Recent Report on the Hydrothermal Growth of LiFePO4 as a Cathode Material
Abstract
:1. Li-Ion Batteries–When All Started
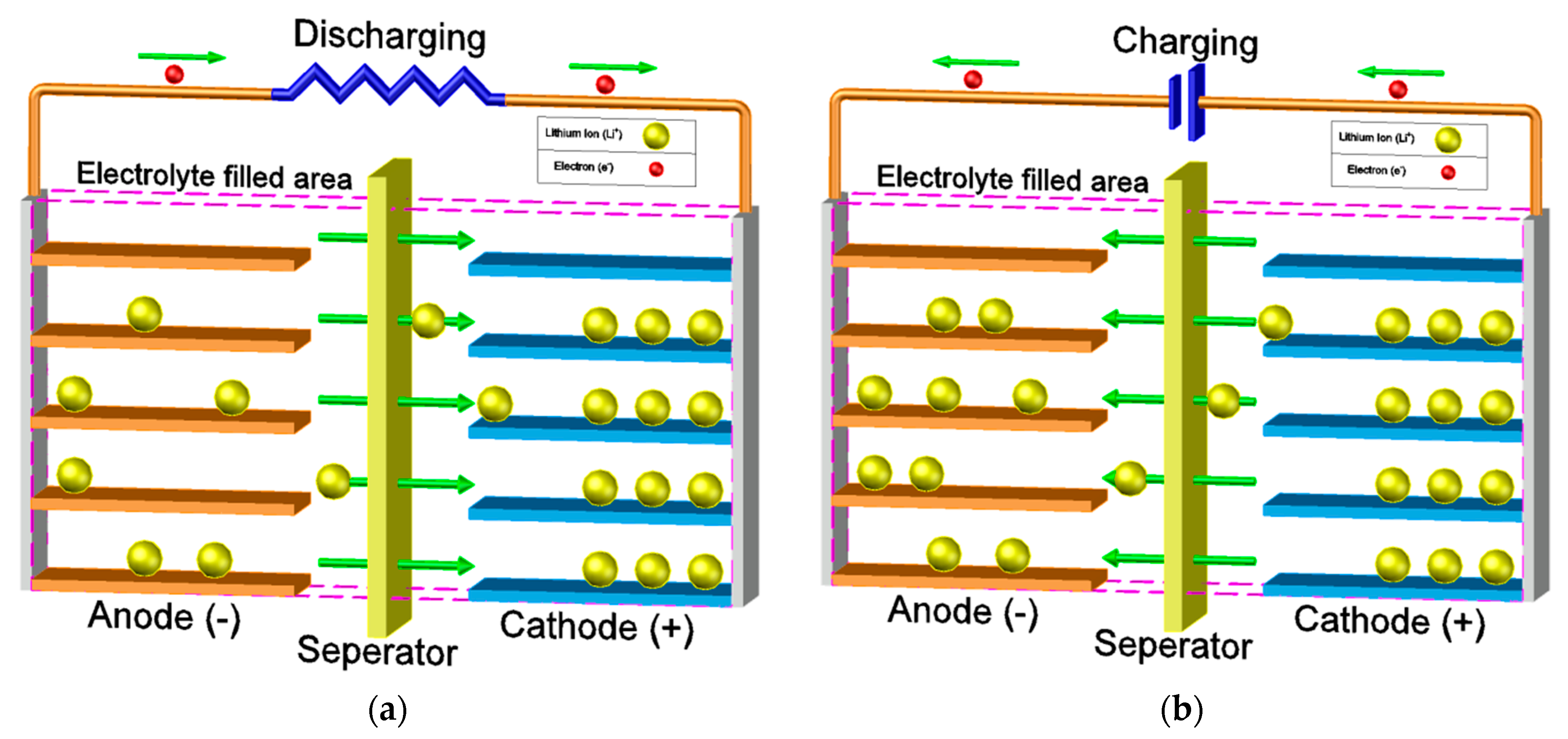
2. Basic Principles of Li-Ion Batteries
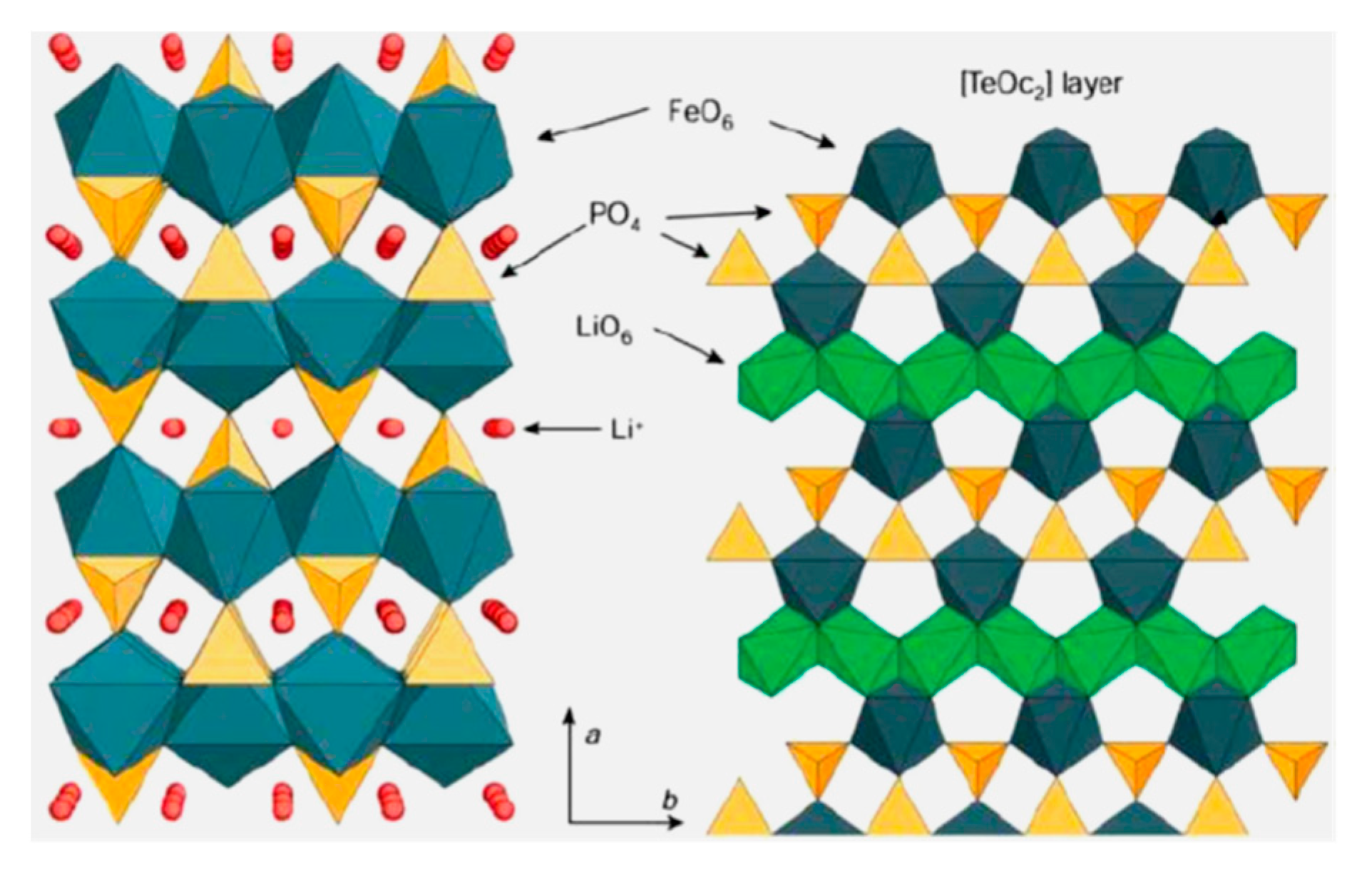
3. Features of LiFePO4
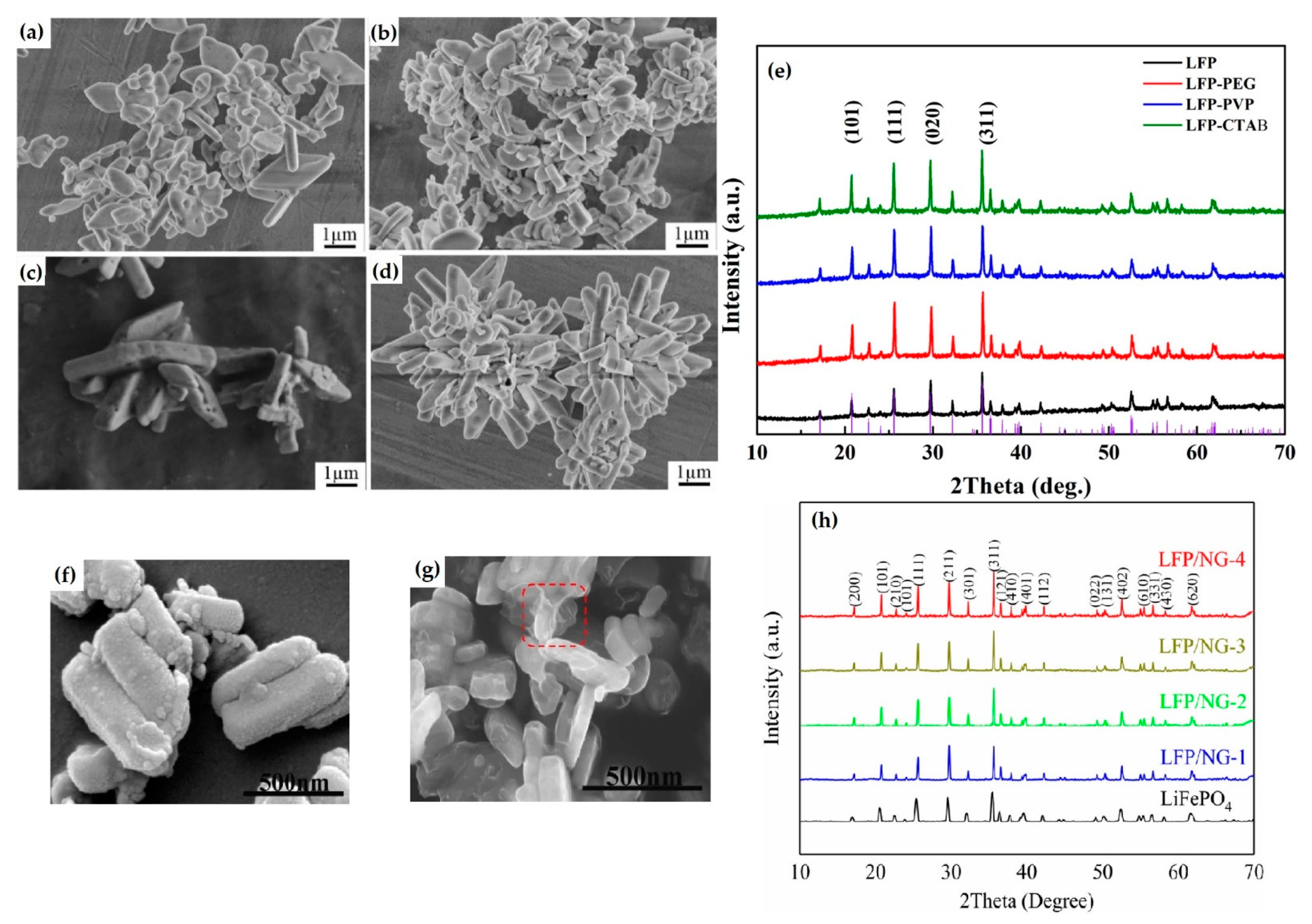
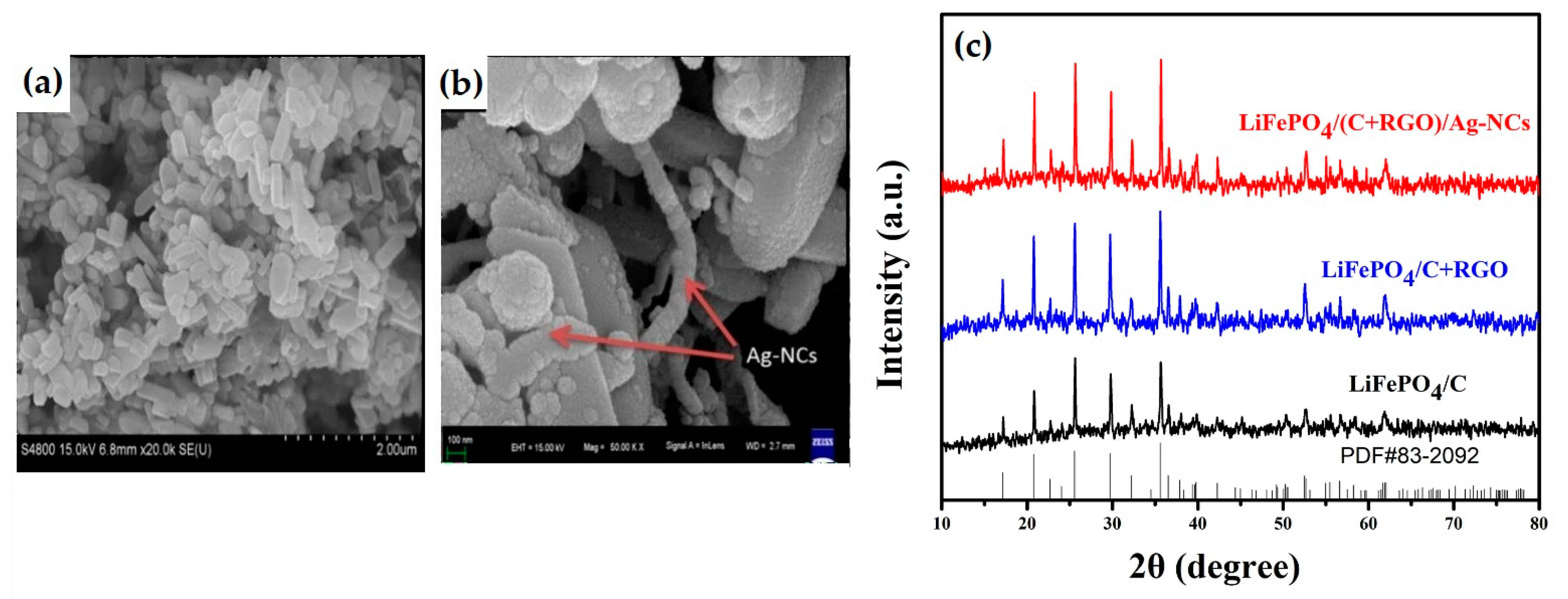
3.1. Hydrothermal Synthesis of LiFePO4
General Remarks
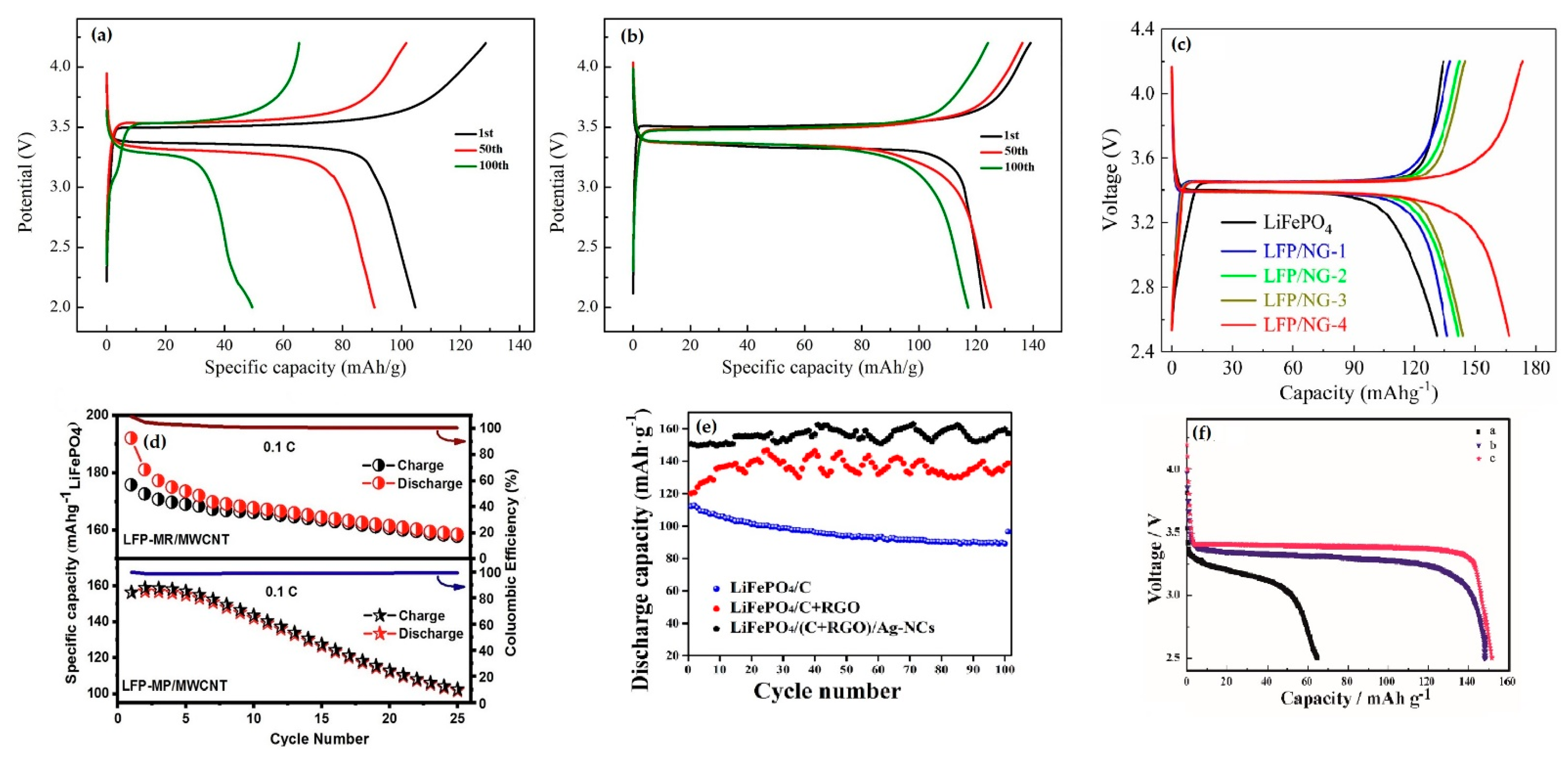
3.2. Electrochemical Evaluation of LiFePO4
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whittingham, M.S.; Gamble, F.R. The lithium intercalates of the transition metal dichalcogenides. Mater. Res. Bull. 1975, 10, 363–371. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x < −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar]
- Yoshino, A.; Sanechika, K.; Nakajima, T. Secondary Battery. JP Patent 1989293, 10 May 1985. [Google Scholar]
- Yazami, R.; Touzan, P. A reversible graphite-lithium negative electrode for electrochemical generators. J. Power Sources 1983, 9, 365–371. [Google Scholar] [CrossRef]
- Zhang, S.-D.; Qi, M.-Y.; Guo, S.-J.; Sun, Y.-G.; Tan, X.-X.; Ma, P.-Z.; Li, J.-Y.; Yuan, R.-Z.; Cao, A.-M.; Wan, L.-J. Advancing to 4.6 V review and prospect in developing high-energy-density LiCoO2 cathode for lithium-ion batteries. Small 2022, 6, 2200148. [Google Scholar] [CrossRef]
- Ritchie, A.G. Recent development and future prospects for lithium rechargeable batteries. J. Power Sources 2001, 96, 1–4. [Google Scholar] [CrossRef]
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.G.; et al. Formation of the spinel in the layered composite cathode used in Li-ion batteries. ACS Nano 2013, 7, 760–767. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, W.; Wang, Y.; Liang, S.; Gan, Y.; Huang, H.; Zhang, J.; Xia, Y.; Liang, C. Lithium sulfide as cathode materials for lithium-ion batteries: Advances and challenges. J. Chem. 2020, 2020, 6904517. [Google Scholar] [CrossRef] [Green Version]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Wu, F.; Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 2017, 10, 435–459. [Google Scholar] [CrossRef]
- Wu, F.; Magasinski, A.; Yushin, G. Nanoporous Li2S and MWCNT-linked Li2S powder cathodes for lithium-sulfur and lithium-ion battery chemistries. J. Mater. Chem. A 2014, 2, 6064–6070. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. NCA, NCM811, and the route to Ni-richer lithium-ion batteries. Energies 2020, 13, 6363. [Google Scholar] [CrossRef]
- Sturman, J.W.; Baranova, E.A.; Abu-Lebdeh, Y. Review: High-entropy materials for lithium-ion battery electrodes. Front. Energy Res. 2022, 10, 862551. [Google Scholar] [CrossRef]
- Liu, Q.; Su, X.; Lei, D.; Qin, Y.; Wen, J.; Guo, F.; Wu, Y.A.; Rong, Y.; Kou, R.; Xiao, X.; et al. Approaching the capacity limit of lithium coablat oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.C.; Hinokuma, K. Optimized LiFePO4 for lithium battery cathodes. J. Electrochem. Soc. 2001, 148, A224–A229. [Google Scholar] [CrossRef]
- Lee, M.-J.; Lee, S.; Oh, P.; Kim, Y.; Cho, J. High performance LiMn2O4 cathode materials grown with epitaxial layered nanostructure for Li-ion batteries. Nano Lett. 2014, 14, 993–999. [Google Scholar] [CrossRef]
- Cho, J.; Kim, Y.-W.; Kim, B.; Lee, J.-G.; Park, B. A breakthrough in the safety of lithium secondary batteries by coating the cathode material with AlPO4 nanoparticles. Angew. Int. Ed. Chem. 2003, 42, 1618–1621. [Google Scholar] [CrossRef]
- Li, Z.; Li, A.; Zhang, H.; Ning, F.; Li, W.; Zangiabadi, A.; Cheng, Q.; Borovials, J.J.; Chen, Y.; Zhang, H.; et al. Multi-scale stabilization of high-voltage LiCoO2 enabled by nanoscale solid electrolyte coating. Energy Storage Mater. 2020, 29, 71–77. [Google Scholar] [CrossRef]
- Flamary-Mespoulie, F.; Boulineau, A.; Martinez, H.; Suchomel, M.R.; Delmas, C.; Pecquenard, B.; Le Cras, F. Lithium-rich layered titanium sulfides: Cobalt- and nickel-free high capacity cathode materials for lithium-ion batteries. Energy Storage Mater. 2020, 26, 213–222. [Google Scholar] [CrossRef]
- Wu, B.; Ren, Y.; Li, N. LiFePO4 cathode material. In Electric Vehicles-The Benefits and Batteries; Soylu, S., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Mahmud, S.; Rahman, M.; Kamruzzaman, M.; Ali, M.O.; Emon, M.S.A.; Khatun, H.; Ali, M.R. Recent advances in lithium-ion battery materials for improved electrochemical performance: A review. Results Eng. 2022, 15, 100472. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Li, X.; Xiao, M.; Choe, S.-Y.; Joe, W.T. Modeling and analysis of LiFePO4/carbon battery considering two-phase transition during galvanostatic charging/discharging. Electrochim. Acta 2015, 155, 447–457. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, W.; Wang, Z.; Hu, H.; Shao, G. Self-consistent assessment of Li+ ion cathodes: Theory vs. experiments. J. Energy Chem. 2021, 59, 229–241. [Google Scholar] [CrossRef]
- Liu, H.; Strobridge, F.C.; Borkiewicz, O.J.; Wiaderek, K.M.; Chapman, K.W.; Chupas, P.J.; Grey, C.P. Batteries. Capturing metastable structures during high-rate cycling of LiFePO4 nanoparticle electrodes. Science 2014, 344, 1252817. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chand, P.; Kumar, A.; Anand, H. Effect of different aqueous electrolytes on electrochemical behavior of LiFePO4 as a cathode material: Lithium ion battery and renewable energy nexus. Energy Nexus 2021, 1, 100005. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.; Reddy, M.V.; Balaya, P.; Gong, H.; Chowdari, B.V.R.; Vittal, J.J. Storage performance of LiFePO4 nanoplates. J. Mater. Chem. 2009, 19, 605–610. [Google Scholar] [CrossRef]
- Bilecka, I.; Hintennach, A.; Rossell, M.D.; Xie, D.; Novák, P.; Niederberger, M. Microwave-assisted solution synthesis of doped LiFePO4 with high specific charge and outstanding cycling performance. J. Mater. Chem. 2011, 21, 5881–5890. [Google Scholar] [CrossRef]
- Shi, Y.; Chou, S.-L.; Wang, J.-Z.; Wexler, D.; Li, H.-J.; Liu, H.-K.; Wu, Y. Graphene wrapped LiFePO4/C composites as cathode materials for Li-ion batteries with enhanced rate capability. J. Mater. Chem. 2012, 22, 16465–16470. [Google Scholar] [CrossRef] [Green Version]
- Saliman, M.A.; Okawa, H.; Takai, M.; Ono, Y.; Kato, T.; Sugawara, K.; Sato, M. Improved battery performance using Pd nanoparticles synthesized on the surface of LiFePO4/C by ultrasound irradiation. Jpn. J. Appl. Phys. 2016, 55, 07KE05. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Yang, C.P.; Rui, X.H.; Cheng, T.; Chen, C.H. V2O3 modified LiFePO4/C composite with improved electrochemical performance. J. Power Sources 2011, 196, 5623–5630. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, G.; Ma, Z.; Wang, G.; Du, J. Influence of surface modification by vanadium oxide and carbon on the electrochemical performance of LiFePO4/C. Ionics 2013, 19, 1091–1097. [Google Scholar] [CrossRef]
- Bezerra, C.A.G.; Davoglio, R.A.; Biaggio, S.R.; Bocchi, N.; Rocha-Filho, R.C. High-purity LiFePO4 prepared by a rapid one-step microwave-assisted hydrothermal synthesis. J. Mater. Sci. 2021, 56, 10018–10029. [Google Scholar] [CrossRef]
- Liu, S.; Yan, P.; Li, H.; Zhang, X.; Sun, W. One-step microwave synthesis of micro/nanoscale LiFePO4/Graphene cathode with high performance for lithium-ion batteries. Front. Chem. 2020, 8, 104. [Google Scholar] [CrossRef]
- Guo, F.A.; Kong, Z.; Wang, T.; Liu, X.; Xu, Z.G.; Fu, A.; Li, Y.; Guo, P.; Guo, Y.-G.; Li, H. Porous microspheres consisting of carbon-modified LiFePO4 grains prepared by a spray-drying assisted approach using cellulose as carbon source. Ionics 2020, 26, 2737–2746. [Google Scholar] [CrossRef]
- Yang, M.-R.; Teng, T.-H.; Wu, S.-H. LiFePO4/carbon cathode materials prepared by ultrasonic spray pyrolysis. J. Power Sources 2006, 159, 307–311. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.J. Nonaqueous sol-gel synthesis of high-performance LiFePO4. Electrochem. Solid-State Lett. 2004, 7, A515–A518. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, L.; Lai, Q.; Ji, X. A PEG assisted sol-gel synthesis of LiFePO4 as cathodic material for lithium ion cells. Mater. Res. Bull. 2007, 42, 883–891. [Google Scholar] [CrossRef]
- Alsamet, M.A.M.M.; Burgaz, E. Synthesis and characterization of nanosized LiFePO4 by using consecutive combination of sol-gel and hydrothermal methods. Electrochim. Acta 2021, 367, 137530. [Google Scholar] [CrossRef]
- Zhu, H.; Miao, C.; Guo, R.; Liu, Y.; Wang, X. A simple and low-cost synthesis strategy of LiFePO4 nanoparticles as cathode materials for lithium ion batteries. Int. J. Electrochem. Sci. 2021, 16, 210331. [Google Scholar] [CrossRef]
- Trapatseli, M.; Vernardou, D.; Tzanetakis, P.; Spanakis, E. Field emission properties of low-temperature, hydrothermally grown tungsten oxide. ACS Appl. Mater. Interfaces 2011, 3, 2726–2731. [Google Scholar] [CrossRef] [PubMed]
- Vernardou, D.; Vlachou, K.; Spanakis, E.; Stratakis, E.; Katsarakis, N.; Kymakis, E.; Koudoumas, E. Influence of solution chemistry on the properties of hydrothermally grown TiO2 for advanced applications. Catal. Today 2009, 144, 172–176. [Google Scholar] [CrossRef]
- Qian, J.; Zhou, M.; Cao, Y.; Ai, X.; Yang, H. Template-free hydrothermal synthesis of nanoembossed mesoporous LiFePO4 microspheres for high-performance lithium-ion batteries. J. Phys. Chem. C 2010, 114, 3477–3482. [Google Scholar] [CrossRef]
- Recham, N.; Armand, M.; Laffont, L.; Tarascon, J.-M. Eco-efficient synthesis of LiFePO4 with different morphologies for Li-ion batteries. Electrochem. Solid-State Lett. 2008, 12, A39–A44. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, H.; Hakuta, Y. Hydrothermal synthesis of metal oxide nanoparticles in supercritical water. Materials 2010, 3, 3794–3817. [Google Scholar] [CrossRef] [Green Version]
- Tajimi, S.; Ikeda, Y.; Uematsu, K.; Toda, K.; Sato, M. Enhanced electrochemical performance of LiFePO4 prepared by hydrothermal reaction. Solid State Ion. 2004, 175, 287–290. [Google Scholar] [CrossRef]
- Jugović, D.; Uskoković, D. A review of recent developments in the synthesis procedures of lithium iron phosphate powders. J. Power Sources 2009, 190, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huo, Q.-Y.; Du, P.-P.; Wang, L.-Z.; Zhang, A.-Q.; Song, Y.-H.; Lv, Y.; Li, G.-Y. Advances in new cathode material LiFePO4 for lithium-ion batteries. Synth. Met. 2012, 162, 1315–1326. [Google Scholar] [CrossRef]
- Satyavani, T.V.S.L.; Kumar, A.S.; Subba Rao, P.S.V. Methods of synthesis and performance improvement of lithium iron phosphate for high rate Li-ion batteries: A review. Eng. Sci. Technol. Int. J. 2016, 19, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.-F.; Zhang, S.-Q.; Hu, M.-Y.; Wang, C.; Jiang, G.-H. Recent advances in LiFePO4 cathode materials for lithium-ion batteries. First—Principles research. Int. J. Electrochem. Sci. 2021, 16, 211226. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Guang, T.; Fan, B.; Zhu, K.; Wang, X. Controlled hydrothermal/solvothermal synthesis of high-performance LiFePO4 for lithium-ion batteries. Small Methods 2021, 5, 2100193. [Google Scholar] [CrossRef]
- Chen, S.P.; Lv, D.; Chen, J.; Zhang, Y.-H.; Shi, F.-N. Review on defects and modification methods of LiFePO4 cathode material for lithium-ion batteries. Energy Fuels 2022, 36, 1232–1251. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, C.; Wen, Z.; Xu, J.; Han, T.; Li, G.; Huang, D.; Liang, X.; Lan, Z.; Ning, H.; et al. Effects of defect chemistry and kinetic behavior on electrochemical properties for hydrothermal synthesis of LiFePO4/C cathode materials. Mater. Chem. Phys. 2019, 227, 56–63. [Google Scholar] [CrossRef]
- Rajoba, S.J.; Jadhav, L.D.; Kalubarme, R.S.; Patil, P.S.; Varma, S.; Wani, B.N. Electrochemical performance of LiFePO4/GO composite for Li-ion batteries. Ceram. Int. 2018, 44, 6886–6893. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, G.Y.; Gu, Y.J.; Wu, F.Z.; Mai, Y.; Dai, X.Y. Study on the preparation of LiFePO4 by hydrothermal method. IOP Conf. Ser. Mater. Sci. Eng. 2019, 761, 012004. [Google Scholar] [CrossRef]
- Geng, J.; Zhang, S.; Zou, Z.; Liu, J.; Zhong, S. Enhanced electrochemical performance of LiFePO4 of cathode materials for lithium-ion batteries synthesized by surfactant-assisted solvothermal method. J. Solid State Electrochem. 2021, 25, 2527–2537. [Google Scholar] [CrossRef]
- Luo, G.-Y.; Gu, Y.-J.; Liu, Y.; Chen, Z.-L.; Huo, Y.-I.; Wu, F.-Z.; Mai, Y.; Dai, X.-Y.; Deng, Y. Electrochemical performance of in situ LiFePO4 modified by N-doped graphene for Li-ion batteries. Ceram. Int. 2021, 47, 11332–11339. [Google Scholar] [CrossRef]
- Kanagaraj, A.B.; Chaturvedi, P.; Kim, H.J.; Choi, D.S. Controllable synthesis of LiFePO4 microrods and its superior electrochemical performance. Mater. Lett. 2021, 283, 128737. [Google Scholar] [CrossRef]
- Wen, L.; Liu, J.; Hou, J.; Zhao, S.; Liu, J.; Zheng, Z.; Bei, F. A high performance LiFePO4 cathode material with 3D conduction network connected by 1D helix-like Ag nanochains. Int. J. Electrochem. Sci. 2021, 16, 151002. [Google Scholar] [CrossRef]
- Hu, L.-H.; Wu, F.-Y.; Lin, C.-T.; Khlobystov, A.N.; Li, L.-J. Graphene-modified LiFePO4 cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Wu, G.; Wu, J.; Yang, H.; Wang, J.; Gao, G.; Cai, R.; Yan, Q. Multiwalled carbon nanotubes-V2O5 integrated composite with nanosized architecture as a cathode material for high performance lithium ion batteries. J. Mater. Chem. A 2013, 1, 15459–15468. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Z.; Huang, J.; Deng, W.; Li, X.; Zhang, H.; Wen, Z. Graphene-decorated carbon-coated LiFePO4 nanospheres as a high-performance cathode material for lithium-ion batteries. Carbon 2018, 127, 149–157. [Google Scholar] [CrossRef]
- Bazzi, K.; Nazri, M.; Naik, V.M.; Garg, V.K.; Oliveira, A.C.; Vaishnava, P.P.; Nazri, G.A.; Naik, R. Enhancement of electrochemical behavior of nanostructured LiFePO4/carbon cathode material with excess Li. J. Power Sources 2016, 306, 17–23. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Lou, J.J.; Teng, X.J.; Lu, X.X.; Liu, S.Y.; Chi, H.Z.; Ji, Z.G. Controllable synthesis of N-C@LiFePO4 nanospheres as advanced cathode of lithium ion batteries. J. Alloys Compd. 2018, 743, 377–382. [Google Scholar] [CrossRef]
- Han, B.; Meng, X.; Ma, L.; Nan, J. Nitrogen-doped carbon decorated LiFePO4 composite synthesized via a microwave heating route using polydopamine as carbon-nitrogen precursor. Ceram. Int. 2016, 42, 2789–2797. [Google Scholar] [CrossRef]
- Zhang, K.; Lee, J.T.; Li, P.; Kang, B.; Kim, J.H.; Yi, G.R.; Park, J.H. Conformal coating strategy comprising N-doped carbon and conventional graphene for achieving ultrahigh power and cyclability of LiFePO4. Nano Lett. 2015, 15, 6756–6763. [Google Scholar] [CrossRef]
- Lei, X.; Zhang, H.; Chen, Y.; Wang, W.; Ye, Y.; Zheng, C.; Deng, P.; Shi, Z. A three-dimensional LiFePO4/carbon nanotubes/graphene composite as a cathode material for lithium-ion batteries with superior high-rate performance. J. Alloys Compd. 2015, 626, 280–286. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, F.; Zhang, B.; Yu, W.J.; Dai, Q.; Hu, S.; He, W.; Tong, H.; Zheng, J.; Liao, J. (010) Facets dominated LiFePO4 nano-flakes confined in 3D porous graphene network as a high-performance Li-ion battery cathode. Ceram. Int. 2018, 44, 18181–18188. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Z.S.; Chen, J.J.; Zhang, C. Synthesis and electrochemical performance of LiFePO4/graphene composites by solid-state reaction. Mater. Lett. 2012, 71, 54–56. [Google Scholar] [CrossRef]
- Rao, Y.; Wang, K.; Zeng, H. The effect of phenol-formaldehyde resin on the electrochemical properties of carbon-coated LiFePO4 materials in pilot scale. Ionics 2015, 21, 1525–1531. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Gu, H.-B. LiFePO4 batteries with enhanced lithium-ion-diffusion ability due to graphene addition. J. Appl. Electrochem. 2014, 44, 1153–1163. [Google Scholar] [CrossRef]
- Liu, N.; Gao, M.; Li, Z.; Li, C.; Wang, W.; Zhang, H.; Yu, Z.; Huang, Y. Effect of gelatin concentration on the synthetize of the LiFePO4/C composite for lithium ion batteries. J. Alloys Compd. 2014, 599, 127–130. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, H.; Xu, Q.; Zhang, X.; Tang, Z.; Miao, C. Effect of graphene nanosheets content on the morphology and electrochemical performance of LiFePO4 particles in lithium ion batteries. Electrochim. Acta 2014, 135, 311–318. [Google Scholar]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
| Cathode | LiFePO4 | LiMn2O4 | LiCoO2 | Li2TiS3 |
|---|---|---|---|---|
| Specific capacity/mAh·g−1 | 170 [15] | 148 [16] | 274 [17,18] | 339 [19] |
| Specific energy/Wh·kg−1 | 590 [20] | 560 [20] | 980 [20] | 810 [19] |
| LiFePO4 | Advantages | Disadvantages |
|---|---|---|
| Microwave assisted synthesis | pure products, control over reaction parameters, green raw materials (H2O, alcohols) | expensive equipment unfeasible reaction monitoring |
| Spray pyrolysis | narrow particle size distribution, homogeneous preparation | plethora of parameters to control (solute concentration, temperature, temperature gradients, residence time in furnace and carrier gases) |
| Sol-gel | homogeneous and high adhesion products, low temperature processing | safety matters concerned since countable amounts of by-products are released in calcination step long growth period |
| Hydrothermal method | simple, easy and low-cost method, production of high-quality nanostructures through an easy control of growth parameters | long growth period |
| LiFePO4 | Precursors | Molar Ratio of Reactants | Fe Source |
|---|---|---|---|
| disc form [26] | LiOOCCH3, Fe(NO3)3·9H2O, NaH2PO4, CTAB | - | Fe(NO3)3·9H2O |
| Nanoparticles [42] | Naphthenic acid, isooctyl alcohol, FeSO4·7H2O, LiOH, H3PO4 | LiOH:H3PO4, 1.2:1 | FeSO4·7H2O |
| Nanoparticles [57] | LiOH·H2O, FeSO4·7H2O, H3PO4, glucose | - | FeSO4·7H2O |
| Flower-like morphology [58] | LiOH·H2O, FeSO4·7H2O, H3PO4, CTAB | LiOH:FeSO4:H3PO4 = 3:1:1 | FeSO4·7H2O |
| Flat rhombohedron-like shape [58] | LiOH·H2O, FeSO4·7H2O, H3PO4, PEG | LiOH:FeSO4:H3PO4 = 3:1:1 | FeSO4·7H2O |
| Porous structure [58] | LiOH·H2O, FeSO4·7H2O, H3PO4, PVP | LiOH:FeSO4:H3PO4 = 3:1:1 | FeSO4·7H2O |
| 3D conductive network structure [59] | LiOH·H2O, FeSO4·7H2O, H3PO4, graphite powder, H2SO4, KMnO4, melamine | - | FeSO4·7H2O |
| Microparticles [60] | LiOH·H2O, FeCl2, H3PO4, ascorbic acid, ethylene glycol | LiOH:FeCl2:H3PO4, 3:1:1 | FeCl2 |
| Microrods [60] | LiOH·H2O, FeSO4, H3PO4, ascorbic acid, ethylene glycol | LiOH:FeSO4:H3PO4, 3:1:1 | FeSO4 |
| 3D conduction network connected by 1D helix-like Ag nanochains [61] | LiOH, FeSO4, H3PO4, NH3·H2O AgNO3, CH3CHO | LiOH: FeSO4: H3PO4, 3:1:1 | FeSO4 |
| Cathode Materials | Synthesis Process | Specific Capacity (mAh·g−1) | Capacity Retention |
|---|---|---|---|
| LFP/GO [56] | Solution combustion/colloidal | 162 at 0.1 C | 96% after 40 cycles at 0.2 C |
| LFP-microrods/MWCNT [60] | Hydrothermal method | 192 at 0.1 C | ~97% after 600 cycles at 10 C |
| LFP@C/G [64] | Rheological phase/solid state | 163.8 at 0.1 C | 92% after 500 cycles at 10 C |
| C-L1.05FP [65] | Sol-gel | 155 at C/30 | Excellent cycling stability after 100 cycles |
| N-C@LFP [66] | Hydrothermal plus chemical polymerization | 162.1 at 1 C | 100% after 100 cycles at 10 C |
| LFP/CN [67] | Microwave heating route | 160 at 0.2 C | 97.9% after 50 cycles at 0.1 C |
| LFP NR@N-C@RGO [68] | Surfactant-assisted synthesis | 172 at 0.1 C | 95.8% after 1000 cycles at 10 C |
| LFP-CNT-G [69] | Solid state | 168.9 at 0.2 C | 98% after 100 cycles at 0.2 C |
| LFP@G [70] | Solvothermal/freeze-drying | 163 at 0.2 C | 99.8% after 600 cycles at 10 C |
| LFP/G [71] | Solid state | 161 at 0.1 C | 70 mAh·g−1 after 44 cycles at 50 C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernardou, D. Recent Report on the Hydrothermal Growth of LiFePO4 as a Cathode Material. Coatings 2022, 12, 1543. https://doi.org/10.3390/coatings12101543
Vernardou D. Recent Report on the Hydrothermal Growth of LiFePO4 as a Cathode Material. Coatings. 2022; 12(10):1543. https://doi.org/10.3390/coatings12101543
Chicago/Turabian StyleVernardou, Dimitra. 2022. "Recent Report on the Hydrothermal Growth of LiFePO4 as a Cathode Material" Coatings 12, no. 10: 1543. https://doi.org/10.3390/coatings12101543
APA StyleVernardou, D. (2022). Recent Report on the Hydrothermal Growth of LiFePO4 as a Cathode Material. Coatings, 12(10), 1543. https://doi.org/10.3390/coatings12101543







