Effect of Nanodiamond Content in the Plating Solution on the Corrosion Resistance of Nickel–Nanodiamond Composite Coatings Prepared on Annealed 45 Carbon Steel
Abstract
:1. Introduction
2. Preparation for Plating
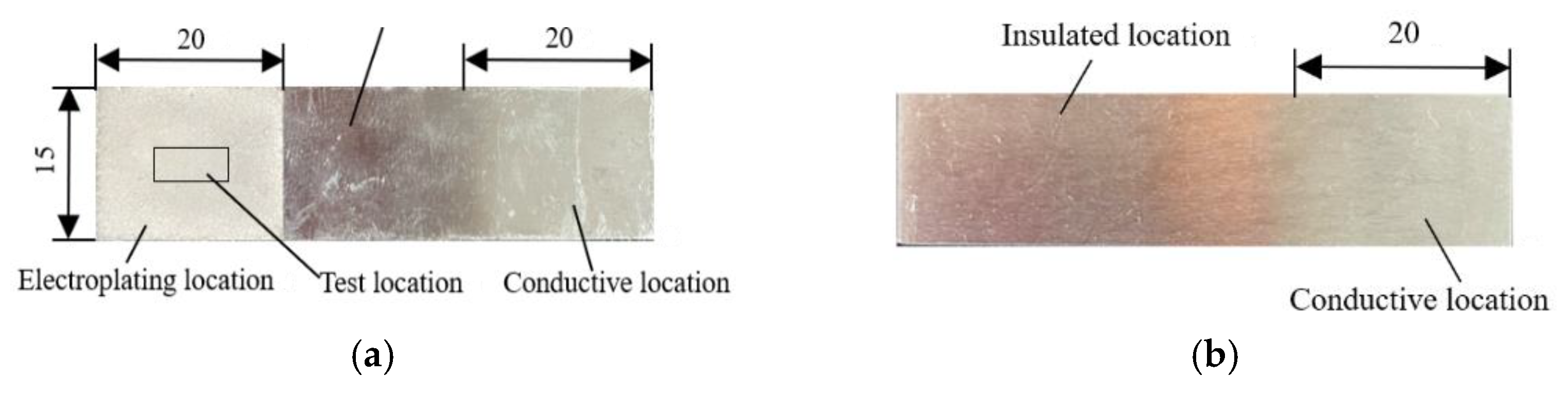
2.1. Substrate Specifications
2.2. Pre-Plating Treatment
2.3. Testing of Cathodic Polarization Behavior and Tafel Curves
2.4. Plating Solution Components and Process Parameters
2.5. Tests on the Performance of the Composite Coating
3. Analysis of Results
3.1. Effect of Nanodiamond in the Plating Solution on the Cathodic Polarization Behavior of Nickel Ions
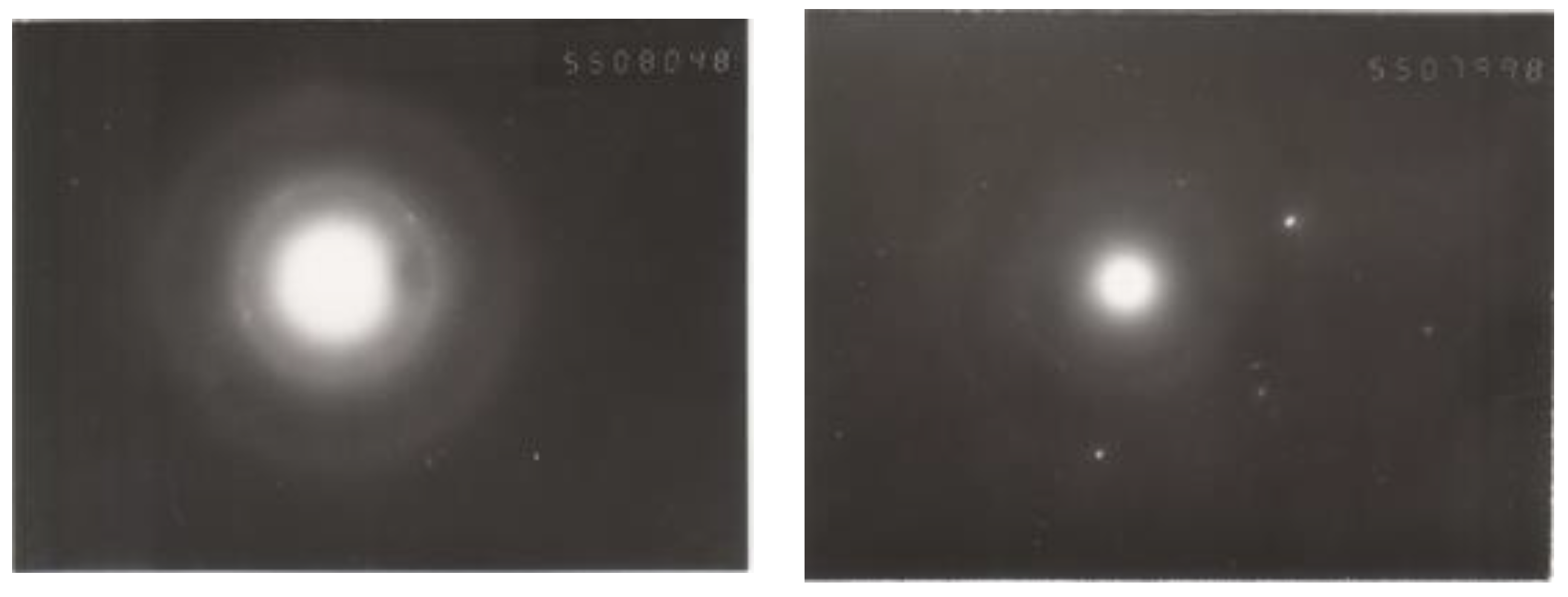
3.2. Testing of Nanodiamond Particles
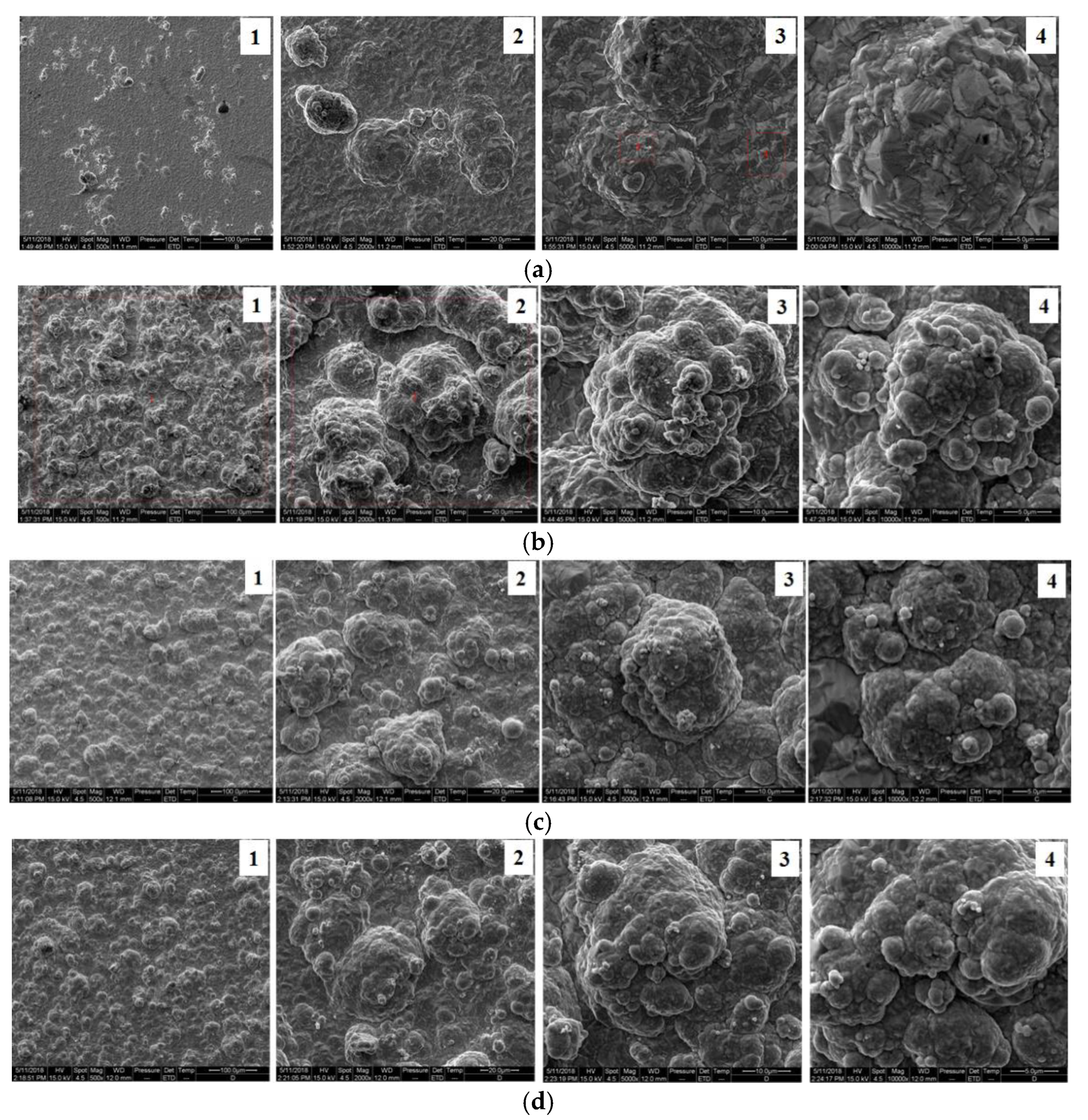
3.3. Surface Morphology Analysis of the Composite Coating
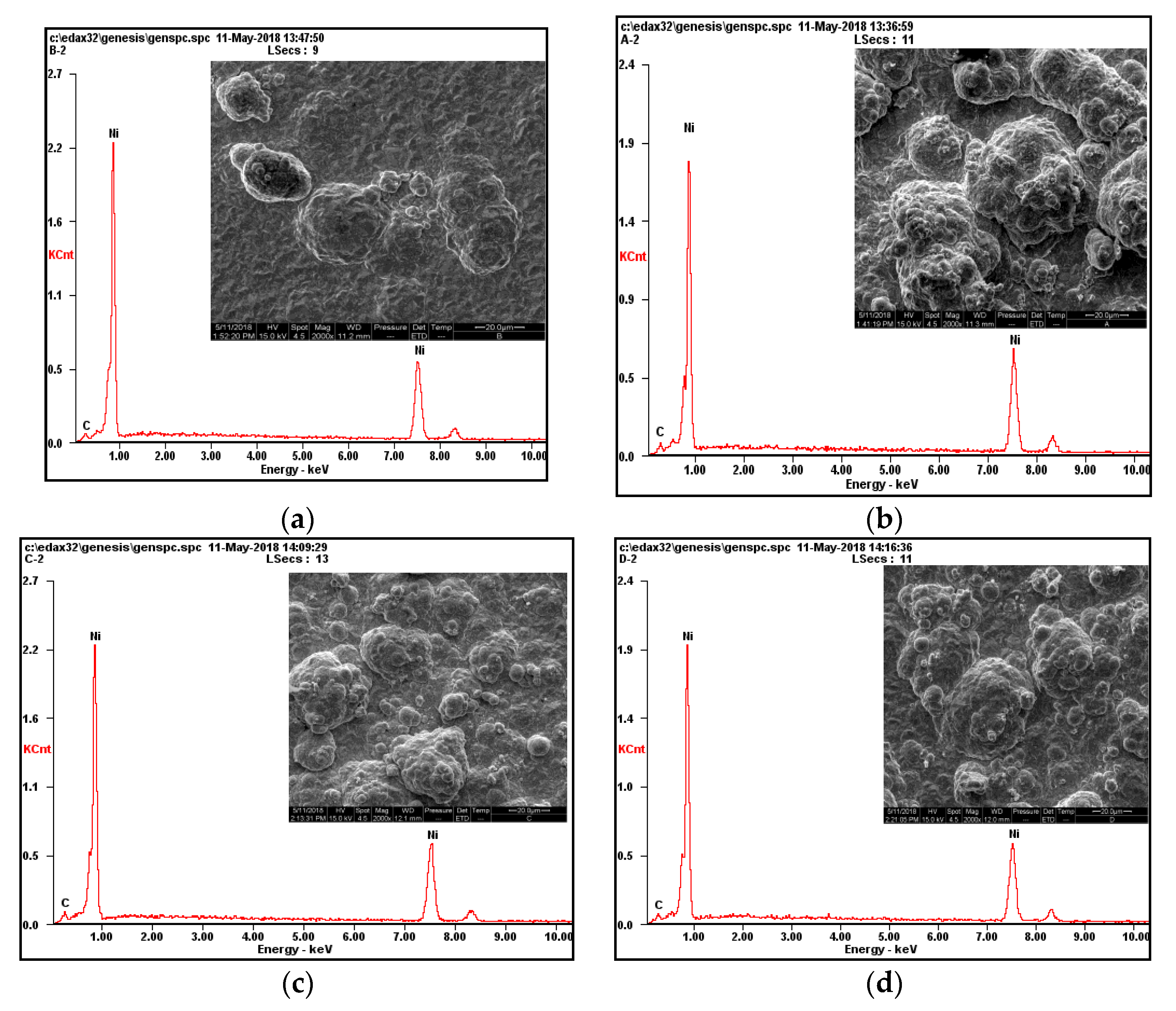
3.4. Analysis of the Surface Composition of the Composite Coating
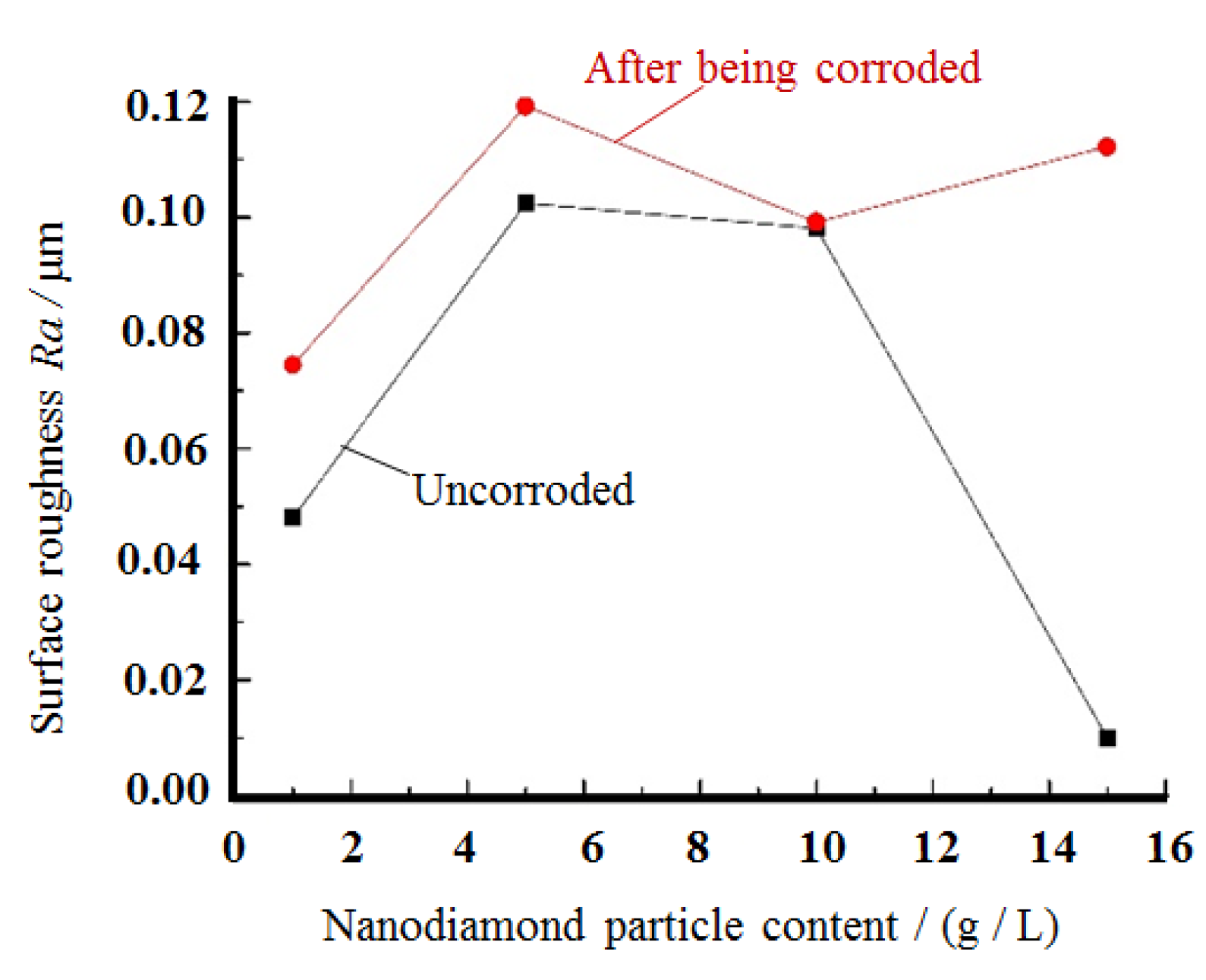
3.5. Analysis of the Corrosion Resistance and Surface Roughness of the Composite Plating
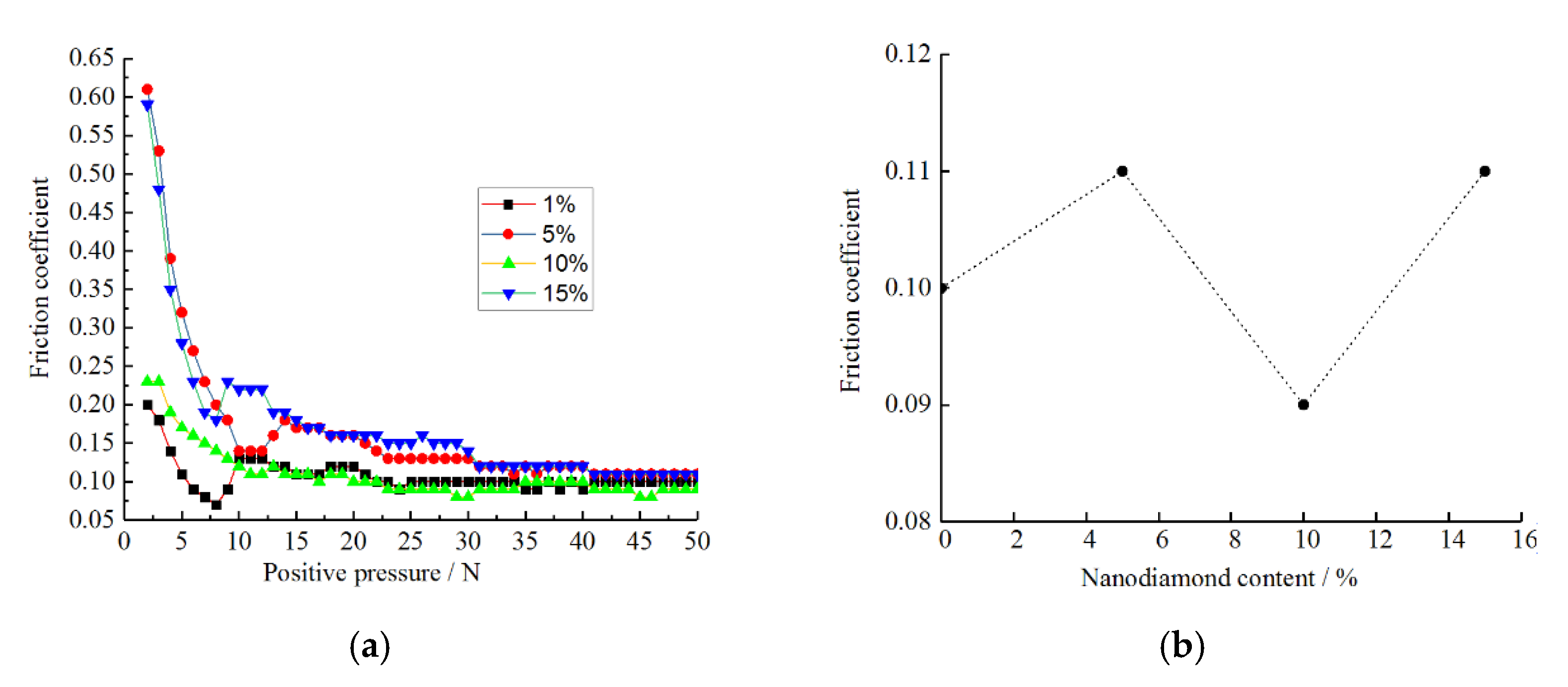
3.6. Analysis of the Friction Properties of the Composite Coating
4. Conclusions
- (1)
- The addition of nanodiamond particles to the plating solution affects the electrochemical behavior of nickel ions. The positively charged nanodiamond particles on the surface facilitate the reduction reaction at the cathode. The main reason is that the nanodiamond particles are positively charged in the wattage plating solution and the cathodic polarization is enhanced during electrodeposition. Among the results, when the content of nanodiamond in the plating solution is 10 g/L, the polarization potential is the smallest, and the nickel particles in the composite plating solution can be better electrodeposited on the substrate surface.
- (2)
- The surface of a nickel composite plating containing nanodiamond particles has a typical “cauliflower head” shape. As the content of nanodiamond particles increases, the grain size of the composite coating surface gradually decreases and the surface becomes flatter and denser, which improves the wear resistance of the composite coating. The main reason is that the sphere-like nanodiamond particles provide a large number of crystalline growth points as the crystalline core of non-spontaneous nucleation, which achieves the effect of effective grain refinement. The surface of the composite plating prepared with the content of 10 g/L is flat and smooth, and the grains are small and uniformly distributed. According to the scanning electron microscope energy spectrum, the highest C content of 1.99% was found on the surface of the composite coating.
- (3)
- The corrosion resistance of the nickel–nanodiamond composite coating was analyzed based on the results of the Tafel curves and by comparing the changes of the surface roughness parameter Ra before and after the composite coating was corroded. The results show that the nanodiamond particles alter the self-corrosion potential of the nickel composite plating. The corrosion current of monolithic nickel plating is higher than that of composite plating and is corroded faster. The general trend is that the corrosion potential shows a positive shift as the content of nanodiamond particles in the plating solution increases. The general trend is that the corrosion potential shows a positive shift as the content of nanodiamond particles in the plating solution increases. This may be related to the fineness and denseness of the grains on the surface of the composite coating due to the addition of nanodiamond.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Fei, J.Y.; Li, B.; Peng, Q.Y. Reseach progress of Ni based diamond compound coating. Hot Work. Technol. 2016, 8, 25–29. [Google Scholar]
- Xu, Z.L.; Xia, L. Engineering Materials; Huazhong University of Science and Technology Press: Wuhan, China, 2020; Volume 8. [Google Scholar]
- Wu, G.H.; Shen, J.X.; Zhuang, L. Metal Materials & Heat Treatment; Beijing University of Technology Press: Beijing, China, 2018; Volume 8. [Google Scholar]
- Zhang, M.X. Preparation and Tribological Properties of Electroless Ni-B Coating on 45# Steel Substrates. Master’s Thesis, Qingdao University of Technology, Qingdao, China, 2015. [Google Scholar]
- Lin, C.S.; Hsu, P.C.; Chang, L.; Chen, C.H. Properties and microstructure of nickel electrodeposited from a sulfamate bath containing ammonium ions. J. Appl. Electrochem. 2001, 31, 925–933. [Google Scholar] [CrossRef]
- Prasanta, S.; Suman, K.D. Tribology of electroless nickel coatings—A review. Mater. Des. 2011, 32, 1760–1775. [Google Scholar] [CrossRef]
- Bai, X.C.; Liang, G.X.; Lv, M. Micromorphology and wear resistance of selective electrochemically deposited nichel coating. Heat Treat. Met. 2021, 46, 142–148. [Google Scholar]
- Yuan, X.T.; Wang, Y.; Sun, D.B.; Yu, H.Y. Influence of pulse parameters on the microstructure and microhardness of nickel electrodeposits. Surf. Coat. Technol. 2008, 202, 1895–1903. [Google Scholar] [CrossRef]
- Walsh, F.C.; Ponce de Leon, C. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology. Trans. IMF 2014, 92, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Peeters, P.; Hoorn, G.V.D.; Daenen, T.; Kurowski, A.; Staikov, G. Properties of electroless and electroplated Ni–P and its application in microgalvanics. Electrochim. Acta 2001, 47, 161–169. [Google Scholar] [CrossRef]
- Guo, C.; Zuo, Y.; Zhao, X.; Zhao, J.; Xiong, J. Effects of surfactants on electrodeposition of nickel-carbon nanotubes composite coatings. Surf. Coat. Technol. 2008, 202, 3385–3390. [Google Scholar] [CrossRef]
- Chou, M.C.; Ger, M.D.; Ke, S.T.; Huang, Y.R.; Wu, S.T. The Ni-P-SiC composite produced by electro-codeposition. Mater. Chem. Phys. 2005, 92, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Zhu, X.B.; Zheng, G.Q.; Yuan, Y.N.; Huang, X.Q.; Cao, F.H.; Yang, J.F.; Zhang, Z. Electrodeposition and characterization of nano-structured Ni–SiC composite films. Surf. Coat. Technol. 2011, 205, 3448–3454. [Google Scholar] [CrossRef]
- Gang, W.; Ning, L.; Derui, Z.; Kurachi, M. Electrodeposited Co-Ni-Al2O3 composite coatings. Surf. Coat. Technol. 2004, 176, 157–164. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Perasinjaroen, T.; Aeksen, W.; Das, M.K.; Hao, R.; Zhang, B.; Wangyao, P.; Boonyongmaneerat, Y.; Limpanart, S.; et al. Preparation and hardness of pulse electrodeposited Ni-W- diamond composite coatings. Surf. Coat. Technol. 2015, 276, 228–232. [Google Scholar] [CrossRef]
- Vaezi, M.R.; Sadrnezhaad, S.K.; Nikzad, L. Electrodeposition of Ni–SiC nano-composite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 176–182. [Google Scholar] [CrossRef]
- Soleimani, R.; Mahboubi, F.; Kazemi, M.; Arman, S.Y. Corrosion and tribological behaviour of electroless Ni-P/nano-SiC composite coating on aluminium 6061. Surf. Eng. 2015, 31, 714–721. [Google Scholar] [CrossRef]
- Luo, H.; Leitch, M.; Behnamian, Y.; Ma, Y.; Zeng, H.; Luo, J.L. Development of electroless Ni-P/nano-WC composite coatings and investigation on its properties. Surf. Coat. Technol. 2015, 277, 99–106. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low, C.T.J.; Bello, O. Influence of surfactants on electrodeposition of a Ni- nanoparticulate SiC composite coating. Trans. IMF 2015, 93, 147–156. [Google Scholar] [CrossRef]
- Tamilarasan, T.R.; Rajendran, R.; Rajagopa, G.; Sudagar, J. Effect of surfactants on the coating properties and corrosion behavior of Ni-P-nano-TiO2 coatings. Surf. Coat. Technol. 2015, 276, 320–326. [Google Scholar] [CrossRef]
- Grosjean, A.; Rezrazi, M.; Takadoum, J.; Bercot, P. Hardness, friction and wear characteristics of nickel-SiC electroless composite deposits. Surf. Coat. Technol. 2001, 137, 92–96. [Google Scholar] [CrossRef]
- Hamed, M.; Saeed, A. Deposition characterization and electrochemical evaluation of Ni-P-nano diamond composite coatings. Appl. Surf. Sci. 2012, 258, 4574–4580. [Google Scholar] [CrossRef]
- Luan, X.W.; Wang, M.Z.; Zhao, Y.C. Study on electroplated nickel-nanodiamond composite coating. Diam. Abras. Eng. 2005, 150, 20–22. [Google Scholar]
- Wang, J.; Zhang, F.L.; Zhang, T.; Liu, W.G.; Li, W.X.; Zhou, Y.M. Preparation of Ni-P-diamond coatings with dry friction characteristics and abrasive wear resistance. Int. J. Refract. Met. Hard Mater. 2018, 70, 32–38. [Google Scholar] [CrossRef]
- Petrov, I.; Detkov, P.; Drovosekov, A.; Ivanov, M.V.; Tyler, T.; Shenderova, O.; Voznecova, N.P.; Toporov, Y.P.; Schulz, D. Nickel galvanic coatings co-deposited with fractions of detonation nanodiamond. Diam. Relat. Mater. 2006, 15, 2035–2038. [Google Scholar] [CrossRef]
- Bi, X.Q.; Wei, Y.L. Effetcs of addition of nano-diamond on structure and properties of Ni-P composite coating of magnesium alloy. Surf. Technol. 2016, 12, 68–72. [Google Scholar]




| Parameter Settings | Process Parameters | |
|---|---|---|
| Positive Process Parameters | Reverse Process Parameters | |
| On time | 0.2 ms | 0.1 ms |
| Turn-off time | 0.8 ms | 0.9 ms |
| Working time | 100 ms | 10 ms |
| Current density | 0.48 A | 0.03 A |
| Duty cycle | 20% | 10% |
| Plating solution temperature | 40 ℃ | |
| Plating solution pH | 3–3.5 | |
| Plating time | 70 min | |
| Stirring | Air pump stops and turns on every 30 s | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Li, F.; Liu, M.; Zhang, W.; Yu, X.; Da, W. Effect of Nanodiamond Content in the Plating Solution on the Corrosion Resistance of Nickel–Nanodiamond Composite Coatings Prepared on Annealed 45 Carbon Steel. Coatings 2022, 12, 1558. https://doi.org/10.3390/coatings12101558
Wang D, Li F, Liu M, Zhang W, Yu X, Da W. Effect of Nanodiamond Content in the Plating Solution on the Corrosion Resistance of Nickel–Nanodiamond Composite Coatings Prepared on Annealed 45 Carbon Steel. Coatings. 2022; 12(10):1558. https://doi.org/10.3390/coatings12101558
Chicago/Turabian StyleWang, Dongai, Feihui Li, Meihua Liu, Wengang Zhang, Xiaohan Yu, and Wei Da. 2022. "Effect of Nanodiamond Content in the Plating Solution on the Corrosion Resistance of Nickel–Nanodiamond Composite Coatings Prepared on Annealed 45 Carbon Steel" Coatings 12, no. 10: 1558. https://doi.org/10.3390/coatings12101558




