Facile Synthesis of Dual Modal Pore Structure Aerogel with Enhanced Thermal Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Characterizations
3. Results and Discussion
3.1. Performance Analysis
3.1.1. Thermal Conductivity
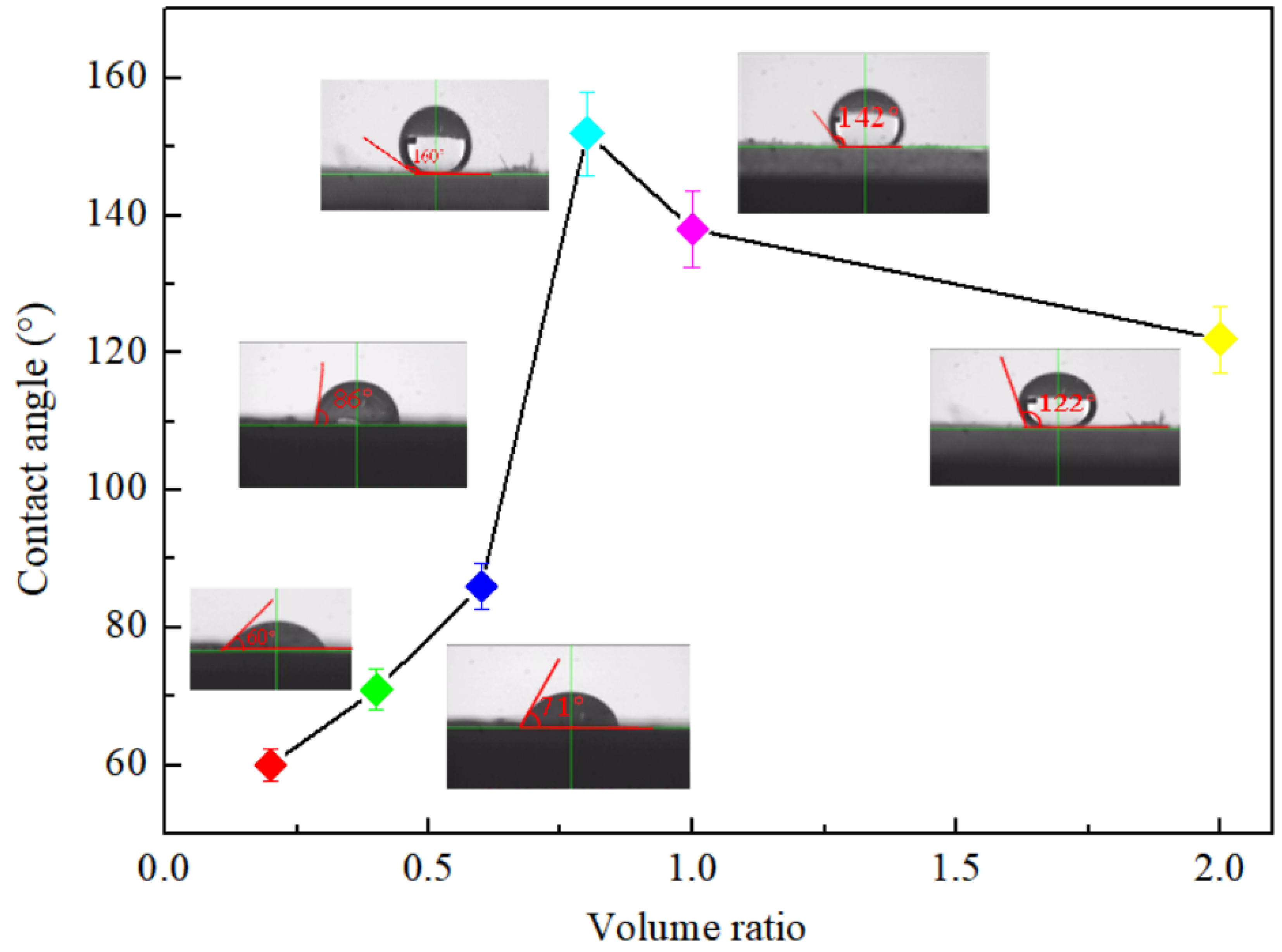
3.1.2. Hydrophobicity
3.1.3. Comparison
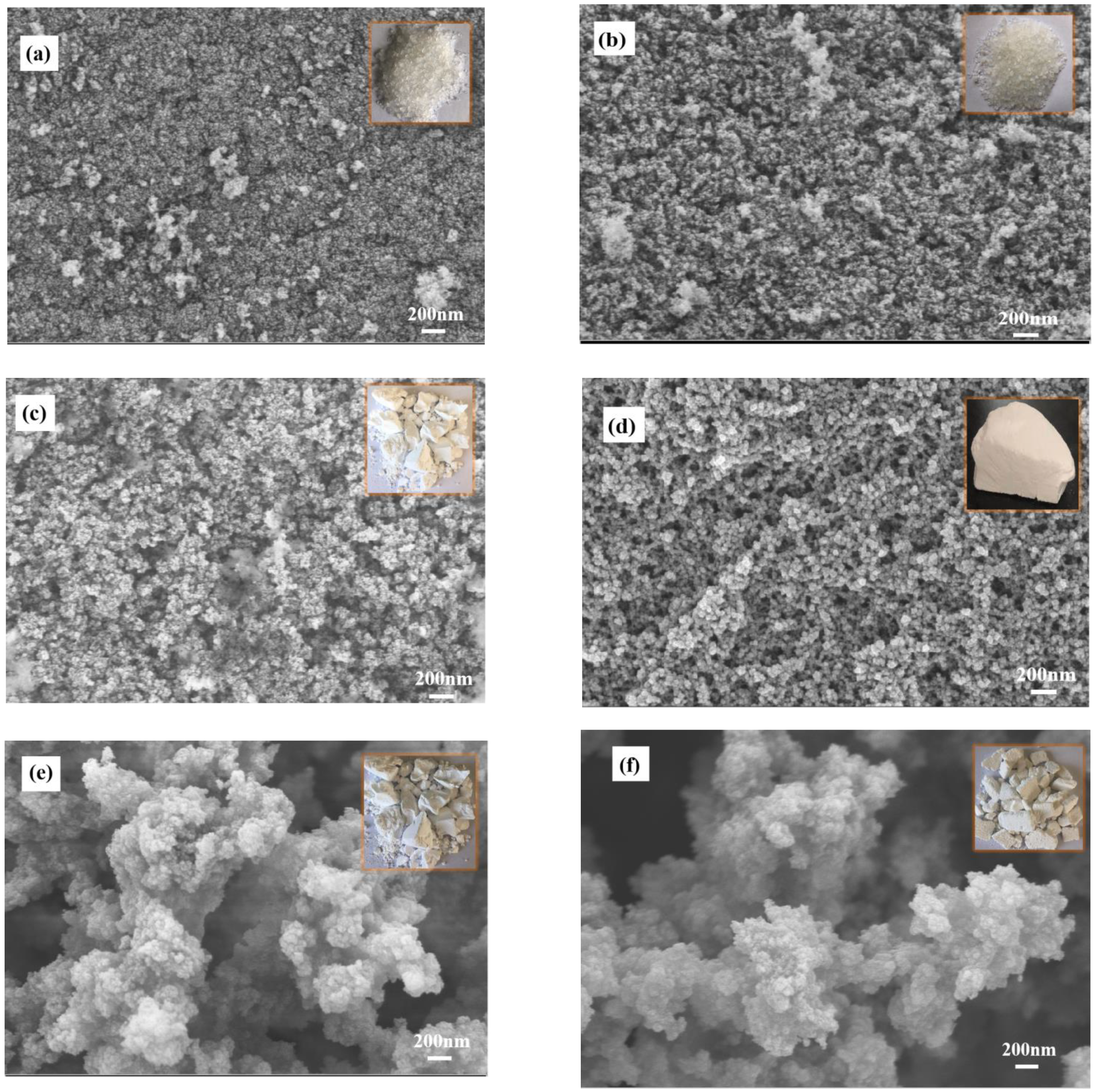
3.2. Microstructure
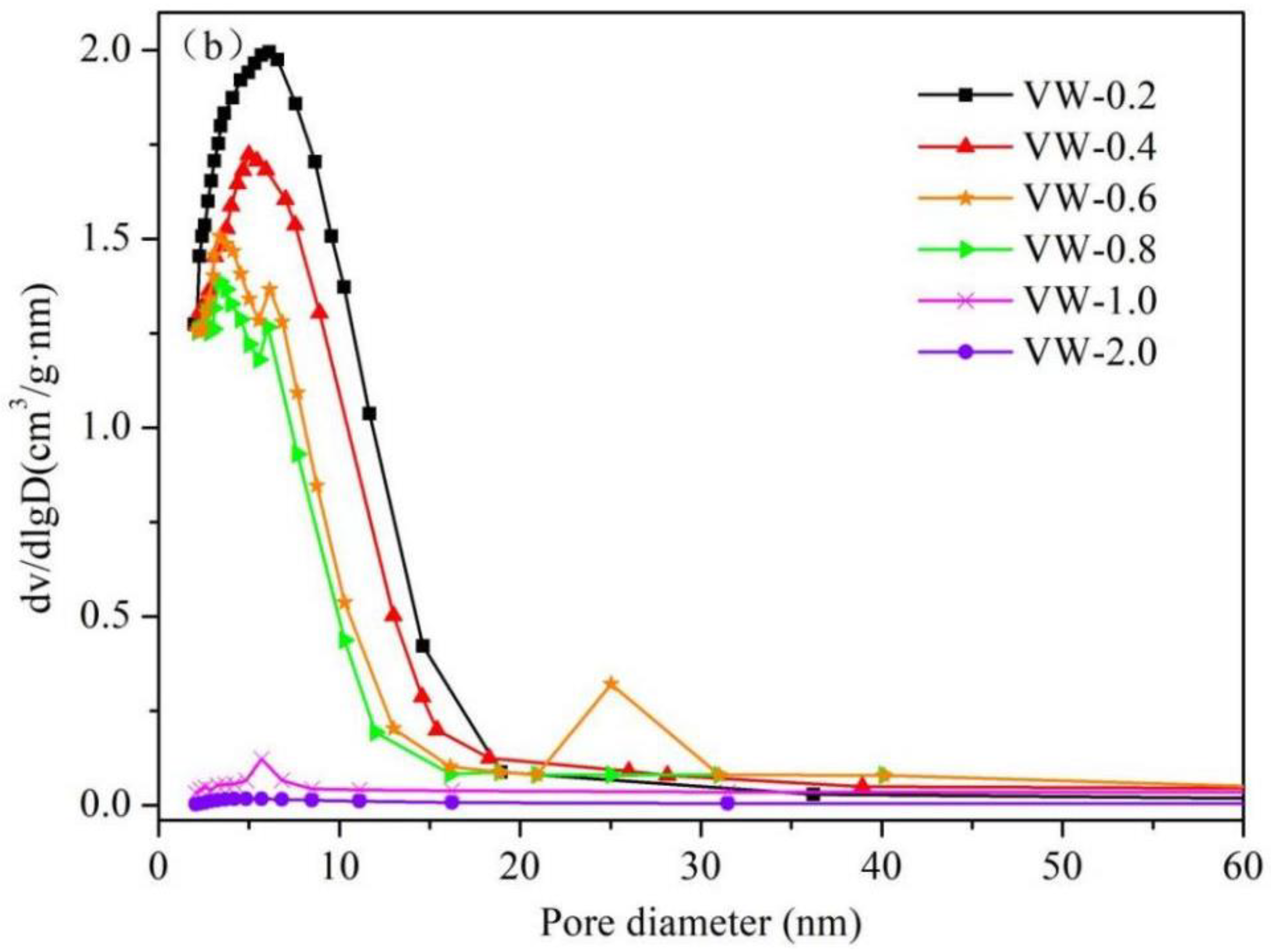
3.3. Pore Characteristics
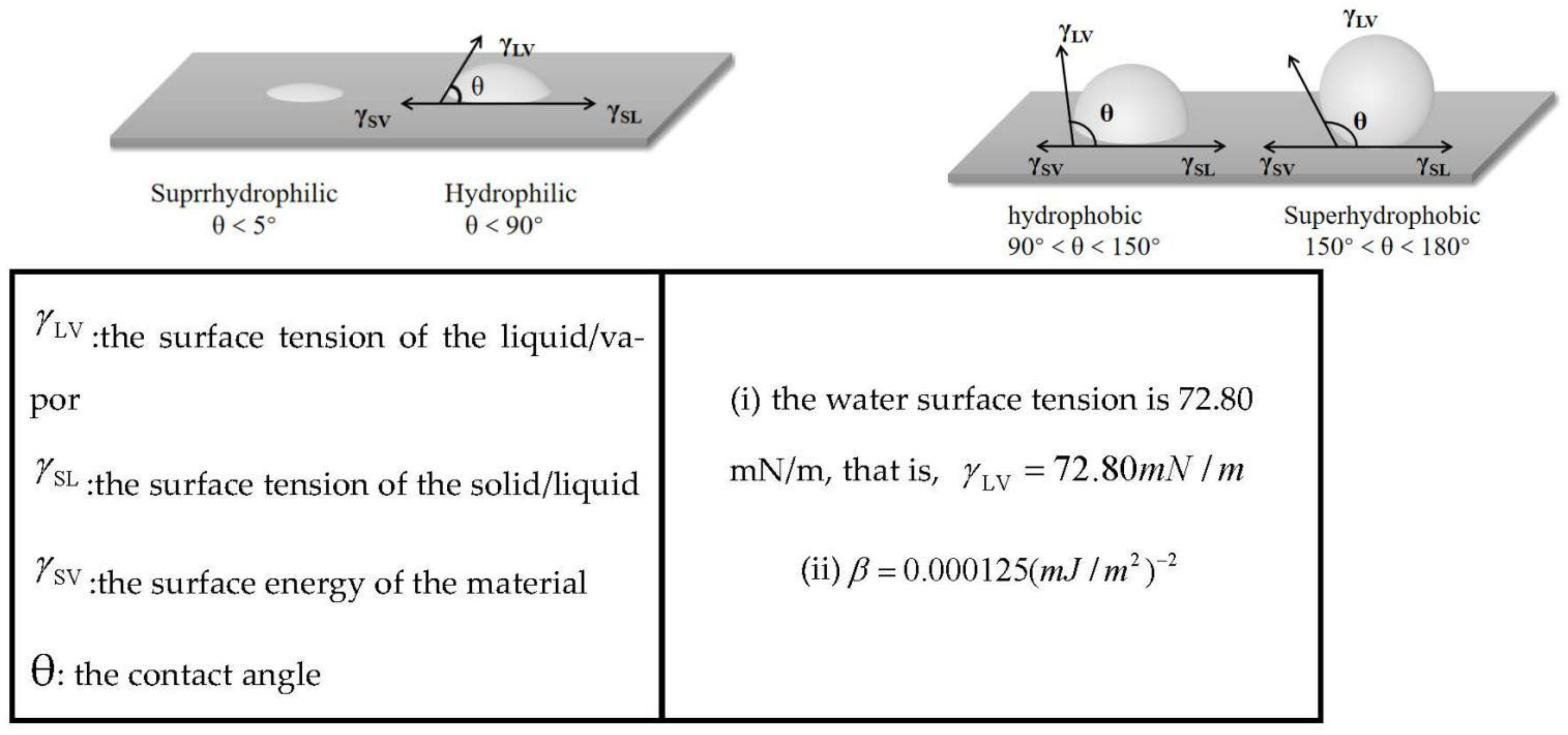
3.4. Capillary Pressure and Surface Energy
3.4.1. Capillary Pressure
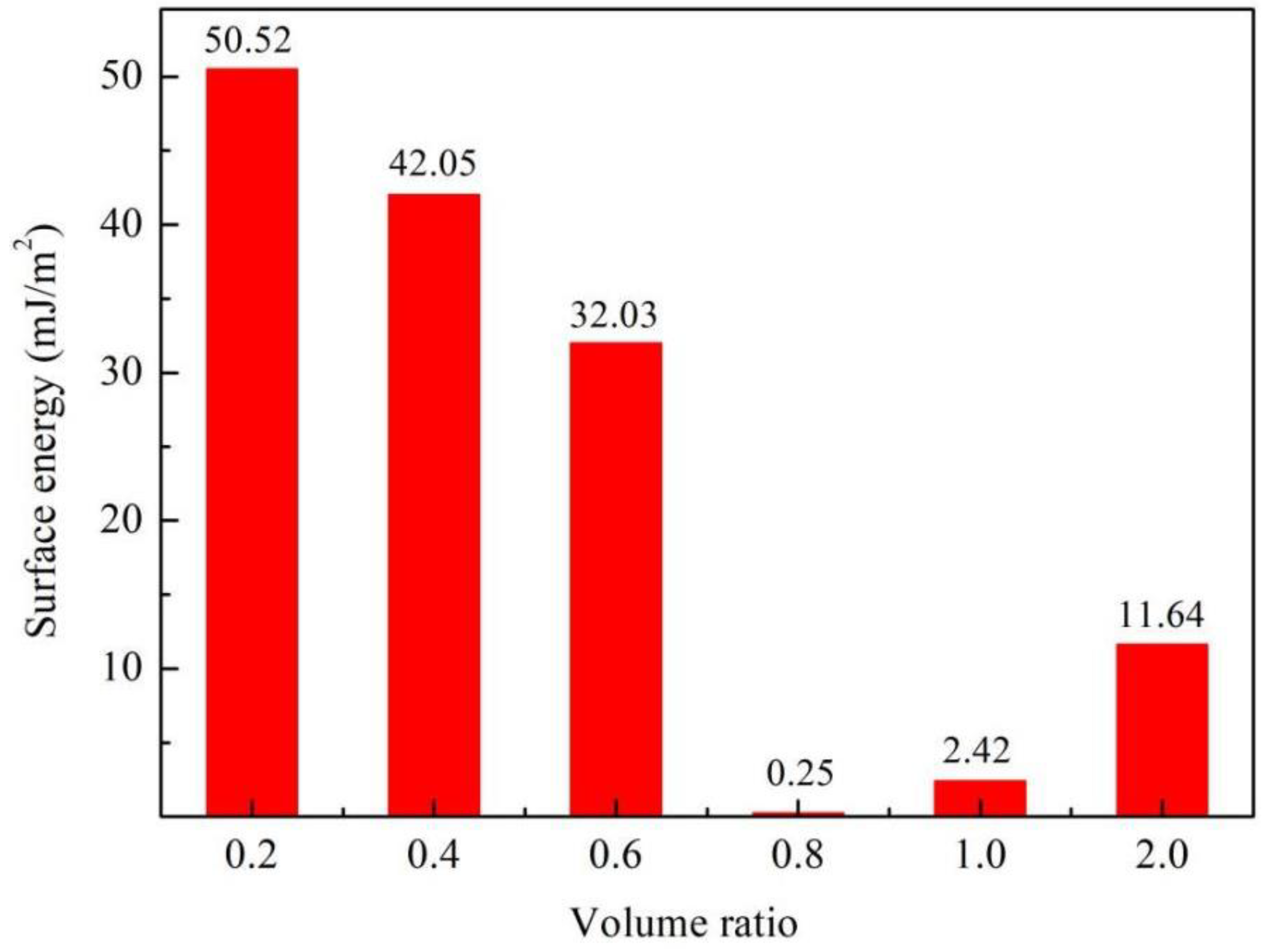
3.4.2. Surface Energy
3.5. Chemical Structure
3.6. Solid-State NMR
3.7. Synthesis Mechanism
4. Conclusions
- (1)
- The performances of VF-SiO2 aerogel vary with the ratio of V:W. When V:W = 0.8, the prepared aerogel has low thermal conductivity (0.0254 W/(m·K)), low density (0.087 g/cm3), superhydrophilicity (160°), high surface area (890.76 m2/g), and high porosity (96.82%).
- (2)
- When V:W = 0.2 or 0.4, the particles connected to the skeleton are compact and the porosity is low; when V:W = 1.0 or 2.0, the particles connected to the skeleton have a chain structure with high porosity. However, when V:W = 0.6 or 0.8, the skeleton is composed of nanoparticles with high cross-linking degree, and it has a dual modal pore structure composed of both small (6–8 nm) and large (20–30 nm) mesopores. Therefore, the promotion of aerogel performance can be attributed to the improvement of pore structure.
- (3)
- By changing the content of VTES, the surface energy of aerogel can be adjusted in a wide range (50.52 mJ/m2–0.25 mJ/m2). Thus, the addition of VTES can reduce capillary force and improve skeleton stability during drying. This is one of the key reasons that VTES can prevent the collapse of aerogel skeleton under APD conditions.
- (4)
- VTES can rapidly condense on the surface of water glass to form a high degree of cross-linking reaction, thereby increasing the reaction degree of the system and improving the performance of aerogel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazrouei-Sebdani, Z.; Begum, H.; Schoenwald, S.; Horoshenkov, K.V.; Malfait, W.J. A review on silica aerogel-based materials for acoustic applications. J. Non-Cryst. Solids 2021, 562, 120770. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, H.; Lu, X.; Li, W.; He, G.; Shen, W.; Shearing, P.R.; Brett, D.J. Porous 3D graphene aerogel co-doped with nitrogen and sulfur for high-performance supercapacitors. Nanotechnology 2021, 32, 195405–195415. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Jiang, Y.; Feng, J.; Li, L.; Feng, J. Facile synthesis of silica aerogel composites via ambient-pressure drying without surface modification or solvent exchange. Vacuum 2020, 173, 109117. [Google Scholar] [CrossRef]
- Cheng, X.; Li, C.; Shi, X.; Li, Z.; Gong, L.; Zhang, H. Rapid synthesis of ambient pressure dried monolithic silica aerogels using water as the only solvent. Mater. Lett. 2017, 204, 157–160. [Google Scholar] [CrossRef]
- Kariper, A. Effect of acids on thermal insulation of solid powder silica aerogels. Ceram. Int. 2020, 46, 8669–8674. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, R.; Fang, D. Superelastic, fatigue resistant and heat insulated carbon nanofiber aerogels for piezoresistive stress sensors. Ceram. Int. 2020, 46, 2122–2127. [Google Scholar] [CrossRef]
- Keshavarz, L.; Ghaani, M.R.; MacElroy, J.D.; English, N.J. A comprehensive review on the application of aerogels in CO2-adsorption: Materials and characterisation. Chem. Eng. J. 2021, 412, 128604. [Google Scholar] [CrossRef]
- Manzocco, L.; Mikkonen, K.S.; García-González, C.A. Aerogels as porous structures for food applications: Smart ingredients and novel packaging materials. Food Struct. 2021, 28, 100188. [Google Scholar] [CrossRef]
- Lermontov, S.A.; Malkova, A.N.; Sipyagina, N.A.; Baranchikov, A.E.; Kopitsa, G.P.; Bespalov, A.S. Hydrophobization of organic resorcinol-formaldehyde aerogels by fluoroacylation. J. Fluor. Chem. 2021, 244, 109742. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Zhang, Z.; Wang, X.; Shen, J. Adsorption of cationic dyes from aqueous solution using hydrophilic silica aerogel via ambient pressure drying. Chin. J. Chem. Eng. 2020, 28, 2467–2473. [Google Scholar] [CrossRef]
- Berardi, U.; Zaidi, M. Characterization of commercial aerogel-enhanced blankets obtained with supercritical drying and of a new ambient pressure drying blanket. Energy Build. 2019, 198, 542–552. [Google Scholar] [CrossRef]
- Torres, R.B.; Vareda, J.P.; Lamy-Mendes, A.; Durães, L. Effect of different silylation agents on the properties of ambient pressure dried and supercritically dried vinyl-modified silica aerogels. J. Supercrit. Fluids 2019, 147, 81–89. [Google Scholar] [CrossRef]
- Ding, J.; Zhong, K.; Liu, S.; Wu, X.; Shen, X.; Cui, S.; Chen, X. Flexible and super hydrophobic polymethylsilsesquioxane based silica aerogel for organic solvent adsorption via ambient pressure drying technique. Powder Technol. 2020, 373, 716–726. [Google Scholar] [CrossRef]
- Stojanovic, A.; Comesaña, S.P.; Rentsch, D.; Koebel, M.M.; Malfait, W.J. Ambient pressure drying of silica aerogels after hydrophobization with mono-, di- and tri-functional silanes and mixtures thereof. Microporous Mesoporous Mater. 2019, 284, 289–295. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, D.; Li, H. A chitosan-assisted co-assembly synthetic route to low-shrinkage Al2O3–SiO2 aerogel via ambient pressure drying. Microporous Mesoporous Mater. 2020, 293, 109781. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Zhang, W.; Yan, H.; Wang, Y.; Li, M.; Liu, Q. Rapid synthesis and characterization of ambient pressure dried monolithic silica aerogels in ethanol/water co-solvent system. J. Non-Cryst. Solids 2019, 503–504, 214–223. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Zhang, J.; Ye, X.; Cui, S. Hydrophobic silica aerogels prepared by microwave irradiation. Chem. Phys. Lett. 2021, 762, 138127. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Koebel, M.M.; Malfait, W.J. Silica aerogels with tailored chemical functionality. Mater. Des. 2020, 193, 108833. [Google Scholar] [CrossRef]
- Guo, T.; Yun, S.; Li, Y.; Chen, Z.; Cao, C.; Gao, Y. Facile synthesis of highly flexible polymethylsilsesquioxane aerogel monoliths with low density, low thermal conductivity and superhydrophobicity. Vacuum 2020, 183, 109825. [Google Scholar] [CrossRef]
- Huang, S.; Wu, X.; Li, Z.; Shi, L.; Zhang, Y.; Liu, Q. Rapid synthesis and characterization of monolithic ambient pressure dried MTMS aerogels in pure water. J. Porous Mater. 2020, 27, 1241–1251. [Google Scholar] [CrossRef]
- Dirè, S.; Tagliazucca, V.; Callone, E.; Quaranta, A. Effect of functional groups on condensation and properties of sol–gel silica nanoparticles prepared by direct synthesis from organoalkoxysilanes. Mater. Chem. Phys. 2011, 126, 909–917. [Google Scholar] [CrossRef]
- Guy, S.; Flecher, X.; Sharma, A.; Argenson, J.N.; Ollivier, M. Highly cross-linked polyethylene can reduce wear rate in THA for high demand patients: A matched-paired controlled study. J. Arthroplast. 2021, 36, 3226–3232. [Google Scholar] [CrossRef]
- Xu, A.; Roland, S.; Colin, X. Thermal ageing of a silane-crosslinked polyethylene stabilised with a thiodipropionate antioxidant. Polym. Degrad. Stab. 2020, 181, 109276. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, J.; Zhang, F.; Zhang, H. Crosslinked poly(ethylene oxide)-based membrane electrolyte consisting of polyhedral oligomeric silsesquioxane nanocages for all-solid-state lithium ion batteries. J. Power Sources 2019, 449, 227541. [Google Scholar] [CrossRef]
- Lebedev, A.E.; Menshutina, N.V.; Khudeev, I.I.; Kamyshinsky, R.A. Investigation of alumina aerogel structural characteristics at different «precursor-water-ethanol» ratio. J. Non-Cryst. Solids 2021, 553, 120475. [Google Scholar] [CrossRef]
- He, X.; Tang, B.; Cheng, X.; Zhang, Y.; Huang, L. Preparation of the methyltriethoxysilane based aerogel monolith with an ultra-low density and excellent mechanical properties by ambient pressure -drying. J. Colloid Interface Sci. 2021, 600, 746–774. [Google Scholar] [CrossRef]
- Mahadik, D.; Wang, Q.; Meti, P.; Lee, K.-Y.; Gong, Y.-D.; Park, H.-H. Synthesis of multi-functional porous superhydrophobic trioxybenzene cross-linked silica aerogels with improved textural properties. Ceram. Int. 2020, 46, 17969–17977. [Google Scholar] [CrossRef]
- Zhang, F.; Su, D.; He, J.; Sang, Z.; Liu, Y.; Ma, Y.; Liu, R.; Yan, X. Methyl modified SiO2 aerogel with tailored dual modal pore structure for adsorption of organic solvents. Mater. Lett. 2018, 238, 202–205. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, L.; Shen, Q.; Li, X.; Wu, T.; Zhang, J.; Nie, C. Rapid synthesis of dual-mesoporous silica aerogel with excellent adsorption capacity and ultra-low thermal conductivity. J. Non-Cryst. Solids 2021, 555, 120547. [Google Scholar] [CrossRef]
- Lei, C.; Li, J.; Sun, C.; Yang, H.; Xia, T.; Hu, Z.; Zhang, Y. A Co-Precursor Approach Coupled with a Supercritical Modification Method for Constructing Highly Transparent and Superhydrophobic Polymethylsilsesquioxane Aerogels. Molecules 2018, 23, 797. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Yu, H.; Li, X.; Ding, H.; Ji, H. Hyperelastic and hydrophobic silica aerogels with enhanced compressive strength by using VTES/MTMS as precursors. J. Non-Cryst. Solids 2019, 525, 119677. [Google Scholar] [CrossRef]
- Yao, C.; Dong, X.; Gao, G.; Sha, F.; Xu, D. Microstructure and Adsorption Properties of MTMS / TEOS Co-precursor Silica Aerogels Dried at Ambient Pressure. J. Non-Cryst. Solids 2021, 562, 120778. [Google Scholar] [CrossRef]
- Wu, X.; Zhong, K.; Ding, J.; Shen, X.; Cui, S.; Zhong, Y.; Ma, J.; Chen, X. Facile synthesis of flexible and hydrophobic polymethylsilsesquioxane based silica aerogel via the co-precursor method and ambient pressure drying technique. J. Non-Cryst. Solids 2020, 530, 119826. [Google Scholar] [CrossRef]
- Haq, E.U.; Zaidi SF, A.; Zubair, M.; Karim MR, A.; Padmanabhan, S.K.; Licciulli, A. Padmanabhan, Hydrophobic Silica Aerogel Glass-Fibre Composite with Higher Strength and Thermal Insulation based on Methyltrimethoxysilane (MTMS) Precursor. Energy Build. 2017, 151, 494–500. [Google Scholar] [CrossRef]
- Çok, S.S.; Gizli, N. Hydrophobic silica aerogels synthesized in ambient conditions by preserving the pore structure via two-step silylation. Ceram. Int. 2020, 46, 27789–27799. [Google Scholar] [CrossRef]
- Namvar, M.; Mahinroosta, M.; Allahverdi, A.; Mohammadzadeh, K. Preparation of monolithic amorphous silica aerogel through promising valorization of silicomanganese slag. J. Non-Cryst. Solids 2022, 586, 121561. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, Y.; Feng, J.; Zhang, S.; Peng, F.; Xiao, Y.; Feng, J. Preparation of silica aerogels with high temperature resistance and low thermal conductivity by monodispersed silica sol. Mater. Des. 2020, 191, 108640–108655. [Google Scholar] [CrossRef]
- Başgöz, Ö.; Güler, Ö. The unusually formation of porous silica nano-stalactite structure by high temperature heat treatment of SiO2 aerogel synthesized from rice hull. Ceram. Int. 2020, 46, 370–380. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Li, J.; Wu, Z.; Yang, C. Silica nanowires reinforced self-hydrophobic silica aerogel derived from crosslinking of propyltriethoxysilane and tetraethoxysilane. J. Sol-Gel Sci. Technol. 2017, 83, 545–554. [Google Scholar] [CrossRef]
- El Rassy, H.; Pierre, A.C. NMR and IR spectroscopy of silica aerogels with different hydrophobic characteristics. J. Non-Cryst. Solids 2005, 351, 1603–1610. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Kanamori, K.; Aizawa, M.; Nakanishi, K.; Hanada, T. New Transparent Methylsilsesquioxane Aerogels and Xerogels with Improved Mechanical Properties. Adv. Mater. 2007, 19, 1589–1593. [Google Scholar] [CrossRef]





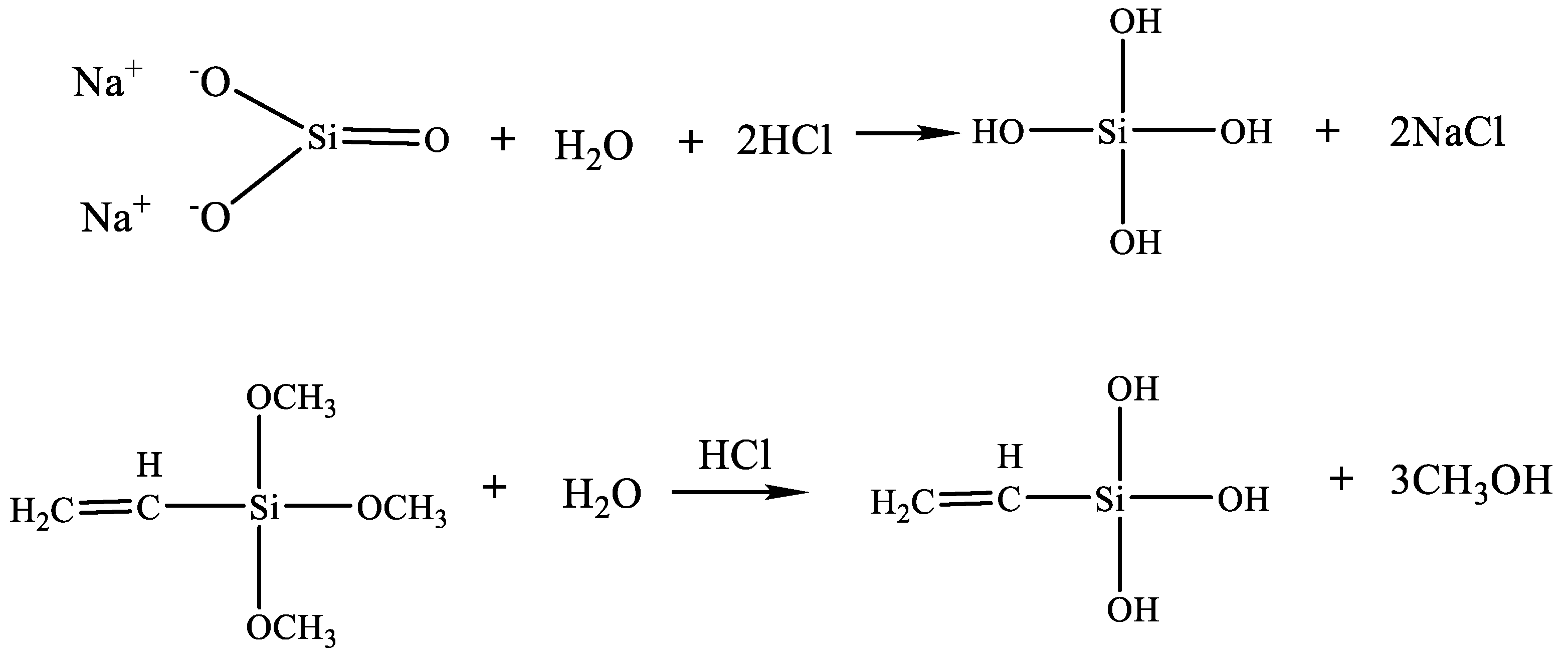


| Precursor | Drying Method | Preparation Time | Thermal Conductivity | Hydrophobicity | Published Time |
|---|---|---|---|---|---|
| MTES + TEOS [28] | Supercritical drying | / | / | 148° | 2018 |
| MTMS + CATB [16] | APD | 10 h | 0.0370 W/(m·K) | / | 2019 |
| MTMS + DMDMS [12] | APD | / | 0.0384 W/(m·K) | / | 2020 |
| MTES + TEOS [17] | Microwave irradiation | 19 h | / | 168° | 2021 |
| MTES + TEOS [29] | APD (silane strengthening) | 20 h | 0.0223 W/(m·K) | / | 2021 |
| MTMS + TMOS [30] | Supercritical drying | 4 d | / | 141° | 2018 |
| VTES + MTES [31] | APD | 7.5 d | 0.0243 W/(m·K) | 141° | 2019 |
| MTMS + TEOS [32] | APD (silane strengthening) | 25 h | / | 128° | 2021 |
| MTMS + TEOS [33] | APD (surface modification) | 54 h | / | 153.9° | 2020 |
| MTES [26] | APD | 88 h | 0.0526 ± 0.0008 W/(m·K) | / | 2021 |
| VTES + water glass (this work) | APD | 9 h | 0.0254 W/(m·K) | 160° ± 2° | / |
| Surface Area (m2/g) | Pore Volume (cm3/g) | Mean Pore Size (nm) | Porosity (%) | |
|---|---|---|---|---|
| VW-0.2 | 485.47 ± 9.5 | 0.970 ± 0.04 | 4.599 ± 0.4 | 40.12 ± 0.3 |
| VW-0.4 | 641.68 ± 6.7 | 1.174 ± 0.36 | 6.273 ± 0.2 | 49.28 ± 0.1 |
| VW-0.6 | 843.79 ± 4.4 | 1.872 ± 0.01 | 8.155 ± 1.3 | 78.43 ± 0.2 |
| VW-0.8 | 890.76 ± 7.9 | 2.413 ± 0.13 | 10.597 ± 2.8 | 96.82 ± 0.1 |
| VW-1.0 | 458.47 ± 8.8 | 1.122 ± 0.38 | 8.629 ± 0.4 | 94.35 ± 0.04 |
| VW-2.0 | 125.84 ± 11.0 | 0.435 ± 0.16 | 8.230 ± 1.0 | 94.15 ± 0.04 |
| VW-0.2 | VW-0.4 | VW-0.6 | VW-0.8 | VW-1.0 | VW-2.0 | |
|---|---|---|---|---|---|---|
| PC | - | - | - | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Si, Z.; Yang, G.; Cao, L.; Liu, X.; Mu, Y.; Tian, C.; Zhang, X.; Luo, Z. Facile Synthesis of Dual Modal Pore Structure Aerogel with Enhanced Thermal Stability. Coatings 2022, 12, 1566. https://doi.org/10.3390/coatings12101566
Zhang M, Si Z, Yang G, Cao L, Liu X, Mu Y, Tian C, Zhang X, Luo Z. Facile Synthesis of Dual Modal Pore Structure Aerogel with Enhanced Thermal Stability. Coatings. 2022; 12(10):1566. https://doi.org/10.3390/coatings12101566
Chicago/Turabian StyleZhang, Meng, Zhengkai Si, Guangjun Yang, Linfang Cao, Xiaohai Liu, Yuandong Mu, Chongfei Tian, Xinsheng Zhang, and Zhongtao Luo. 2022. "Facile Synthesis of Dual Modal Pore Structure Aerogel with Enhanced Thermal Stability" Coatings 12, no. 10: 1566. https://doi.org/10.3390/coatings12101566
APA StyleZhang, M., Si, Z., Yang, G., Cao, L., Liu, X., Mu, Y., Tian, C., Zhang, X., & Luo, Z. (2022). Facile Synthesis of Dual Modal Pore Structure Aerogel with Enhanced Thermal Stability. Coatings, 12(10), 1566. https://doi.org/10.3390/coatings12101566






