Effect of Al2O3 Content on High-Temperature Oxidation Resistance of Ti3SiC2/Al2O3
Abstract
1. Introduction
2. Experimental Procedure
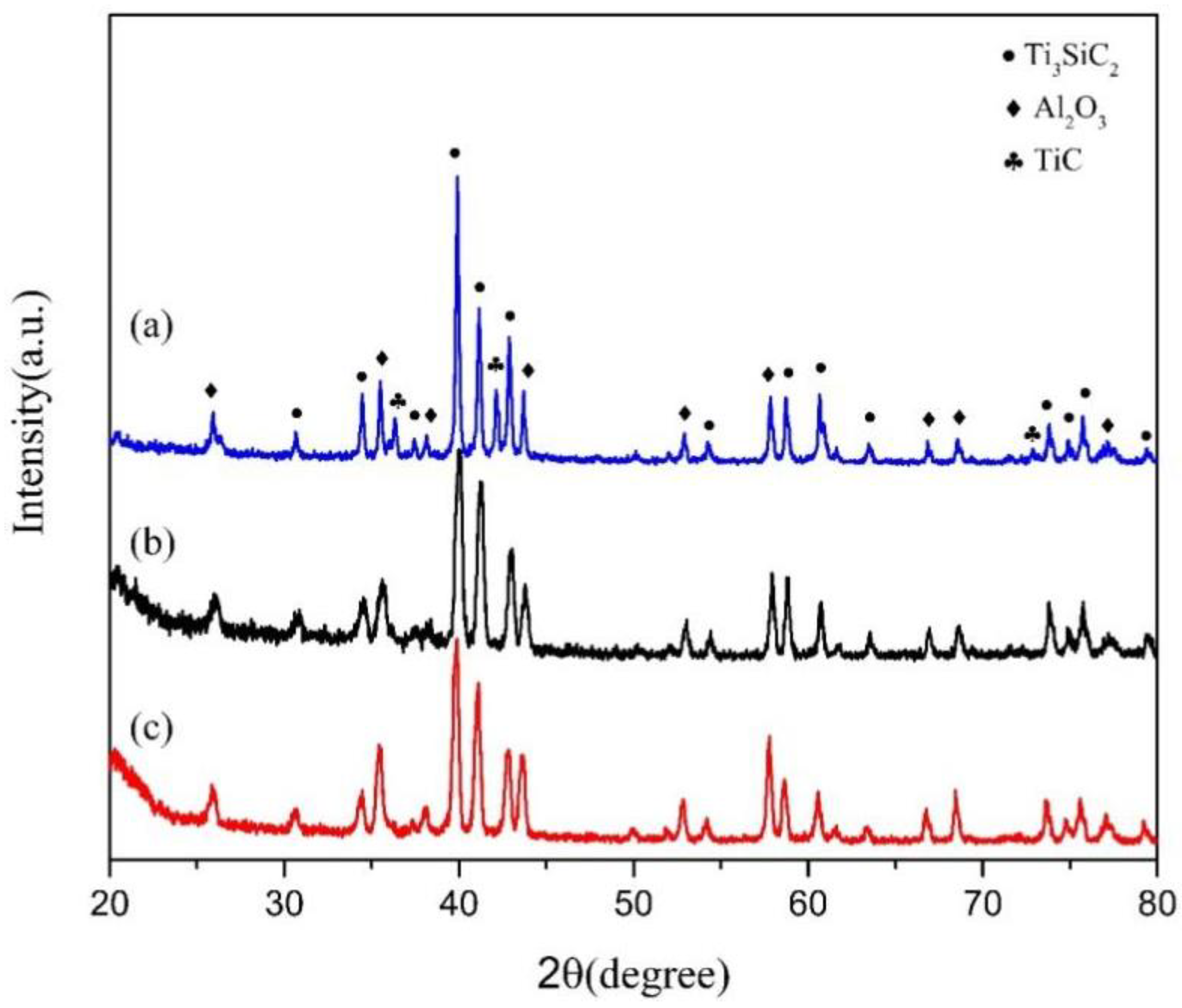
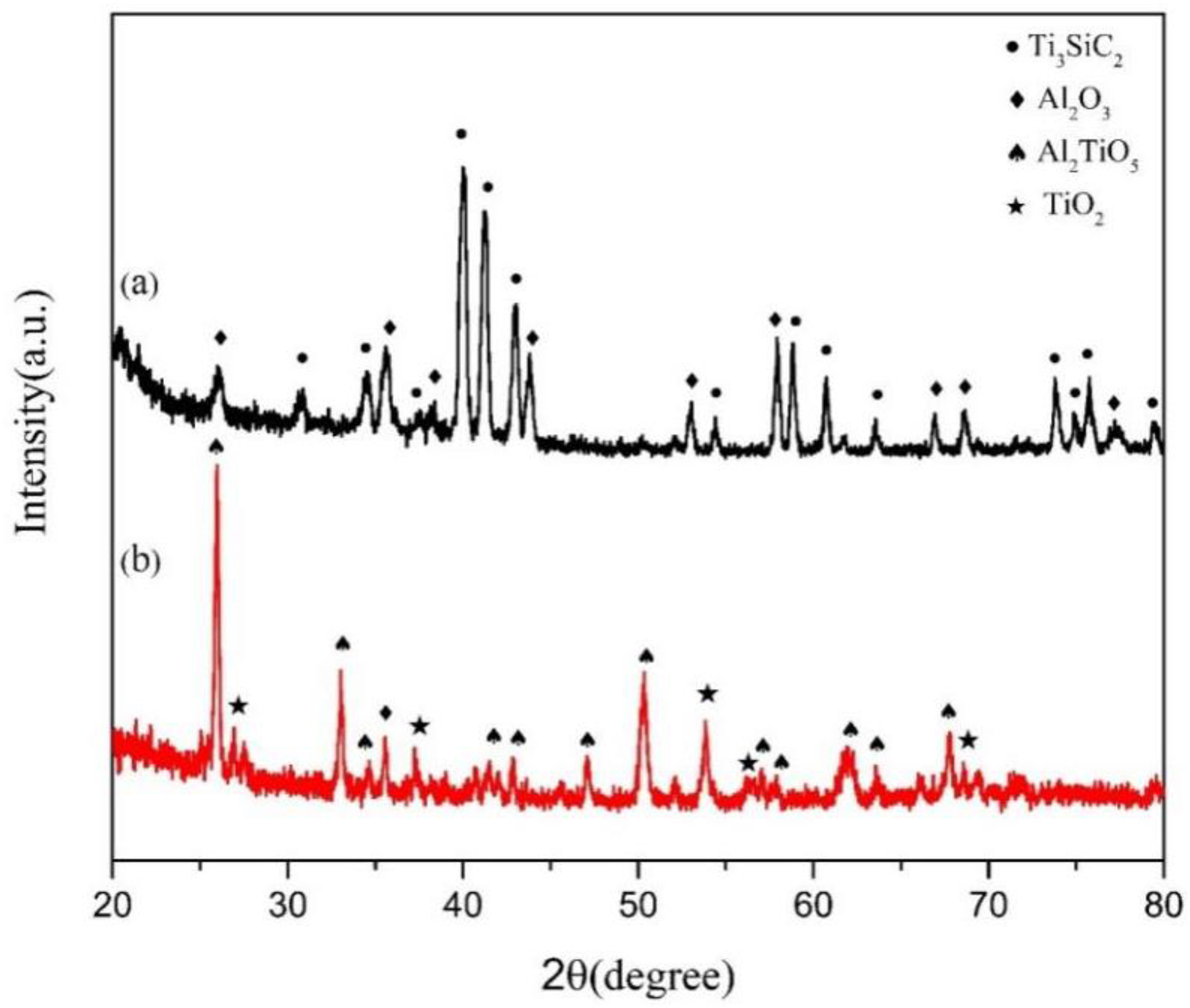
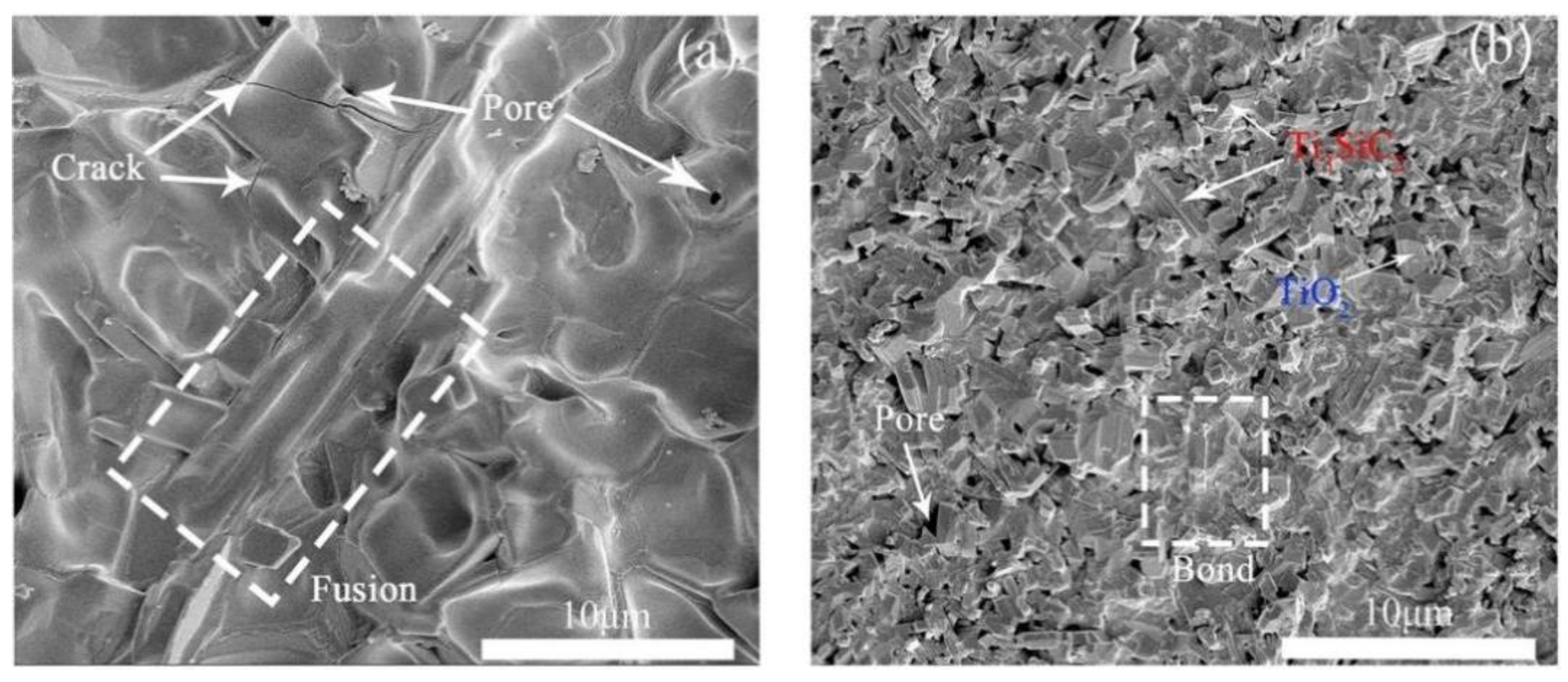
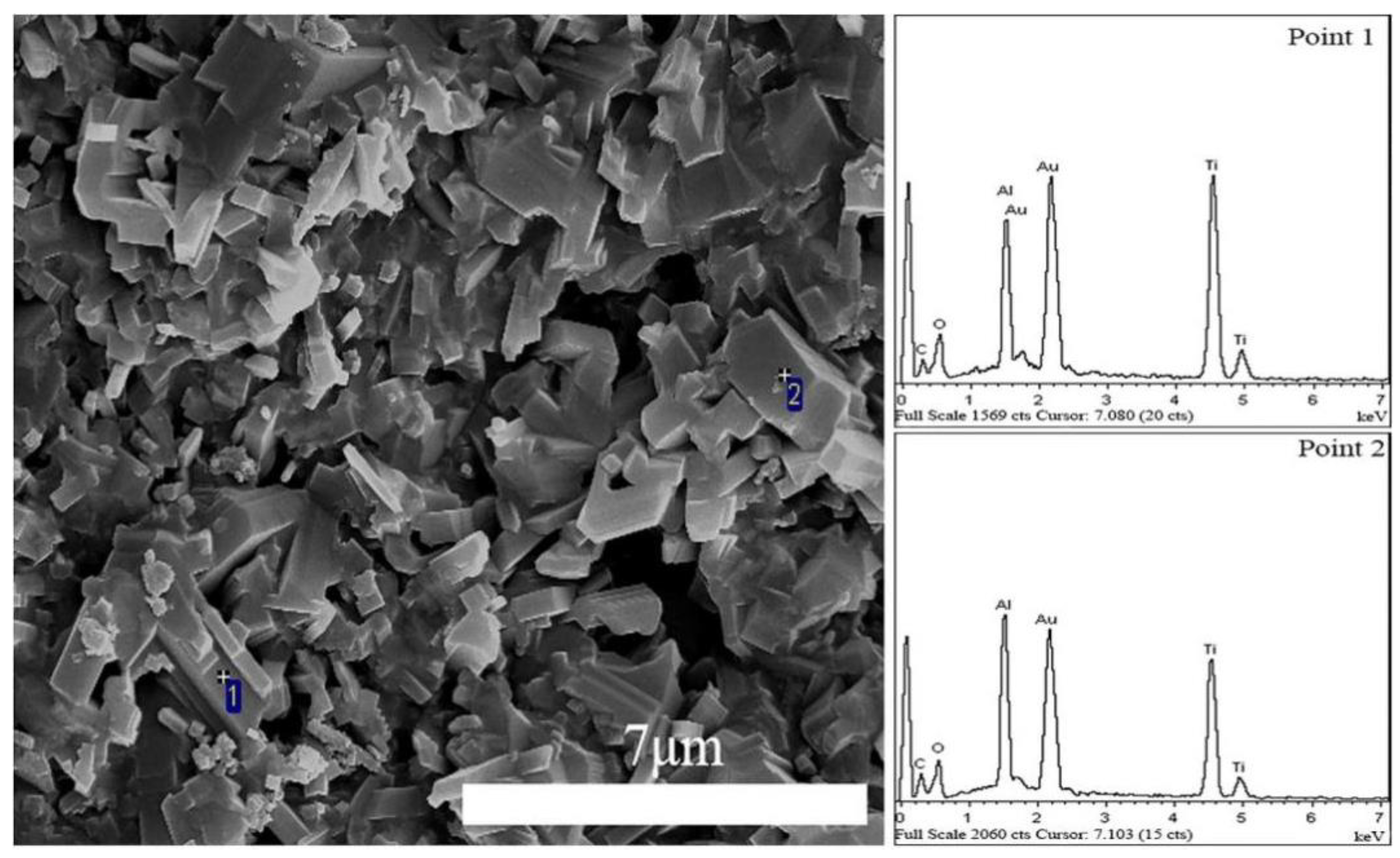
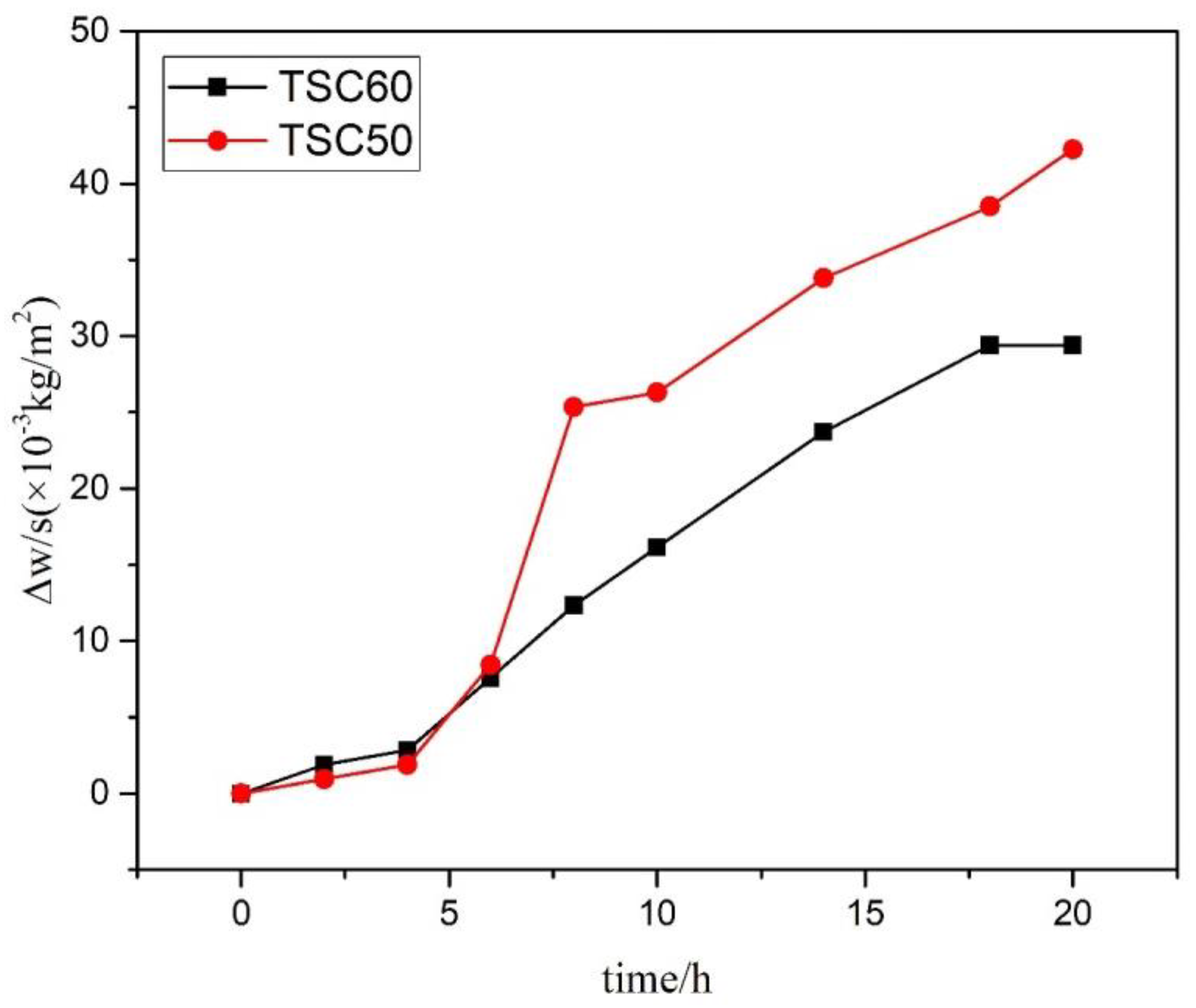
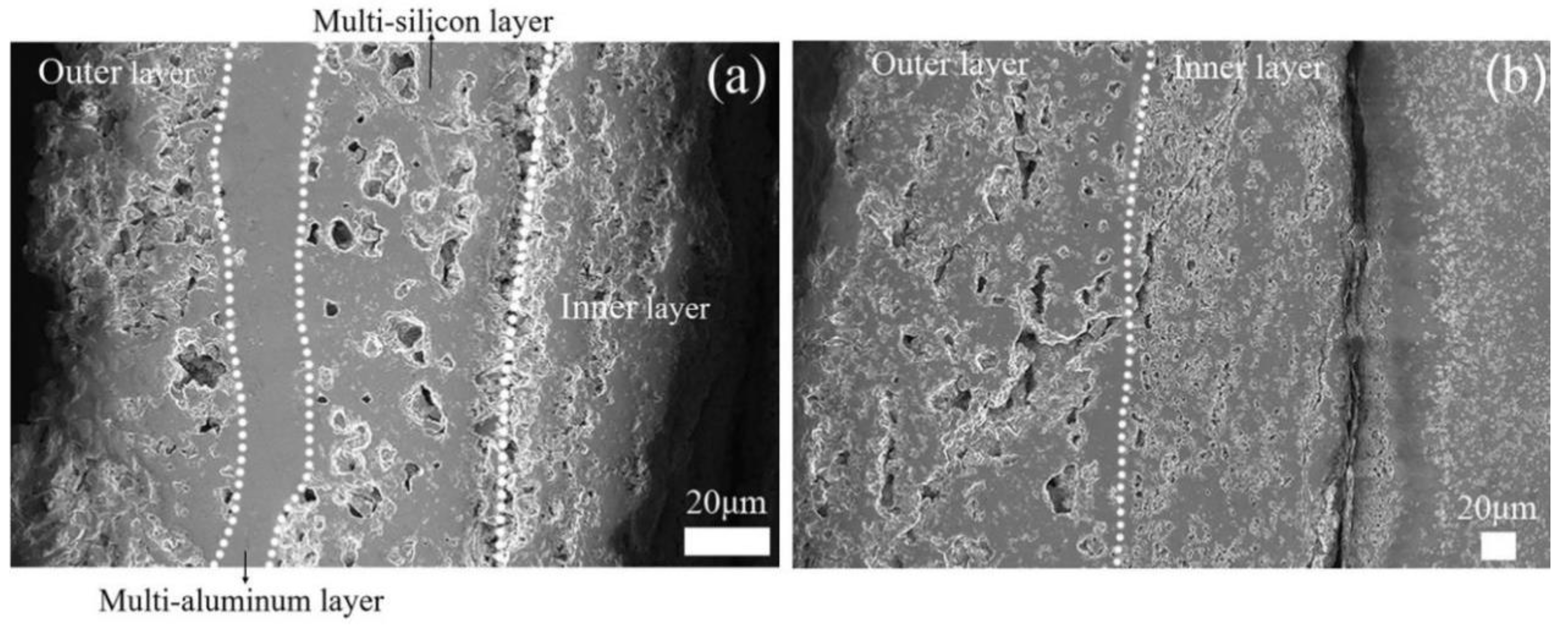
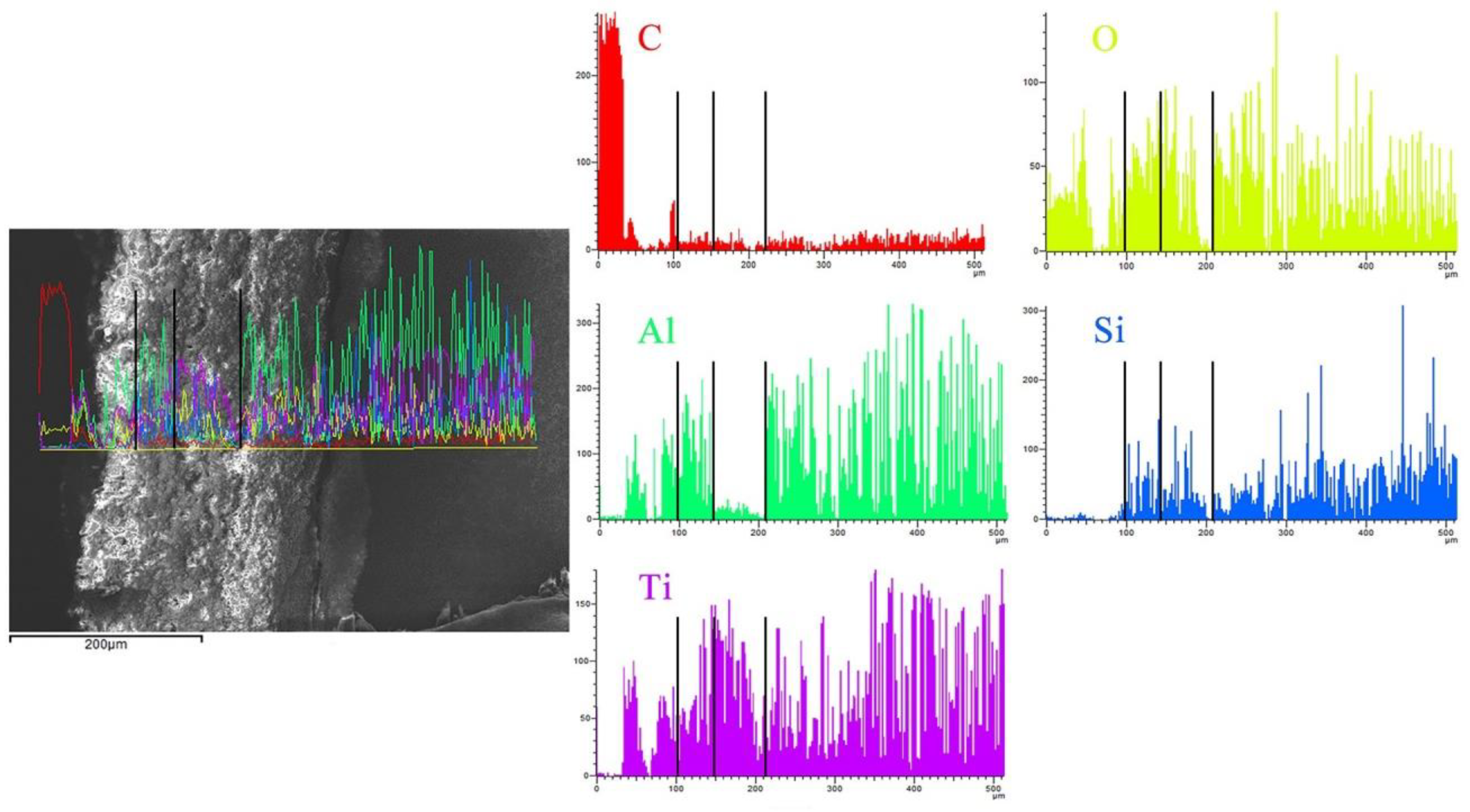
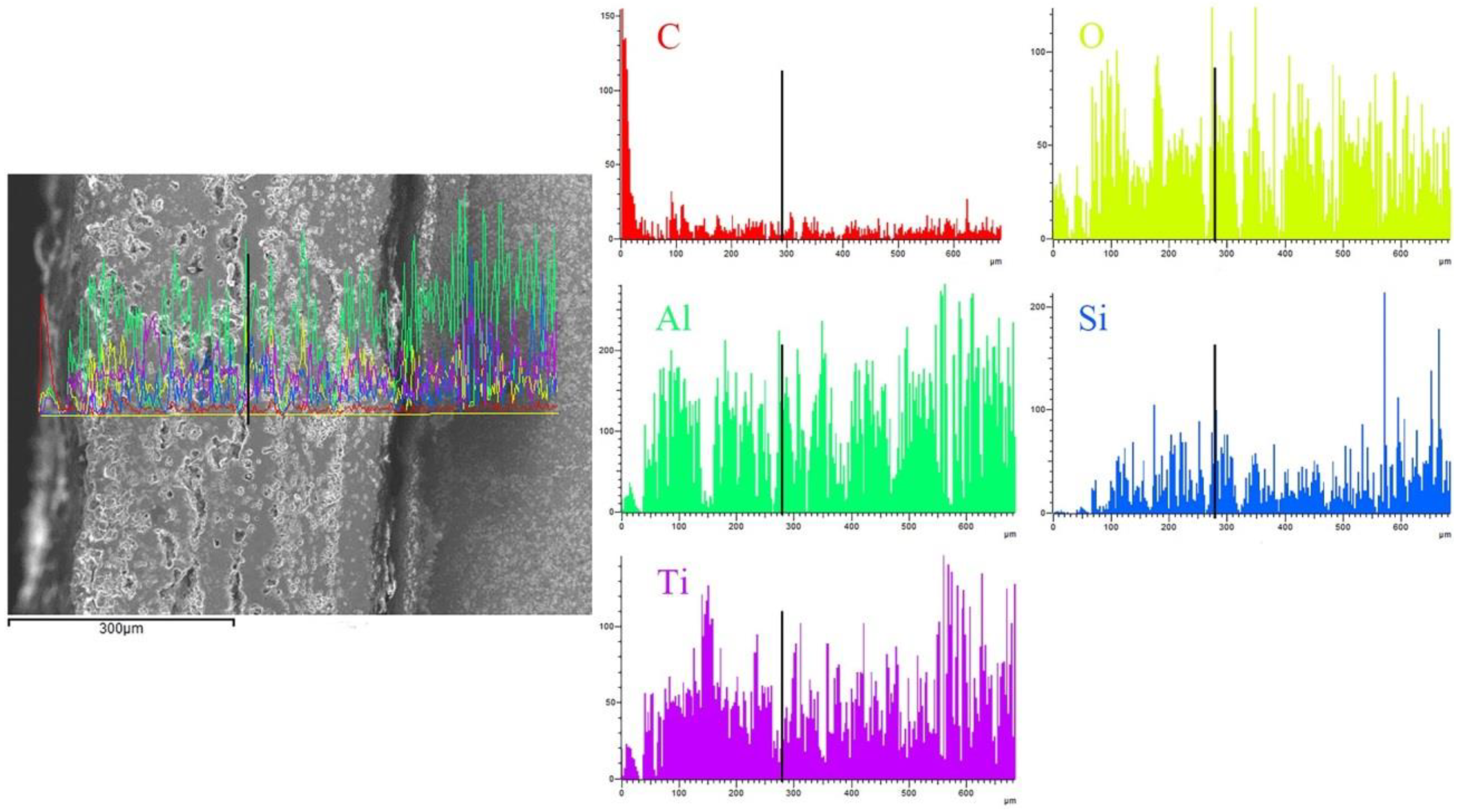
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.M. Progress in research and development on MAX phases: A family of layered ternary compounds. Int. Mater. Rev. 2011, 56, 143–166. [Google Scholar] [CrossRef]
- Atazadeh, N.; Heydari, M.S.; Baharvandi, H.R.; Ehsani, N. Reviewing the effects of different additives on the synthesis of the Ti3SiC2 MAX phase by mechanical alloying technique. Int. J. Refract. Met. Hard Mater. 2016, 61, 67–78. [Google Scholar] [CrossRef]
- Qin, J.; He, D. Phase stability of Ti3SiC2 at high pressure and high temperature. Ceram. Int. 2013, 39, 9361–9367. [Google Scholar] [CrossRef]
- Dezellus, O.; Gardiola, B.; Andrieux, J.; Lay, S. Experimental evidence of copper insertion in a crystallographic structure of Ti3SiC2 MAX phase. Scr. Mater. 2015, 104, 17–20. [Google Scholar] [CrossRef]
- Islak, B.Y.; Ayas, E. Evaluation of properties of spark plasma sintered Ti3SiC2 and Ti3SiC2/SiC composites. Ceram. Int. 2019, 45, 12297–12306. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Jiang, Y.; He, Y. Characterization and application of porous Ti3SiC2 ceramic prepared through reactive synthesis. Mater. Des. 2015, 79, 94–98. [Google Scholar] [CrossRef]
- El Saeed, M.A.; Deorsola, F.A.; Rashad, R.M. Optimization of the Ti3SiC2 MAX phase synthesis. Int. J. Refract. Met. Hard Mater. 2012, 35, 127–131. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Cheng, L.F. Effect of positioning impregnation on the oxidation behaviour of Ti3SiC2/SiC functionally graded materials at 1400 °C. J. Alloy. Compd. 2018, 742, 180–190. [Google Scholar] [CrossRef]
- Yang, J.S.; Zhang, X.Y. Fabrication of Ti3SiC2 powders using TiH2 as the source of Ti. Ceram. Int. 2012, 38, 3509–3512. [Google Scholar] [CrossRef]
- Zheng, L.-L.; Sun, L.-C.; Li, M.-S.; Zhou, Y.-C. Improving the high-temperature oxidation resistance of Ti3(SiAl)C2 by Nb-doping. J. Am. Ceram. Soc. 2011, 94, 3579–3586. [Google Scholar] [CrossRef]
- Li, X.; Qian, Y.; Zheng, L.; Xu, J.; Li, M. Determination of the critical content of Al for selective oxidation of Ti3AlC2 at 1100 °C. J. Eur. Ceram. Soc. 2016, 36, 3311–3318. [Google Scholar] [CrossRef]
- Li, X.; Zheng, L.; Qian, Y.; Xu, J.; Li, M. Breakaway oxidation of Ti3AlC2 during long-term exposure in air at 1100 °C. Corros. Sci. 2016, 104, 112–122. [Google Scholar] [CrossRef]
- Gong, Y.; Tian, W.; Zhang, P.; Chen, J.; Zhang, Y.; Sun, Z. Slip casting and pressureless sintering of Ti3AlC2. J. Adv. Ceram. 2019, 8, 367–376. [Google Scholar] [CrossRef]
- Qi, F.F.; Wang, Z. Improved mechanical properties of Al2O3 ceramic by in-suit generated Ti3SiC2 and TiC via hot pressing sintering. Ceram. Int. 2017, 43, 10691–10697. [Google Scholar] [CrossRef]
- Shi, S.L.; Pan, W. Toughening of Ti3SiC2 with 3Y-TZP addition by spark plasma sintering. Mater. Sci. Eng. A 2007, 447, 303–306. [Google Scholar] [CrossRef]
- Islak, B.Y.; Candar, D. Synthesis and properties of TiB2/Ti3SiC2 composites. Ceram. Int. 2021, 47, 1439–1446. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Jiang, W.; Chen, L. High temperature oxidation behavior and mechanism of Ti3SiC2-SiC nanocomposites in air. Compos. Sci. Technol. 2008, 68, 1531–1538. [Google Scholar] [CrossRef]
- Li, S.; Song, G.M.; Zhou, Y. A dense and fine-grained SiC/Ti3Si(Al)C2 composite and its high-temperature oxidation behavior. J. Eur. Ceram. Soc. 2012, 32, 3435–3444. [Google Scholar] [CrossRef]
- Xu, X.; Ngai, T.L.; Li, Y. Synthesis and characterization of quarternary Ti3Si(1-x)AlxC2 MAX phase materials. Ceram. Int. 2015, 41, 7626–7631. [Google Scholar] [CrossRef]
- Guedouar, B.; Hadji, Y. Oxidation behavior of Al-doped Ti3SiC2-20wt.%Ti5Si3 composite. Ceram. Int. 2021, 47, 33622–33631. [Google Scholar] [CrossRef]
- Heider, B.; Scharifi, E.; Engler, T.; Oechsner, M.; Steinhoff, K. Influence of heated forming tools on corrosion behavior of high strength aluminum alloys. Mater. Sci. Eng. Technol. 2021, 52, 145–151. [Google Scholar] [CrossRef]
- Popov, A.I.; Lushchik, A.; Shablonin, E.; Vasil’chenko, E.; Kotomin, E.A.; Moskina, A.M.; Kuzovkov, V.N. Comparison of the F-type center thermal annealing in heavy-ion and neutron irradiated Al2O3 single crystals. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 433, 93–97. [Google Scholar] [CrossRef]
- Averback, R.S.; Ehrhart, P.; Popov, A.I. Defects in ion implanted and electron irradiated Mgo and Al2O3. Radiat. Eff. Defects Solids 1995, 136, 169–173. [Google Scholar] [CrossRef]
- Shablonin, E.; Popov, A.I.; Prieditis, G.; Vasil’chenko, E.; Lushchik, A. Thermal annealing and transformation of dimer F centers in neutron-irradiated Al2O3 single crystals. J. Nucl. Mater. 2021, 543, 152600. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, Y.; Li, M. High temperature oxidation behavior of Ti3SiC2-based material in air. Acta Mater. 2001, 49, 4347–4353. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Y.; Wang, R.; Wang, X.; Li, Y. Study on Al2TiO5-SiO2-Al2O3 composites. Bull. Chin. Ceram. Soc. 2008, 27, 649–653. [Google Scholar]
- Zhang, H.B.; Shen, S.Y. Oxidation behavior of porous Ti3SiC2 prepared by reactive synthesis. Trans. Nonferrous Met. Soc. China 2018, 28, 1774–1783. [Google Scholar] [CrossRef]
- Zhang, H.B.; Zhou, Y.C.; Bao, Y.W.; Li, M.S. Improving the oxidation resistance of Ti3SiC2 by forming a Ti3Si0.9Al0.1C2 solid solution. Acta Mater. 2004, 52, 3631–3637. [Google Scholar] [CrossRef]
- Gao, H.; Benitez, R.; Son, W.; Arroyave, R.; Radovic, M. Structural, physical and mechanical properties of Ti3(Al1−xSix)C2 solid solution with x = 0–1. Mater. Sci. Eng. A 2016, 676, 197–208. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Li, Q.; Chen, S.; Ma, D.; Pan, B.; Zhang, Z.; Li, J. Effect of Al2O3 Content on High-Temperature Oxidation Resistance of Ti3SiC2/Al2O3. Coatings 2022, 12, 1641. https://doi.org/10.3390/coatings12111641
Du Y, Li Q, Chen S, Ma D, Pan B, Zhang Z, Li J. Effect of Al2O3 Content on High-Temperature Oxidation Resistance of Ti3SiC2/Al2O3. Coatings. 2022; 12(11):1641. https://doi.org/10.3390/coatings12111641
Chicago/Turabian StyleDu, Yuhang, Qinggang Li, Sique Chen, Deli Ma, Baocai Pan, Zhenyu Zhang, and Jinkai Li. 2022. "Effect of Al2O3 Content on High-Temperature Oxidation Resistance of Ti3SiC2/Al2O3" Coatings 12, no. 11: 1641. https://doi.org/10.3390/coatings12111641
APA StyleDu, Y., Li, Q., Chen, S., Ma, D., Pan, B., Zhang, Z., & Li, J. (2022). Effect of Al2O3 Content on High-Temperature Oxidation Resistance of Ti3SiC2/Al2O3. Coatings, 12(11), 1641. https://doi.org/10.3390/coatings12111641






