Structure-Property Correlation in Sodium Borophosphate Glasses Modified with Niobium Oxide
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
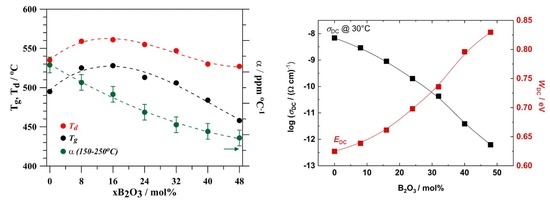
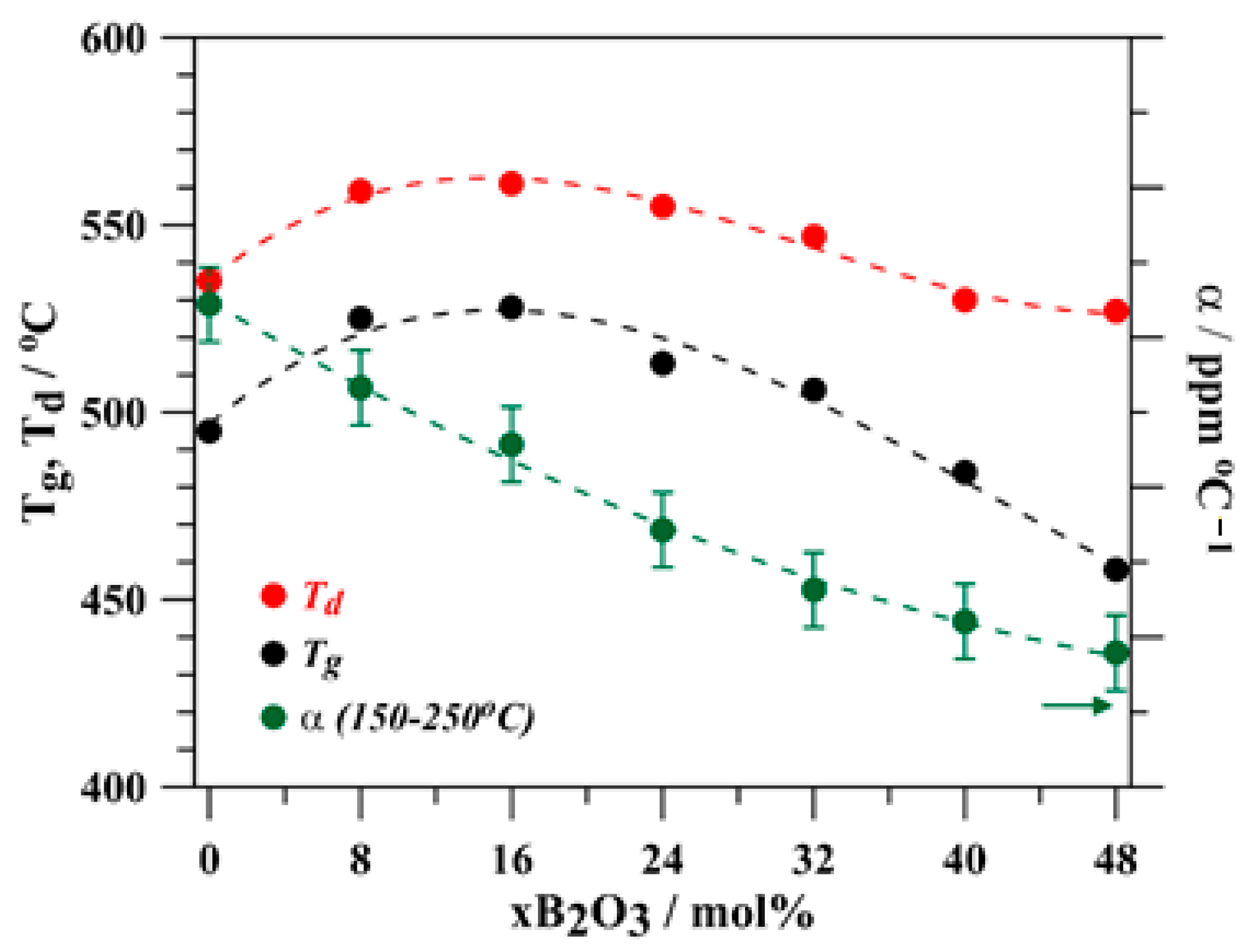
3.1. Glass Properties
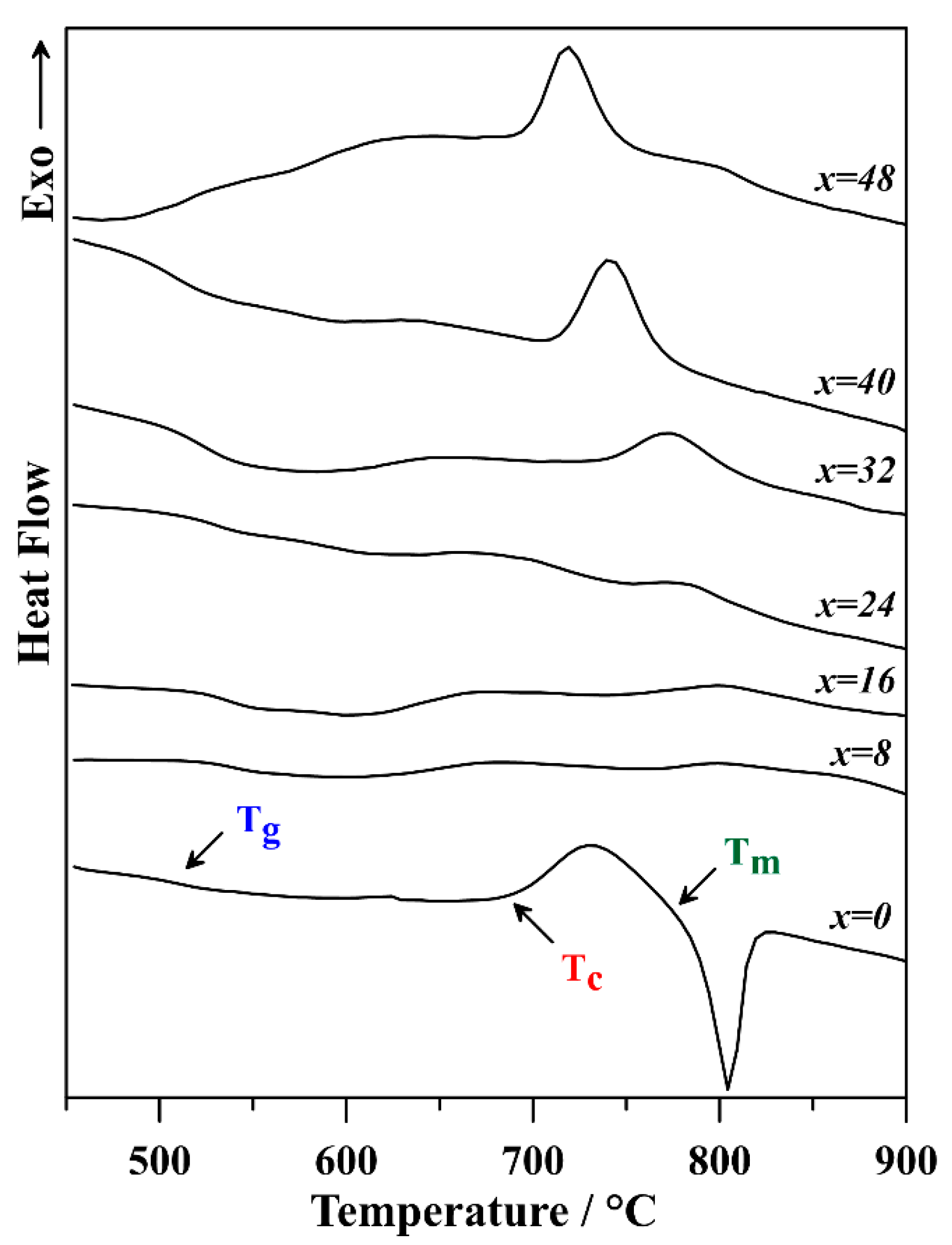
3.2. Thermal Behavior
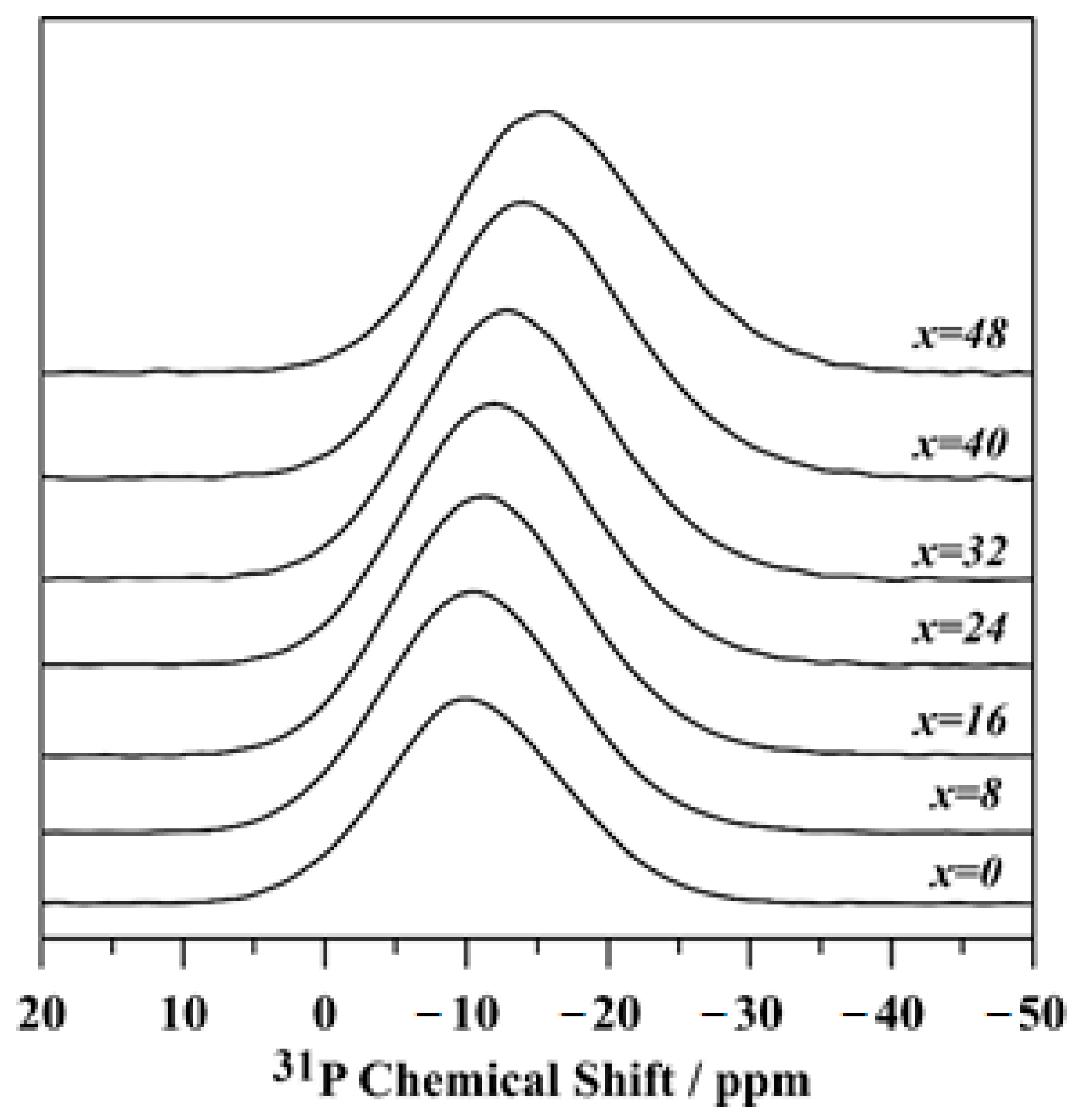
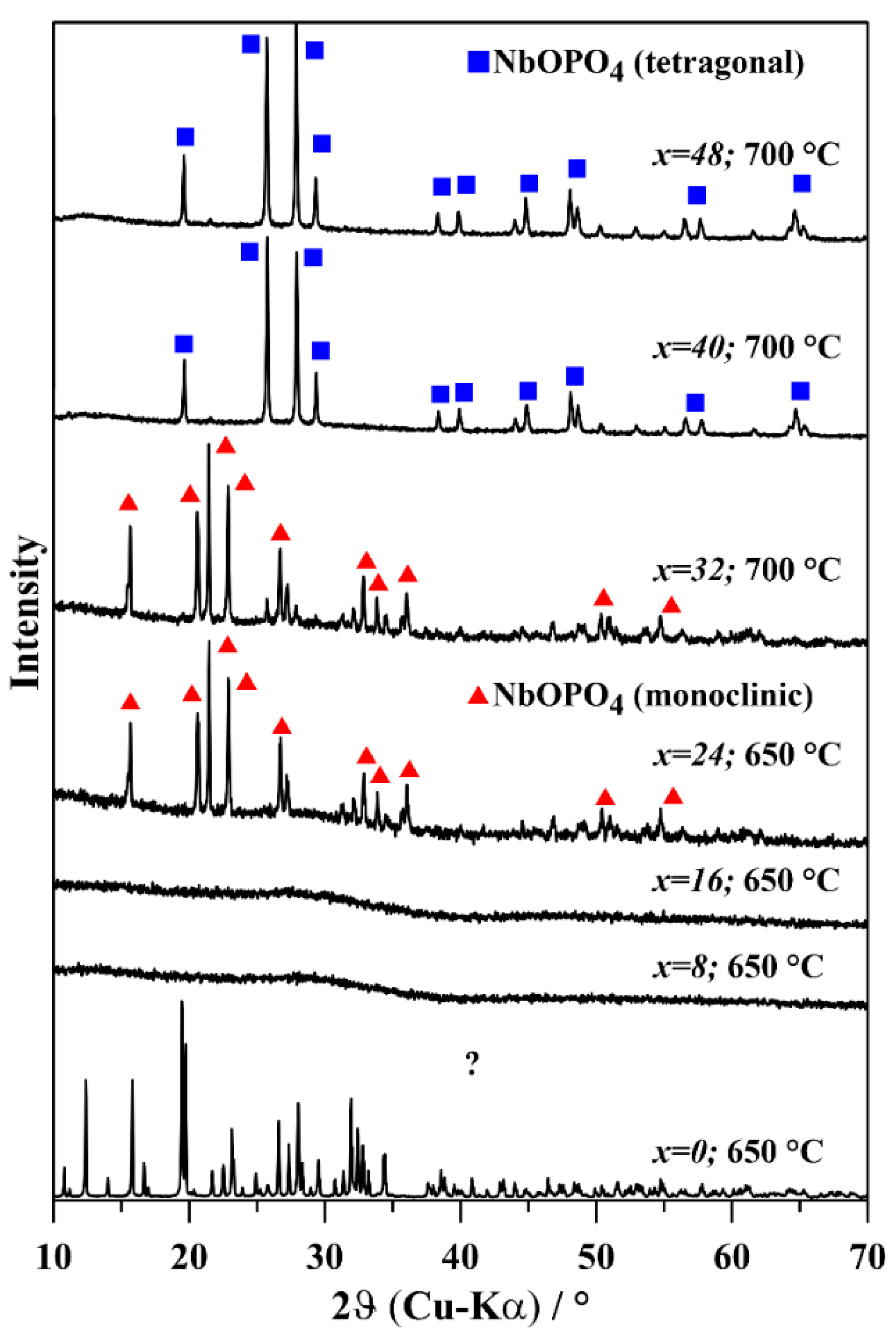
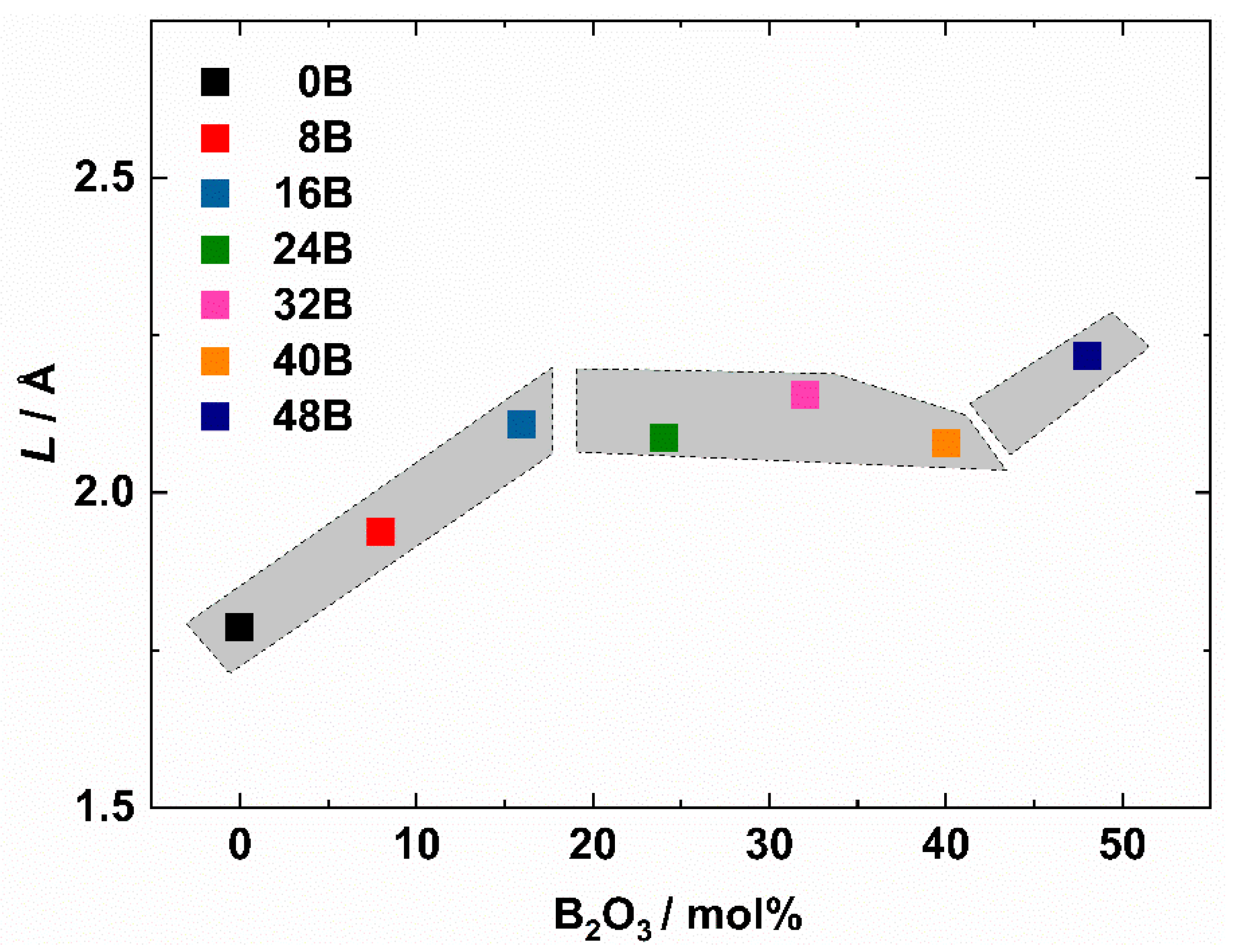
3.3. Glass Structure
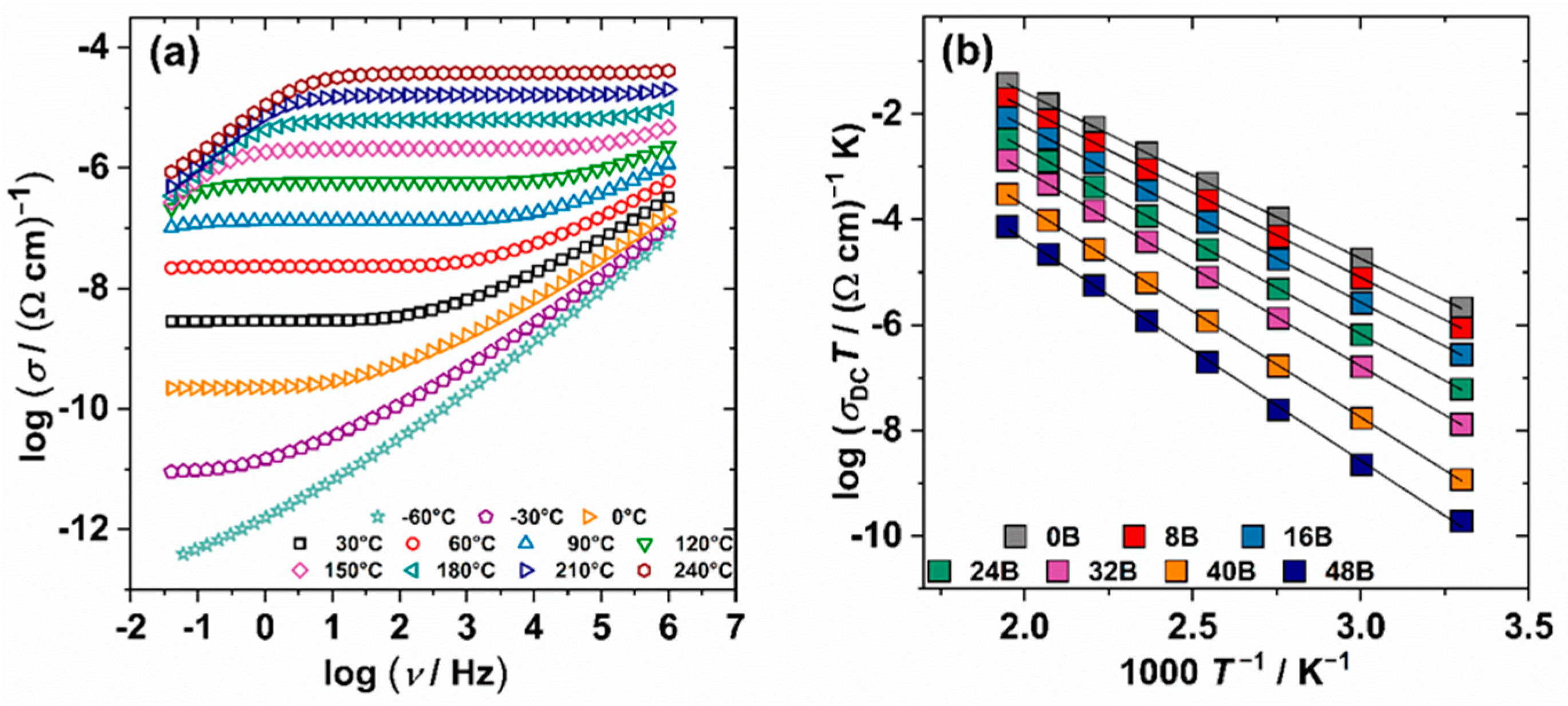
3.4. Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ducel, J.F.; Videau, J.J. Physical and chemical characterizations of sodium borophosphate glass. J. Non-Cryst. Solids 1992, 13, 271–274. [Google Scholar] [CrossRef]
- Petit, L.; Cardinal, T.; Videau, J.J.; Guyot, Y.; Boulon, G.; Couzi, M.; Buffeteau, T. Erbium luminescence properties of niobium-rich oxide glasses. J. Non-Cryst. Solids 2005, 351, 2076–2084. [Google Scholar] [CrossRef]
- Petit, L.; Cardinal, T.; Videau, J.J.; Smektala, F.; Jouan, T.; Richardson, K.; Schulte, A. Fabrication and characterization of new Er3+ doped niobium borophosphate glass fiber. Mater. Sci. Eng. B 2005, 117, 283–286. [Google Scholar] [CrossRef]
- Scagliotti, M.; Villa, M.; Chiodelli, G. Short range order in the network of the borophosphate glasses: Raman results. J. Non-Cryst. Solids 1987, 93, 350–360. [Google Scholar] [CrossRef]
- Villa, M.; Scagliotti, M.; Chiodelli, G. Short range order in the network of the borophosphate glasses: A 31P NMR-MAS (Magic Angle Spinning) study. J. Non-Cryst. Solids 1987, 94, 101–121. [Google Scholar] [CrossRef]
- Brow, R.K.; Tallant, D.R. Structural design of sealing glasses. J. Non-Cryst. Solids 1997, 222, 396–406. [Google Scholar] [CrossRef]
- Zielniok, D.; Cramer, C.; Eckert, H. Structure/property correlations in ion-conducting mixed network-former glasses: Solid-state NMR studies of the system Na2O-B2O3-P2O5. Chem. Mater. 2007, 19, 3162–3170. [Google Scholar] [CrossRef]
- Carta, D.; Qiu, D.; Guerry, P.; Ahmed, I.; Abou Neal, A.A.; Knowles, J.C.; Smith, M.E.; Newport, R.J. The effect of composition on the structure of sodium borophosphate glasses. J. Non-Cryst. Solids 2008, 354, 3671–3677. [Google Scholar] [CrossRef]
- Rascar, D.; Rinke, M.T.; Eckert, H. The mixed-network former effect in phosphate glasses: NMR and XPS studies of the connectivity distribution in the glass system (NaPO3)1-x(B2O3)x. J. Phys. Chem. C 2008, 112, 12530–12539. [Google Scholar] [CrossRef]
- Rinke, M.T.; Eckert, H. The mixed network former effect in glasses: Solid state NMR and XPS structural studies of the glass system (Na2O)x(BPO4)1-x. Phys. Chem. Chem. Phys. 2011, 13, 6552–6555. [Google Scholar] [CrossRef]
- Christensen, R.; Byer, J.; Olson, G.; Martin, S.W. The densities of mixed glass former 0,35Na2O+0,65[xB2O3+(1-x)P2O5] glasses related to the atomic fractions and volumes of short range structures. J. Non-Cryst. Solids 2012, 358, 583–589. [Google Scholar] [CrossRef]
- Christensen, R.; Byer, J.; Olson, G.; Martin, S.W. The glass transition temperature of mixed glass former 0,35Na2O+0,65[xB2O3+(1-x)P2O5] glasses. J. Non-Cryst. Solids 2012, 358, 826–831. [Google Scholar] [CrossRef]
- Christensen, R.; Olson, G.; Martin, S.W. Structural studies of mixed glass former 0,35Na2O+0,65[xB2O3+(1-x)P2O5] glasses by Raman and 11B and 31P magic angle spinning nuclear magnetic spectroscopies. J. Phys. Chem. B 2013, 117, 2169–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, R.; Olson, G.; Martin, S.W. Ionic conductivity of mixed glass former 0,35Na2O+0,65[xB2O3+(1-x)P2O5] glasses. J. Phys. Chem B 2013, 117, 16577–16586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raguenet, B.; Tricot, G.; Silly, G.; Ribes, M.; Padel, A. Revisiting the ‘mixed glass former effect’ in ultra-fast quenched borophosphate glasses by advanced 1D/2D solid state NMR. J. Mater. Chem. 2011, 21, 17693–17704. [Google Scholar] [CrossRef]
- Moguš-Milanković, A.; Sklepić, K.; Blažanović, H.; Mošner, P.; Vorokhta, M.; Koudelka, L. Influence of germanium oxide addition on the electrical properties of Li2OB2O3-P2O5 glasses. J. Power Sources 2013, 242, 91–98. [Google Scholar] [CrossRef]
- Koudelka, L.; Pospíšil, J.; Mošner, P.; Montagne, L.; Delevoye, L. Structure and properties of potassium niobato-borophosphate glasses. J. Non-Cryst. Solids 2008, 354, 129–133. [Google Scholar] [CrossRef]
- International Centre of Diffraction Data. Joint Committee on Powder Diffraction Standards; International Centre of Diffraction Data: Swarthmore, PA, USA, 2022. [Google Scholar]
- Cardinal, T.; Fargin, E.; le Flem, G.; Couzi, M.; Canioni, L.; Segonds, P.; Sarger, L.; Ducasse, A.; Adamietz, F. Nonlinear optical properties of some niobium (V) oxide glasses. Eur. J. Solid State Inorg. Chem. 1996, 33, 597–605. [Google Scholar]
- Raguenet, B.; Tricot, G.; Silly, G.; Ribes, M.; Padel, A. The mixed glass former effect in twin-roller quenched lithium borophosphate glasses. Solid State Ion. 2012, 208, 25–30. [Google Scholar] [CrossRef]
- Chenu, S.; Werner-Zwanziger, U.; Calahoo, C.; Zwanziger, J.W. Structure and properties of NaPO3–ZnO–Nb2O5–Al2O3 glasses. J. Non-Cryst. Solids 2012, 358, 1795–1805. [Google Scholar] [CrossRef]
- Sene, F.F.; Martinelli, J.R.; Gomes, L. Synthesis and characterization of niobium phosphate glasses containing barium and potassium. J. Non-Cryst. Solids 2004, 348, 30–37. [Google Scholar] [CrossRef]
- Hidi, I.J.; Melinte, G.; Stefan, R.; Bindea, M.; Baia, L. The study of the structure and bioactivity of the B2O3 • Na2O•P2O5 system. J. Raman Spectrosc. 2013, 44, 1187–1194. [Google Scholar] [CrossRef]
- Flambard, A.; Videau, J.J.; Delevoye, L.; Cardinal, T.; Labrugère, C.; Rivero, C.A.; Couzi, M.; Montagne, L. Structure and nonlinear optical properties of sodium–niobium phosphate glasses. J. Non-Cryst. Solids 2008, 354, 3540–3547. [Google Scholar] [CrossRef]
- Longo, J.M.; Kierkegaard, P. The crystal structure of NbOPO4. Acta Chem. Scand. 1966, 20, 72–78. [Google Scholar] [CrossRef]
- Leclaire, A.; Chahboun, H.; Groult, D.; Raveau, B. The crystal structure of β-NbPO5. Z. Kristallogr. 1986, 177, 277–286. [Google Scholar] [CrossRef]
- Komatsu, T.; Honma, T.; Tasheva, T.; Dimitrov, V. Structural role of Nb2O5 in glass-forming ability, electronic polarizability and nanocrystallization in glasses: A review. J. Non-Cryst. Solids 2022, 581, 121414. [Google Scholar] [CrossRef]
- Razum, M.; Pavić, L.; Ghussn, L.; Moguš-Milanković, A.; Šantić, A. Transport of potassium ions in niobium phosphate glasses. J. Am. Ceram. Soc. 2021, 104, 4669–4678. [Google Scholar] [CrossRef]
- Summerfield, S. Universal low-frequency behaviour in the a.c. hopping conductivity of disordered systems. Philos. Mag. B 1985, 52, 9–22. [Google Scholar] [CrossRef]
- Summerfield, S.; Butcher, P.N. Universal behaviour of AC hopping conductivity in disordered systems. J. Non-Cryst. Solids 1985, 77–78, 135–138. [Google Scholar] [CrossRef]
- Roling, B.; Happe, A.; Funke, K.; Ingram, M.D. Carrier concentrations and relaxation spectroscopy: New information from scaling properties of conductivity spectra in ionically conducting glasses. Phys. Rev. Lett. 1997, 78, 2160–2163. [Google Scholar] [CrossRef]
- Roling, B. Scaling properties of the conductivity spectra of glasses and supercooled melts. Solid State Ion. 1998, 105, 185–193. [Google Scholar] [CrossRef]
- Pavić, L.; Fazinić, S.; Ertap, H.; Karabulut, M.; Moguš-Milanković, A.; Šantić, A. Polaronic conductivity in iron phosphate glasses containing B2O3. Materials 2020, 13, 2505. [Google Scholar] [CrossRef] [PubMed]
- Banhatti, R.D.; Cramer, C.; Zielniok, D.; Robertson, A.J.; Ingram, M.D. Insights into ion-network interactions and ion transport in glass. Z. Für Phys. Chem. 2009, 223, 1201–1215. [Google Scholar] [CrossRef]
- Sidebottom, D.L.; Green, P.F.; Brow, R.K. Structural correlations in the ac conductivity of ion-containing glasses. J. Non-Cryst. Solids 1997, 222, 354–360. [Google Scholar] [CrossRef]





| Na2O | P2O5 | Nb2O5 | B2O3 | ρ ± 0.02 | VM ± 0.5 | Tg ± 2 | Td ± 1 | α ± 0.5 | nd ± 0.005 |
|---|---|---|---|---|---|---|---|---|---|
| Batch/mol% | g∙cm−3 | cm3∙mol−1 | °C | ppm/°C | [-] | ||||
| 40 | 40 | 20 | 0 | 3.06 | 44.01 | 496 | 539 | 16.4 | 1.655 |
| 36.8 | 36.8 | 18.4 | 8 | 3.05 | 42.5 | 525 | 559 | 15.3 | 1.658 |
| 33.6 | 33.6 | 16.8 | 16 | 3.01 | 41.23 | 528 | 561 | 14.6 | 1.658 |
| 30.4 | 30.4 | 15.2 | 24 | 2.96 | 40.22 | 513 | 555 | 13.4 | 1.656 |
| 27.2 | 27.2 | 13.6 | 32 | 2.92 | 39.7 | 510 | 547 | 12.6 | 1.644 |
| 24 | 24 | 12 | 40 | 2.84 | 38.87 | 484 | 530 | 12.2 | 1.629 |
| 20.8 | 20.8 | 10.4 | 48 | 2.73 | 37.88 | 458 | 534 | 11.8 | 1.618 |
| Raman Shift cm−1 | Assignment | Ref. |
|---|---|---|
| 248–261 | O–P–O and O–Nb–O coupled deformation mode | [21] |
| 400–470 | O–P–O and O–Nb–O coupled mode | [21,22] |
| 600–700 | P–O–Bsym stretch and P–O–Psym stretch modes | [13,23] |
| 770–824 | P–O–B stretching modes | [19,23] |
| 771 | P–O–P stretching mode | [21,23] |
| 928–934 | Nb–O bonds in NbO6 octahedra | [21,24] |
| 1211 | P–O–P (Q2) stretching vibration | [21,23] |
| Sample | N a | W | σDCb |
|---|---|---|---|
| cm3 | eV | (Ωcm)−1 | |
| B0 | 1.08 × 1022 | 0.62 | 6.88 × 10−9 |
| B8 | 1.04 × 1022 | 0.64 | 2.89 × 10−9 |
| B16 | 9.77 × 1021 | 0.66 | 9.04 × 10−10 |
| B24 | 9.10 × 1021 | 0.69 | 1.98 × 10−10 |
| B32 | 8.28 × 1021 | 0.74 | 4.30 × 10−11 |
| B40 | 7.50 × 1021 | 0.79 | 3.86 × 10−12 |
| B48 | 6.61 × 1021 | 0.83 | 6.26 × 10−13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mošner, P.; Hostinský, T.; Koudelka, L.; Razum, M.; Pavić, L.; Montagne, L.; Revel, B. Structure-Property Correlation in Sodium Borophosphate Glasses Modified with Niobium Oxide. Coatings 2022, 12, 1626. https://doi.org/10.3390/coatings12111626
Mošner P, Hostinský T, Koudelka L, Razum M, Pavić L, Montagne L, Revel B. Structure-Property Correlation in Sodium Borophosphate Glasses Modified with Niobium Oxide. Coatings. 2022; 12(11):1626. https://doi.org/10.3390/coatings12111626
Chicago/Turabian StyleMošner, Petr, Tomáš Hostinský, Ladislav Koudelka, Marta Razum, Luka Pavić, Lionel Montagne, and Bertrand Revel. 2022. "Structure-Property Correlation in Sodium Borophosphate Glasses Modified with Niobium Oxide" Coatings 12, no. 11: 1626. https://doi.org/10.3390/coatings12111626
APA StyleMošner, P., Hostinský, T., Koudelka, L., Razum, M., Pavić, L., Montagne, L., & Revel, B. (2022). Structure-Property Correlation in Sodium Borophosphate Glasses Modified with Niobium Oxide. Coatings, 12(11), 1626. https://doi.org/10.3390/coatings12111626