Structural and Magnetic Specificities of Fe-B Thin Films Obtained by Thermionic Vacuum Arc and Magnetron Sputtering
Abstract
1. Introduction
2. Experimental Details
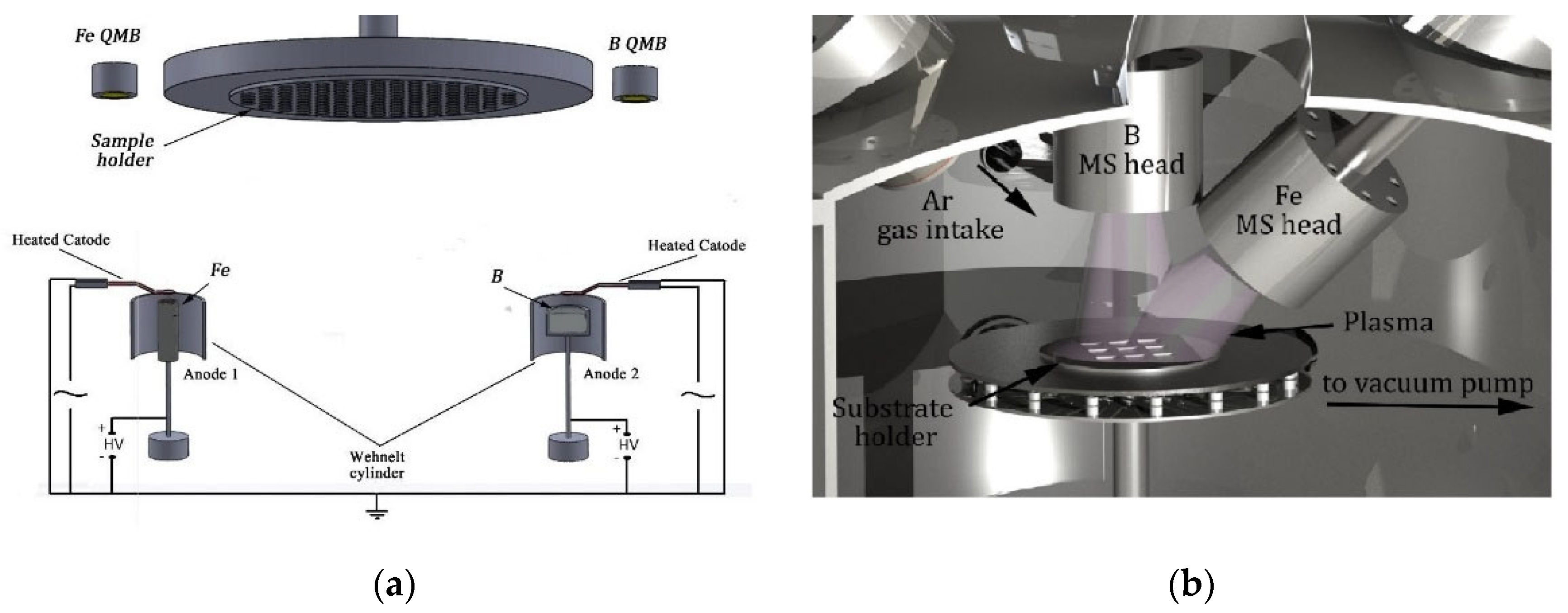
2.1. Preparation by Thermionic Vacuum Arc versus Magnetron Sputtering
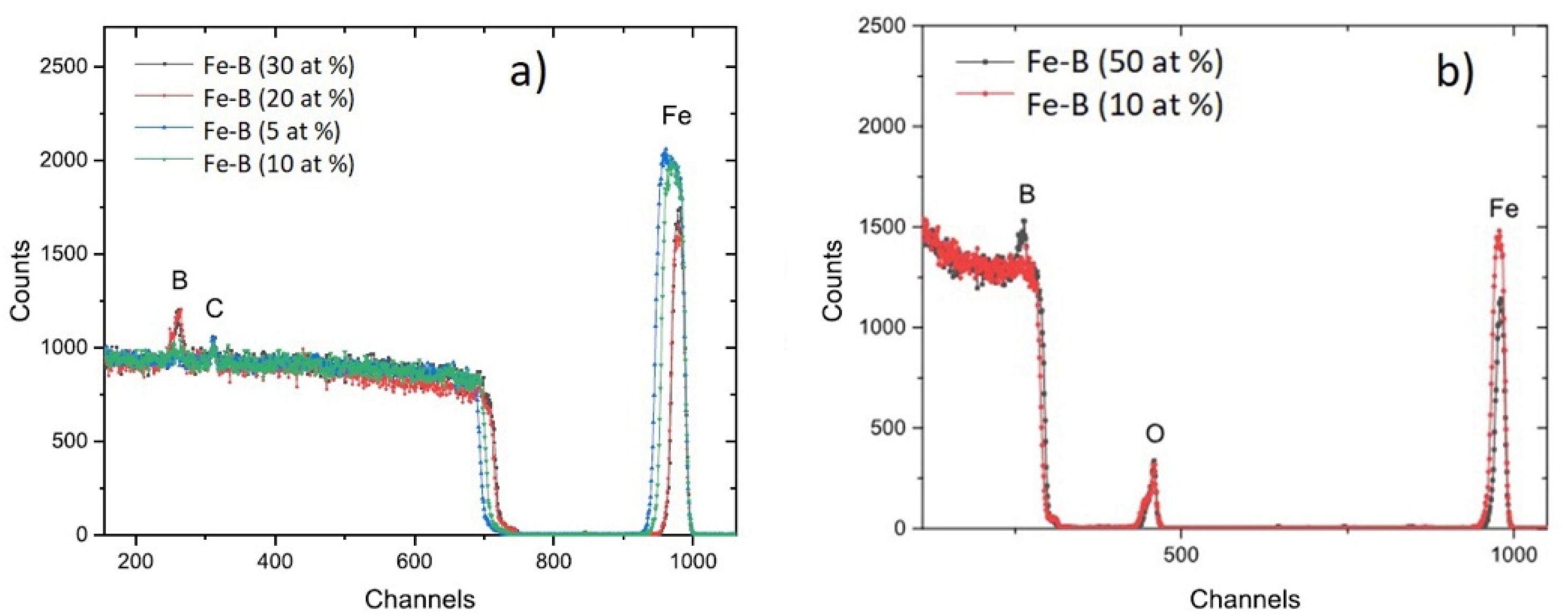
2.2. Characterization
3. Results and Discussion
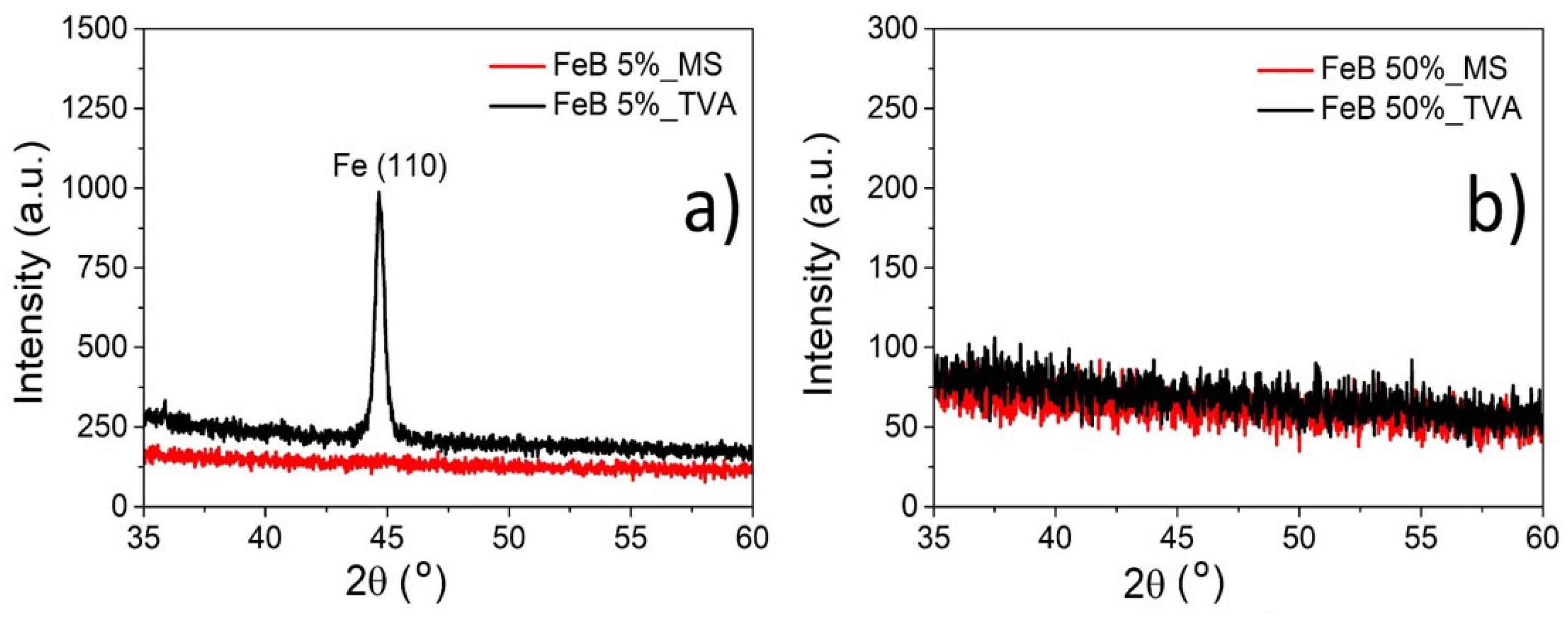
3.1. Morphology and Structure of Fe-B Films
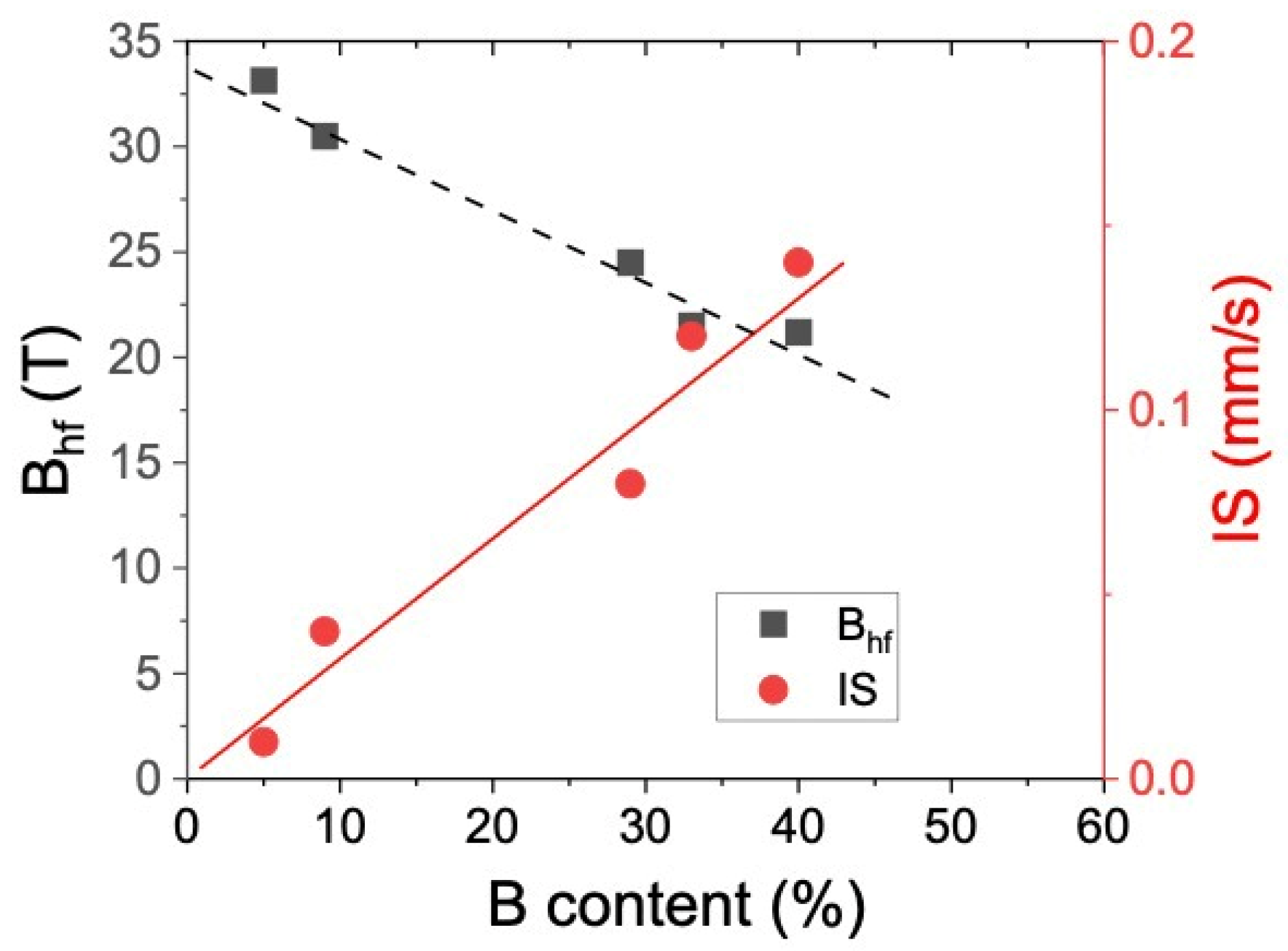
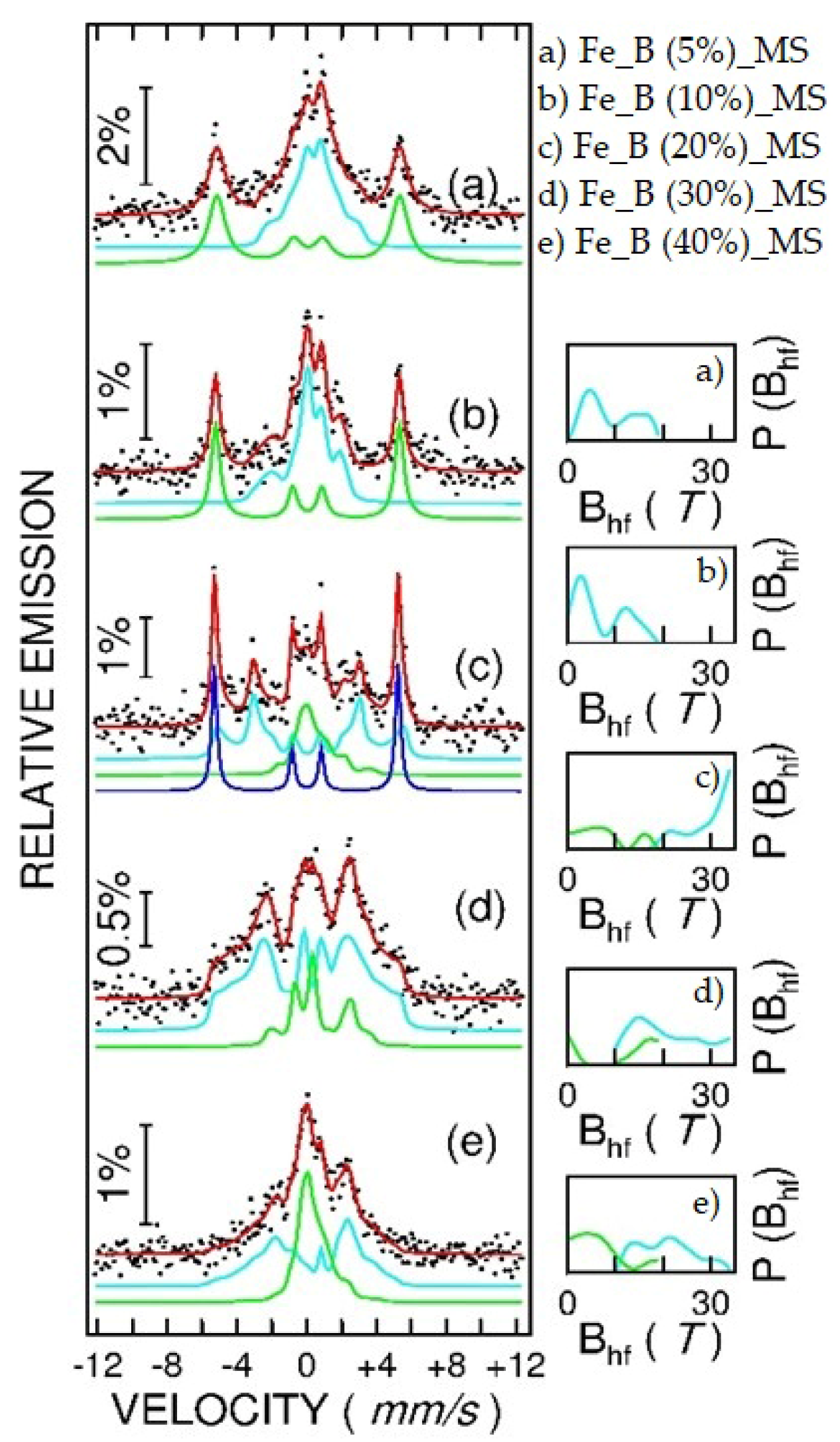
3.2. Mössbauer Spectroscopy
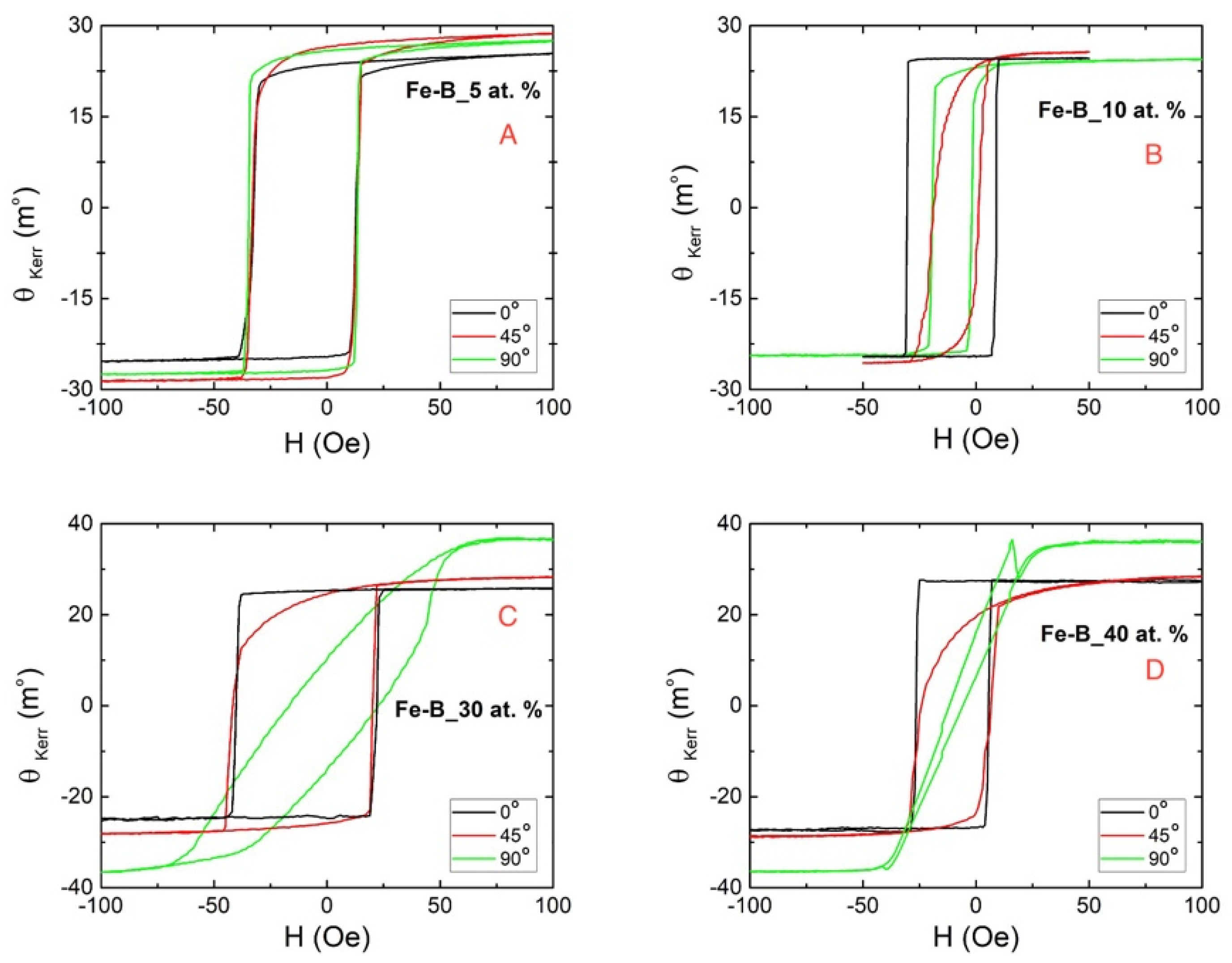
3.3. Magnetic Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.-Y.; Tien, H.-Y.; Chin, T.-S. Soft magnetic ternary iron-boron-based bulk metallic glasses. Appl. Phys. Lett. 2005, 86, 162501. [Google Scholar] [CrossRef]
- Nagamatsu, J.; Nakagawa, N.; Muranaka, T.; Zenitani, Y.; Akimitsu, J. Superconductivity at 39 K in magnesium diboride. Nature 2001, 410, 63–64. [Google Scholar] [CrossRef]
- Tan, X.; Chai, P.; Thompson, C.M.; Shatruk, M. Magnetocaloric Effect in AlFe2B2: Toward Magnetic Refrigerants from Earth-Abundant Elements. J. Am. Chem. Soc. 2013, 135, 9553–9557. [Google Scholar] [CrossRef]
- Fokwa, B.P.T. Borides: Solid-state chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 16 June 2014; Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781119951438.eibc0022.pub2 (accessed on 25 May 2022).
- Fokwa, B.P.T. Transition-metal-rich borides−fascinating crystal structures and magnetic properties. Eur. J. Inorg. Chem. 2010, 2010, 3075–3092. [Google Scholar] [CrossRef]
- Albert, B.; Hillebrecht, H. Boron: Elementary Challenge for Experimenters and Theoreticians. Angew. Chem. Int. Ed. 2009, 48, 8640–8668. [Google Scholar] [CrossRef]
- Mori, T. Boron-Based Materials in Materials Aspect of Thermoelectricity; Uher, C., Akopov, G., Yeung, M.T., Kaner, R.B., Eds.; CRC Press: Boca Raton, FL, USA, 2016; Available online: https://www.routledge.com/Materials-Aspect-of-Thermoelectricity/Uher/p/book/9781498754903 (accessed on 25 May 2022).
- Herbst, J.F.; Croat, J.J.; Pinkerton, F.E.; Yelon, W.B. Relationships between crystal structure and magnetic properties in Nd2Fe14B. Phys. Rev. B 1984, 29, 4176–4178. [Google Scholar] [CrossRef]
- Chung, H.Y.; Weinberger, M.B.; Levine, J.B.; Cumberland, R.W.; Kavner, A.; Yang, J.M.; Tolbert, S.H.; Kaner, R.B. Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure. Science 2007, 316, 436–439. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Weinberger, M.B.; Yang, J.-M.; Tolbert, S.H.; Kaner, R.B. Correlation between hardness and elastic moduli of the ultraincompressible transition metal diborides RuB2, OsB2, and ReB2. Appl. Phys. Lett. 2008, 92, 261904. [Google Scholar] [CrossRef]
- Gu, Q.; Krauss, G.; Steurer, W. Transition Metal Borides: Superhard versus Ultra-incompressible. Adv. Mater. 2008, 20, 3620–3626. [Google Scholar] [CrossRef]
- Weinberger, M.B.; Levine, J.B.; Chung, H.Y.; Cumberland, R.W.; Rasool, H.I.; Yang, J.M.; Kaner, R.B.; Tolbert, S.H. Incompressibility and Hardness of Solid Solution Transition Metal Diborides: Os1−xRuxB2. Chem. Mater. 2009, 21, 1915–1921. [Google Scholar] [CrossRef]
- Levine, J.B.; Tolbert, S.H.; Kaner, R.B. Advancements in the Search for Superhard Ultra-Incompressible Metal Borides. Adv. Funct. Mater. 2009, 19, 3519–3533. [Google Scholar] [CrossRef]
- Sanchez, F.H.; Budnick, J.I.; Zhang, Y.D.; Hines, W.A.; Choi, M.; Hasegawa, R. Mössbauer study of the local atomic environments in metastable crystalline Fe-B alloys. Phys. Rev. B 1986, 34, 4738–4743. [Google Scholar] [CrossRef]
- Hesse, J.; Rubartsch, A.J. Model independent evaluation of overlapped Mossbauer spectra. J. Phys. E Sci. Instrum. 1974, 7, 526. [Google Scholar] [CrossRef]
- Kuncser, V.; Filoti, G.; Serbanescu, M.D.; Jianu, A.; Cristea, L.; Dormann, J.L.; Noguès, M.; Maknani, J. Hyperfine fields and magnetic anisotropy in Fe1000−xGdx thin films and Fe80−xGdxB20 (0 ≤ x ≤ 24) thin films and ribbons. Hyperfine Interact. 1994, 94, 2323–2326. [Google Scholar] [CrossRef]
- Marcu, A.; Ticoş, C.M.; Grigoriu, C.; Jepu, I.; Porosnicu, C.; Lungu, A.M.; Lungu, C.P. Simultaneous carbon and tungsten thin film deposition using two thermionic vacuum arcs. Thin Solid Film. 2011, 519, 4074–4077. [Google Scholar] [CrossRef]
- Kuncser, V.; Schinteie, G.; Palade, P.; Jepu, I.; Mustata, I.; Lungu, C.P.; Miculescu, F.; Filoti, G. Magnetic properties of Fe-Co ferromagnetic layers and Fe-Mn/Fe-Co bilayers obtained by thermionic vacuum arc. J. Alloy. Compd. 2010, 499, 23–29. [Google Scholar] [CrossRef]
- Jepu, I.; Porosnicu, C.; Lungu, C.P.; Mustata, I.; Luculescu, C.; Kuncser, V.; Iacobescu, G.; Manin, A.; Ciupina, V. Combinatorial Fe-Co thin film magnetic structures obtained by thermionic vacuum arc method. Surf. Coat. Technol. 2014, 240, 344–352. [Google Scholar] [CrossRef][Green Version]
- Miculescu, F.; Jepu, I.; Stan, G.E.; Miculescu, M.; Voicu, S.I.; Cotrut, C.; Pisu, T.M.; Ciuca, S. Tailoring the electric and magnetic properties of submicron-sized metallic multilayered systems by TVA atomic inter-diffusion engineered processes. Appl. Surf. Sci. 2015, 358, 619–626. [Google Scholar] [CrossRef]
- Jepu, I.; Porosnicu, C.; Mustata, I.; Lungu, C.P.; Kunkser, V.; Osiac, M.; Iacobescu, G.; Ionescu, V.; Tudor, T. Simultaneously Thermionic Vacuum Arc Discharges In Obtaining Ferromagnetic Thin Films, Romanian Reports. Physics 2011, 63, 804–816. [Google Scholar]
- Brand, R.A. Improving the validity of hyperfine field distributions from magnetic alloys: Part I: Unpolarized source. Nucl. Instr. Meth. Phys. Res. B 1987, 28, 398–416. [Google Scholar] [CrossRef]
- Mayer, M. SIMNRA User’s Guide Technical Report IPP 9/133; MPI für Plasmaphysik: Garching, Germany, 1997. [Google Scholar]
- Nastasi, M.; Mayer, J.W.; Wang, Y. Ion Beam Analysis: Fundamentals and Applications; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2015. [Google Scholar]
- IBANDL. Available online: https://www-nds.iaea.org/exfor/ibandl.htm (accessed on 25 May 2022).
- Seqqat, M.; Nogués, M.; Crisan, O.; Kuncser, V.; Cristea, L.; Jianu, A.D.; Filoti, G.; Dormann, J.L.; Sayah, D.; Godinho, M. Magnetic properties of Fe100−xSmx thin films and Fe80−xSmxB20 thin films and ribbons. J. Magn. Magn. Mater. 1996, 157, 225–226. [Google Scholar] [CrossRef]
- Stanciu, A.E.; Schinteie, G.; Kuncser, A.; Iacob, N.; Trupina, L.; Ionita, I.; Crisan, O.; Kuncser, V. Unexpected magneto-functionalities of amorphous Fe-Gd thin films crossing the magnetization compensation point. J. Magn. Magn. Mater. 2020, 498, 166173. [Google Scholar] [CrossRef]
- Miglierini, M.B. Radiation Effects in Amorphous Metallic Alloys as Revealed by Mössbauer Spectrometry: Part I. Neutron Irradiation. Metals 2021, 11, 845. [Google Scholar] [CrossRef]
- Kuncser, V.; Palade, P.; Kuncser, A.; Greculeasa, S.; Schinteie, G. Size Effects in Nanostructures, Basics and Applications; Kuncser, V., Miu, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 169–237. [Google Scholar]
- Locovei, C.; Radu, C.; Kuncser, A.; Iacob, N.; Schinteie, G.; Stanciu, A.; Iftimie, S.; Kuncser, V. Relationship between the Formation of Magnetic Clusters and Hexagonal Phase of Gold Matrix in AuxFe1−x Nanophase Thin Films. Nanomaterials 2022, 12, 1176. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, A.E.; Schinteie, G.; Kuncser, A.C.; Locovei, C.; Trupina, L.; Iacob, N.; Leca, A.; Borca, B.; Kuncser, V. Magnetic Properties of Nanosized Fe and FeCo Systems on Trenched Mo Templates. Coatings 2022, 12, 1366. [Google Scholar] [CrossRef]




| No. | Concentration (at. %) | Fe (nm) | B (nm) |
|---|---|---|---|
| 1 | Fe + B (5%) | 96.9 | 3.3 |
| 2 | Fe + B (10%) | 97.3 | 7.0 |
| 3 | Fe + B (20%) | 86.5 | 14.0 |
| 4 | Fe + B (30%) | 79.3 | 22.0 |
| 5 | Fe + B (40%) | 70.0 | 30.0 |
| 6 | Fe + B (50%) | 60.0 | 40.0 |
| Sample Name | Samples Obtained by TVA | Samples Obtained by MS |
|---|---|---|
| Fe-B (10 at. %) | Fe0.91B0.0797O0.01W0.0003 | Fe0.51B0.045C0.045O0.37H0.03 |
| Fe-B (20 at. %) | Fe0.6997B0.28O0.02W0.0003 | Fe0.536B0.13C0.05O0.24H0.044 |
| Fe-B (30 at. %) | Fe0.6197B0.3O0.07W0.0003 | Fe0.48B0.2C0.03O0.26H0.03 |
| Fe-B (40 at. %) | Fe0.5597B0.38O0.05W0.0003 | Fe0.43B0.255C0.01O0.28H0.025 |
| Fe-B (50 at. %) | Fe0.4497B0.45O0.05W0.0003 | Fe0.33B0.3C0.02O0.32H0.03 |
| Sample | TVA |
|---|---|
| Fe-B (10 at. %) | Fe0.91B0.09 |
| Fe-B (20 at. %) | Fe0.71B0.29 |
| Fe-B (30 at. %) | Fe0.67B0.33 |
| Fe-B (40 at. %) | Fe0.60B0.40 |
| Fe-B (50 at. %) | Fe0.50B0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staicu, C.; Locovei, C.; Dinu, A.A.; Burducea, I.; Dincă, P.; Butoi, B.; Pompilian, O.G.; Porosnicu, C.; Lungu, C.P.; Kuncser, V. Structural and Magnetic Specificities of Fe-B Thin Films Obtained by Thermionic Vacuum Arc and Magnetron Sputtering. Coatings 2022, 12, 1592. https://doi.org/10.3390/coatings12101592
Staicu C, Locovei C, Dinu AA, Burducea I, Dincă P, Butoi B, Pompilian OG, Porosnicu C, Lungu CP, Kuncser V. Structural and Magnetic Specificities of Fe-B Thin Films Obtained by Thermionic Vacuum Arc and Magnetron Sputtering. Coatings. 2022; 12(10):1592. https://doi.org/10.3390/coatings12101592
Chicago/Turabian StyleStaicu, Cornel, Claudiu Locovei, Andrei Alexandru Dinu, Ion Burducea, Paul Dincă, Bogdan Butoi, Oana Gloria Pompilian, Corneliu Porosnicu, Cristian Petrica Lungu, and Victor Kuncser. 2022. "Structural and Magnetic Specificities of Fe-B Thin Films Obtained by Thermionic Vacuum Arc and Magnetron Sputtering" Coatings 12, no. 10: 1592. https://doi.org/10.3390/coatings12101592
APA StyleStaicu, C., Locovei, C., Dinu, A. A., Burducea, I., Dincă, P., Butoi, B., Pompilian, O. G., Porosnicu, C., Lungu, C. P., & Kuncser, V. (2022). Structural and Magnetic Specificities of Fe-B Thin Films Obtained by Thermionic Vacuum Arc and Magnetron Sputtering. Coatings, 12(10), 1592. https://doi.org/10.3390/coatings12101592







