized λ virus phage [34,35].
The time-dependent airborne concentration calibration curve of the testing chamber was established by continuously delivering bacterial and virus aerosols and collecting samples in 30 min intervals to evaluate the initial airborne bioaerosol concentration.
The natural decay constant (kn) was defined as the first-order decay coefficient of bioaerosol concentrations without using the CNT plasma apparatus across the various ACH parameters (diluted only with mechanical ventilation) set in the testing chamber. On the other hand, the first-order decay coefficients of bioaerosol concentrations when using CNT plasma apparatus were defined as kCNT, which is explored in the second experimental part of this study. The third part, in addition, we installed the CNT coated plasma apparatus in an otorhinolaryngology clinic to investigate its air-cleaning performance in field indoor medical environments, which have more interfering factors, e.g., number of personnel in the clinic and stress-resistant bacterial species, may cause fluctuation in bioaerosol concentration.
4. Results and Discussions
4.1. The Initial Bioaerosol Concentration in the Environmental-Controlled Chamber
Figure 4 presents the calibration curves of E. coli bacterial and λ virus bioaerosol concentration with continuous nebulizing delivery to the environment-controlled chamber. The linear relationships between nebulizing time and bioaerosol concentration indicate stable accumulation of the two testing bioaerosols in the environment-controlled chamber. For the E. coli bioaerosols, the airborne concentration increased to 3 × 104 CFU/m3 after 50 min of nebulization. On the other hand, the λ virus bioaerosol, airborne concentration of 3 × 104 PFU/m3 was reached after about 80 min of nebulization in the environment-controlled chamber. Hence, the subsequent mechanical ventilation dilution and CNT-coated plasma inactivation experiments in the test chamber all applied the initial bioaerosol concentration of 3 × 104 CFU/m3 (to E. coli) and PFU/m3 (to λ virus) for simulating a sufficiently contagious indoor environment.
Figure 4.
Calibration curves of testing bioaerosol nebulization in the environmental-controlled chamber.
4.2. The Mechanical Ventilation Dilution on Bioaerosols
The E. coli bacterial- and λ virus-bioaerosol dilution induced by mechanical ventilation (made-up with clean air) are shown in Figure 5 and Table 1, respectively.
Figure 5.
Mechanical ventilation of E. coli bioaerosol in the chamber.

Table 1.
The natural decay behaviour of bioaerosols in the environmental-controlled testing chamber.
According to the results under ACH = 0 h−1 (make-up air was turned off, simulating a fully-closed indoor environment without any ventilation), in this environment-controlled chamber test, the relatively low kn value of the tested bioaerosols (0.02 min−1 to E. coli bacterial (R2 = 0.98, p = 0.0041) and 0.01 to λ virus bioaerosols (R2 = 0.98, p = 0.0047) indicated that wall loss and gravity deposition were not significantly impacting the airborne concentration of the tested bioaerosols. Over 90% of tested bioaerosols remained well-mixed suspended in the air for 30 min after being nebulized in the environment-controlled chamber. This meant that the interference due to wall loss and gravity deposition characteristics on testing bioaerosols could be neglected in the environment-controlled chamber for conducting the next CNT-coated plasma treatment.
When the fresh air make-up in the environment-controlled chamber was set at ACH = 0.5 (medium rate) and 1.0 (high rate) h−1, the kn value of the E. coli bioaerosol was 0.09 and 0.15 min−1, respectively (R2 = 0.99, p < 0.0001). These results indicated the kn value increased obviously with ACH. The increasing fresh intake air resulted in an obvious dilution effect on airborne bacterial bioaerosols inside the environmental-controlled chamber. In addition, for the λ virus bioaerosol, the kn values were 0.08 and 0.14 min−1 with the ACH set at 0.5 and 1.0 h−1, respectively (R2 = 0.99, p < 0.0001, shown in Table 1). These λ virus bioaerosol data also revealed similar trends of ventilation dilution as the E. coli bacterial bioaerosol presented in the environment-controlled chamber. This means that ventilation dilution (ACH) is a non-negligible mechanical removal mechanism in natural decay’s affecting parameters. Therefore, under the next CNT-coated plasma tests, the bioaerosol removal ability should deduct the ventilation dilution effect.
4.3. The Inactivation Efficiency of CNT-Coated Plasma Treatment on E. coli Bioaerosol
Table 2 and Figure 6 show the inactivation efficiency of the E. coli bioaerosol using the CNT-coated plasma treatment (voltage = 6.0 kV and flow rate = 7.5 Lpm). When the ACH of the environment-controlled chamber was set at medium (0.5 h−1), the kCNT value was 0.24 min−1. Compared to the kn value of the E. coli bioaerosol under the same ACH set (kn = 0.09, ACH = 0.5 h−1, shown in Figure 6) without using the plasma treatment apparatus, the CNT-coated plasma treatment demonstrated a significant inactivation effect on the E. coli bioaerosol. The survival rate of the E. coli bioaerosol decreased to less than 10% of the initial concentration (3 × 104 CFU/m3) within 10 min of the CNT-coated plasma treatment. After 30 min of using the plasma-treatment apparatus, the E. coli bioaerosol was neutralized to nearly 0%. The results indicated that the CNT-coated plasma treatment apparatus was capable of air cleaning even under medium ventilation dilution (R2 = 0.99, p < 0.0001, shown in Figure 6).

Table 2.
The inactivation efficiency of CNT-coated plasma treating bioaerosols in the environmental-controlled testing chamber.
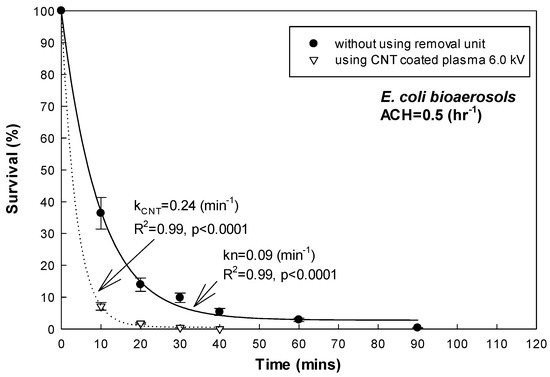
Figure 6.
Inactivation efficiency of E. coli bioaerosol using CNT-coated plasma (ACH = 0.5 h−1).
Furthermore, Table 2 shows the inactivation efficiency on the E. coli bioaerosol using the CNT-coated plasma treatment (voltage = 6.0 kV and flow rate = 7.5 Lpm) under higher ventilation dilution (ACH = 1.0 h−1). The kCNT value was 0.31 min−1 (R2 = 0.99, p < 0.0001) while the ACH of the environment-controlled chamber was set at medium (1.0 h−1). Compared with the same high mechanical ventilation (kn = 0.15 min−1 under ACH = 1.0 h−1, R2 = 0.99, p < 0.0001) condition, using the CNT-coated plasma treatment apparatus yielded an additional inactivation effect on the E. coli bioaerosol. According to previous studies, the major antimicrobial mechanisms were the oxygen-containing radicals and UV-rays generated during plasma processing. These oxygen-containing radicals and UV-rays damage the cell membrane or DNA of the microorganisms [25,28].
4.4. The Inactivation Efficiency of CNT-Coated Plasma Treatment on λ Virus Nioaerosol
Table 2 and Figure 7 show the inactivation efficiency of the λ virus bioaerosol using CNT-coated plasma treatment (voltage = 6.0 kV and flow rate = 7.5 Lpm). The kCNT value on the λ virus was 0.230 min−1 while the ACH of the environment-controlled chamber was set at medium (0.5 h−1). Compared to the kn value of the λ virus bioaerosol under the same ACH settings (kn = 0.08, R2 = 0.99, p < 0.0001, ACH = 0.5 h−1, shown in Figure 7) without using the plasma treatment apparatus, the CNT-coated plasma treatment had a significant inactivation effect on the λ virus bioaerosol. Furthermore, over 90% of the λ virus bioaerosol was neutralized from the initial concentration (3 × 104 PFU/m3) within 10 min of CNT-coated plasma treatment. After 30 min using the plasma treatment apparatus, the λ-virus bioaerosol was neutralized to nearly 0%. The result indicated that the CNT-coated plasma treatment apparatus was capable of performing air cleaning on the λ virus bioaerosol (R2 = 0.99, p < 0.0001, shown in Figure 7), similar to the results for the E. coli bioaerosol.
Figure 7.
Inactivation efficiency on λ virus bioaerosol using CNT-coated plasma (ACH = 0.5 h−1).
Table 2 shows the efficiency of using CNT-coated plasma treatment (voltage = 6.0 kV and flow rate = 7.5 Lpm) with high ventilation dilution (ACH = 1.0 h−1) to inactivate the λ virus bioaerosol. The kCNT value was 0.29 min−1 (R2 = 0.99, p < 0.0001) while the ACH of the environment-controlled chamber was set at medium (1.0 h−1). Compared with the same high mechanical ventilation (kn = 0.14 min−1 under ACH = 1.0 h−1, R2 = 0.99, p < 0.0001) condition, using the CNT-coated plasma treatment apparatus yielded an additional inactivation effect on the λ virus bioaerosol, similar to results for the E. coli bioaerosol. Moreover, our CNT-coated plasma had inactivating ability on the λ virus bioaerosol.
4.5. Effect of Operating Voltage of CNT-Coated Plasma Treatment Apparatus on Inactivation Efficiency for Bioaerosols
Figure 8 and Table 3 show the inactivation efficiency of CNT-coated plasma treatment on the λ virus bioaerosol caused by changing the operating voltage. The mechanical ventilation of the environment-controlled chamber was set at medium (ACH = 0.5 h−1). A higher inactivation efficiency (kCNT = 0.29 min−1, R2 = 0.99, p < 0.0001) was observed when the operating voltage of the CNT-coated plasma treatment apparatus was set at 7.5 kV, compared with the lower voltage setting of 6.0 kV (kCNT = 0.29 min−1, R2 = 0.99, p < 0.0001). When the operating voltage of the CNT-coated plasma treatment apparatus was reduced to 4.5 kV, the inactivation efficiency decreased to kCNT = 0.20 min−1 (R2 = 0.99, p < 0.0001).
Figure 8.
Inactivation efficiency on λ virus bioaerosol using CNT-coated plasma with various operating voltages (ACH = 0.5 hr−1).

Table 3.
The inactivation efficiency of CNT-coated plasma with different operating voltages for treating bioaerosols in the environmental-controlled chamber.
Furthermore, Table 3 shows the inactivation efficiency of the CNT-coated plasma treatment on the E. coli bioaerosol under different operating voltages (under ACH = 0.5 h−1). At the operating voltages of 7.5, 6.0, and 4.5 kV, the inactivation efficiency (kCNT) of the CNT-coated plasma treatment was 0.21, 0.24, and 0.31 min−1 (R2 = 0.99, p < 0.0001), respectively. These results were similar to the results for the λ virus bioaerosol.
4.6. Effect of Operating Flow Rate on Inactivation Efficiency for Bioaerosols
Figure 9 and Table 4 show the effect on the inactivation efficiency of the CNT-coated plasma treatment on the λ virus bioaerosol caused by changing the operating cross flow rate (7.5, 15.1, and 22.1 Lpm, the face velocities through the cross-sectional area were equal to 0.1, 0.2, and 0.3 m/s). The mechanical ventilation of the environment-controlled chamber was set at medium (ACH = 0.5 h−1). Lower inactivation efficiency (kCNT = 0.19 min−1, R2 = 0.99, p < 0.0001) was observed when the operating flow of the CNT-coated plasma treatment apparatus was set at 15.1 Lpm, compared with the lower operating flow rate (7.5 Lpm, kCNT = 0.23 min−1, R2 = 0.99, p < 0.0001). In addition, when the operating flow of the CNT-coated plasma treatment apparatus was increased to 22.1 Lpm, the inactivation efficiency decreased to kCNT = 0.18 min−1 (R2 = 0.99, p < 0.0001).
Figure 9.
Inactivation efficiency on λ virus bioaerosol using CNT-coated plasma with various operating cross flowrates (ACH = 0.5 h−1).

Table 4.
The inactivation efficiency of CNT-coated plasma with various operating flowrates for testing bioaerosols.
Furthermore, Table 4 shows the inactivation efficiency of the CNT-coated plasma treatment on the E. coli bioaerosol under various operating cross flow rates (operating voltage = 6.0 kV and ACH = 0.5 h−1). At operating cross flow rates of 7.5, 15.1, and 22.1 Lpm, the inactivation efficiency (kCNT) of the CNT-coated plasma treatment was 0.18, 0.20, and 0.24 min−1 (R2 = 0.99, p < 0.0001), respectively. These results were similar to the results of the λ virus bioaerosol.
In the experimental results on airborne bacteria inactivation conducted by Perhn et al. [36] and Timmeran et al. [37], plasma-generated reactive species, e.g., ions and hydroxyl radicals, are the major antimicrobial agent. Lower airflow velocity may increase the opportunity for bioaerosols to be exposed to antimicrobial agents.
4.7. Application of CNT-Coated Plasma Treatment in Indoor Medical Environments
The CNT-coated plasma system was utilized in a medical environment to evaluate its capability to inactivate bioaerosols. Our hand-fabricated CNT-coated plasma treatment apparatus was deployed in an otorhinolaryngology clinic (air-conditioned, generally with windows and doors closed). The operating flow rate and voltage of the CNT-coated plasma treatment apparatus were set at 7.5 Lpm and 6.0 kV, in accordance with previous experimental data, to obtain the expected bioaerosol inactivation efficiency and energy savings. Since the activity of personnel is highly associated with the concentration of indoor bacterial bioaerosols, we planned a 1-day, two-round experiment to align with the physician’s work duty in the clinic. The CNT-coated plasma treatment apparatus was used for neutralization for two-rounds (turned on from 10:00 a.m. to 4:00 p.m.) for approximately 2 h, starting from 1 h after a physician’s diagnosis and treatment task in the clinic (from 9:00 a.m. to 3:00 p.m.). This two-round, 2 h experiment allowed the indoor bioaerosols to accumulate (brought by incoming and waiting patients) to a sufficient concentration before applying the CNT-coated plasma treatment to evaluate the air-cleaning efficiency in a medical environment.
The total indoor bacterial bioaerosols were collected at 1.5 m height from the floor by the sampler at 0 min, 10 min, and then at intervals of 30 min from when the CNT-coated plasma treatment apparatus was turned on. All the bacterial bioaerosol collection and cultivation processes were followed with the Taiwan Environmental Analysis Laboratory guideline (NIEA E301.15C) using a BioStage sampler in triplicate, as described in Section 3.3. The number of personnel in the clinic (including one physician, one assistant, and several patients) was recorded at the scene of sampling. Figure 10 shows the neutralizing efficiency of the CNT-coated plasma treatment apparatus in the real otorhinolaryngology clinic. In the first-round (apparatus turned on at 10:00 a.m.) within 60 min of CNT-coated plasma treatment, the indoor total bacterial bioaerosol concentration was significantly reduced to an average of 2695 CFU/m3 from 1333 CFU/m3. This result indicated that the CNT-coated plasma treatment apparatus yielded, roughly, a 70% to 80% air-cleaning effect in the medical environment. The bacterial bioaerosol concentration did not recurrent in the next four samples (1 h durations).
Figure 10.
Survival of bacterial bioaerosols by using CNT-coated plasma treatment in the otorhinolaryngology clinic.
In the second round (apparatus turned at 4:00 p.m.), the CNT-coated plasma treatment significantly neutralized the total indoor bacterial bioaerosols. With an initial average concentration of 848 CFU/m3, the bacterial bioaerosols were reduced to about 228 CFU/m3, yielding a 70% to 80% air-cleaning effect, similar to the result obtained in the first round. Likewise, a recurrence of bacterial bioaerosols was not observed in the next four samples (1 h duration). During the field evaluation experiment, the average relative humidity was 55.5% and the average temperature was 25.6 °C. Furthermore, the number of personnel did not significantly vary during the experiment (6–10 and 5–9 personnel in the morning and afternoon rounds, respectively). This means that the bacterial bioaerosols were continuously brought into the clinic and were unlikely to decrease without an air-cleaning intervention. The field evaluation results indicate that only 20% to 30% of bacteria bioaerosols can survive exposure to the CNT-coated plasma treatment. This preliminary, two-round study shows that CNT-coated plasma treatment has the potential to improve air quality in an indoor medical environment.
Non-thermal plasma technology has been used in bioaerosol removal. However, when this technology is applied to indoor bioaerosol removal, its safety, environmental friendliness, and cost should be considered. In this work, we attempted to enhance bioaerosol inactivating ability by means of using the plasma with a CNT-coated discharge electrode. This CNT coated plasma system had a significant antimicrobial effect (oxygen-containing radicals or UV-rays) on both bacterial and viral bioaerosols when using the low operating discharging voltage range of 4.5–7.5 kV. Moreover, the CNT coated plasma air-cleaning device showed potential in removing bacterial bioaerosols in the medical field environment.
In the review paper discussing the biomedical application of plasma technology, Inactivation of microorganisms by plasma treatment is potentially wide-ranging because it is eco-friendly and effective to inactivate common infectious microorganisms. Moreover, plasma technology is unlikely to enhance multidrug resistance in bacteria, which is an emerging crisis in healthcare facilities [38].
5. Conclusions
In this study, three essential operating parameters influencing CNT-coated plasma inactivating ability were evaluated in an environmental-controlled chamber. Under the higher ACH condition, the CNT-coated plasma performed higher bioaerosol purifying efficiency, even when accompanied by the mechanical dilution of clean air exchange. Increasing the flowrate and operating voltage could increase the inactivating ability of CNT-coated plasma apparatus. In addition, this apparatus demonstrated its satisfactory cleaning performance to suppress the airborne bacteria concentration in a field medical environment. The methods for preparing a CNT-coated plasma will be improved, with an emphasis on energy savings and cost reduction. Investigation of the inactivating effects of the CNT-coated plasma on various microorganisms will also be conducted to expand the applicability of the emerging technology.
Author Contributions
Conceptualization, S.Y.; methodology, S.Y., Y.-T.D. and Y.-F.H.; software, S.Y. and C.-Y.C.; validation, S.Y. and Y.-F.H.; formal analysis, P.-C.H.; investigation, S.Y. and Y.-T.D.; resources, S.Y.; data curation, S.Y. and C.-Y.C.; writing—original draft preparation, S.Y.; writing—review and editing, S.Y. and C.-Y.C.; visualization, S.Y. and C.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Institute of Labor, Occupational Safety and Health, Ministry of Labor.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the results in the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank the Institute of Labor, Occupational Safety and Health, Ministry of Labor for financially supporting this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Can-Güven, E. The current status and future needs of global bioaerosol research: A bibliometric analysis. Int. J. Environ. Sci. Technol. 2022, 19, 7857–7868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yao, M.; Zhang, X.; Hu, B.; Li, X.; Chen, H.; Zhang, L.; Liu, Y.; Du, M.; Sun, B.; et al. Breath-, air- and surface-borne SARS-CoV-2 in hospitals. J. Aerosol. Sci. 2021, 152, 105693. [Google Scholar] [CrossRef] [PubMed]
- Pease, L.F.; Wang, N.; Kulkarni, G.R.; Flaherty, J.E.; Burns, C.A. A missing layer in COVID-19 studies: Transmission of enveloped viruses in mucus-rich droplets. Int. J. Heat Mass. Transf. 2022, 131, 105746. [Google Scholar] [CrossRef]
- Grimalt, J.O.; Vílchez, H.; Fraile-Ribot, P.A.; Marco, E.; Campins, A.; Orfila, J.; van Drooge, B.L.; Fanjul, F. Spread of SARS-CoV-2 in hospital areas. Environ. Res. 2022, 204, 112074. [Google Scholar] [CrossRef] [PubMed]
- Jalili, D.; Dehghani, M.; Fadaei, A.; Alimohammadi, M. Assessment of airborne bacterial and fungal communities in Shahrekord hospitals. Int. J. Environ. Res. Public. Health 2021, 2021, 8864051. [Google Scholar] [CrossRef]
- Munyeshyaka, E.; Cyuzuzo, P.; Yadufashije, C.; Karemera, J. Contribution of medical wards contamination to wound infection among patients attending Ruhengeri Referral hospital. Int. J. Food. Microbiol. 2021, 2021, 7838763. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; White, J.K.; Nielsen, J.L.; Keskin, M.E.; Tendal, K.; Frederiksen, M.W. A cross sectional study on airborne inhalable microorganisms, endotoxin, and particles in pigeon coops—Risk assessment of exposure. Environ. Res. 2022, 204, 112404. [Google Scholar] [CrossRef] [PubMed]
- Noorimotlagh, Z.; Mirzaee, S.A.; Jaafarzadeh, N.; Maleki, M.; Kalvandi, G.; Karami, C. A systematic review of emerging human coronavirus (SARS-CoV-2) outbreak: Focus on disinfection methods, environmental survival, and control and prevention strategies. Environ. Sci. Pollut. Res. 2021, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Wang, Y.C. Surface germicidal effects of ozone for microorganisms. Am. Ind. Hyg. Assoc. J. 2003, 64, 533–537. [Google Scholar] [CrossRef]
- Lin, C.H.; Li, C.S. Effectiveness of titanium dioxide photocatalyst filters for controlling bioaerosol. Aerosol. Sci. Technol. 2003, 37, 162–170. [Google Scholar] [CrossRef]
- Lin, C.Y.; Li, C.S. Control effectiveness of ultraviolet germicidal irradiation on bioaerosols. Aerosol. Sci. Tech. 2002, 36, 474–478. [Google Scholar] [CrossRef]
- Ghosh, D.; Dhibar, S.; Dey, A.; Mukherjee, S.; Joardar, N.; Babu, S.P.S.; Dey, B. Graphene oxide dispersed supramolecular hydrogel capped benign green silver nanoparticles for anticancer, antimicrobial, cell attachment and intracellular imaging applications. J. Mol. Liq. 2019, 282, 1–12. [Google Scholar] [CrossRef]
- Dey, B.; Mukherjee, S.; Mukherjee, N.; Mondal, R.K.; Satpati, B.; Senapati, D.; Babu, S.P.S. Green silver nanoparticles for drug transport, bioactivities and a bacterium (Bacillus subtilis)-mediated comparative nano-patterning feature. RSC. Adv. 2016, 6, 46573–46581. [Google Scholar] [CrossRef]
- Majumdar, S.; Ghosh, M.; Mukherjee, S.; Satpati, B.; Dey, B. DNA mediated graphene oxide (GO)-nanosheets dispersed supramolecular GO-DNA hydrogel: An efficient soft-milieu for simplistic synthesis of Ag-NPs@GO-DNA and Gram + ve/−ve bacteria-based Ag-NPs@GO-DNA-bacteria nano-bio composites. J. Mol. Liq. 2021, 342, 117482. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Yang, S.; Chang, M.Y.; Huang, H.C.; Luo, C.H.; Hung, P.C.; Fang, W. Inactivation efficiency to Bacillus subtilis and Escherichia coli bacterial aerosols of spraying neutral electrolyzed water. J. Air Waste. Manag. Assoc. 2013, 63, 1447–1456. [Google Scholar] [CrossRef]
- Hsu, Y.F.; Chuang, C.Y.; Yang, S. Evaluation of the bioaerosol inactivation ability of chitosan-coated antimicrobial filters. Int. J. Environ. Res. Publ. Health 2021, 18, 7183. [Google Scholar] [CrossRef]
- Masuda, S.; Hosokawa, S.; Tu, X.; Wang, Z. Novel plasma chemical technologies—PPCP and SPCP for control of gaseous pollutants and air toxics. J. Electrostat. 1995, 34, 415–438. [Google Scholar] [CrossRef]
- Yan, K.; Van Heesch, E.J.M.; Pemen, A.J.M.; Huijbrechts, P.A.H.J. Elements of pulsed corona induced non-thermal plasmas for pollution control and sustainable development. J. Electrostat. 2001, 51, 218–224. [Google Scholar] [CrossRef]
- Sato, M.; Ohgiyama, O.; Clements, J.S. Formation of chemical species and their effects on microorganisms using a pulsed high voltage discharge in water. IEEE Trans. Ind. Appl. 1996, 32, 106–112. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process. Polymer 2005, 2, 391–400. [Google Scholar]
- Laroussi, M. Nonthermal decontamination of biological media by atmospheric-pressure plasmas: Review, analysis, and prospects. IEEE. Trans. Plasma. Sci. 2002, 30, 1409–1415. [Google Scholar] [CrossRef]
- Locke, B.R.; Sato, M.; Sunka, P.; Hoffmann, M.R.; Chang, J.S. Electrohydraulic discharge and nonthermal plasma for water treatment. Ind. Eng. Chem. Res. 2006, 45, 882–905. [Google Scholar] [CrossRef]
- Gadri, R.B.; Roth, J.R.; Montie, T.C.; Kelly-Wintenberg, K.; Tsai, P.P.Y.; Helfritch, D.J.; Feldman, P.; Sherman, D.M.; Karakaya, F.; Chen, X. UTK Plasma Sterilization Team, Sterilization and plasma processing of room temperature surfaces with one atmosphere uniform glow discharge plasma (OAUGDP). Surf. Coat. Technol. 2000, 131, 528–542. [Google Scholar] [CrossRef]
- Sladek, R.E.J.; Stoffels, E. Deactivation of Escherichia coli by plasma needle. J. Appl. Physiol. 2005, 38, 1716–1721. [Google Scholar]
- Gallagher, M.J., Jr.; Vaze, N.; Gangoli, S.; Vasilets, V.N.; Gutsol, A.F.; Milovanova, T.N.; Anandan, S.; Murasko, D.M.; Fridman, A.A. Rapid inactivation of airborne bacteria using atmospheric pressure dielectric barrier grating discharge. IEEE Trans. Plasma. Sci. 2007, 35, 1501–1510. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Y.; Sun, K.; Chen, Q.; Shen, F.; Zhang, J.; Yao, M.; Zhu, T.; Fang, J. Rapid inactivation of biological species in the air using atmospheric pressure nonthermal plasma. Environ. Sci. Technol. 2012, 46, 3360–3368. [Google Scholar] [CrossRef]
- Helbich, S.; Dobslaw, D.; Schulz, A.; Engesser, K.H. Styrene and bioaerosol removal from waste air with a combined biotrickling filter and DBD–Plasma system. Sustainability 2020, 12, 9240. [Google Scholar] [CrossRef]
- Bisag, A.; Isabelli, P.; Laghi, G.; Laurita, R.; Dirani, G.; Taddei, F.; Bucci, C.; Capelli, F.; Gherardi, M.; Paglianti, A.; et al. Colombo, V. Cold atmospheric plasma decontamination of SARS-CoV-2 bioaerosols. Plasma. Process. Polym. 2022, 19, e2100133. [Google Scholar] [CrossRef]
- Iijima, S. Helical Microtubes of Graphite Carbon. Nature 1991, 354, 56. [Google Scholar] [CrossRef]
- Heer, W.A.D.; Chatelain, A.; Ugarte, D. A carbon nanotube field-emission electron source. Science 1995, 270, 1179–1180. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.C.; Luo, C.H.; Lin, Y.C.; Huang, J.W.; Chuang, C.P.J.; Chen, C.J.; Fang, W.; Chuang, C.Y. Inactivation efficiency of bioaerosols using carbon nanotube plasma. Clean 2011, 39, 201–205. [Google Scholar] [CrossRef]
- Machado, G.T.; Pinto, C.R.C.; da Fonseca, L.A.V.; Ramos, T.; Paggi, T.F.P.; Spira, B. Bacteriophages as surrogates for the study of viral dispersion in open air. Arch. Microbiol. 2021, 203, 4041–4049. [Google Scholar] [CrossRef] [PubMed]
- Hogan, C.J., Jr.; Kettleson, E.M.; Lee, M.H.; Ramaswami, B.; Angenent, L.T.; Biswas, P. Sampling methodologies and dosage assessment techniques for submicrometre and ultrafine virus aerosol particles. J. Appl. Microbiol. 2005, 99, 1422–1434. [Google Scholar] [CrossRef]
- Tseng, C.C.; Li, C.S. Collection efficiencies of aerosol samplers for virus-containing aerosols. J. Aerosol. Sci. 2005, 36, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.P.; Lee, G.W.M.; Lin, S.Y.; Huang, C.P. Removal of bioaerosols by the combination of a photocatalytic filter and negative air ions. J. Aerosol. Sci. 2008, 39, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Prehn, F.; Timmermann, E.; Kettlitz, M.; Schaufler, K.; Günther, S.; Hahn, V. Inactivation of airborne bacteria by plasma treatment and ionic wind for indoor air cleaning. Plasma. Process. Polym. 2020, 17, 2000027. [Google Scholar] [CrossRef]
- Timmermann, E.; Prehn, F.; Schmidt, M.; Höft, H.; Brandenburg, R.; Kettlitz, M. Indoor air purification by dielectric barrier discharge combined with ionic wind: Physical and microbiological investigations. J. Phys. D 2018, 51, 164003. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).