Controlled Electroplating of Noble Metals on III-V Semiconductor Nanotemplates Fabricated by Anodic Etching of Bulk Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Anodization
2.2. Electroplating
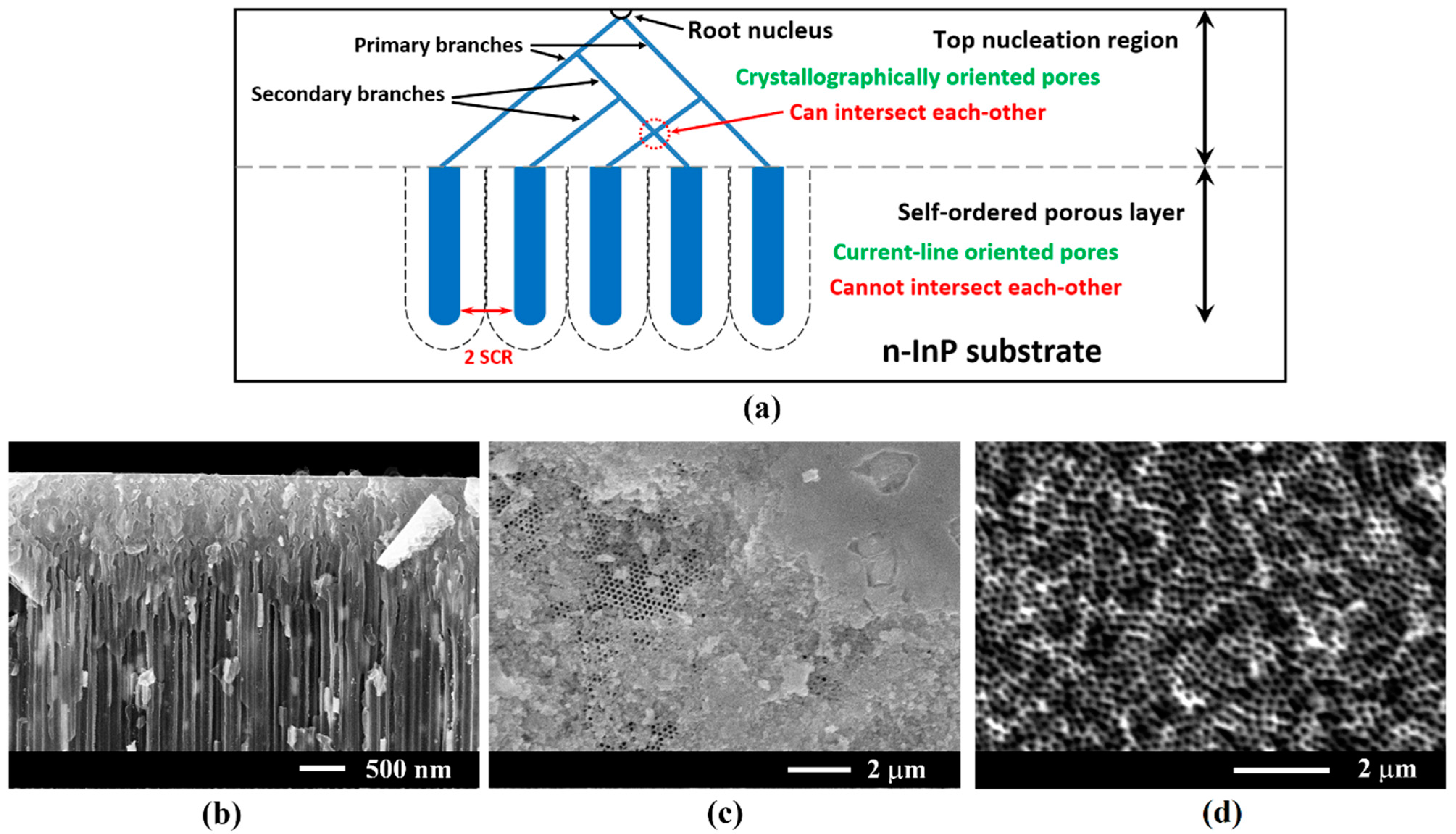
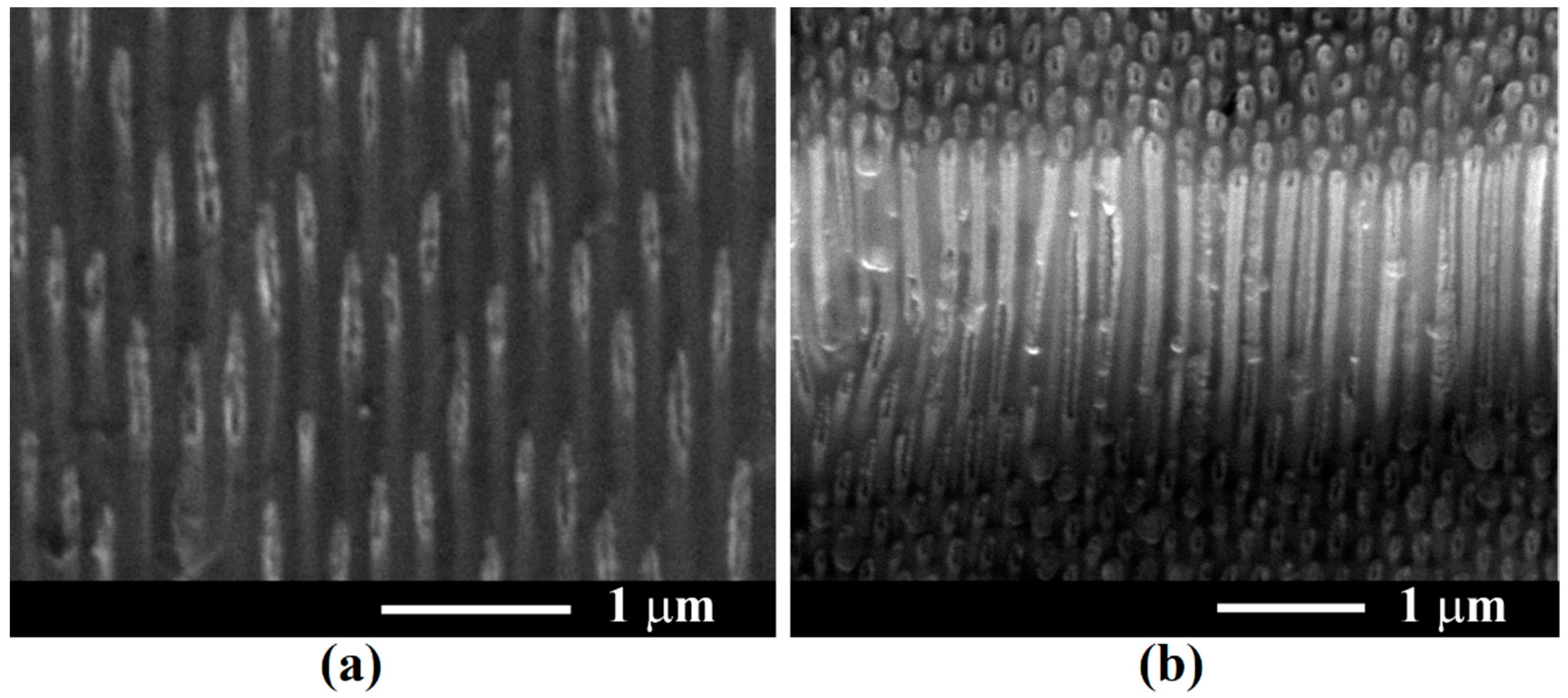
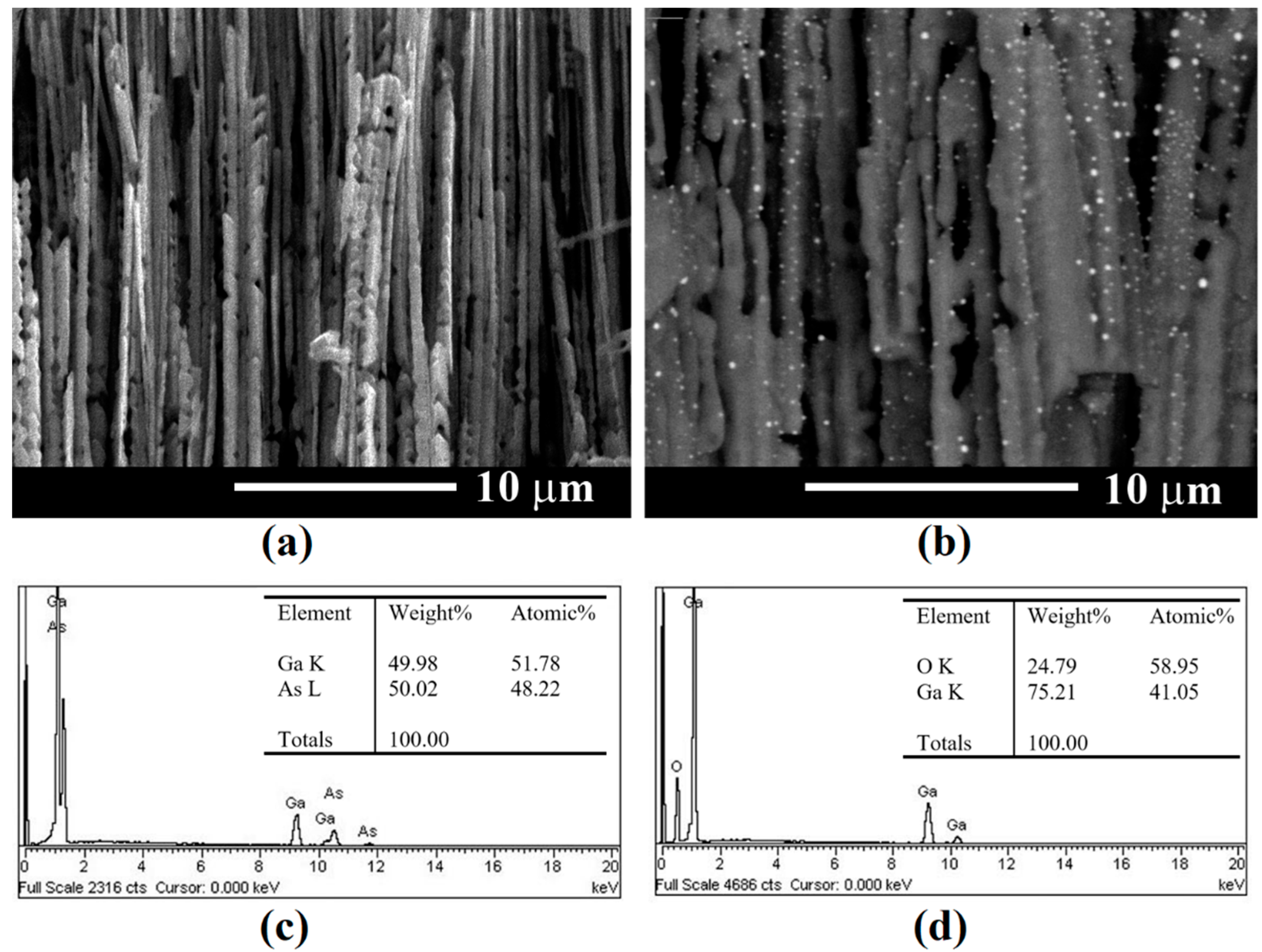
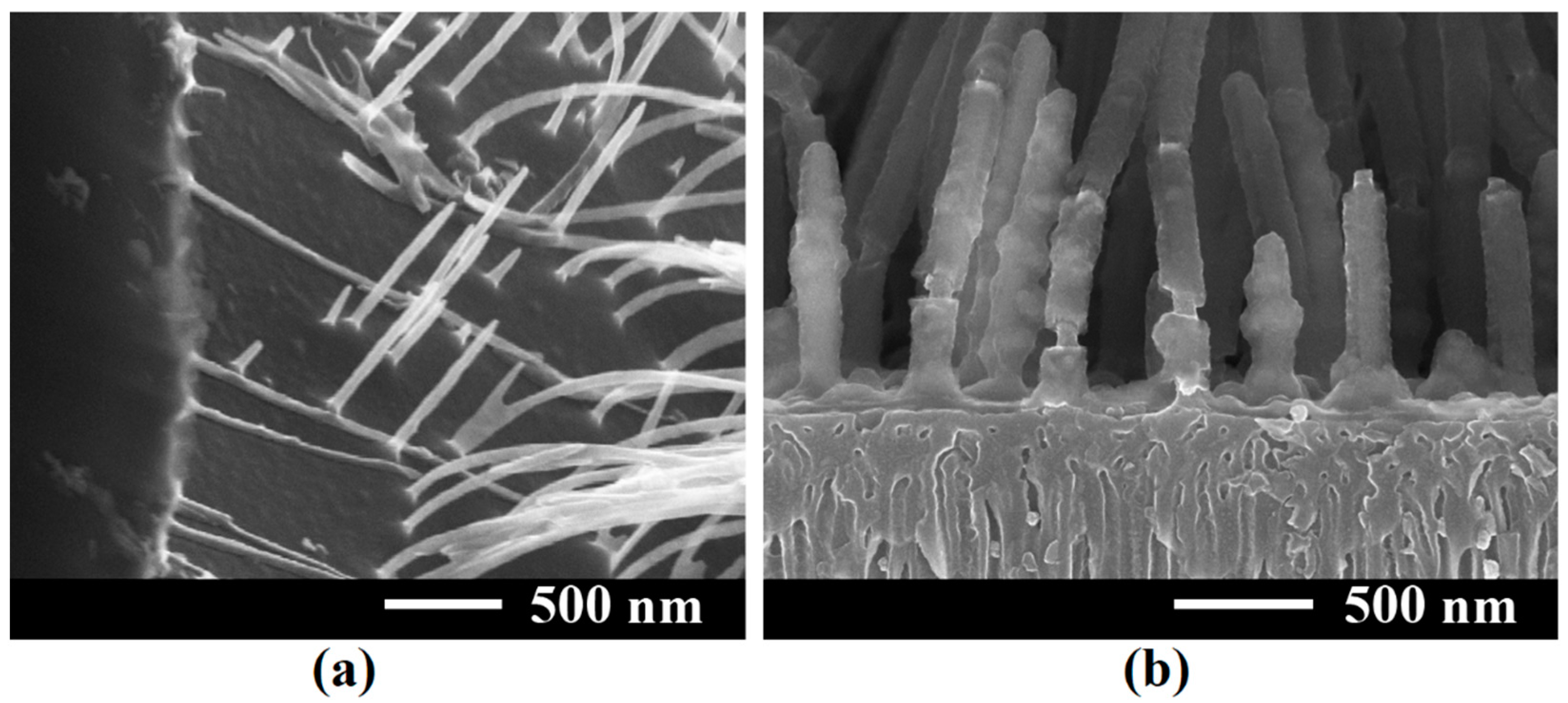
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent Progress of Magnetic Nanoparticles in Biomedical Applications: A Review. Nano Sel. 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Li, K.; Xu, J.; Li, P.; Fan, Y. A Review of Magnetic Ordered Materials in Biomedical Field: Constructions, Applications and Prospects. Compos. Part B Eng. 2022, 228, 109401. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Mohammed, H.; Moreno, J.A.; Kosel, J. Advanced Fabrication and Characterization of Magnetic Nanowires; IntechOpen: London, UK, 2017; ISBN 978-1-78923-679-8. [Google Scholar]
- Shah, K.W.; Xiong, T. Multifunctional Metallic Nanowires in Advanced Building Applications. Materials 2019, 12, 1731. [Google Scholar] [CrossRef] [PubMed]
- Proenca, M.P.; Sousa, C.T.; Ventura, J.; Araújo, J.P. 6—Cylindrical Magnetic Nanotubes: Synthesis, Magnetism and Applications. In Magnetic Nano- and Microwires, 2nd ed.; Vázquez, M., Ed.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Sawston, UK, 2020; pp. 135–184. ISBN 978-0-08-102832-2. [Google Scholar]
- Staňo, M.; Fruchart, O. Chapter 3—Magnetic Nanowires and Nanotubes. In Handbook of Magnetic Materials; Brück, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 27, pp. 155–267. [Google Scholar]
- Sharma, M.; Kuanr, B.K. Electrodeposition of Ferromagnetic Nanostructures; IntechOpen: London, UK, 2015; ISBN 978-953-51-2213-5. [Google Scholar]
- Gerein, N.J.; Haber, J.A. Effect of AC Electrodeposition Conditions on the Growth of High Aspect Ratio Copper Nanowires in Porous Aluminum Oxide Templates. J. Phys. Chem. B 2005, 109, 17372–17385. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Zhang, Y.; Du, J. Preparation of Anodic Aluminum Oxide Tubular Membranes with Various Geometries. Mater. Chem. Phys. 2011, 128, 187–190. [Google Scholar] [CrossRef]
- Enculescu, I. Nanowires and Nanotubes Prepared Using Ion Track Membrane Templates. Dig. J Nanomater. Biostructures 2006, 1, 15–20. [Google Scholar]
- Enculescu, I.; Siwy, Z.; Dobrev, D.; Trautmann, C.; Toimil Molares, M.E.; Neumann, R.; Hjort, K.; Westerberg, L.; Spohr, R. Copper Nanowires Electrodeposited in Etched Single-Ion Track Templates. Appl. Phys. A 2003, 77, 751–755. [Google Scholar] [CrossRef]
- Martin, C.R. Nanomaterials: A Membrane-Based Synthetic Approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef]
- Sulka, G.; Zaraska, L.; Stępniowski, W. Anodic Porous Alumina as a Template for Nanofabrication. In Encyclopedia of Nanoscience and Nanotechnology; American Scientific Publishers: Valencia, CA, USA, 2011; Volume 11, pp. 261–349. [Google Scholar]
- Tiginyanu, I.; Ursaki, V.; Monaico, E. Template Assisted Formation of Metal Nanotubes. In Nanostructures and Thin Films for Multifunctional Applications: Technology, Properties and Devices; Tiginyanu, I., Topala, P., Ursaki, V., Eds.; NanoScience and Technology; Springer International Publishing: Cham, Switzerland, 2016; pp. 473–506. ISBN 978-3-319-30198-3. [Google Scholar]
- Langa, S.; Carstensen, J.; Tiginyanu, I.M.; Christophersen, M.; Föll, H. Formation of Tetrahedron-Like Pores during Anodic Etching of (100) Oriented n-GaAs. Electrochem. Solid-State Lett. 2001, 5, C14. [Google Scholar] [CrossRef]
- Monaico, E.; Tiginyanu, I.; Ursaki, V. Porous Semiconductor Compounds. Semicond. Sci. Technol. 2020, 35, 103001. [Google Scholar] [CrossRef]
- Tiginyanu, I.M.; Ursaki, V.V.; Monaico, E.; Enachi, M.; Sergentu, V.V.; Colibaba, G.; Nedeoglo, D.D.; Cojocaru, A.; Föll, H. Quasi-Ordered Networks of Metal Nanotubes Embedded in Semiconductor Matrices for Photonic Applications. J. Nanoelectron. Optoelectron. 2011, 6, 463–472. [Google Scholar] [CrossRef]
- Belov, A.N.; Gavrilov, S.A.; Shevyakov, V.I.; Redichev, E.N. Pulsed Electrodeposition of Metals into Porous Anodic Alumina. Appl. Phys. A 2011, 102, 219–223. [Google Scholar] [CrossRef]
- Abbondanza, G.; Larsson, A.; Linpé, W.; Hetherington, C.; Carlá, F.; Lundgren, E.; Harlow, G.S. Templated Electrodeposition as a Scalable and Surfactant-Free Approach to the Synthesis of Au Nanoparticles with Tunable Aspect Ratios. Nanoscale Adv. 2022, 4, 2452–2467. [Google Scholar] [CrossRef] [PubMed]
- Tiginyanu, I.; Monaico, E.; Monaico, E. Ordered Arrays of Metal Nanotubes in Semiconductor Envelope. Electrochem. Commun. 2008, 10, 731–734. [Google Scholar] [CrossRef]
- Dela Pena, E.M.; Roy, S. Electrodeposited Copper Using Direct and Pulse Currents from Electrolytes Containing Low Concentration of Additives. Surf. Coat. Technol. 2018, 339, 101–110. [Google Scholar] [CrossRef]
- Boukhouiete, A.; Creus, J. Nickel Deposits Obtained by Continuous and Pulsed Electrodeposition Processes. J. Mater. Environ. Sci. 2015, 6, 1840–1844. [Google Scholar]
- Renner, F.U.; Ankah, G.N.; Bashir, A.; Ma, D.; Biedermann, P.U.; Shrestha, B.R.; Nellessen, M.; Khorashadizadeh, A.; Losada-Pérez, P.; Duarte, M.J.; et al. Star-Shaped Crystallographic Cracking of Localized Nanoporous Defects. Adv. Mater. 2015, 27, 4877–4882. [Google Scholar] [CrossRef]
- Plesco, I.; Ciobanu, V.; Braniste, T.; Ursaki, V.; Rasch, F.; Sarua, A.; Raevschi, S.; Adelung, R.; Dutta, J.; Tiginyanu, I. Highly Porous and Ultra-Lightweight Aero-Ga2O3: Enhancement of Photocatalytic Activity by Noble Metals. Materials 2021, 14, 1985. [Google Scholar] [CrossRef]
- Enachi, M.; Guix, M.; Postolache, V.; Ciobanu, V.; Fomin, V.M.; Schmidt, O.G.; Tiginyanu, I. Light-Induced Motion of Microengines Based on Microarrays of TiO2 Nanotubes. Small 2016, 12, 5497–5505. [Google Scholar] [CrossRef]
- Monaico, E.; Tiginyanu, I.M.; Ursaki, V.V.; Sarua, A.; Kuball, M.; Nedeoglo, D.D.; Sirkeli, V.P. Photoluminescence and Vibrational Properties of Nanostructured ZnSe Templates. Semicond. Sci. Technol. 2007, 22, 1115–1121. [Google Scholar] [CrossRef]
- Irmer, G.; Monaico, E.; Tiginyanu, I.M.; Gärtner, G.; Ursaki, V.V.; Kolibaba, G.V.; Nedeoglo, D.D. Fröhlich Vibrational Modes in Porous ZnSe Studied by Raman Scattering and Fourier Transform Infrared Reflectance. J. Phys. Appl. Phys. 2009, 42, 045405. [Google Scholar] [CrossRef]
- Monaico, E.V.; Tiginyanu, I.M.; Ursaki, V.V.; Nielsch, K.; Balan, D.; Prodana, M.; Enachescu, M. Gold Electroplating as a Tool for Assessing the Conductivity of InP Nanostructures Fabricated by Anodic Etching of Crystalline Substrates. J. Electrochem. Soc. 2017, 164, D179. [Google Scholar] [CrossRef]
- Tiginyanu, I.; Monaico, E.; Nielsch, K. Self-Assembled Monolayer of Au Nanodots Deposited on Porous Semiconductor Structures. ECS Electrochem. Lett. 2015, 4, D8. [Google Scholar] [CrossRef]
- Tiginyanu, I.; Monaico, E.; Ursaki, V. Two-Dimensional Metallo-Semiconductor Networks for Electronic and Photonic Applications. ECS Trans. 2012, 41, 67. [Google Scholar] [CrossRef]
- Tiginyanu, I.; Monaico, E.; Sergentu, V.; Tiron, A.; Ursaki, V. Metallized Porous GaP Templates for Electronic and Photonic Applications. ECS J. Solid State Sci. Technol. 2015, 4, P57. [Google Scholar] [CrossRef]
- Monaico, E.; Tiginyanu, I.; Volciuc, O.; Mehrtens, T.; Rosenauer, A.; Gutowski, J.; Nielsch, K. Formation of InP Nanomembranes and Nanowires under Fast Anodic Etching of Bulk Substrates. Electrochem. Commun. 2014, 47, 29–32. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaico, E.I.; Monaico, E.V.; Ursaki, V.V.; Tiginyanu, I.M. Controlled Electroplating of Noble Metals on III-V Semiconductor Nanotemplates Fabricated by Anodic Etching of Bulk Substrates. Coatings 2022, 12, 1521. https://doi.org/10.3390/coatings12101521
Monaico EI, Monaico EV, Ursaki VV, Tiginyanu IM. Controlled Electroplating of Noble Metals on III-V Semiconductor Nanotemplates Fabricated by Anodic Etching of Bulk Substrates. Coatings. 2022; 12(10):1521. https://doi.org/10.3390/coatings12101521
Chicago/Turabian StyleMonaico, Elena I., Eduard V. Monaico, Veaceslav V. Ursaki, and Ion M. Tiginyanu. 2022. "Controlled Electroplating of Noble Metals on III-V Semiconductor Nanotemplates Fabricated by Anodic Etching of Bulk Substrates" Coatings 12, no. 10: 1521. https://doi.org/10.3390/coatings12101521
APA StyleMonaico, E. I., Monaico, E. V., Ursaki, V. V., & Tiginyanu, I. M. (2022). Controlled Electroplating of Noble Metals on III-V Semiconductor Nanotemplates Fabricated by Anodic Etching of Bulk Substrates. Coatings, 12(10), 1521. https://doi.org/10.3390/coatings12101521





