Research on the Thickness and Microstructure of Plate-like TiO2 by the Nanosheet-Seeding Growth Technique
Abstract
:1. Introduction
2. Experiment
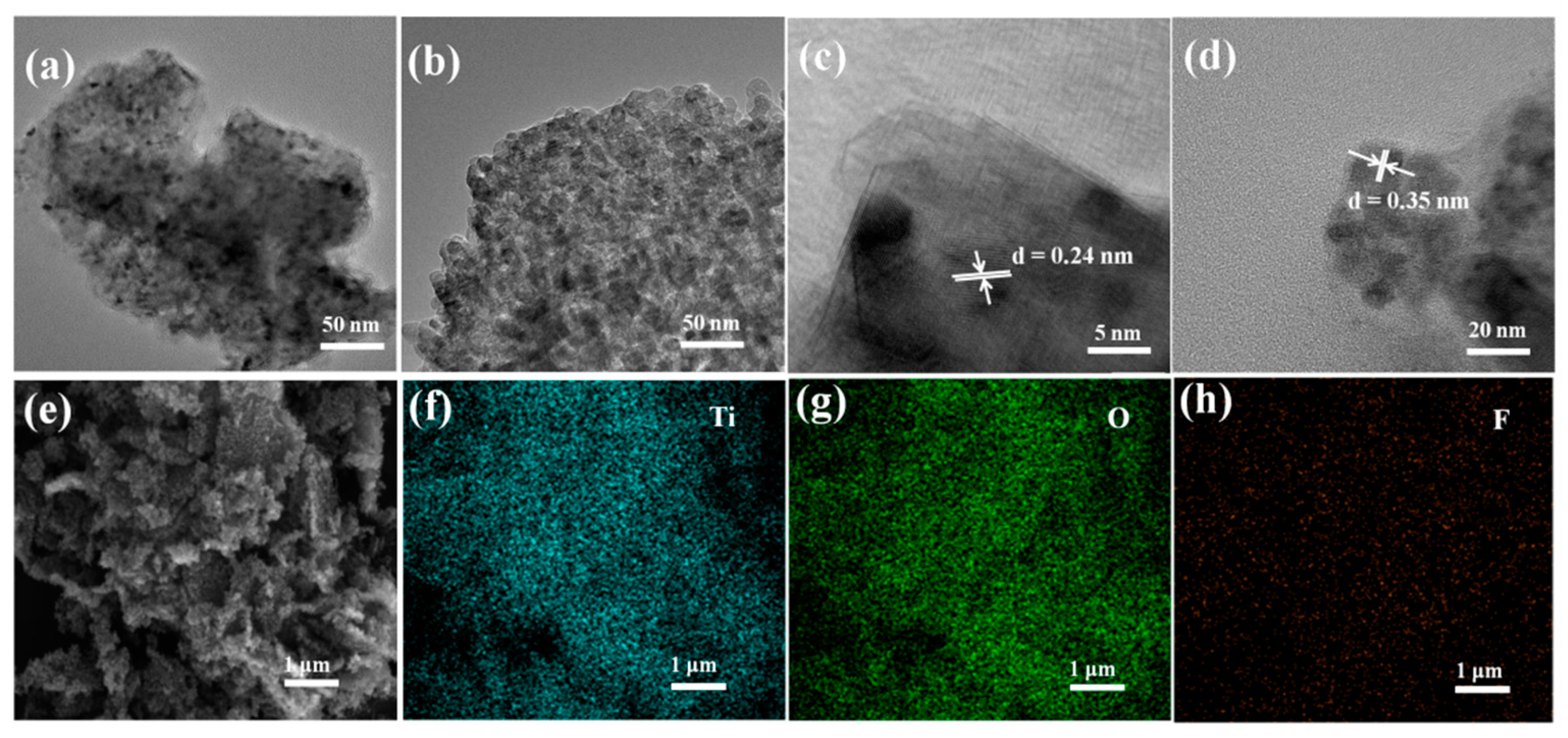
2.1. Preparation of Ti0.87O2 Nanosheets
2.2. Preparation of Anatase TiO2 Nanoplates
2.3. Characterization
3. Results and Discussion
3.1. The Effect of Synthesis Time
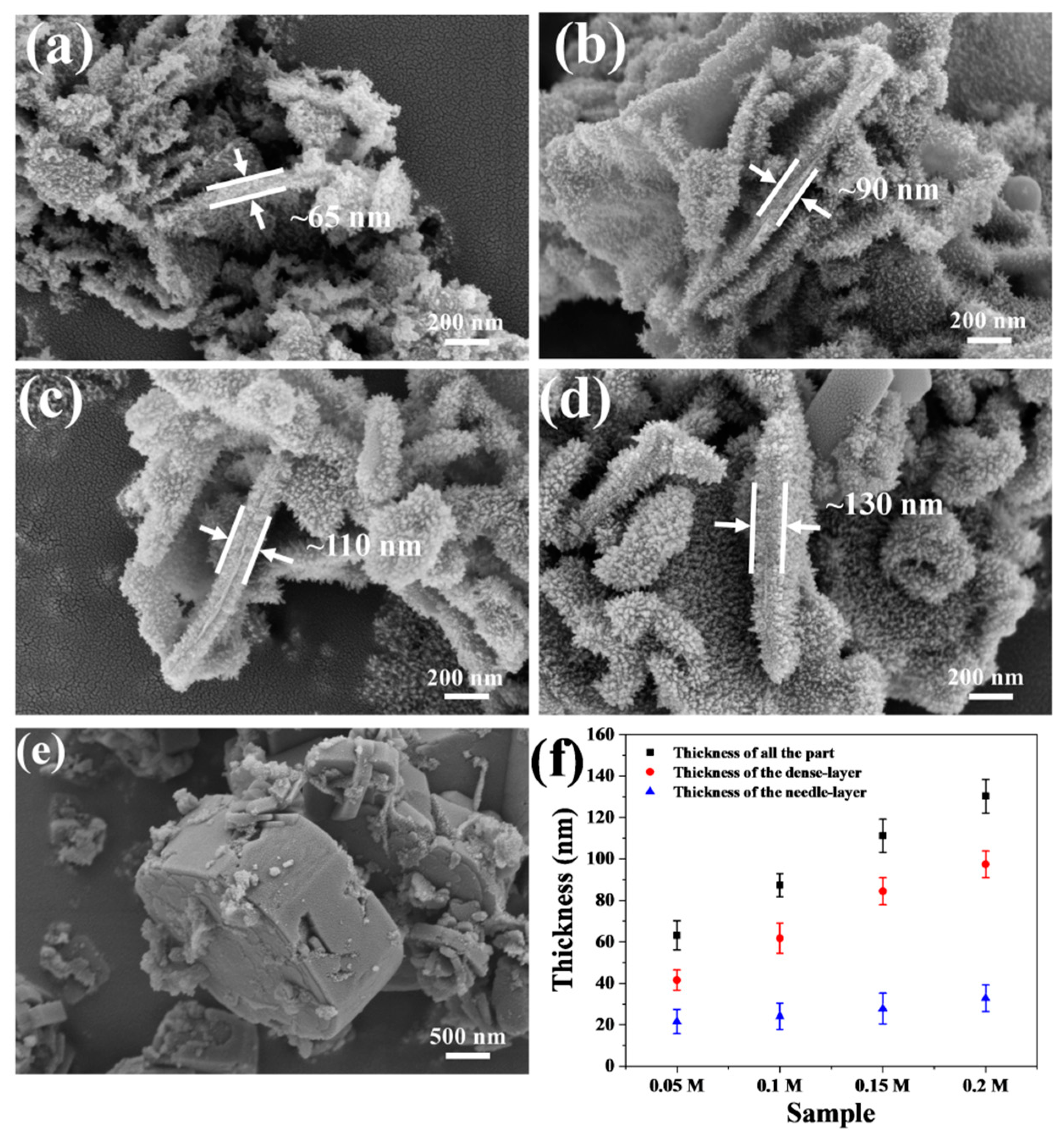
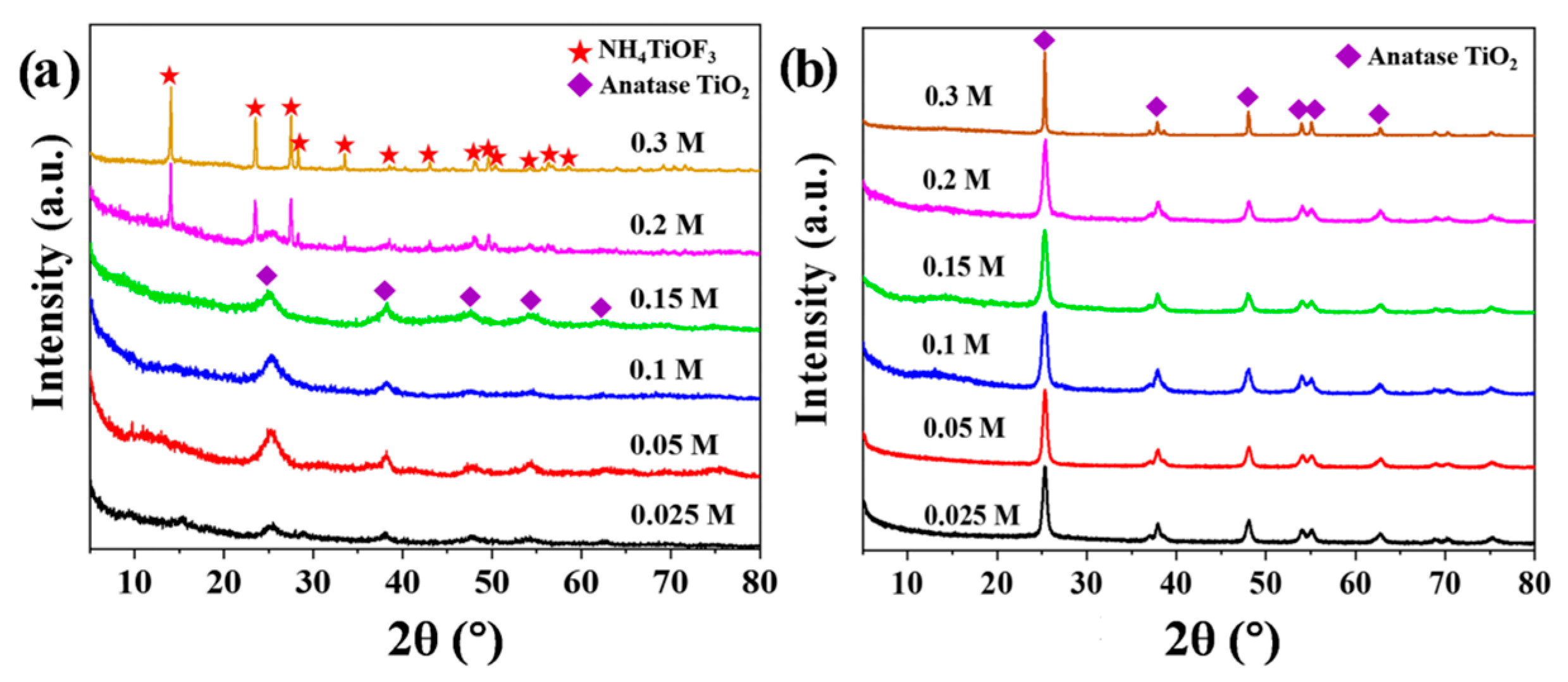
3.2. The Influence of (NH4)2TiF6 Concentration
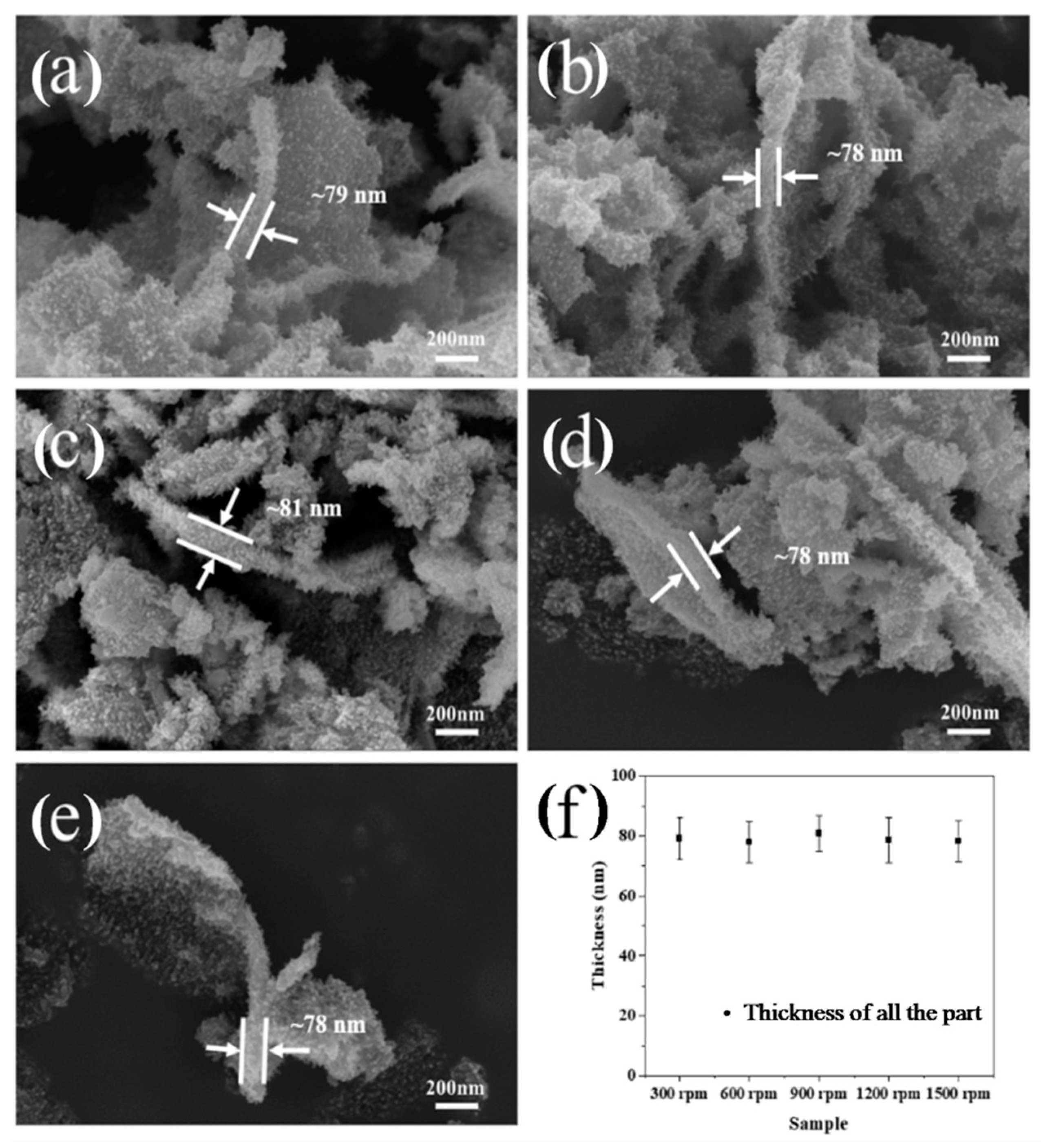
3.3. The Influence of Stirring Rate
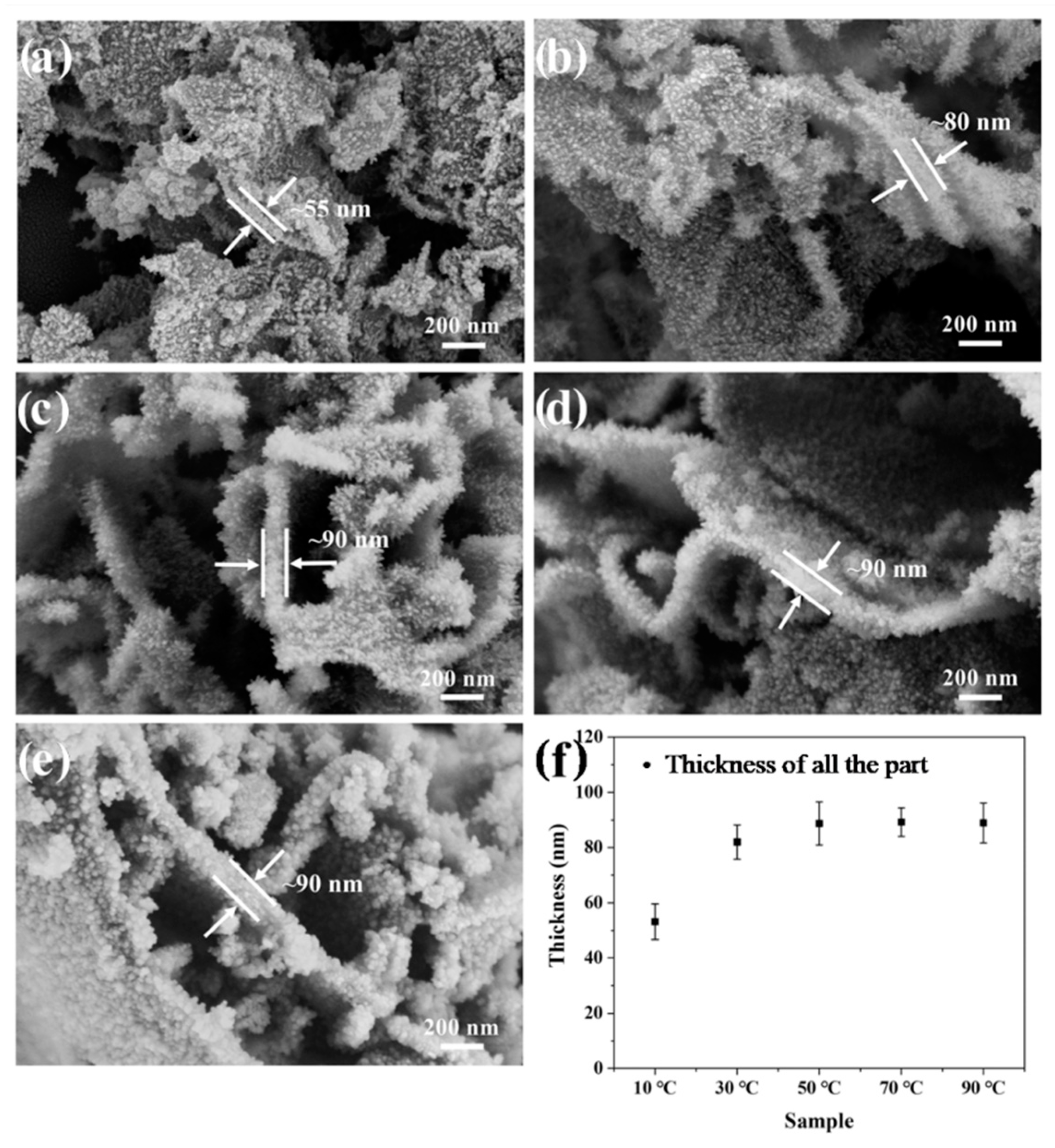
3.4. The Effect of the Synthesis Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Mai, Y.; Wu, D.; Zhang, F.; Feng, X. Two-dimensional soft nanomaterials: A fascinating world of materials. Adv. Mater. 2015, 27, 403–427. [Google Scholar] [CrossRef]
- Sun, Z.; Chang, H. Graphene and graphene-like two-dimensional materials in photodetection: Mechanisms and methodology. ACS Nano 2014, 8, 4133–4156. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, A.; Liu, F.; Cao, J.; Wang, L.; Hu, Q. Carbon dioxide adsorption of two-dimensional carbide MXenes. J. Adv. Ceram. 2018, 7, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Yan, F.; Cai, C.; Wu, Z.; Zhang, C.; Yin, T.; Hu, L. Freeze-drying and hot-pressing strategy to embed two-dimensional Ti0.87O2 monolayers in commercial polypropylene films with enhanced dielectric properties. J. Adv. Ceram. 2021, 10, 368–376. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Osada, M.; Sasaki, T. Nanosheet architectonics: A hierarchically structured assembly for tailored fusion materials. Polym. J. 2014, 47, 89–98. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Stranol, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Chen, K.; Sun, S.; Zhang, J.; Cui, W.; Xie, Z.; Liu, G. Crystalline boron nitride nanosheets by sonication-assisted hydrothermal exfoliation. J. Adv. Ceram. 2019, 8, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Zhou, A.; Wu, J.; Chen, Y.; Lan, X.; Bai, H.; Li, L. Bottom-up preparation of ultrathin 2D aluminum oxide nanosheets by duplicating graphene oxide. Adv. Mater. 2016, 28, 1703–1708. [Google Scholar] [CrossRef]
- Yuan, H.; Han, K.; Dubbink, D.; Mul, G.; Ten Elshof, J.E. Modulating the external facets of functional nanocrystals enabled by two-dimensional oxide crystal templates. ACS Catal. 2017, 7, 6858–6863. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, J.; Bai, X.; Liu, R.; Yang, D.; Xu, T.; Shan, C.; Yuan, H. Suppressing photocarrier recombination in anatase TiO2 nanoplates via thickness optimization for enhanced photocatalytical H2 generation. Appl. Surf. Sci. 2021, 566, 150698. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, I.S.; Li, Z.; Xia, X.; Lee, W.I.; Dai, S.; Bahnemann, D.W.; Pan, J.H. Nanoporous TiO2 spheres with tailored textural properties: Controllable synthesis, formation mechanism, and photochemical applications. Prog. Mater. Sci. 2020, 109, 100620. [Google Scholar] [CrossRef]
- Badmus, K.; Wewers, F.; Al-Abri, M.; Shahbaaz, M.; Petrik, L. Synthesis of oxygen deficient TiO2 for improved photocatalytic efficiency in solar radiation. Catalysts 2021, 11, 904. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Airinei, A.; Olaru, N.; Rosca, I.; Koudoumas, E.; Suchea, M.P. Innovative Ag–TiO2 nanofibers with excellent photocatalytic and antibacterial actions. Catalysts 2021, 11, 1234. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of poly(titanium oxide) on the viscoelastic and thermophysical properties of interpenetrating polymer networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Qamar, S.; Lei, F.; Liang, L.; Gao, S.; Liu, K.; Sun, Y.; Ni, W.; Xie, Y. Ultrathin TiO2 flakes optimizing solar light driven CO2 reduction. Nano Energy 2016, 26, 692–698. [Google Scholar] [CrossRef]
- Nijland, M.; Kumar, S.; Lubbers, R.; Blank, D.H.A.; Rijnders, G.; Koster, G.; Ten Elshof, J.E. Local control over nucleation of epitaxial thin films by seed layers of inorganic nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 2777. [Google Scholar] [CrossRef]
- Sasaki, T.; Kooli, F.; Iida, M.; Michiue, Y.; Takenouchi, S.; Yajima, Y.; Izumi, F.; Chakoumakos, B.C.; Watanabe, M. A mixed alkali metal titanate with the lepidocrocite-like layered structure. Preparation, crystal structure, protonic form, and acid−base intercalation properties. Chem. Mater. 1998, 10, 4123–4128. [Google Scholar] [CrossRef]
- Tanaka, T.; Ebina, Y.; Takada, K.; Kurashima, K.; Sasaki, T. Oversized titania nanosheet crystallites derived from flux-grown layered titanate single crystals. Chem. Mater. 2003, 15, 3564–3568. [Google Scholar] [CrossRef]
- Yuan, H.; Lubbers, R.; Besselink, R.; Nijland, M.; Ten Elshof, J.E. Improved langmuir-blodgett titanate films via in situ exfoliation study and optimization of deposition parameters. ACS Appl. Mater. Interfaces 2014, 6, 8567–8574. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Dubbink, D.; Besselink, R.; Ten Elshof, J.E. The rapid exfoliation and subsequent restacking of layered titanates driven by an acid-base reaction. Angew. Chem. Int. Ed. 2015, 54, 9239–9243. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, H.; Cheng, B.; Zhao, X.; Yu, J.; Ho, W. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Kishimoto, H.; Takahama, K.; Hashimoto, N.; Aoi, Y.; Deki, S. Photocatalytic activity of titanium oxide prepared by liquid phase deposition (LPD). J. Mater. Chem. 1998, 8, 2019–2024. [Google Scholar] [CrossRef]
- Carbone, L.; Cozzoli, P.D. Colloidal heterostructured nanocrystals: Synthesis and growth mechanisms. Nano Today 2010, 5, 449–493. [Google Scholar] [CrossRef]
- Casavola, M.; Buonsanti, R.; Caputo, G.; Cozzoli, P.D. Colloidal strategies for preparing oxide-based hybrid nanocrystals. Eur. J. Inorg. Chem. 2008, 2008, 837–854. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Q.; Wu, X.J.; Li, L.; Liu, J.; Zhang, H. Wet-chemical synthesis and applications of semiconductor nanomaterial-based epitaxial heterostructures. Nano-Micro. Lett. 2019, 11, 86. [Google Scholar] [CrossRef] [Green Version]
- Deki, S.; Aoi, Y.; Hiroi, O.; Kajinami, A. Titanium (IV) oxide thin films prepared from aqueous solution. Chem. Lett. 1996, 1996, 433–434. [Google Scholar] [CrossRef]
- Gong, J.; Yang, C.; Pu, W.; Zhang, J. Liquid phase deposition of tungsten doped TiO2 films for visible light photoelectrocatalytic degradation of dodecyl-benzenesulfonate. Chem. Eng. J. 2011, 167, 190–197. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.; Pu, W.; Tan, Y.; Yang, K.; Zhang, J. Liquid phase deposition of WO3/TiO2 heterojunction films with high photoelectrocatalytic activity under visible light irradiation. Electrochim. Acta 2014, 148, 180–186. [Google Scholar] [CrossRef]
- Zhou, L.; Smyth-Boyle, D.; O’Brien, P. A facile synthesis of uniform NH4TiOF3mesocrystals and their conversion to TiO2mesocrystals. J. Am. Chem. Soc. 2008, 130, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Masuda, Y.; Koumoto, K. Microstructure-controlled deposition of SrTiO3 thin film on self-assembled monolayers in an aqueous solution of (NH4)2TiF6-Sr(NO3)2-H3BO3. Chem. Mater. 2003, 15, 2399–2410. [Google Scholar] [CrossRef]
- Saito, N.; Haneda, H.; Seo, W.S.; Koumoto-Langmuir, K. Selective deposition of ZnF(OH) on self-assembled monolayers in ZnNH4F aqueous solutions for micropatterning of zinc oxide. Langmuir 2001, 17, 1461–1469. [Google Scholar] [CrossRef]
- Liotta, S. Kinetics of precipitation. J. Am. Chem. Soc. 1965, 87, 1414. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.; Fu, X.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Nowotny, J. Titanium dioxide-based semiconductors for solar-driven environmentally friendly applications: Impact of point defects on performance. Energy Environ. Sci. 2008, 1, 565–572. [Google Scholar] [CrossRef]
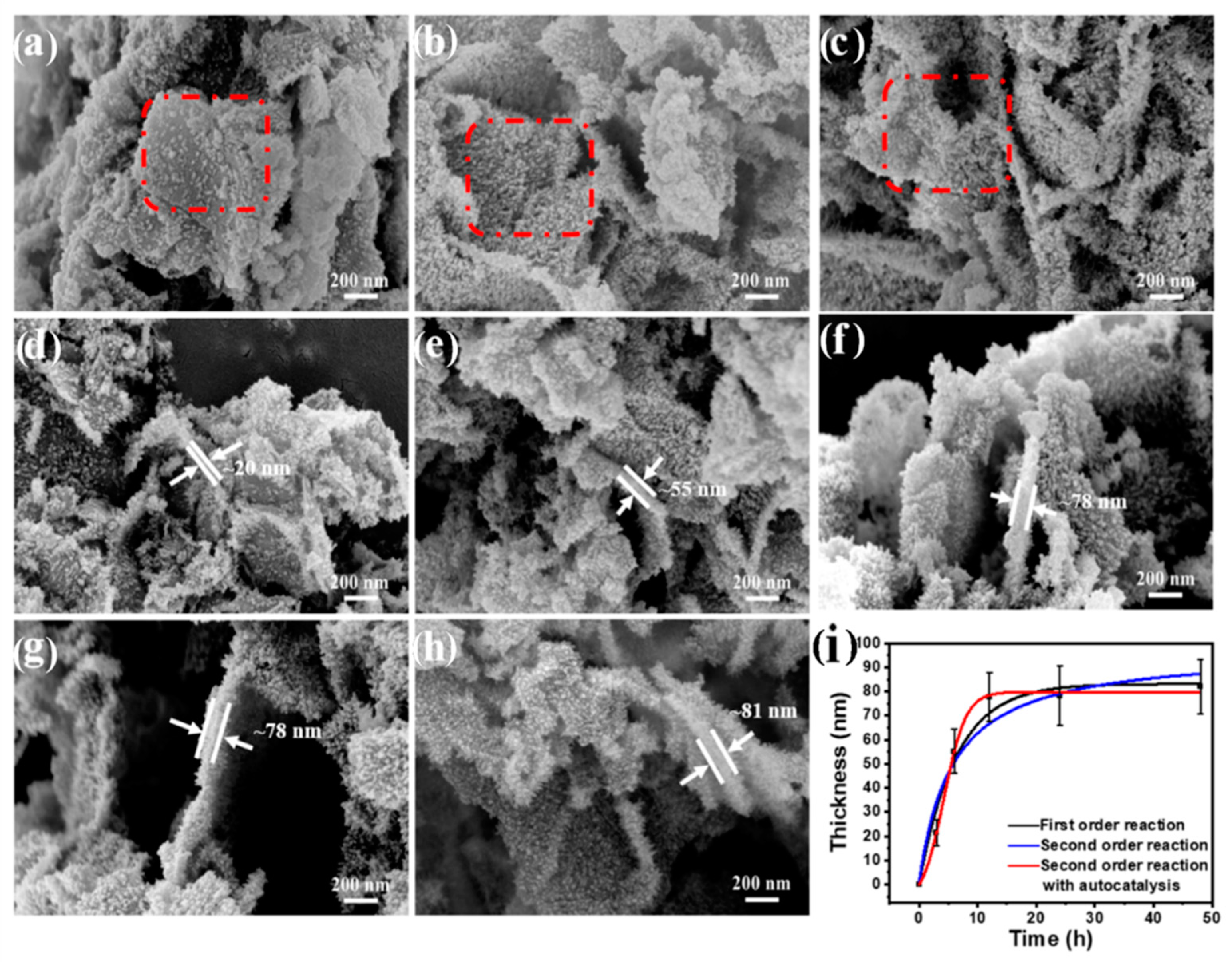
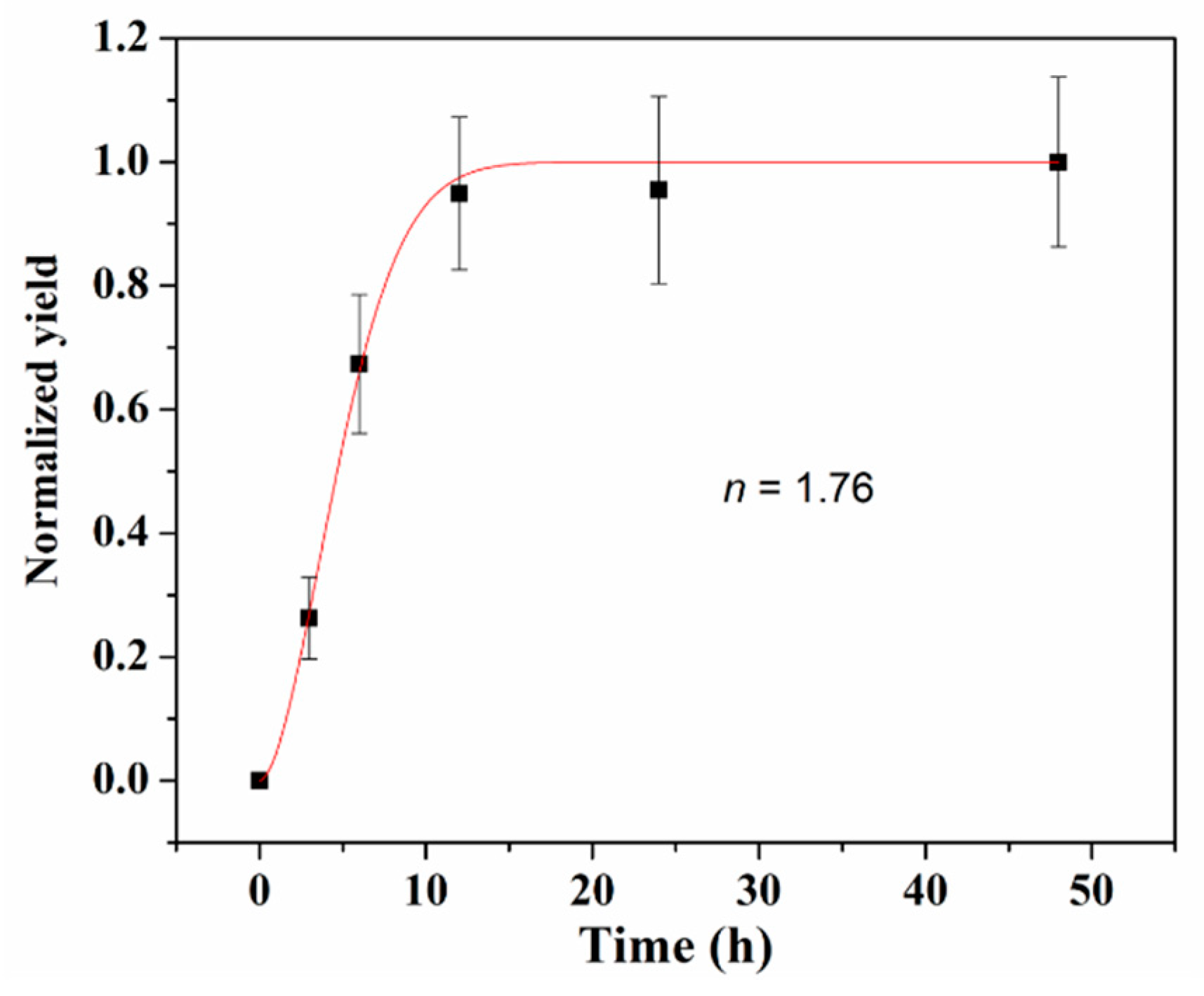

| Reaction Kinetics | Kinetic Equation | k Value | R2 |
|---|---|---|---|
| First-order reaction | k = 4.45 × 10−5 s−1 | 0.97009 | |
| Second-order reaction | k = 5.16 × 10−7 s−1 | 0.94305 | |
| Second-order reaction with autocatalysis | k = 1.68 × 10−6 s−1 | 0.99871 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, H.; Cui, J.; Bai, X.; Yang, D.; Yuan, H.; Wang, B. Research on the Thickness and Microstructure of Plate-like TiO2 by the Nanosheet-Seeding Growth Technique. Coatings 2022, 12, 1673. https://doi.org/10.3390/coatings12111673
Zhang Y, Liu H, Cui J, Bai X, Yang D, Yuan H, Wang B. Research on the Thickness and Microstructure of Plate-like TiO2 by the Nanosheet-Seeding Growth Technique. Coatings. 2022; 12(11):1673. https://doi.org/10.3390/coatings12111673
Chicago/Turabian StyleZhang, Yanyan, Hao Liu, Junyan Cui, Xiaosong Bai, Daoyuan Yang, Huiyu Yuan, and Baoming Wang. 2022. "Research on the Thickness and Microstructure of Plate-like TiO2 by the Nanosheet-Seeding Growth Technique" Coatings 12, no. 11: 1673. https://doi.org/10.3390/coatings12111673
APA StyleZhang, Y., Liu, H., Cui, J., Bai, X., Yang, D., Yuan, H., & Wang, B. (2022). Research on the Thickness and Microstructure of Plate-like TiO2 by the Nanosheet-Seeding Growth Technique. Coatings, 12(11), 1673. https://doi.org/10.3390/coatings12111673






