Effects of Sintering Temperature on MoOx Target and Film
Abstract
:1. Introduction
2. Experimental Methods
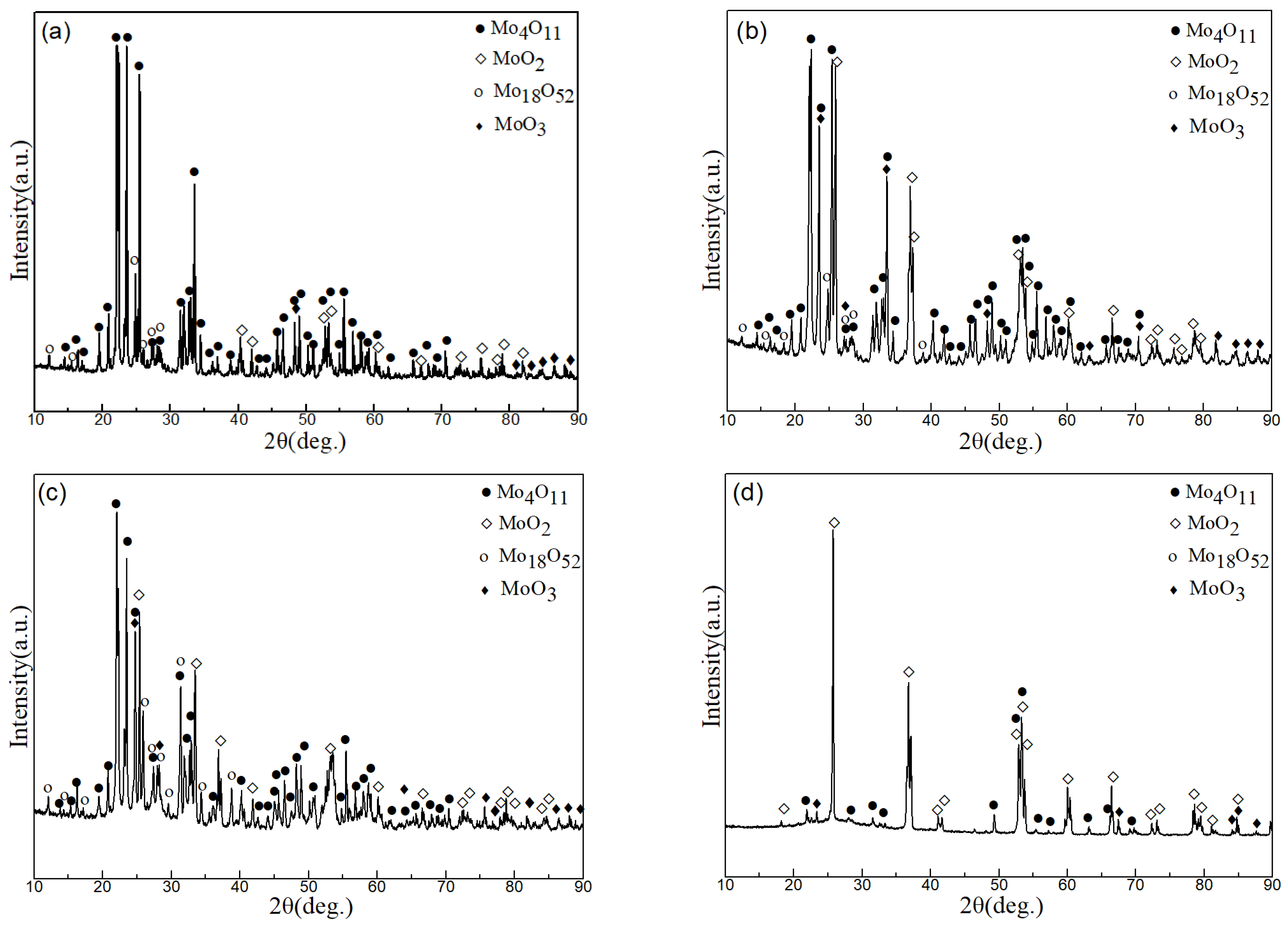
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bullock, J.; Hettick, M.; Geissbühler, J.; Ong, A.J.; Allen, T.; Sutter-Fella, C.M.; Chen, T.; Ota, H.; Schaler, E.W.; De Wolf, S.; et al. Efficient silicon solar cells with dopant-free asymmetric heterocontacts. Nat. Energy 2016, 1, 15031. [Google Scholar] [CrossRef]
- Gerling, L.G.; Mahato, S.; Morales-Vilches, A.; Masmitja, G.; Ortega, P.; Voz, C.; Alcubilla, R.; Puigdollers, J. Transition metal oxides as hole-selective contacts in silicon heterojunctions solar cells. Sol. Energy Mater. Sol. Cells 2016, 145, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Bessonov, A.A.; Kirikova, M.N.; Petukhov, D.; Allen, M.; Ryhänen, T.; Bailey, M.J.A. Layered memristive and memcapacitive switches for printable electronics. Nat. Mater. 2015, 14, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Cauduro, A.L.; Dos Reis, R.; Chen, G.; Schmid, A.K.; Methivier, C.; Rubahn, H.-G.; Bossard-Giannesini, L.; Cruguel, H.; Witkowski, N.; Madsen, M. Crystalline molybdenum oxide thin-films for application as interfacial layers in optoelectronic devices. ACS Appl. Mater. Interfaces 2017, 9, 7717–7724. [Google Scholar] [CrossRef] [Green Version]
- Bin, X.; Tian, Y.; Luo, Y.; Sheng, M.; Luo, Y.; Ju, M.; Que, W. High-performance flexible and free-standing N-doped Ti3C2Tx/MoOx films as electrodes for supercapacitors. Electrochim. Acta 2021, 389, 138774. [Google Scholar] [CrossRef]
- Jiang, Y.; Cao, S.; Lu, L.; Du, G.; Lin, Y.; Wang, J.; Yang, L.; Zhu, W.; Li, D. Post-annealing Effect on Optical and Electronic Properties of Thermally Evaporated MoOX Thin Films as Hole-Selective Contacts for p-Si Solar Cells. Nanoscale Res. Lett. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Chen, D.; Tang, S.; Zhang, J.; Wang, Y.; Han, G.; Xu, S.; Hao, Y. Flexible ITO-Free Organic Solar Cells Based on MoO3/Ag Anodes. IEEE Photonics J. 2015, 7, 1–9. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Douvas, A.M.; Georgiadou, D.G.; Palilis, L.C.; Kennou, S.; Sygellou, L.; Soultati, A.; Kostis, I.; Papadimitropoulos, G.; Davazoglou, D.; et al. The Influence of Hydrogenation and Oxygen Vacancies on Molybdenum Oxides Work Function and Gap States for Application in Organic Optoelectronics. J. Am. Chem. Soc. 2012, 134, 16178–16187. [Google Scholar] [CrossRef]
- Wang, S.; Guo, H.; Song, Y.; Ma, G.; Li, S.; Zhang, L.; Shao, X. P-63: Lower Reflective TFT Materials and Technology Innovation. SID Symp. Dig. Tech. Pap. 2017, 48, 1478–1481. [Google Scholar] [CrossRef]
- Shiming, W.; Xianghui, Z.; Zheli, Z.; Haiyu, G.; Xiaolai, S.; Baohui, L. Electrode Structure, Touch Pad and Touch Device. CN201721924180.8 25 September 2008. [Google Scholar]
- Pachlhofer, J.M.; Martín-Luengo, A.T.; Franz, R.; Franzke, E.; Köstenbauer, H.; Winkler, J.; Bonanni, A.; Mitterer, C. Non-reactive dc magnetron sputter deposition of Mo-O thin films from ceramic MoOx targets. Surf. Coat. Technol. 2017, 332, 80–85. [Google Scholar] [CrossRef]
- Pachlhofer, J.M.; Martín-Luengo, A.T.; Franz, R.; Franzke, E.; Köstenbauer, H.; Winkler, J.; Bonanni, A.; Mitterer, C. Industrial-scale sputter deposition of molybdenum oxide thin films: Microstructure evolution and properties. J. Vac. Sci. Technol. A Vac. Surf. Film. 2017, 35, 021504. [Google Scholar] [CrossRef]
- Magnéli, A. Structures of the ReO3-type with recurrent dislocations of atoms: ‘homologous series’ of molybdenum and tungsten oxides. Acta Crystallogr. 1953, 6, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Fan, H.; Zhang, M.; Ma, J.; Du, Z.; Yan, B.; Li, H.; Jiang, X. Simple electrodeposition of MoO3 film on carbon cloth for high-performance aqueous symmetric supercapacitors. Chem. Eng. J. 2020, 390, 124477. [Google Scholar] [CrossRef]
- Alex, K.V.; Prabhakaran, A.; Jayakrishnan, A.R.; Kamakshi, K.; Silva, J.P.B.; Sekhar, K.C. Charge coupling enhanced photocatalytic activity of BaTiO3/MoO3 heterostructures. ACS Appl. Mater. Interfaces 2019, 11, 40114–40124. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Robertson, J. Origin of the high work function and high conductivity of MoO3. Appl. Phys. Lett. 2014, 105, 222110. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, A.; De Melo, O.; Dutt, A.; Santana, G. Study of optoelectronic properties of thin MoOx films for application in silicon solar cells. In Proceedings of the 2019 IEEE 46th Photovoltaic Specialists Conference (PVSC), Chicago, IL, USA, 16–21 June 2019; pp. 2655–2658. [Google Scholar]
- Liu, X.; Bhatia, S.R. Laponite® and Laponite®-PEO hydrogels with enhanced elasticity in phosphate-buffered saline. Polym. Adv. Technol. 2015, 26, 874–879. [Google Scholar] [CrossRef]
- Fernandes Cauduro, A.L.; Fabrim, Z.E.; Ahmadpour, M.; Fichtner, P.F.P.; Hassing, S.; Rubahn, H.-G.; Madsen, M. Tuning the optoelectronic properties of amorphous MoOx films by reactive sputtering. Appl. Phys. Lett. 2015, 106, 202101. [Google Scholar] [CrossRef] [Green Version]
- Meng, B.; Wang, J.; Yang, L.; Chen, M.; Zhu, S.; Wang, F. On the rumpling mechanism in nanocrystalline coatings: Improved by reactive magnetron sputtering with oxygen. J. Mater. Sci. Technol. 2022, 132, 69–80. [Google Scholar] [CrossRef]
- Lin, T.; Chen, P.; Wang, Z.; Zhuang, K.; Chiu, S.; Peng, C.-H.; Lee, S.-W. Tailoring transparence in MoOx/Ag/MoOx electrode through Ag by O2/Ar plasma exposure. Ceram. Int. 2017, 43, 308–315. [Google Scholar] [CrossRef]
- Hong, R.; Li, Z.; Liu, Q.; Sun, W.; Deng, C.; Wang, Q.; Lin, H.; Tao, C.; Zhang, D. Fabrication and photocatalytic property of MoOx nano-particle films from Mo target by laser ablation at ambient conditions. Opt. Mater. 2020, 99, 109589. [Google Scholar] [CrossRef]
- Gretener, C.; Perrenoud, J.; Kranz, L.; Baechler, C.; Yoon, S.; Romanyuk, Y.; Buecheler, S.; Tiwari, A. Development of MoOx thin films as back contact buffer for CdTe solar cells in substrate configuration. Thin Solid Film. 2013, 535, 193–197. [Google Scholar] [CrossRef]
- Tsu, D.V.; Muehle, M.; Köstenbauer, H.; Linke, C.; Winkler, J. Optical properties of Mo and amorphous MoOx, and application to antireflection coatings for metals. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2022, 40, 22209. [Google Scholar] [CrossRef]
- Schmidt, H.; Köstenbauer, H.; Köstenbauer, J.; Linke, C.; Franzke, E.; Winkler, J. Sputtered Molybdenum-Oxide for Anti-Reflection Layers in Displays: Optical Properties and Thermal Stability. SID Symp. Dig. Tech. Pap. 2018, 49, 225–229. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, M.; Yang, Y.; Zhang, F. Thermodynamic modeling and first-principles calculations of the Mo–O system. Calphad 2014, 45, 178–187. [Google Scholar] [CrossRef]
- Huang, L.; Pan, Y.; Zhang, J.; Du, Y.; Zhang, Y. Remanufacturing of the waste refractory Mo–10Nb sputtering target by spark plasma sintering technology. Vacuum 2022, 200, 111050. [Google Scholar] [CrossRef]
- Min, K.H.; Kang, S.P.; Kim, D.-G.; Kim, Y.D. Sintering characteristic of Al2O3-reinforced 2xxx series Al composite powders. J. Alloy. Compd. 2005, 400, 150–153. [Google Scholar] [CrossRef]
- De Campos, M.F. Diffusion coefficients of interest for the simulation of heat treatment in rare-earth transition metal magnets. Mater. Sci. Forum 2012, 727–728, 163–168. [Google Scholar] [CrossRef]
- Tang, T.; Chang, S. Sintering Theory and Practice Sintering Theory and Practice 272, 1996. ISIJ Int. 2008, 48, 1473–1477. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Xiong, H.; Xu, J. Effects of Sintering Temperature on MoOx Target and Film. Coatings 2022, 12, 1624. https://doi.org/10.3390/coatings12111624
Zhou X, Xiong H, Xu J. Effects of Sintering Temperature on MoOx Target and Film. Coatings. 2022; 12(11):1624. https://doi.org/10.3390/coatings12111624
Chicago/Turabian StyleZhou, Xianjie, Hanqing Xiong, and Jiwen Xu. 2022. "Effects of Sintering Temperature on MoOx Target and Film" Coatings 12, no. 11: 1624. https://doi.org/10.3390/coatings12111624
APA StyleZhou, X., Xiong, H., & Xu, J. (2022). Effects of Sintering Temperature on MoOx Target and Film. Coatings, 12(11), 1624. https://doi.org/10.3390/coatings12111624




