Sol-Gel Materials for Electrochemical Applications: Recent Advances
Abstract
:1. Introduction
2. Sol-Gel Process
3. Sol-Gel Materials for Supercapacitors Application
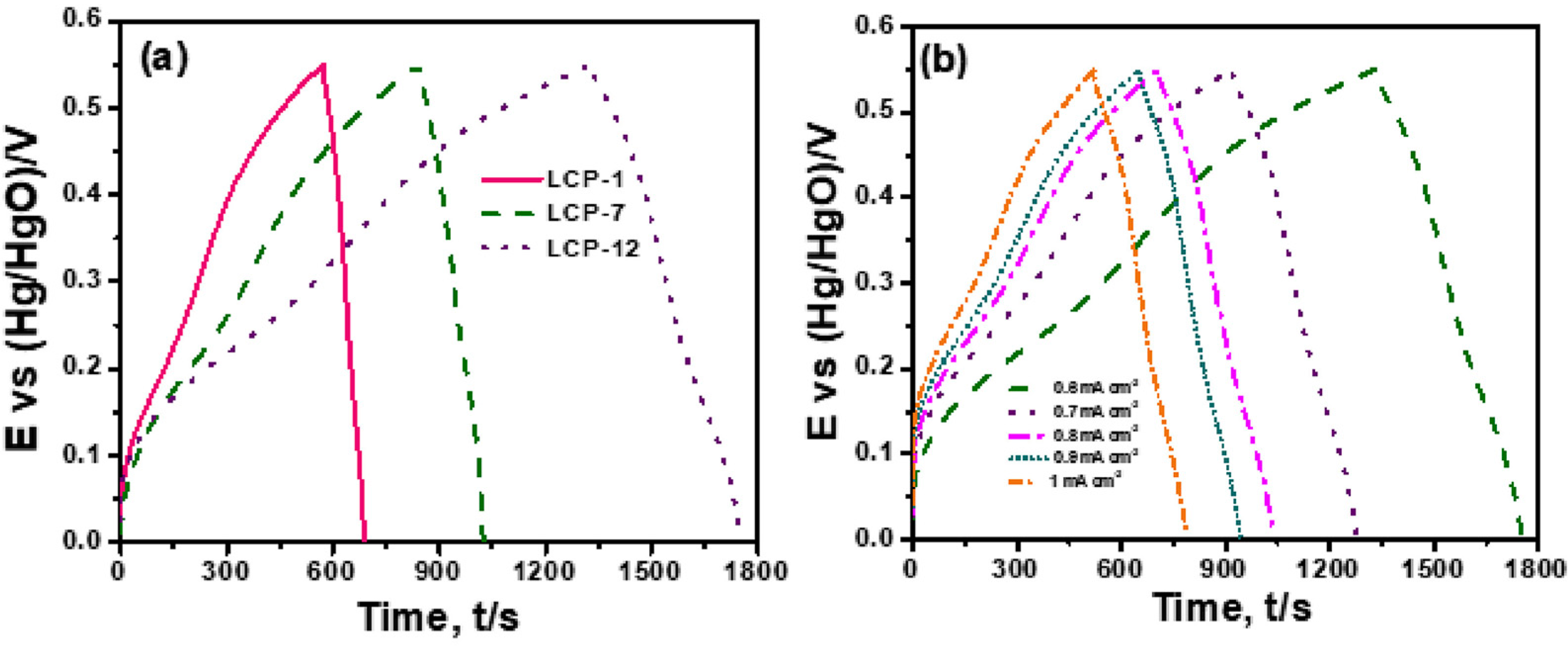
3.1. Olivine-Type Materials
3.2. Sol-Gel Metal-Oxide-Based Materials
3.2.1. Ni-Based Materials
3.2.2. Mn-Based Materials
3.2.3. Others
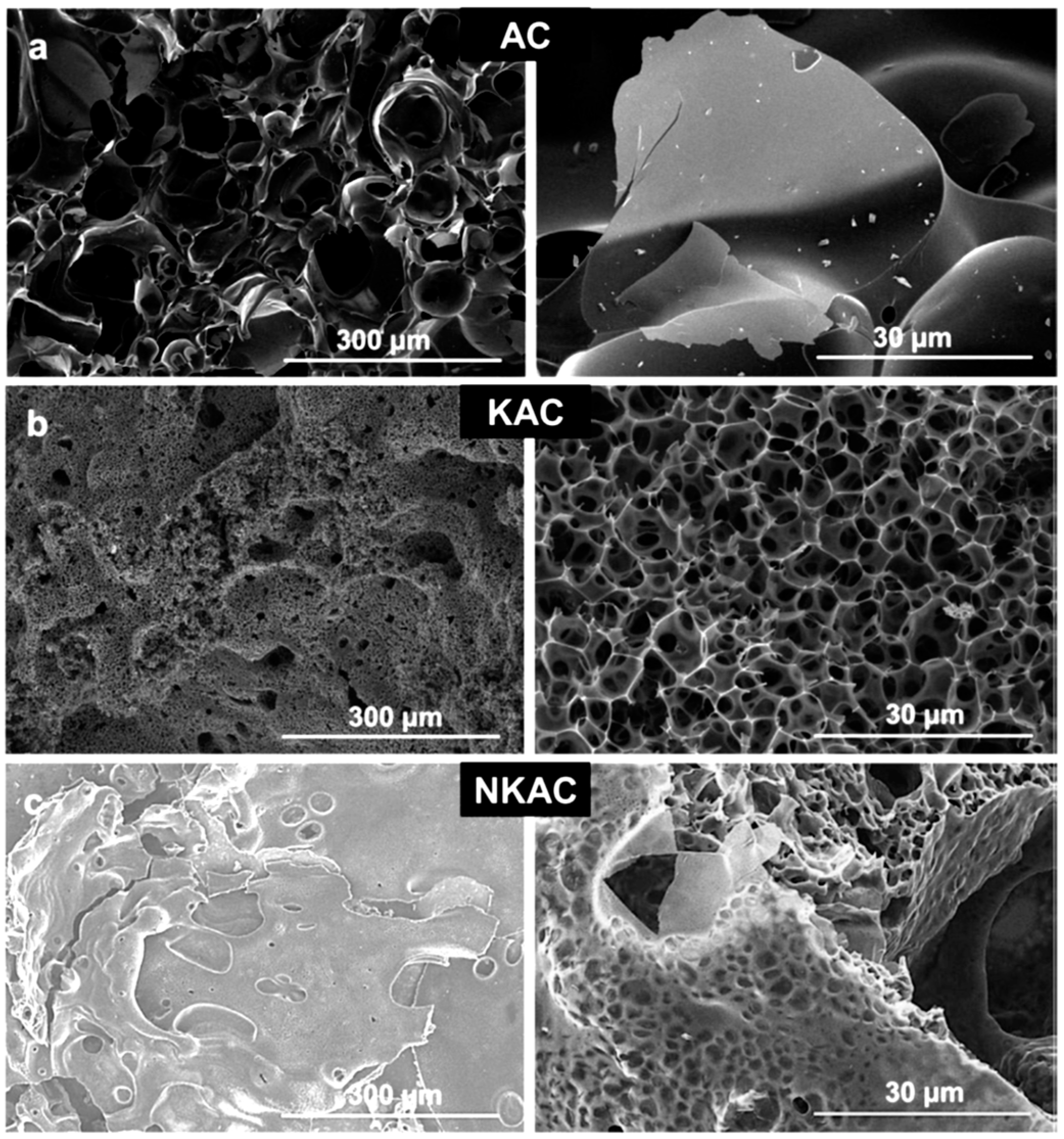
3.3. Carbon Aerogels
3.4. Perovskites
| Material | Electrode Capacitance | Current/Scan Rate | Stability, Cycles | Ref. |
|---|---|---|---|---|
| LiCoPO4 | 631 F/g | 0.6 mA/cm2 | 5000 cycles (two electrode cells) | [29] |
| N-doped porous carbon aerogels | 400 F/g | 0.5 A/g | 100,000 (96% retention) | [54] |
| V2O5@rGO nanocomposite (V2O5 sol-gel) | 289 F/g | 0.01 A/g | 1000 (85% retention) | [42] |
| NiCo2O4 | 140.1 mAh/g | 0.5 A/g | 5000 (66.7% retention) | [24] |
| Fe3O4/3D-graphene (3D-graphene sol-gel route) | 388.8 F/g | 1 A /g | 5000 (88% retention) | [43] |
| LiNiPO4 | 417 F/g | 1 mA/cm2 | 89% being retained after 2000 cycles (AC‖LiNiPO4 supercapacitor) | [30] |
| La1−xCaxMnO3, (perovskite) | 170 F/g | 1 A/g | Poor stability | [67] |
| N-doped carbon hollow microspheres | 146 F/g (80 Mv/s) | - | - | [69] |
| NiMn2O4 | 303 F/g | 0.5 A/ g | 5000 cycles | [38] |
| MnO2 nanorod-loaded activated carbon | 213.1 F/g | 1 A/g | 5000 cycles (106% retention) | [41] |
| Zn1−xNixMn2O4 (x = 0.00, 0.25, 0.50, 0.75 and 1.00) nanospheres | 161.27 F/g | 5 mV/s | - | [40] |
| BiFeO3– Graphene nanocomposite | 64 F/g (two-electrode) | 20 mV/s | 2000 (95% retention) | [44] |
| LiFePO4 + AC (LiFePO4Sol-gel) | 36 mA h/g | 100 μA /cm2. | - | [31] |
| NiO | 871 F/g | 5 mV/s | 10,000(86.5% retention) | [36] |
| TiO2-ZnO/MCM-41 (MCM-41 sol-gel) | 642.4 F/g | 2 A/g | 5000 (98.7% retention) | [45] |
| MnO2 | 1.6 mF/cm2 (576 F/g) | 0.1 mA/cm2 | - | [39] |
| NiCo2O4/CeO2 | 1355 F/g | 5 A/g | 6000 (4.7% capacity loss) | [37] |
| PrBaCo2O5+ᵟ | 428.2 C/g | 1 mV/s | 2000 (93% retention) | [68] |
| NiTiO3 rods | 542.26 F/g | 5 mV/s | 2100 (91% retention) | [59] |
| LaMnO3 | 392 F /g | 1 A/g | 10,000 (90% retention) | [60] |
| Alumina-embedded SrCo0.95V0.05O3 | 0.4 F/g | 25 mA/g | - | [61] |
| SrCo0.9Mo0.1O3−δ, | 1223.34 F/g | 1 A/g1 | 5000 (93.52% retention) | [62] |
| Aerogel/PPy | 433 F/g | 0.5 A/ g | 5000 (87.2% retention) | [55] |
| starch-derived carbon aerogels | 147 F/g | 1 A/g | 97.0% of the initial discharge capacity after 10,000 cycles. | [56] |
4. Hybrid Sol-Gel Coating for Corrosion Resistance
4.1. Hybrid Sol-Gel Coating for the Corrosive Resistance of Al Alloys
4.2. Hybrid Sol-Gel Coating for the Corrosive Resistance of Steel Substrates: Steel/Mild/Stainless/Low Carbon
4.3. Hybrid Sol-Gel Coating for the Corrosive Resistance of Steel Substrates: Mg Substrates
5. Sol-Gel Technologies in Electrochemical Sensors
5.1. Oxide Materials and Materials Based on Them
5.2. Materials Based on Complex Oxides
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Najafi, A.; Golestani-Fard, F.; Rezaie, H.R.; Saeb, S.P. Sol-Gel synthesis and characterization of SiC–B4C nanopowder. Ceram. Int. 2020, 47, 6376–6387. [Google Scholar] [CrossRef]
- Fakhimi, O.; Najafi, A.; Khalaj, G. A facile route to obtain Al2O3 nanopowder via recycling aluminum cans by sol-gel method. Mater. Res. Express 2020, 7, 045008. [Google Scholar] [CrossRef]
- Gaponenko, N.V. Sol-gel derived films in mesoporous matrices: Porous silicon, anodic alumina, and artificial opals. Synth. Met. 2001, 124, 125–130. [Google Scholar] [CrossRef]
- Molchan, I.S.; Molchan, T.V.; Gaponenko, N.V.; Skeldon, P.; Thompson, G.E. Impurity-driven defect generation in porous anodic alumina. Electrochem. Commun. 2010, 12, 693–696. [Google Scholar] [CrossRef]
- Han, F.; Qian, O.; Meng, G.; Lin, D.; Chen, G.; Zhang, S.; Pan, Q.; Zhang, X.; Zhu, X.; Wei, B. Structurally integrated 3D carbon tube grid-based high-performance filter capacitor. Mater. Sci. 2022, 377, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Yalovega, G.; Myasoedova, T.; Funik, A.; Plugotarenko, N.; Brzhezinskaya, M.; Bahmatskaya, A. Mechanism of the formation of copper-containing fractal-like crystallites in metal-organic thin films: Shape simulation and XANES analysis. Phys. Status Solidi B Basic Res. 2016, 253, 2217–2224. [Google Scholar] [CrossRef]
- Yalovega, G.; Funik, A.; Myasoedova, T.; Brzhezinskaya, M. Copper-oxide metalorganic nanocomposite: Morphological and X-ray spectroscopy studies. J. Phys. Conf. Ser. 2016, 712, 012055. [Google Scholar] [CrossRef] [Green Version]
- Shmatko, V.A.; Yalovega, G.E.; Myasoedova, T.N.; Shtekhin, I.E.; Petrov, V.V. Influence of the surface morphology and structure on the gas-sorption properties of SiO2CuOx nanocomposite materials: X-ray spectroscopy investigations. Phys. Solid State 2015, 57, 399–406. [Google Scholar] [CrossRef]
- Plugotarenko, N.K.; Korolev, A.N.; Petrov, V.V.; Nazarova, T.N. Preparation of sols from water-alcohol solutions of tetraethyl orthosilicate and SnCl4 and the effect of sol composition on the surface morphology of sol-gel films. Inorg. Mater. 2007, 43, 1010–1014. [Google Scholar] [CrossRef]
- Myasoedova, T.N.; Plugotarenko, N.K.; Moiseeva, T.A. Copper-containing films obtained by the simple citrate sol-gel route for NO2 detection: Adsorption and kinetic study. Chemosensors 2020, 8, 79. [Google Scholar] [CrossRef]
- Myasoedova, T.N.; Mikhailova, T.S.; Yalovega, G.E.; Plugotarenko, N.K. Resistive low-temperature sensor based on the SiO2ZrO2 film for detection of high concentrations of NO2 gas. Chemosensors 2018, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Myasoedova, T.N.; Mikhailova, T.S.; Plugotarenko, N.K. A Study on A NO2 Sensor Based on SiO2-ZrO2 Composite Film. In Proceedings of the 14th International Scientific-Technical Conference on Actual Problems of Electronic Instrument Engineering, APEIE 2018, Novosibirsk, Russia, 2–6 October 2018. [Google Scholar]
- Dhere, S. Electrode materials for supercapacitors synthesized by sol-gel process. Curr. Sci. 2018, 115, 436–449. [Google Scholar] [CrossRef]
- Masalovich, M.; Zagrebelnyy, O.; Nikolaev, A.; Shilova, O.; Ivanova, A. Development of pseudocapacitive materials based on cobalt and iron oxide compounds for an asymmetric energy storage device. Electrochim. Acta 2022, 410, 139999. [Google Scholar] [CrossRef]
- Lev, O.; Wu, Z.; Bharathi, S.; Glezer, V.; Modestov, A.; Gun, J.; Rabinovich, L.; Sampath, S. Sol-Gel Materials in Electrochemistry. Chem. Mater. 1997, 9, 2354–2375. [Google Scholar] [CrossRef]
- Il’ina, E.A.; Lyalin, E.D.; Antonov, V.D.; Pankratov, A.A.; Vovkotrub, E.G. Sol-gel synthesis of Al- and Nb-co-doped Li7La3Zr2O12 solid electrolytes. Ionics 2020, 26, 3239–3247. [Google Scholar] [CrossRef]
- Fernandes, M.; de Zea Bermudez, V. Chapter 13—Sol-gel materials for smart electrochromic devices. Chem. Solut. Synth. Mater. Des. Thin Film Device Appl. 2021, 2021, 439–475. [Google Scholar]
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Schem, M.; Schmidt, T.; Gerwann, J.; Wittmar, M.; Veith, M.; Thompson, G.E.; Molchan, I.S.; Hashimoto, T.; Skeldon, P.; Phani, A.R.; et al. CeO2-filled sol-gel coatings for corrosion protection of AA2024-T3 aluminium alloy. Corros. Sci. 2009, 51, 2304–2315. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: London, UK, 1990; p. 903. [Google Scholar]
- Rahman, I.A.; Padavettan, V. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—A review. J. Nanomater. 2012, 2012, 8. [Google Scholar] [CrossRef] [Green Version]
- Catauro, M.; Tranquillo, E.; Poggetto, G.D.; Pasquali, M.; Dell’Era, A.; Vecchio, S.C. Influence of the heat treatment on the particles size and on the crystalline phase of TiO2 synthesized by the sol-gel method. Materials 2018, 11, 2364. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Open Chem. 2012, 10, 279–294. [Google Scholar] [CrossRef]
- Lai, S.; Qu, X.; Zhao, H.; Hong, S.W.; Lee, K. Improved performance in asymmetric supercapacitors utilized by dual ion-buffering reservoirs based on honeycomb-structured NiCo2O4 and 3D rGO-PPy aerogels. Appl. Surf. Sci. 2022, 586, 152847. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Rajendran, V. Synthesis and characterization of nano-TiO2 via different methods. Arch. Appl. Sci. Res. 2012, 4, 1183–1190. [Google Scholar]
- Verma, R.; Mantri, B.; Srivastava, A.K. Shape control synthesis, characterizations, mechanisms and optical properties of large, scaled metal oxide nanostructures of ZnO and TiO2. Adv. Mater. Lett. 2015, 6, 324–333. [Google Scholar] [CrossRef]
- Aparicio, M.; Jitianu, A.; Klein, L.C. Sol-Gel Processing for Conventional and Alternative Energy; Springer: New York, NY, USA, 2012; 397p. [Google Scholar]
- Eftekhari, A. Surface modification of thin-film based LiCoPO4 5 V cathode with metal oxide. J. Electrochem. Soc. 2004, 151, A1456–A1460. [Google Scholar] [CrossRef]
- Priyadharsini, N.; Shanmugapriya, S.; Kasturi, P.R.; Surendran, S.; Selvan, R.K. Morphology-dependent electrochemical properties of sol-gel synthesized LiCoPO4 for aqueous hybrid capacitors. Electrochim. Acta 2018, 289, 516–526. [Google Scholar] [CrossRef]
- Priyadharsini, N.; Kasturi, P.R.; Shanmugavani, A.; Surendran, S.; Shanmugapriya, S.; Selvan, R.K. Effect of chelating agent on the sol-gel thermolysis synthesis of LiNiPO4 and its electrochemical properties for hybrid capacitors. J. Phys. Chem. Solids 2018, 119, 183–192. [Google Scholar] [CrossRef]
- Singh, M.K.; Hashmi, S.A. Performance of solid-state hybrid supercapacitor with LiFePO4/AC composite cathode and Li4Ti5O12 as anode. Ionics 2017, 23, 2931–2942. [Google Scholar] [CrossRef]
- Jiang, Q.; Kurra, N.; Alhabeb, M.; Gogotsi, Y.; Alshareef, H.N. All pseudocapacitive MXene-RuO2 asymmetric supercapacitors. Adv. Energy Mater. 2018, 8, 1703043. [Google Scholar] [CrossRef]
- Zhu, S.; Li, L.; Liu, J.; Wang, H.; Wang, T.; Zhang, Y.; Zhang, L.; Ruoff, R.S.; Dong, F. Structural directed growth of ultrathin parallel birnessite on β-MnO2 for high-performance asymmetric supercapacitors. ACS Nano 2018, 12, 1033–1042. [Google Scholar] [CrossRef]
- Lian, C.; Wang, Z.; Lin, R.; Wang, D.; Chen, C.; Li, Y. An efficient, controllable and facile two-step synthesis strategy: Fe3O4@RGO composites with various Fe3O4 nanodots and their super capacitance properties. Nano Res. 2017, 10, 3303–3313. [Google Scholar] [CrossRef]
- Peng, W.; Chen, K.; Li, S.; Wang, J.; Su, Z. Spherical spinel NiMn2O4 in-situ grown on MWCNT via solvothermal synthesis for supercapacitors. Diam. Relat. Mater. 2022, 128, 109266. [Google Scholar] [CrossRef]
- Dhas, S.D.; Maldar, P.S.; Patil, M.D.; Waikar, M.R.; Sonkawade, R.G.; Moholcar, A.V. Sol-gel synthesized nickel oxide nanostructures on nickel foam and nickel mesh for a targeted energy storage application. J. Energy Storage 2022, 47, 103658. [Google Scholar] [CrossRef]
- Santhosh, G.; Nayaka, G.P.; Bhatt, A.S. Ultrahigh capacitance of NiCo2O4/CeO2 mixed oxide material for supercapacitor applications. J. Alloy. Compd. 2022, 899, 163312. [Google Scholar] [CrossRef]
- Vamsi Krishna, B.N.; Bhagwan, J.; Yu, J.S. Sol-Gel Routed NiMn2O4 Nanofabric Electrode Materials for Supercapacitors. J. Electrochem. Soc. 2019, 166, A1950–A1955. [Google Scholar] [CrossRef]
- Jin, S.; Ryu, I.; Choe, G.; Song, S.W.; Kim, H.M.; Hong, D.; Yim, S. Trade-off between areal capacitance and optical transmittance of highly transparent MnO2 electrodes for supercapacitors. J. Energy Storage 2022, 50, 104641. [Google Scholar] [CrossRef]
- Hasan, M.; Zawar, S.; Mustafa, G.M.; Ghaffar, F.; Razaq, A.; Atiq, S. Porous Architecture of Ni substituted ZnMn2O4 nanospheres as an electrode material for supercapacitor applications. Phys. B Phys. Condens. Matter. 2022, 633, 413767. [Google Scholar] [CrossRef]
- Kour, S.; Tanwar, S.; Sharma, A.L. MnO2 nanorods loaded activated carbon for high-performance supercapacitors. J. Alloy. Compd. 2022, 910, 164834. [Google Scholar] [CrossRef]
- Kiruthiga, R.; Nithya, C.; Karvembu, R.; Venkata Rami Reddy, B. Reduced Graphene Oxide Embedded V2O5 Nanorods and Porous Honey Carbon as High-Performance Electrodes for Hybrid Sodium-ion Supercapacitors. Electrochim. Acta 2017, 256, 221–231. [Google Scholar]
- Zhao, X.; Jia, Y.; Liu, Z.-H. GO-graphene ink-derived hierarchical 3D-graphene architecture supported Fe3O4 nanodots as high-performance electrodes for lithium/sodium storage and supercapacitors. J. Colloid Interface Sci. 2019, 536, 463–473. [Google Scholar] [CrossRef]
- Nayak, S.; Soam, A.; Nanda, J.; Mahender, C.; Singh, M.; Mohapatra, D.; Kumar, R. Sol-gel synthesized BiFeO3–Graphene nanocomposite as an efficient electrode for supercapacitor application. J. Mater. Sci. Mater. Electron. 2018, 29, 9361–9368. [Google Scholar] [CrossRef]
- Ehsani, A.; Bigdeloo, M.; Alamgholiloo, H.; Asgari, E.; Sheikhmohammadi, A.; Nazari, S.; Hashemzadeh, B.; Ghasemian, N. Ternary nanocomposite of TiO2-ZnO/MCM-41: Synthesis and electrochemical performance in supercapacitors. J. Energy Storage 2022, 50, 104633. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, T.; Li, M.; Yu, M.; Luo, Y.; Tong, Y.; Li, Y. Multiscale pore network boosts capacitance of carbon electrodes for ultrafast charging. Nano Lett. 2017, 17, 3097–3104. [Google Scholar] [CrossRef]
- Wan, C.C.; Jiao, Y.; Li, J. Flexible, highly conductive, and free-standing reduced graphene oxide/polypyrrole/cellulose hybrid papers for supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 3819–3831. [Google Scholar] [CrossRef]
- Ko, Y.; Kwon, M.; Bae, W.K.; Lee, B.; Lee, S.W.; Cho, J. Flexible supercapacitor electrodes based on real metal-like cellulose papers. Nat. Commun. 2017, 8, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzel, G.K.; Deveci, H. Synergistic effects of silica aerogels/xerogels on properties of polymer composites: A review. J. Ind. Eng. Chem. 2020, 89, 13–27. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhang, X.T.; Xu, J.B.; Shen, Y.; Wang, C.A.; Li, F.W.; Zhang, Z.H.; Chen, J.; Ye, Y.H.; Shen, R.Q. Fabrication and Characterization of Al-CuO Nanocomposites Prepared by Sol-Gel Method. Def. Tech. 2020, 1507, 022024. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Niu, P.; Wang, S.L.; Shi, J.; Li, L. Porous two-dimensional materials for photocatalytic and electrocatalytic applications. Matter 2020, 2, 1377–1413. [Google Scholar] [CrossRef]
- Qian, M.; Wang, Z.; Li, Z.; Xu, J.; Sun, P.; Lin, J.; Lin, T.; Huang, F. Sol-gel assisted chemical activation for nitrogen-doped porous carbon. Microporous Mesoporous Mater. 2019, 286, 18–24. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.; Li, J.; Wang, L. Magnetic N-doped carbon aerogel from sodium carboxymethyl cellulose/collagen composite aerogel for dye adsorption and electrochemical supercapacitor. Int. J. Biol. Macromol. 2018, 115, 185–193. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, T.; Zhang, J.; Cui, E.; Yue, L.; Jiang, R.; Hou, G. The potassium hydroxide-urea synergy in improving the capacitive energy-storage performance of agar-derived carbon aerogels. Carbon 2019, 147, 451–459. [Google Scholar] [CrossRef]
- Yu, M.; Li, Y.; Xu, S.; Li, J.; Wang, L. Growing spherical polypyrrole nanoparticles onto the magnetic carbon aerogel for improving electrochemical performance for supercapacitors. J. Porous Mater. 2021, 28, 1999–2011. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, B.; Wang, S.; Dong, X.; Zhang, L.; Liu, Z. Carbon aerogels with oxygen-containing surface groups for use in supercapacitors. Solid State Ion 2019, 339, 115005. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, X.; Chen, M. Pore structure improvement of lignin composite carbon aerogels by introducing manganese ion and its application in supercapacitors. Mater. Res. Express 2019, 6, 065036. [Google Scholar] [CrossRef]
- Kostopoulou, A.; Kymakis, E.; Stratakis, E. Perovskite nanostructures for photovoltaic and energy storage devices. J. Mater. Chem. 2018, 6, 9765–9798. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Ma, Y.-R.; Shirage, P.M.; Devan, R.S. Mesoporous perovskite of interlocked nickel titanate nanoparticles for efficient electrochemical supercapacitor electrode. J. Alloy. Compd. 2020, 833, 155134. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Li, D.; Zhu, C.; Zhang, W.; Miao, C. LaMnO3 nanocomposite double network hydrogel electrodes with enhanced electrochemical and mechanical performance for flexible supercapacitors. J. Alloy. Compd. 2021, 888, 161555. [Google Scholar] [CrossRef]
- Salguero Salas, M.A.; De Paoli, J.M.; Pérez Linarez, O.E.; Bajales, N.; Fuertes, V.C. Synthesis and characterization of alumina-embedded SrCo0.95V0.05O3 nanostructured perovskite: An attractive material for supercapacitor devices. Microporous Mesoporous Mater. 2020, 293, 109797. [Google Scholar] [CrossRef]
- Tomar, A.K.; Singh, G.; Sharma, R.K. Fabrication of a Mo-Doped Strontium Cobaltite Perovskite Hybrid Supercapacitor Cell with High Energy Density and Excellent Cycling Life. ChemSusChem 2018, 11, 4123–4130. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Choudhary, R.J.; Phase, D.M.; Devan, R.S. Structural correlation of a nanoparticle-embedded mesoporous CoTiO3 perovskite for an efficient electrochemical supercapacitor. RSC Adv. 2020, 10, 23446. [Google Scholar] [CrossRef]
- Ansari, A.; Siddiqui, V.U.; Siddiqi, W.A. 4—Perovskite-type catalytic materials for water treatment. Hybrid Perovskite Compos. Mater. 2021, 2021, 117–134. [Google Scholar]
- Athayde, D.D.; Souza, D.F.; Silva, A.M.; Vasconcelos, D.; Nunes, E.H.; da Costa, J.C.; Vasconcelos, W.L. Review of perovskite ceramic synthesis and membrane preparation methods. Ceram. Int. 2016, 42, 6555–6571. [Google Scholar] [CrossRef] [Green Version]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Nan, H.; Lang, X.; Liu, S.; Qiao, L.; Hu, X.; Tian, H. Influence of calcium doping on performance of LaMnO3 supercapacitors. Ceram. Int. 2018, 44, 9733–9741. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Chen, Y.; Yang, L.; Wang, Y.; Mingrui, W. A-site cation-ordered double perovskite PrBaCo2O5+q oxide as an anion-inserted pseudocapacitor electrode with outstanding stability. J. Alloy. Compd. 2019, 810, 151830. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Liu, Z.; Zhao, Y.; Liu, Y.; Yue, Q.; Zhu, H.; Deng, Y.; Wu, Y.; Zhao, D. N-doped carbon hollow microspheres for metal-free quasi-solid-state fullsodium-ion capacitors. Nano Energy 2017, 41, 674–680. [Google Scholar] [CrossRef]
- Ćurković, L.; Otmačić Ćurković, H.; Žmak, I.; Mustafa, M.K.; Gabelica, I. Corrosion Behavior of Amorphous Sol–Gel TiO2–ZrO2 Nano Thickness Film on Stainless Steel. Coatings 2021, 11, 988. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Salvado, I.M.; Ferreira, M.G.S. Sol–gel coatings for corrosion protection of metals. J. Mater. Chem. 2005, 15, 5099. [Google Scholar] [CrossRef]
- Wu, L.K.; Wu, J.J.; Wu, W.Y.; Cao, F.H.; Jiang, M.Y. Sol–gel-based coatings for oxidation protection of TiAl alloys. J. Mater. Sci. 2020, 55, 6330–6351. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Arki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Talha, M.; Ma, Y.; Xu, M.; Wang, Q.; Lin, Y.; Kong, X. Recent Advancements in Corrosion Protection of Magnesium Alloys by Silane-Based Sol–Gel Coatings. Ind. Eng. Chem. Res. 2020, 59, 19840–19857. [Google Scholar] [CrossRef]
- Costenaro, H.; Lanzutti, A.; Paint, Y.; Fedrizzi, L.; Terada, M. Corrosion resistance of 2524 Al alloy anodized in tartaric-sulphuric acid at different voltages and protected with a TEOS-GPTMS hybrid sol-gel coating. Surf. Coat. Tech. 2017, 324, 438–450. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, S.; Jin, Z.; Zhang, B.; Liu, N.; Chen, S.; Liu, S.; Sun, X.; Duan, J. Perfect Combination of LBL with Sol–Gel Film to Enhance the Anticorrosion Performance on Al Alloy under Simulated and Accelerated Corrosive Environment. Materials 2020, 13, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussin, M.H. Electrochemical data of single and hybrid sol-gel coating precursors for aluminum alloy corrosion protection in 3.5% NaCl. Data Brief 2019, 22, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Saeid Mersagh, D.; Shanaghi, A.; Baghshahi, S. Investigation of Electrochemical Behavior of Zirconia -Benzotriazole Hybrid Nanostructured Coating Applied on Al 2024 by Sol-Gel Method. Prot. Met. Phys. Chem. Surf. 2018, 54, 1050–1058. [Google Scholar] [CrossRef]
- Genet, C.; Menu, M.-J.; Gavard, O.; Ansart, F.; Gressier, M.; Montpellaz, R. Innovative Formulation Combining Al, Zr and Si Precursors to Obtain Anticorrosion Hybrid Sol-Gel Coating. Molecules 2018, 23, 1135. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Ke, R.; Dong, Y. Characterization and corrosion protection of nano-titanium dioxide doped BTSE-GPTMS sol–gel coating on cast Al–Si alloy. J. Sol-Gel Sci. Tech. 2020, 94, 671–680. [Google Scholar] [CrossRef]
- Peng, D.; Huang, K.; He, Y.; Zhang, Z.; Wang, Y.; Wu, J. Hybrid sol-gel coating incorporated with TiO2 nanosheets and anti-corrosive effects on AA2024-T3. Anti-Corros. Methods Mater. 2019, 66, 215–221. [Google Scholar] [CrossRef]
- Balaji, J.; Sethuraman, M.G. Chitosan-doped-hybrid/TiO2 nanocomposite based sol-gel coating for the corrosion resistance of aluminum metal in 3.5% NaCl medium. Int. J. Biol. Macromol. 2017, 104, 1730–1739. [Google Scholar]
- Nezamdoust, S.; Seifzadeh, D. rGO@APTES/hybrid sol-gel nanocomposite for corrosion protection of 2024 aluminum alloy. Prog. Org. Coat. 2017, 109, 97–109. [Google Scholar] [CrossRef]
- Tarzanagh, Y.J.; Seifzadeh, D.; Rajabalizadeh, Z.; Habibi-Yangjeh, A.; Khoyadari, A.; Sohrabnezhad, S. Sol-gel/MOF nanocomposite for effective protection of 2024 aluminum alloy against corrosion. Surf. Coat. Tech. 2019, 380, 125038. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Sampath, S.; Aruna, S.T. Silica-alumina based sol-gel coating containing cerium oxide nanofibers as a potent alternative to conversion coating for AA2024 alloy. Surf. Coat. Tech. 2021, 411, 127007. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Li, Y.; Yu, M.; Yin, X.; Li, S. Enhancement of active anticorrosion via Ce-doped Zn-Al layered double hydroxides embedded in sol-gel coatings on aluminum alloy. J. Wuhan Univ. Tech.-Mater. Sci. Ed. 2017, 32, 1199–1204. [Google Scholar] [CrossRef]
- Subasri, R.; Soma Raju, K.R.C.; Reddy, D.S.; Jyothirmayi, A.; Ijeri, V.S.; Prakash, O.; Gaydos, S.P. Environmentally friendly Zn–Al layered double hydroxide (LDH)-based sol–gel corrosion protection coatings on AA 2024-T3. J. Coat. Tech. Res. 2019, 16, 1447–1463. [Google Scholar] [CrossRef]
- Rodríguez-Alonso, L.; López-Sánchez, J.; Serrano, A.; Rodríguez de la Fuente, O.; Galván, J.C.; Carmona, N. Hybrid Sol-Gel Coatings Doped with Non-Toxic Corrosion Inhibitors for Corrosion Protection on AZ61 Magnesium Alloy. Gels 2022, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Alibakhshi, E.; Akbarian, M.; Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M. Evaluation of the corrosion protection performance of mild steel coated with hybrid sol-gel silane coating in 3.5 wt.% NaCl solution. Prog. Org. Coats. 2018, 123, 190–200. [Google Scholar] [CrossRef]
- Perrin, F.X.; Ziarelli, F.; Dupuis, A. Relation between the corrosion resistance and the chemical structure of hybrid sol-gel coatings with interlinked inorganic-organic network. Prog. Org. Coat. 2020, 141, 105532. [Google Scholar] [CrossRef]
- Vivar Mora, L.; Naik, S.; Paul, S.; Dawson, R.; Neville, A. Influence of silica nanoparticles on corrosion resistance of sol-gel based coatings on mild steel. Surf. Coat. Tech. 2017, 324, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Khosrav, H.S.; Veerapandiyan, V.K.; Vallant, R.; Reichmann, K. Effect of processing conditions on the structural properties and corrosion behavior of TiO2–SiO2 multilayer coatings derived via the sol-gel method. Ceram. Int. 2020, 46, 17741–17751. [Google Scholar] [CrossRef]
- Krishna, V.; Padmapreetha, R.; Chandrasekhar, S.B.; Murugan, K.; Johnson, R. Oxidation resistant TiO2–SiO2 coatings on mild steel by Sol–Gel. Surf. Coat. Tech. 2019, 378, 125041. [Google Scholar] [CrossRef]
- Shanaghi, A.L.I.; Chu, P.K.; Moradi, H. Effect of Inhibitor Agents Addition on Corrosion Resistance Performance of Titania Sol–Gel Coatings Applied on 304 Stainless Steel. Surf. Rev. Lett. 2016, 24, 1750055. [Google Scholar] [CrossRef]
- Olya, N.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B. Construction of a novel corrosion protective composite film based on a core-shell LDH-Mo@SiO2 inhibitor nanocarrier with both self-healing/barrier functions. J. Taiwan Inst. Chem. Eng. 2020, 113, 406–418. [Google Scholar] [CrossRef]
- Ramezanzadeh, B. Corrosion Protection of Steel with Zinc Phosphate Conversion Coating and Post-Treatment by Hybrid Organic-Inorganic Sol-Gel Based Silane Film. J. Electrochem. Soc. 2017, 164, C224–C230. [Google Scholar] [CrossRef]
- Taheri, M.; Naderi, R.; Mohsen Saremi, M.; Mahdavian, M. Development of an ecofriendly silane sol-gel coating with zinc acetylacetonate corrosion inhibitor for active protection of mild steel in sodium chloride solution. J. Sol.-Gel. Sci. Tech. 2016, 81, 154–166. [Google Scholar]
- Rassouli, L.; Naderi, R.; Mahdavain, M. Study of the impact of sequence of corrosion inhibitor doping in zeolite on the self-healing properties of silane sol–gel film. J. Indust. Eng. Chem. 2018, 66, 221–230. [Google Scholar] [CrossRef]
- Ashrafi-Shahri, S.M.; Ravari, F.; Seifzadeh, D. Smart organic/inorganic sol-gel nanocomposite containing functionalized mesoporous silica for corrosion protection. Prog. Org. Coat. 2019, 133, 44–54. [Google Scholar] [CrossRef]
- Suárez-Vega, A.; Agustín-Sáenz, C.; O’Dell, L.A.; Brusciotti, F.; Somers, A.; Forsyth, M. Properties of hybrid sol-gel coatings with the incorporation of lanthanum 4-hydroxy cinnamate as corrosion inhibitor on carbon steel with different surface finishes. Appl. Surf. Sci. 2021, 561, 149881. [Google Scholar]
- Parhizkar, N.; Ramezanzadeh, B.; Shahrabi, T. Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a sol-gel based silane coating filled with amino and isocyanate silane functionalized graphene oxide nanosheets. Appl. Surf. Sci. 2018, 439, 45–59. [Google Scholar] [CrossRef]
- Qian, Z. Corrosion Behavior Study of AZ31B magnesium Alloy by Sol- Gel Silica-based Hybrid Coating. Inter. J. Electrochem. Sci. 2017, 12, 8269–8279. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D. Application of CeH–V/ sol–gel composite coating for corrosion protection of AM60B magnesium alloy. Transact. Nonferrous Met. Soc. China 2017, 27, 352–362. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. Application of novel sol–gel composites on magnesium alloy. J. Magnes. Alloy. 2019, 7, 419–432. [Google Scholar] [CrossRef]
- Adsul, S.H.; Siva, A.T.; Sathiyanarayanan, S.; Sonawane, S.H.; Subasri, R. Self-healing ability of nanoclay-based hybrid sol-gel coatings on magnesium alloy AZ91D. Surf. Coat. Tech. 2017, 309, 609–620. [Google Scholar] [CrossRef]
- Samadianfard, R.; Seifzadeh, D.; Habibi-Yangjeh, A.; Jafari-Tarzanagh, Y. Oxidized fullerene/sol-gel nanocomposite for corrosion protection of AM60B magnesium alloy. Surf. Coat. Tech. 2020, 385, 125400. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. PTMS/OH-MWCNT sol-gel nanocomposite for corrosion protection of magnesium alloy. Surf. Coat. Tech. 2018, 335, 228–240. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H. Effect of amino acids and montmorillonite nanoparticles on improving the corrosion protection characteristics of hybrid sol-gel coating applied on AZ91 Mg alloy. Mater. Chem. Phys. 2019, 225, 298–308. [Google Scholar] [CrossRef]
- Upadhyay, V.; Bergseth, Z.; Kelly, B.; Battocchi, D. Silica-Based Sol-Gel Coating on Magnesium Alloy with Green Inhibitors. Coatings 2017, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Golli, A.E.; Echabaane, M.; Dridi, C. Development of an electrochemical nanoplatform for non-enzymatic glucose sensing based on Cu/ZnO nanocomposite. Mater. Chem. Phys. 2022, 280, 125844. [Google Scholar] [CrossRef]
- Haque, M.; Fouad, H.; Seo, H.-K.; Alothman, O.Y.; Ansari, Z.A. Cu-Doped ZnO Nanoparticles as an Electrochemical Sensing Electrode for Cardiac Biomarker Myoglobin Detection. IEEE Sens. J. 2020, 20, 8820–8832. [Google Scholar] [CrossRef]
- Zhan, B.; Zhang, Y.; Zhao, X. High sensitive sol-gel based electrochemical immunosensor for Clenbuterol Determination. Int. J. Electrochem. Sci. 2021, 16, 211124. [Google Scholar] [CrossRef]
- Alsaiari, M.; Saleem, A.; Alsaiari, R.; Muhammad, N.; Latif, U.; Tariq, M.; Almohana, A.; Rahim, A. SiO2/Al2O3/C grafted 3-n propylpyridinium silsesquioxane chloride-based non-enzymatic electrochemical sensor for determination of carcinogenic nitrite in food products. Food Chem. 2022, 369, 130970. [Google Scholar] [CrossRef] [PubMed]
- Lete, C.; Chelu, M.; Marin, M.; Mihaiu, S.; Preda, S.; Anastasescu, M.; Calderón-Moreno, J.M.; Dinulescu, S.; Moldovan, C.; Gartner, M. Nitrite electrochemical sensing platform based on tin oxide films. Sens. Actuat. B Chem. 2020, 316, 128102. [Google Scholar] [CrossRef]
- Yin, X.T.; Zhou, W.D.; Li, J.; Wang, Q.; Wu, F.Y.; Dastan, D.; Wang, D.; Garmestani, H.; Wang, X.M.; Ţălu, Ş. A highly sensitivity and selectivity Pt-SnO2 nanoparticles for sensing applications at extremely low level hydrogen gas detection. J. Alloy. Compd. 2019, 805, 229–236. [Google Scholar] [CrossRef]
- Sharma, A.; Arya, S.; Chauhan, D.; Solanki, P.R.; Khajuria, S.; Khosla, A. Synthesis of Au-SnO2 nanoparticles for electrochemical determination of vitamin B12. J. Mater. Res. Tech. 2020, 9, 14321–14327. [Google Scholar] [CrossRef]
- Khatoon, Z.; Fouad, H.; Seo, H.-K.; Alothman, O.Y.; Ansari, Z.A.; Ansari, S.G. Ethyl Acetate Chemical Sensor as Lung Cancer Biomarker Detection Based on Doped Nano-SnO2 Synthesized by Sol-Gel Process. IEEE Sens. J. 2020, 20, 12504–12511. [Google Scholar] [CrossRef]
- Duan, W.; Gunes, M.; Baldi, A.; Gich, M.; Fernandez-Sanchez, C. Compact fluidic electrochemical sensor platform for on-line monitoring of chemical oxygen demand in urban wastewater. Chem. Eng. J. 2022, 449, 137837. [Google Scholar] [CrossRef]
- Bhowmik, B.; Dutta, K.; Bhattacharyya, P. An Efficient Room Temperature Ethanol Sensor Device Based on p-n Homojunction of TiO2 Nanostructures. IEEE Trans. Electron Devices 2019, 66, 1063–1068. [Google Scholar] [CrossRef]
- Ahmad, K.; Kim, H. Design and fabrication of WO3/SPE for dopamine sensing application. Mater. Chem. Phys. 2022, 287, 126298. [Google Scholar] [CrossRef]
- Atchudan, R.; Muthuchamy, N.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R. An ultrasensitive photoelectrochemical biosensor for glucose based on bioderived nitrogen-doped carbon sheets wrapped titanium dioxide nanoparticles. Biosens. Bioelectron. 2019, 126, 160–169. [Google Scholar] [CrossRef]
- Ahmad, K.; Shinde, M.A.; Song, G.; Kim, H. Design and fabrication of MoSe2/WO3 thin films for the construction of electrochromic devices on indium tin oxide based glass and flexible substrates. Ceram. Int. 2021, 47, 34297–34306. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Faisal, M.; Ahmed, J.; Harraz, F.A.; Jalalah, M.; Alsareii, S.A. Sensitive Detection of Aqueous Methanol by Electrochemical Route Using Mesoporous α-Fe2O3 Doped CdSe Nanostructures Modified. J. Electrochem. Soc. 2021, 168, 057525. [Google Scholar] [CrossRef]
- Tareen, A.K.; Khan, K.; Ahmad, W.; Khan, M.F.; Khan, Q.U.; Liu, X. A novel MnO–CrN nanocomposite based nonenzymatic hydrogen peroxide sensor. RSC Adv. 2021, 11, 19316–19322. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, G.A.; Sheikh, M.U.D. Development of Highly Efficient NiO based Composite Materials for Ultra-Sensitive Glucose Sensors. In Proceedings of the 2019 Thirteenth International Conference on Sensing Trchnology (ICST), Nice, France, 27–31 October 2019. [Google Scholar]
- Paramparambath, S.; Shafath, S.; Maurya, M.R.; Cabibihan, J.-J.; Malik, R.A.; Sadasivuni, K.K. Nonenzymatic Electrochemical Sensor Based on CuO-MgO Composite for Dopamine Detection. IEEE Sens. J. 2021, 21, 25597–25606. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, L.; Hao, X.; Li, W.; Wang, B.; Wang, T.; Liang, X.; Liu, F.; Wang, C.; Lu, G. Mixed potential type NH3 sensor based on YSZ solid electrolyte and metal oxides (NiO, SnO2, WO3) modified FeVO4 sensing electrodes. Sens. Actuat. B Chem. 2021, 343, 130043. [Google Scholar] [CrossRef]
- Peng, S.; Lai, T.; Kong, Y.; Ran, Y.; Su, L.; Ma, D.; Xiao, X.; Wang, Y. A novel non-enzymatic glucose electrochemical sensor with high sensitivity and selectivity based on CdIn2O4 nanoparticles on 3D Ni foam substrate. Nanotechnology 2021, 32, 405502. [Google Scholar] [CrossRef]
- Shu, H.; Peng, S.; Lai, T.; Cui, X.; Ren, J.; Chen, T.; Xiao, X.; Wang, Y. Nickel foam electrode decorated with Fe-CdIn2O4 nanoparticles as an effective electrochemical sensor for non-enzymatic glucose detection. J. Electroanal. Chem. 2022, 919, 116524. [Google Scholar] [CrossRef]
- Petruleviciene, M.; Juodkazyte, J.; Savickaja, I.; Karpicz, R.; Morkvenaite-Vilkonciene, I.; Ramanavicius, A. BiVO4-based coatings for non-enzymatic photoelectrochemical glucose determination. J. Electroanalyt. Chem. 2022, 918, 16446. [Google Scholar] [CrossRef]
- Asiabar, B.M.; Karimi, M.A.; Tavallali, H.; Nasrabadi, M.R. Application of MnFe2O4 and AuNPs modified CPE as a sensitive flunitrazepam electrochemical sensor. Microchem. J. 2021, 161, 105745. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Fayazi, M.; Nodehi, M.; Esmaeelnia, A. Electrochemical detection of bisphenol a on a MWCNTs/CuFe2O4 nanocomposite modified glassy carbon electrode. Mater. Chem. Phys. 2021, 261, 124247. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Wang, T.; Li, W.; Hao, X.; Lu, Q.; Yu, H.; Liang, X.; Liu, F.; Liu, F.; et al. Ce0.8Gd0.2O1.95-based mixed potential type triethylamine sensor utilizing La2NiFeO6 sensing electrode. Sens. Actuat. B Chem. 2021, 345, 130438. [Google Scholar] [CrossRef]
- Hao, X.; Ma, C.; Yang, X.; Liu, T.; Wang, B.; Liu, F.; Liang, X.; Yang, C.; Zhu, H.; Lu, G. YSZ-based mixed potential H2S sensor using La2NiO4 sensing electrode. Sens. Actuat. B Chem. 2018, 255, 3033–3039. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Li, J.; Liu, F.; You, R.; Lv, S.; Yang, Z.; He, J.; Hao, Y.; Yan, X.; et al. Stabilized zirconia-based solid state electrochemical gas sensor coupled with CdTiO3 for acetylene detection. Sens. Actuat. B Chem. 2020, 316, 128199. [Google Scholar] [CrossRef]
- Wang, X.T.; Li, B.; Kong, D.R.; Zhang, Z.Y.; Zhang, X.F.; Deng, Z.P.; Huo, L.H.; Gao, S. T- and T-type layered perovskite Ln2CuO4 nanocrystals for enhanced sensing detection of hydrogen peroxide. J. Alloy. Compd. 2022, 911, 165037. [Google Scholar] [CrossRef]

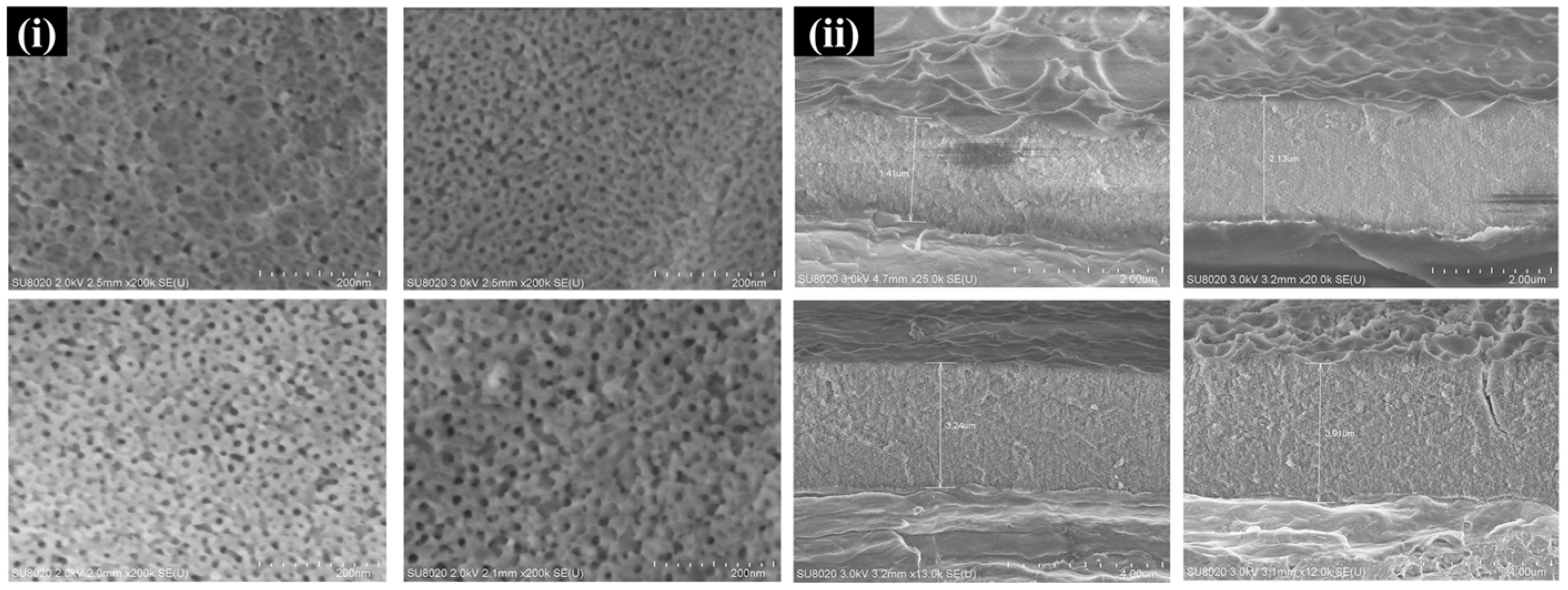
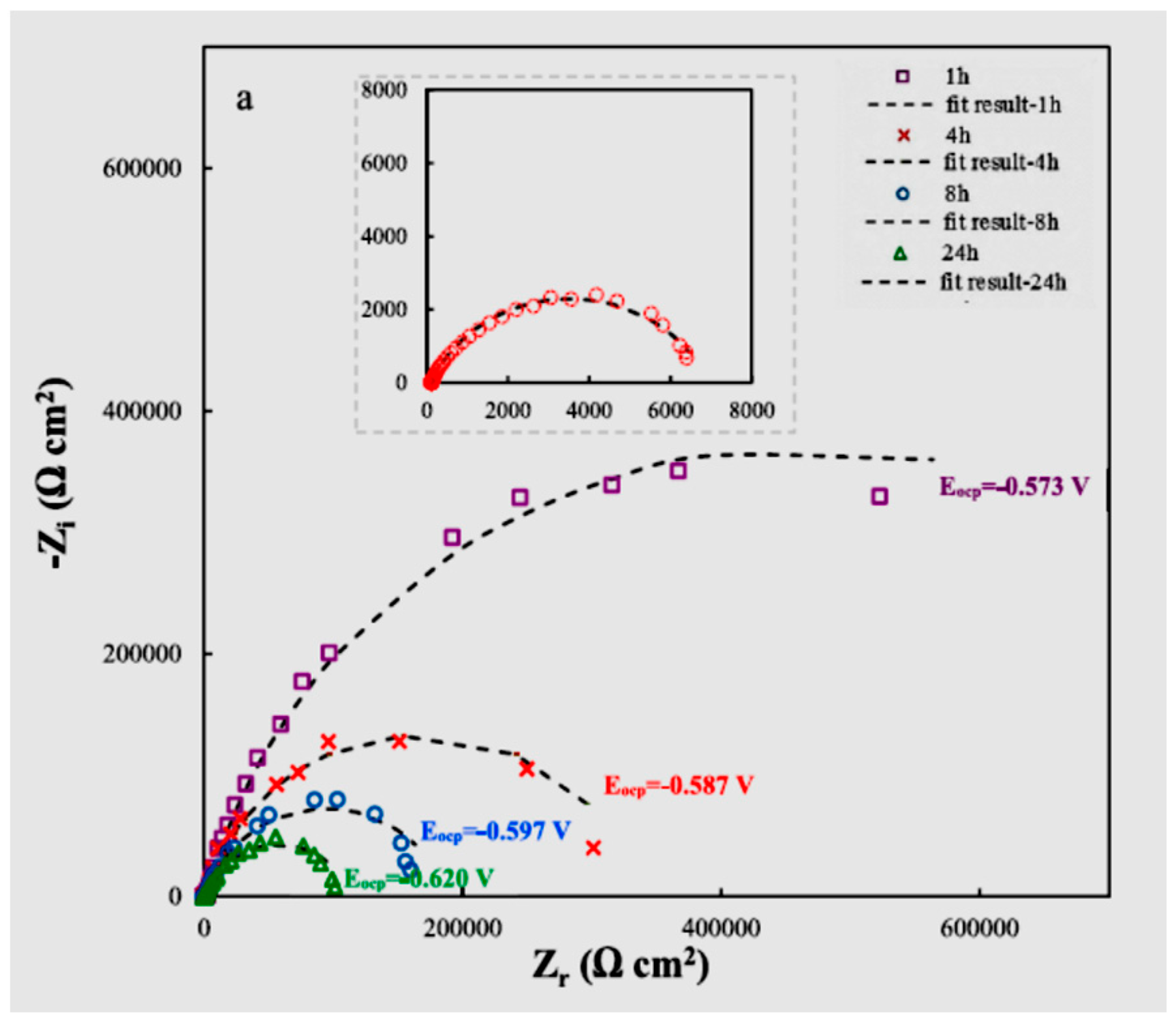
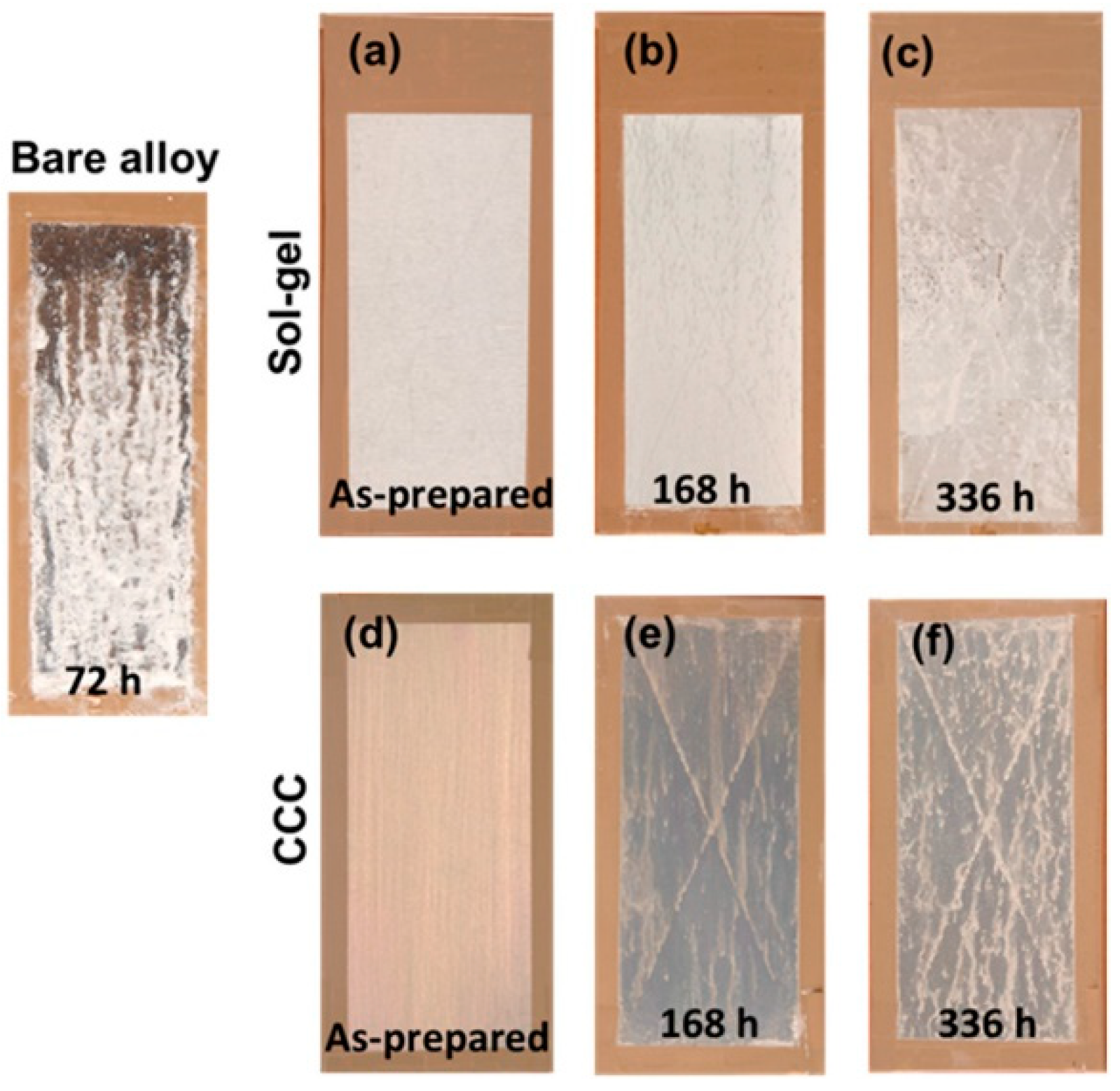


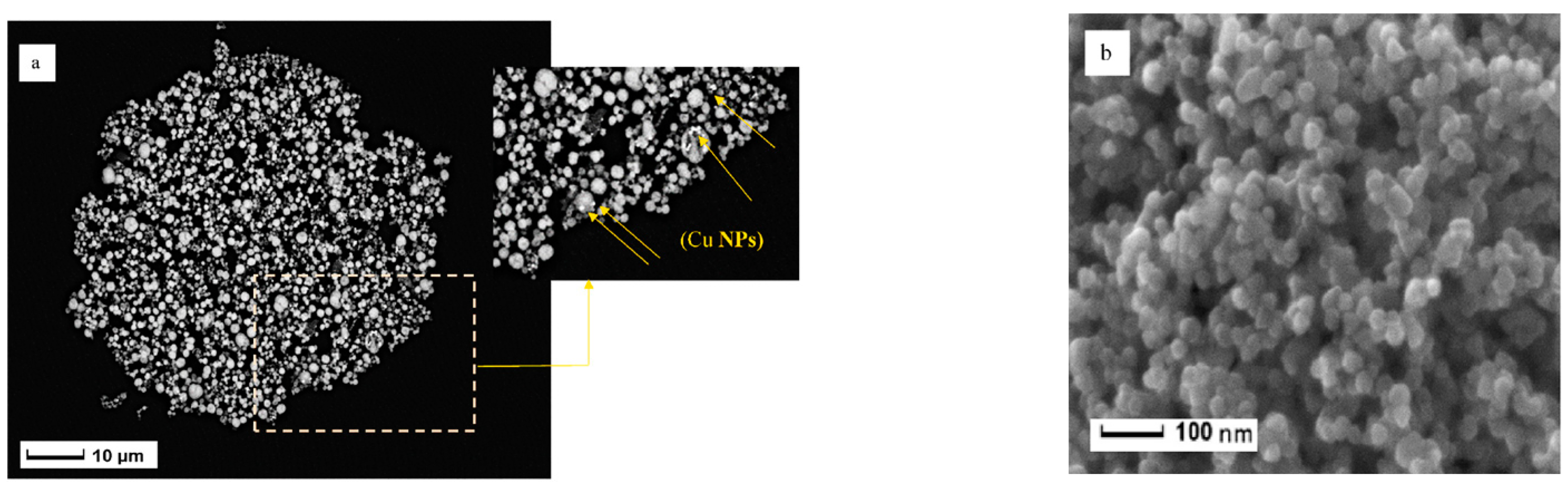
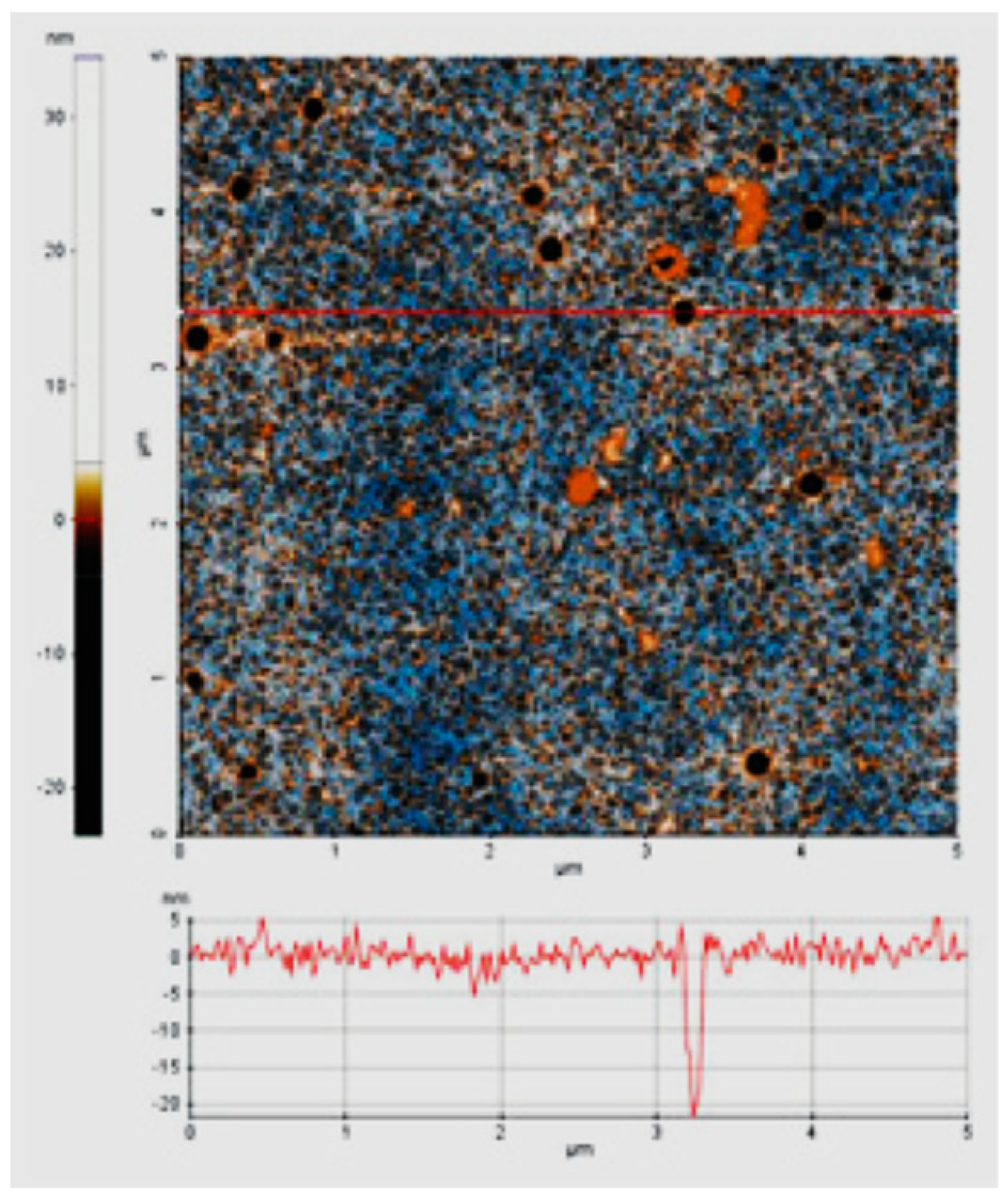

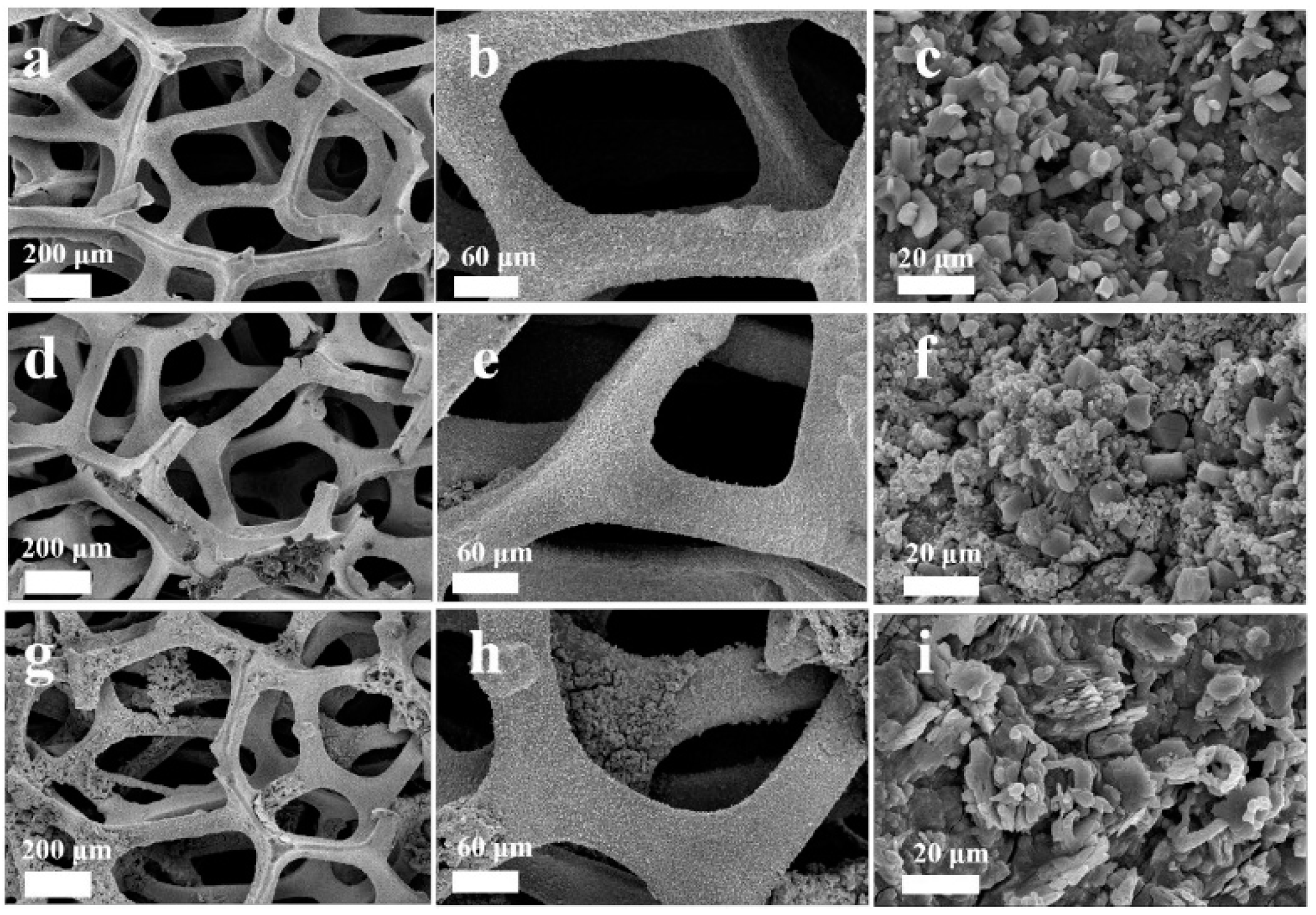
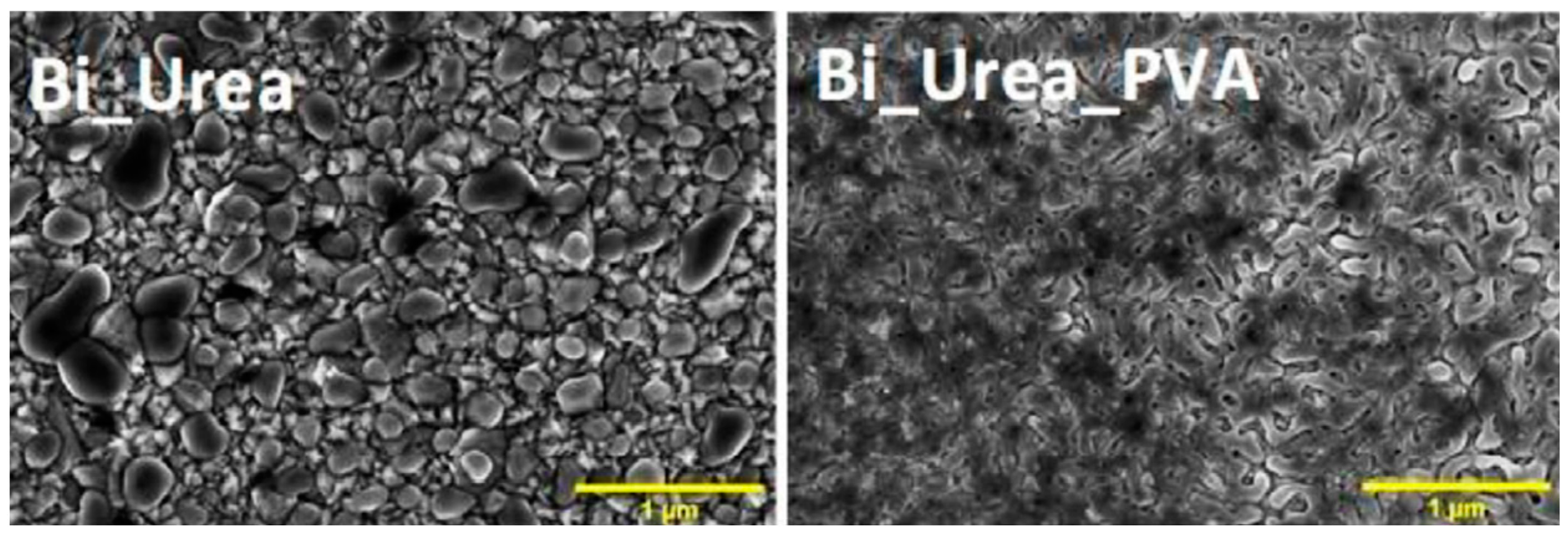
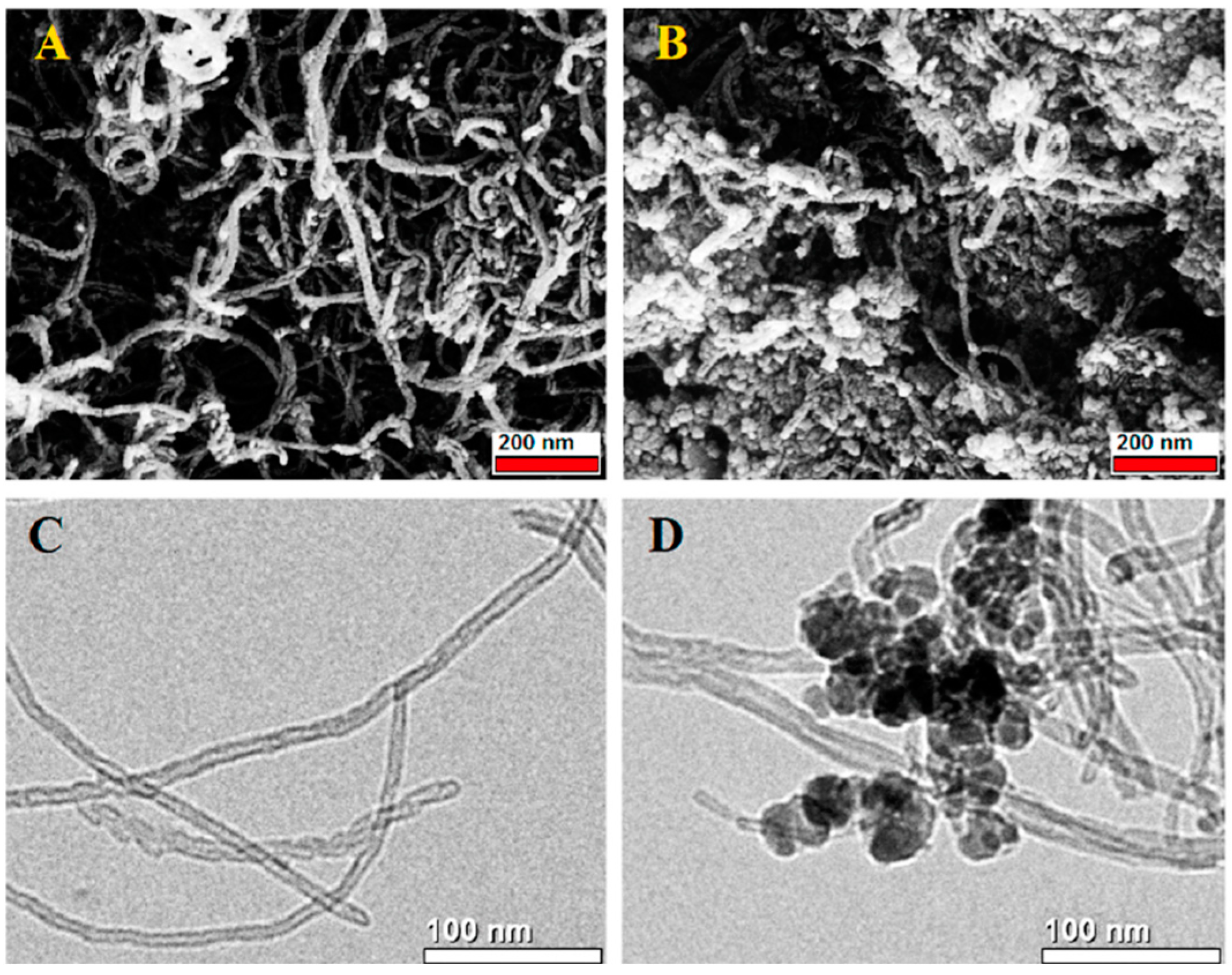
| Material Composition | Silane and Sol Sources | Substrate | Corrosion Medium | Ref. |
|---|---|---|---|---|
| Anodizing different voltage | TEOS, GPTMS | 2524 Al alloy | - | [75] |
| Anodized Al alloy LBL with PAA/PEI | Alumina sol | 2A12 Al alloy | 3.5% NaCl solution | [76] |
| TEOS TEOS-APTES | TEOS, APTES | AA6061 | 3.5% NaCl solution | [77] |
| Zirconia benzotriazole | TMS | Al 2024 | 3.5% NaCl solution | [78] |
| GPTMS/TPOZ GPTMS/ASB GPTMS/TPOZ/ASB | GPTMS, TPOZ, ASB | AA6061 | 5% NaCl solution | [79] |
| nano-TiO2 BTSE + GPTMS BTSE + GPTMS/nano-TiO2 | BTSE, GPTMS | EN AC-43000 Al-Si alloy | 3.5% NaCl solution | [80] |
| TiO2 nanosheets | KH750 | AA2024-T3 | 3.5% NaCl solution | [81] |
| Chitosan/TiO2 | TEOS, GPTMS | Al electrode | 3.5% NaCl solution | [82] |
| rGO@APTES | APTMS, TEOS, GPTMS | 2524 Al alloy | 3.5% NaCl solution | [83] |
| MIL-53 MOF | TEOS, GPTMS | 2524 Al alloy | Harrison’s solution | [84] |
| Cerium oxide nanofiber | GPTMS, ASB | AA2024 | 3.5% NaCl solution | [85] |
| ZnAl-LDH Ce doped ZnAl-LDH | GPTMS, TPOZ | AA2024 | 0.05 M NaCl solution | [86] |
| ZnAl-LDH, Intercalated inhibitor (VO4, MBZ, MoO4, PA, 8HQ) | MPTS, PhTMS, APTMS, GPTMS | AA 2423-T3 | 3.5% NaCl solution | [87] |
| Inhibitors: L-cys, DGM, QUI, 2AP, GS, TiO2-NPs | TEOS, MEMO | AZ61 Mg-Al alloy | - | [88] |
| Material Composition | Silane and Sol Sources | Substrate | Corrosion Medium | Ref. |
|---|---|---|---|---|
| - | TEOS, TMOMS | Mild steel | 3.5% NaCl solution | [89] |
| - | GPTMS, TEOS, TETA | Low carbon steels | 3.5% NaCl solution | [90] |
| SiO2 | TEOS, GPTMS | S-46 (A1008 steel) | 3.5% NaCl solution | [91] |
| TiO2–SiO2 | TBOT, TEOS | C45E steel | 3.5% NaCl solution | [92] |
| TiO2–SiO2 | TTIP, TEOS | Mild steel | [93] | |
| TiO2 hybrid sol Inhibitors: Ce, BTA, and 8H | TBT, GPTMS | 304 stainless steel | 3.5% NaCl solution | [94] |
| ZnAl-MoO4-LDH/SiO2 | TEOS, TMOS | Mild steel plate | - | [95] |
| Zinc phosphate | TMOMS, TEOS | Steel | 3.5% NaCl solution | [96] |
| Zinc acetylacetonate | γ-GPS, TEOS, MTES | Mild steel | 0.1 M NaCl solution | [97] |
| NaX zeolite nanoparticles Inhibitor: zinc nitrate, 2-mercaptobenzimidazole | g-GPS, TEOS, MTES | Mild steel | 3.5% NaCl solution | [98] |
| Meso porous silica Inhibitor: ECBT | TEOS, GPTMS | Mild steel Treated Ti-Zr | 3.5% NaCl solution | [99] |
| La(4-OHCin)3 | TEOS, GPTMS | S355 J2+N carbon steel | 0.005 M NaCl solution | [100] |
| GO, epoxy/polyamide | MTES, TEOS, TEPI, APTMS | Steel | 3.5% NaCl solution | [101] |
| Material Composition | Silane and Sol Sources | Substrate | Corrosion Medium | Ref. |
|---|---|---|---|---|
| - | TEOS, GPTMS | AZ31B | Harrison’s solution | [102] |
| Ce-V | TEOS, GPTMS | AM60B | Harrison’s solution | [103] |
| Ti-Zr-hybrid (TEOS+GPTMS) Ti-Zr-PTMS | TEOS, GPTMS, PTMS | AM60B | 0.05 M NaCl solution | [104] |
| Halloysite nanotube Ce-Zr | GPTMS | AZ91D | 3.5% NaCl solution | [105] |
| C-60 | TEOS, GPTMS | AM60B | 3.5% NaCl solution | [106] |
| OH-MWCNTs | PTMS | AM60B alloy | 3.5% NaCl solution | [107] |
| MMT Inhibitors: L-glutamine, L-methionine, L-alanine | TEOS, MTES | AZ91 | 3.5% NaCl solution | [108] |
| Inhibitors: QDA, BET, DOP, and DZU | GPTMS, TMSPED | AZ31B | 3.5% NaCl solution | [109] |
| Sol-Gel Material | Detectable Substance | Maximum Gas Sensitivity | Linear Range | Ref. |
|---|---|---|---|---|
| Cu/ZnO nanocomposite | glucose | 36.641 μAmM−1cm−2 | 0.01–1, 1–7 mM | [111] |
| Cu-doped ZnO nanoparticles | myoglobin | 2.13–10.14 µAnM−1cm−2 | 3–15 nM | [112] |
| ZnO nanoparticles | clenbuterol | - | 0.3–1000 ng/mL | [113] |
| SiO2/Al2O3/C | nitrite | 410 μAμM−1 | 0.2–280 μM | [114] |
| SnO2 coatings | nitrite | 22.56 μAμM−1 | 10–400 μM | [115] |
| Pt-SnO2 nanoparticles | hydrogen | - | 0.08–500 ppm | [116] |
| Au–SnO2 nanoparticles | vitamin B12 | 110.843 μApM−1 | 0–1500 pM | [117] |
| Cu-doped SnO2 nanoparticles | ethyl acetate | 4.8 µA/ppb | 1–20 ppb | [118] |
| CuNPs on the C/SiO2 working electrode | glucose | - | 53–670 mg/L O2 | [119] |
| p-TiO2 nanoparticles | ethanol | ~50% | 1–100 ppm | [120] |
| WO3 rods | dopamin | 3.66 μAμM−1cm−2 | 1–250 μM | [121] |
| Nitrogen-doped carbon sheets wrapped in SnO2 nanoparticles | glucose | 215 nAμM−1cm−2 | 0.05–10 μM | [122] |
| α-Fe2O3 doped CdSe | aqueous methanol | 0.2744 μAmM−1cm−2 | 0.2–48 mM | [123] |
| MnO–CrN nanocomposite | hydrogen peroxide | 2156.25 μAmM−1cm−2 | 0.33–15 000 μM | [125] |
| NiO nanoporous materials | glucose | 445 μAm−1cm−2 | - | [126] |
| CuO-MgO composite | dopamine | 69 μAmM−1cm−2 | 10–100 μM | [127] |
| WO3@SnO2-20 | ammonia | - | 5–50 ppm | [128] |
| CdIn2O4 nanoparticles | glucose | 3292.5 μAmM−1cm−2 | 1.0 μM–1.0 mM | [129] |
| Fe-CdIn2O4 nanoparticles | glucose | 8992 μAmM−1cm−2 | 0.01–1 mM | [130] |
| BiVO4 | glucose | - | 1–35 mM | [131] |
| MnFe2O4 | flunitrazepam | - | 0.1–100 μM | [132] |
| Multiwalled carbon nanotubes CuFe2O4 | bisphenol | 0.355 μAμM−1 | 0.01–120 μM | [133] |
| La2NiFeO6 | triethylamine | - | 0.5–200 ppm | [134] |
| K2NiF4-type oxides La2NiO4 | hydrogen sulfide | −70 mV | 0.05–2 ppm | [135] |
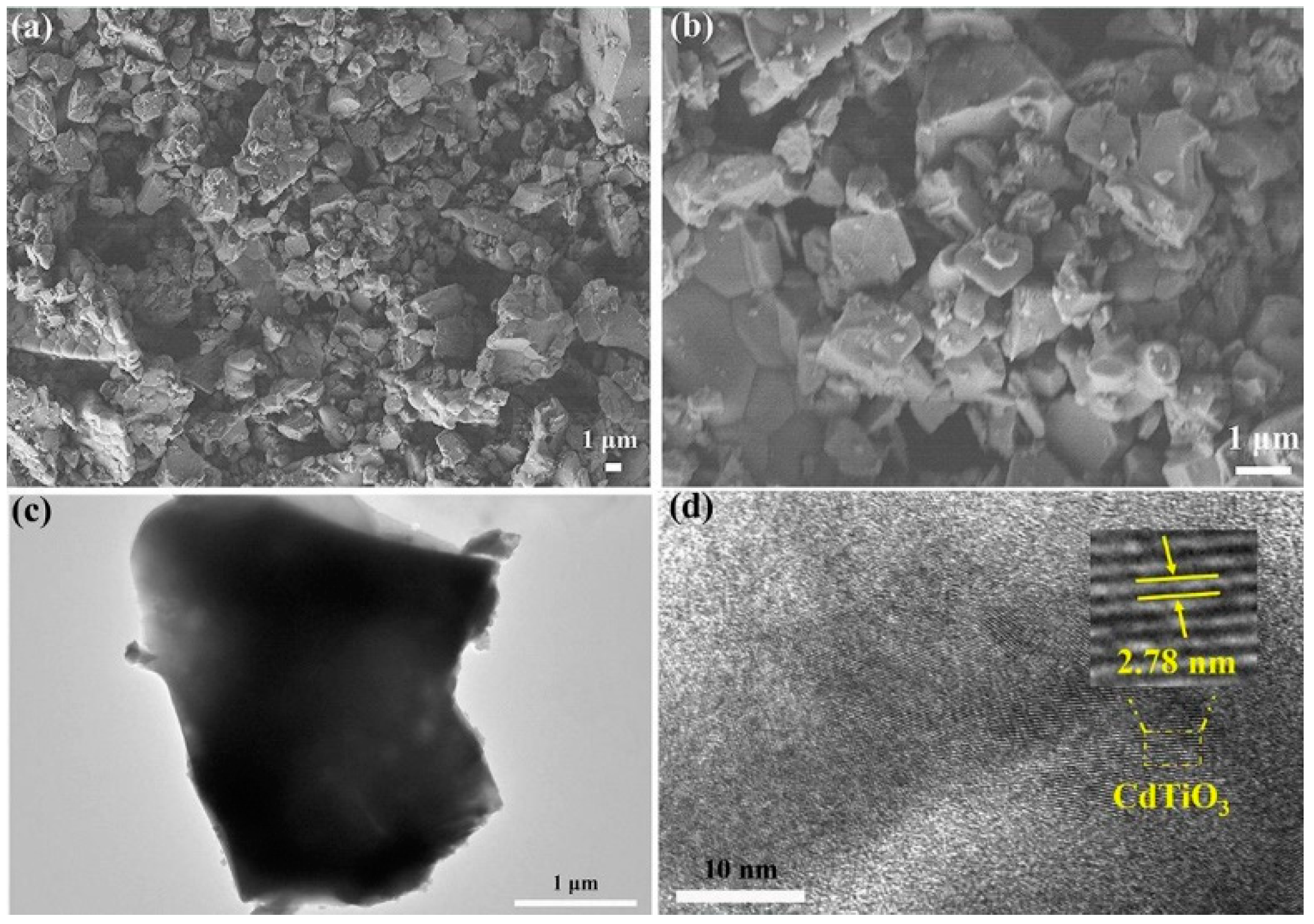
| CdTiO3 | acetylene | −91 mV | 5–100 ppm | [136] |
| Ln2CuO4 nanocrystals | hydrogen peroxide | - | 0.50–15.87 μM | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myasoedova, T.N.; Kalusulingam, R.; Mikhailova, T.S. Sol-Gel Materials for Electrochemical Applications: Recent Advances. Coatings 2022, 12, 1625. https://doi.org/10.3390/coatings12111625
Myasoedova TN, Kalusulingam R, Mikhailova TS. Sol-Gel Materials for Electrochemical Applications: Recent Advances. Coatings. 2022; 12(11):1625. https://doi.org/10.3390/coatings12111625
Chicago/Turabian StyleMyasoedova, Tatiana N., Rajathsing Kalusulingam, and Tatiana S. Mikhailova. 2022. "Sol-Gel Materials for Electrochemical Applications: Recent Advances" Coatings 12, no. 11: 1625. https://doi.org/10.3390/coatings12111625






