Cu/Mn Synergy Catalysis-Based Colorimetric Sensor for Visual Detection of Hydroquinone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Regents and Materials
2.2. Characterization
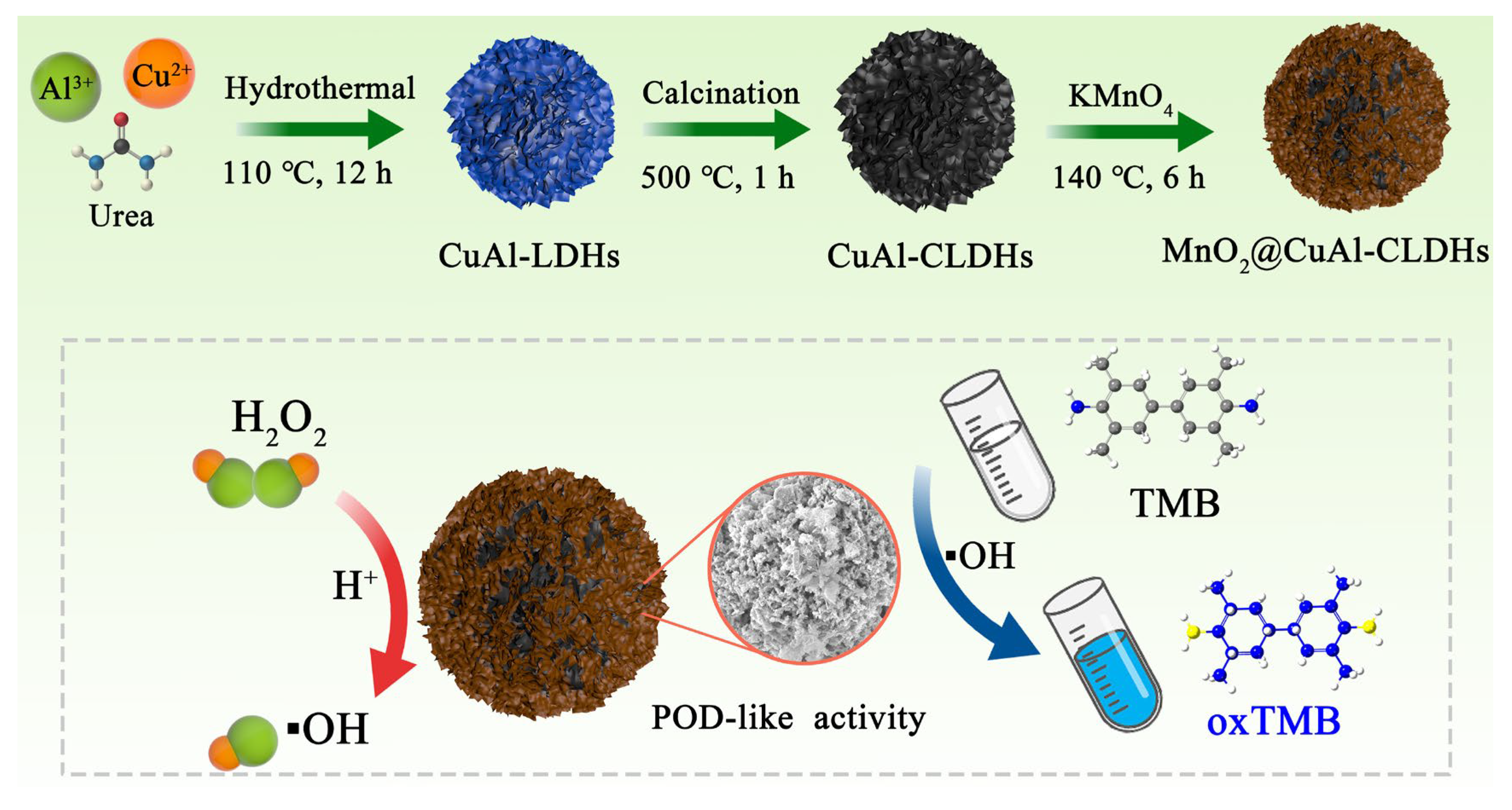
2.3. Synthesis of Flower-like MnO2@CuAl-CLDHs
2.4. Peroxide-like Activity of Flower-Like MnO2@CuAl-CLDHs
2.5. Detection of HQ
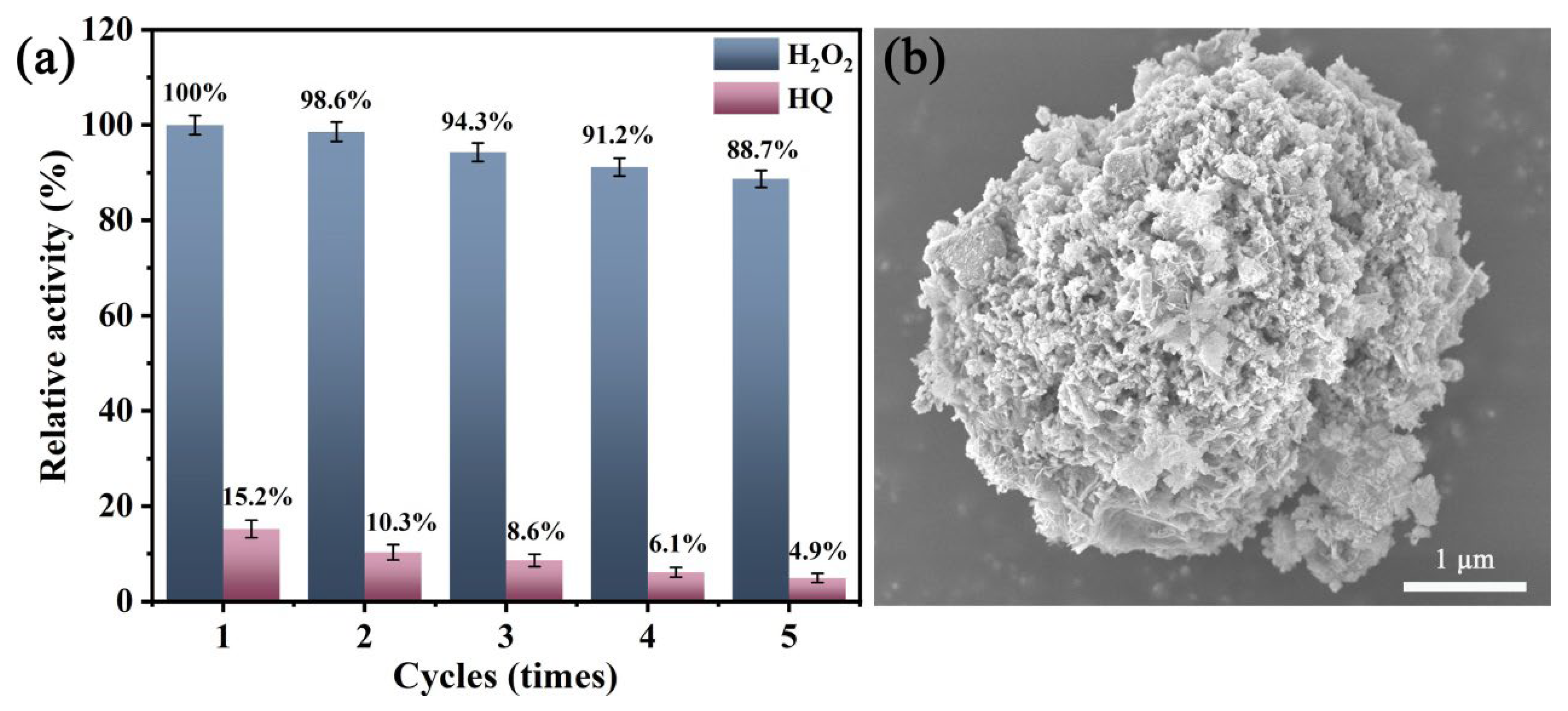
2.6. Reusability of MnO2@CuAl-CLDHs
2.7. Analysis of HQ in Real Samples
3. Results and Discussion
3.1. Design and Preparation of MnO2@CuAl-CLDHs
3.2. Characterization of MnO2@CuAl-CLDHs
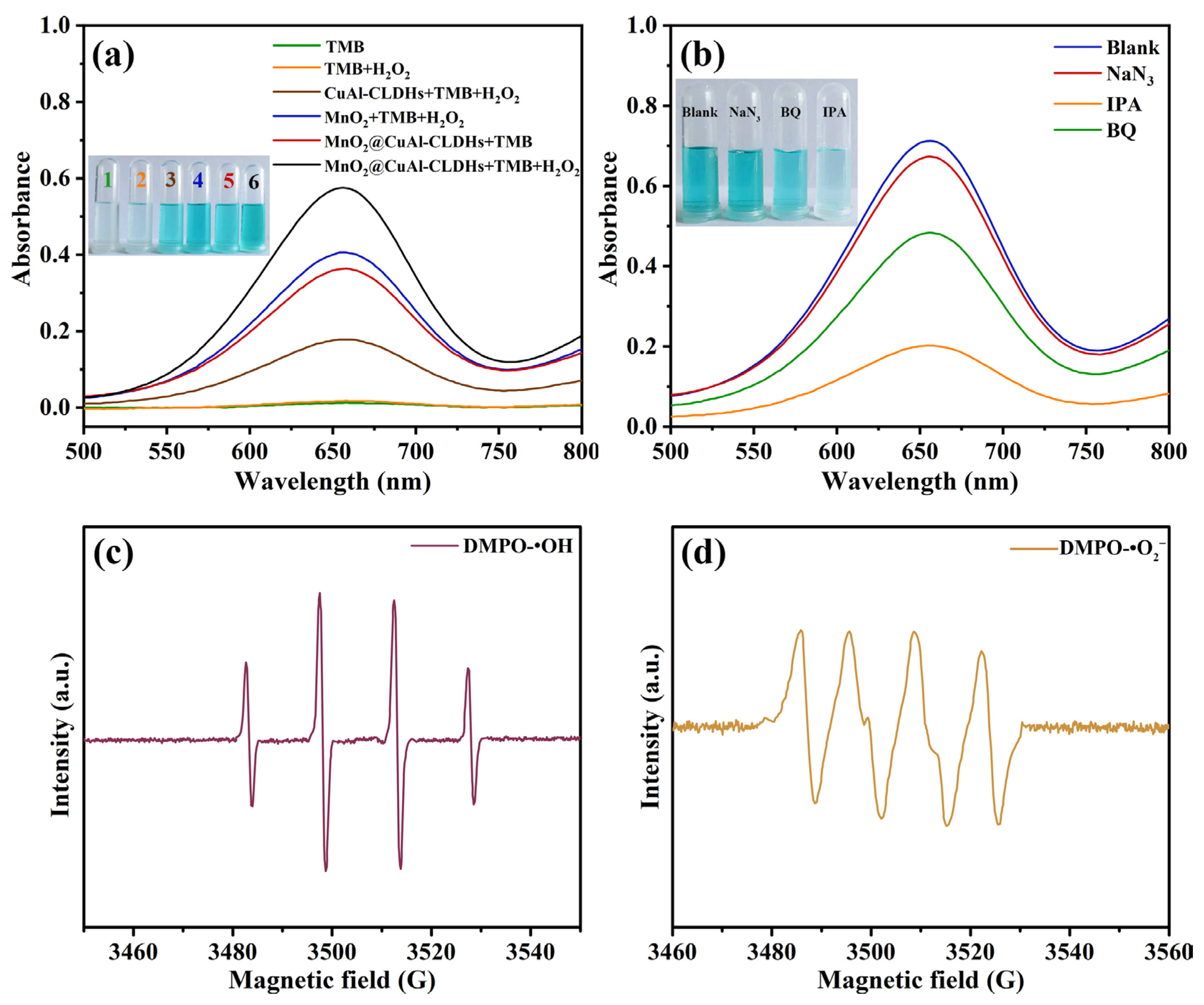
3.3. Peroxide-like Activity of MnO2@CuAl-CLDHs
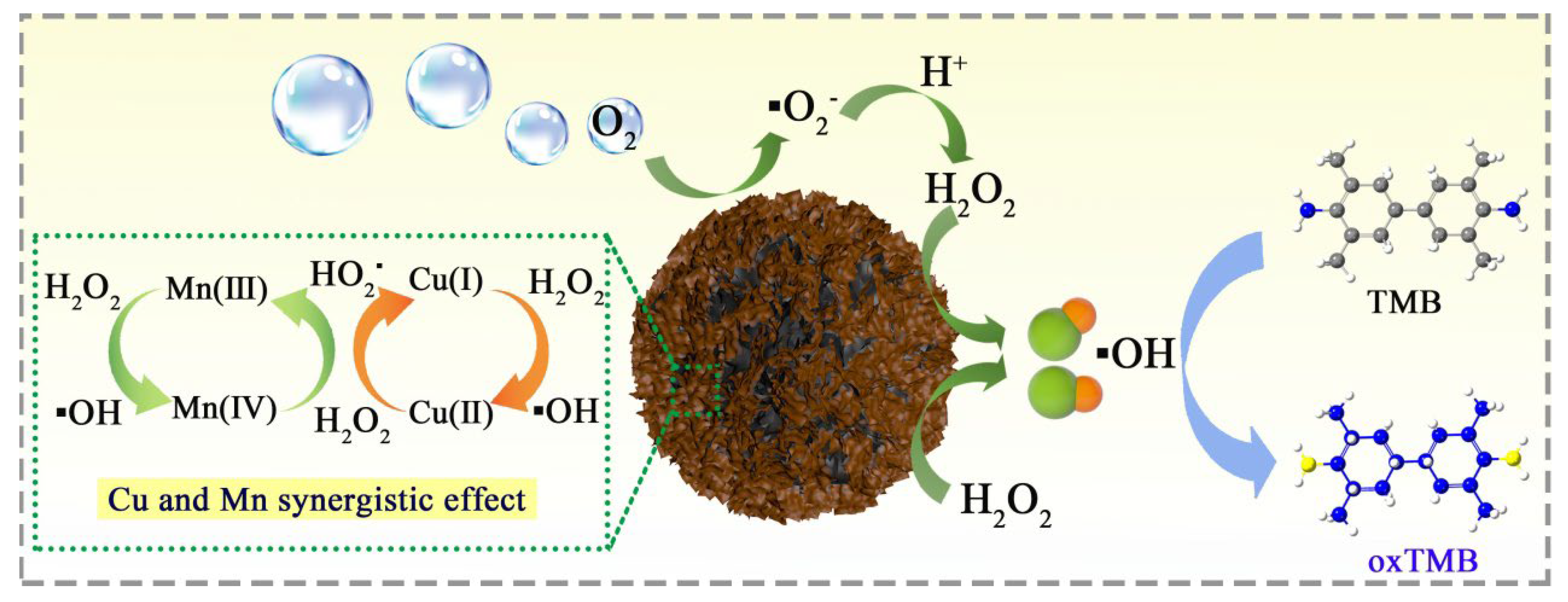
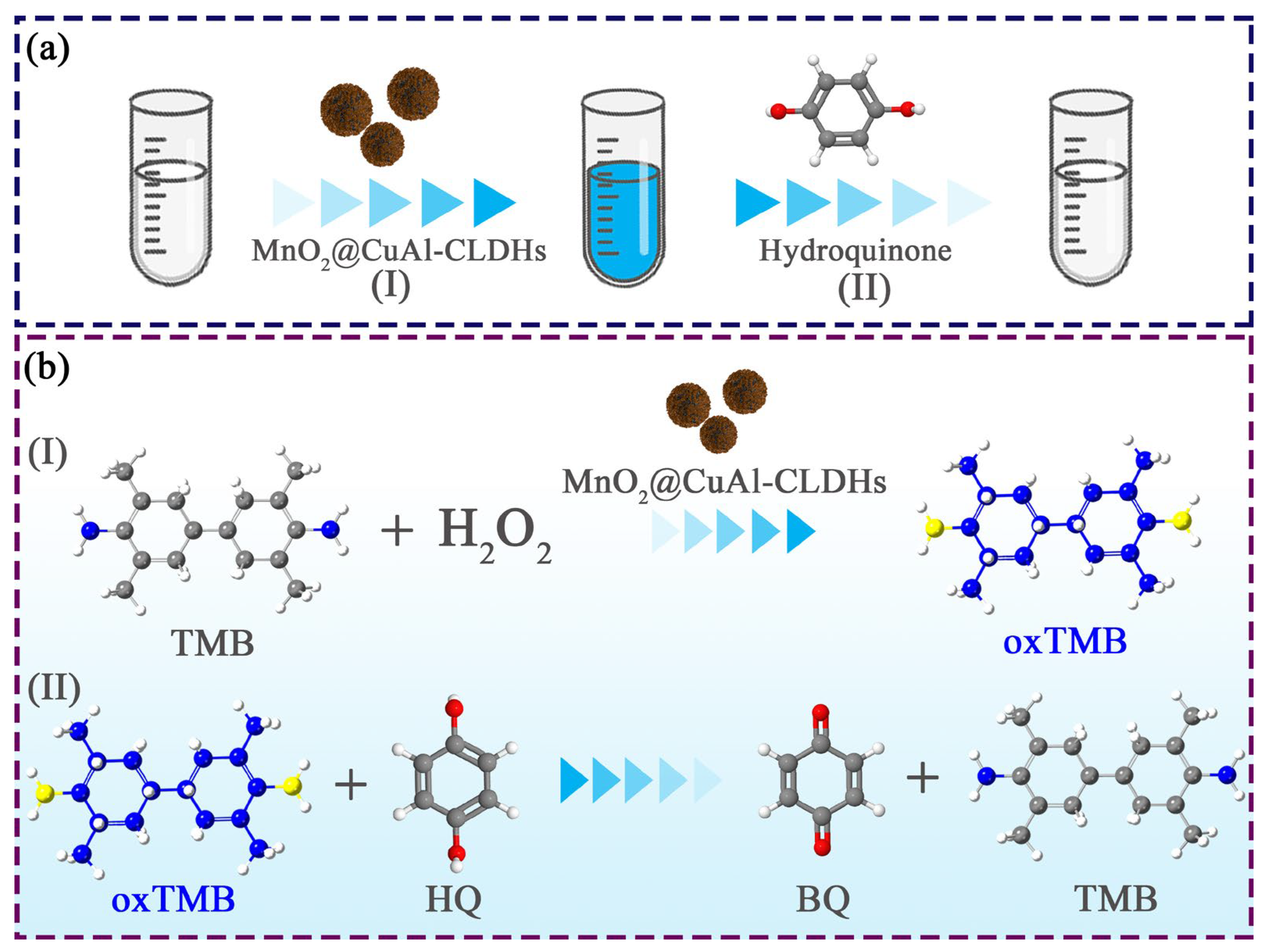
3.4. Enzyme-Like Catalysis Mechanism of MnO2@CuAl-CLDHs
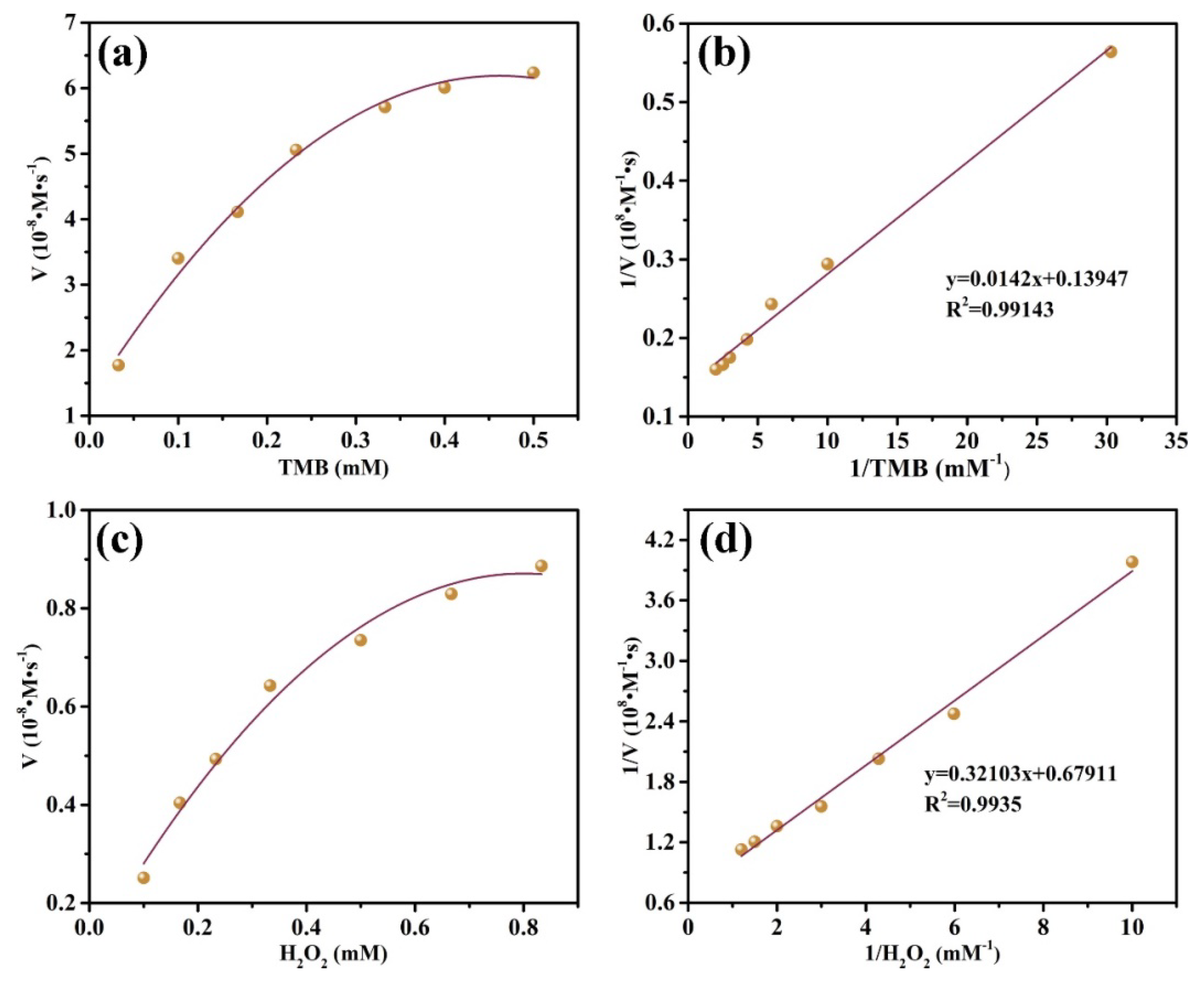
3.5. Steady-State Kinetic Analysis of MnO2@CuAl-CLDHs
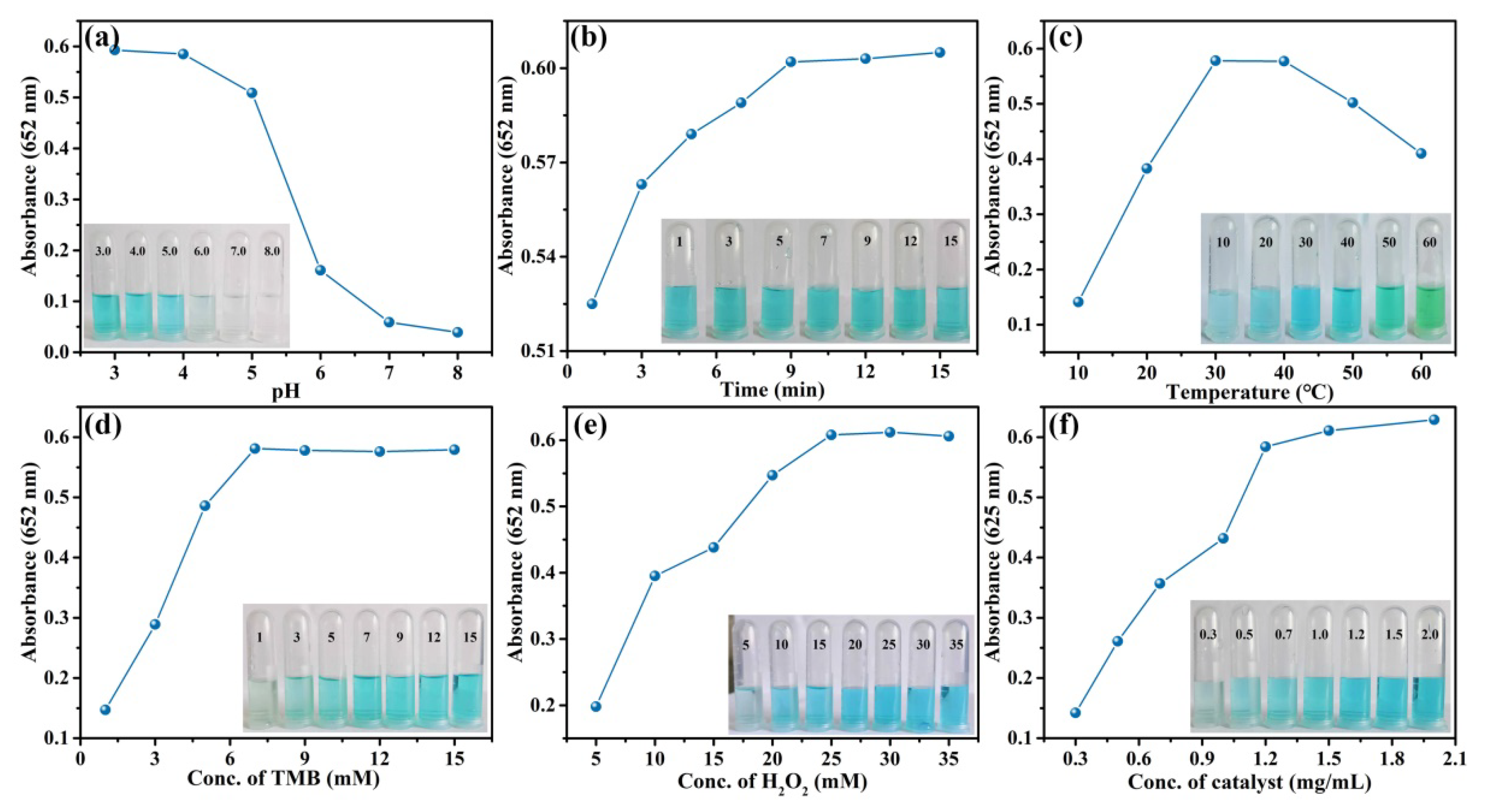
3.6. Optimization of the Catalytic Activity of MnO2@CuAl-CLDHs
3.7. Colorimetric Detection of HQ
3.8. Reusability of MnO2@CuAl-CLDHs and Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, J.; Rahman, M.M.; Siddiquey, I.A.; Asiri, A.M.; Hasnat, M.A. Efficient hydroquinone sensor based on zinc, strontium and nickel based ternary metal oxide (TMO) composites by differential pulse voltammetry. Sens. Actuators B 2018, 256, 383–392. [Google Scholar] [CrossRef]
- Wang, H.; Li, R.; Li, Z. Nanohybrid of Co3O4 and histidine-functionalized graphene quantum dots for electrochemical detection of hydroquinone. Electrochim. Acta 2017, 255, 323–334. [Google Scholar] [CrossRef]
- Liang, N.; Ge, X.; Zhao, Y.; Xia, L.; Song, Z.-L.; Kong, R.-M.; Qu, F. Promoting sensitive colorimetric detection of hydroquinone and Hg2+ via ZIF-8 dispersion enhanced oxidase-mimicking activity of MnO2 nanozyme. J. Hazard. Mater. 2023, 454, 131455. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cao, Y.; Diao, D. Electrochemical activation of graphene sheets embedded carbon films for high sensitivity simultaneous determination of hydroquinone, catechol and resorcinol. Sens. Actuators B 2020, 305, 127495. [Google Scholar] [CrossRef]
- Esteki, M.; Nouroozi, S.; Shahsavari, Z. A fast and direct spectrophotometric method for the simultaneous determination of methyl paraben and hydroquinone in cosmetic products using successive projections algorithm. Int. J. Cosmet. Sci. 2016, 38, 25–34. [Google Scholar] [CrossRef]
- Butwong, N.; Kunawong, T.; Luong, J.H.T. Simultaneous Analysis of Hydroquinone, Arbutin, and Ascorbyl Glucoside Using a Nanocomposite of Ag@AgCl Nanoparticles, Ag2S Nanoparticles, Multiwall Carbon Nanotubes, and Chitosan. Nanomaterials 2020, 10, 1583. [Google Scholar] [CrossRef] [PubMed]
- Marrubini, G.; Calleri, E.; Coccini, T.; Castoldi, A.F.; Manzo, L. Direct Analysis of Phenol, Catechol and Hydroquinone in Human Urine by Coupled-Column HPLC with Fluorimetric Detection. Chroma 2005, 62, 25–31. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Liang, M.; Chen, G.; Kong, R.-M.; Xia, L.; Qu, F. A Boric Acid-Functionalized Lanthanide Metal–Organic Framework as a Fluorescence “Turn-on” Probe for Selective Monitoring of Hg2+ and CH3Hg+. Anal. Chem. 2020, 92, 3366–3372. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, H.; Wu, T.; Zhao, H.; Gao, Y.; Zhu, X.; Liu, Q. Pt deposited on sea urchin-like CuCo2O4 nanowires: Preparation, the excellent peroxidase-like activity and the colorimetric detection of sulfide ions. J. Environ. Chem. Eng. 2022, 10, 107228. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Zhang, F.; Xia, J.; Wang, Z. Flexible enzyme cascade sensing platform based on a G-quadruplex nanofiber biohydrogel for target colorimetric sensing. Anal. Chim. Acta 2020, 1140, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Liu, J.; Wang, C.; Gu, W.; Ran, G.; Liu, B.; Song, Q. Ir nanoparticles with multi-enzyme activities and its application in the selective oxidation of aromatic alcohols. Appl. Catal. B 2020, 267, 118725. [Google Scholar] [CrossRef]
- Sun, L.; Ding, Y.; Jiang, Y.; Liu, Q. Montmorillonite-loaded ceria nanocomposites with superior peroxidase-like activity for rapid colorimetric detection of H2O2. Sens. Actuators B 2017, 239, 848–856. [Google Scholar] [CrossRef]
- Yang, W.-P.; Yang, X.; Zhu, L.-J.; Chu, H.-S.; Li, X.-Y.; Xu, W.-T. Nanozymes: Activity origin, catalytic mechanism, and biological application. Coord. Chem. Rev. 2021, 448, 214170. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, X.; Song, Y.; Huang, J.; Xu, H. Research progress of nanozymes in colorimetric biosensing: Classification, activity and application. Chem. Eng. J. 2024, 487, 150612. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Huang, Z.; Sun, Y.-M.; Xu, Z.-L.; Liu, J. The Most Active Oxidase-Mimicking Mn2O3 Nanozyme for Biosensor Signal Generation. Chem.-A Eur. J. 2021, 27, 9597–9604. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Wu, J.; Zhong, H.; Zhang, Y.; Xu, W.; Wu, Y.; Chen, Y.; Yan, H.; Zhang, Q.; Gu, W.; et al. Densely Isolated FeN4 Sites for Peroxidase Mimicking. ACS Catal. 2020, 10, 6422–6429. [Google Scholar] [CrossRef]
- Tao, L.; Qiao, M.; Jin, R.; Li, Y.; Xiao, Z.; Wang, Y.; Zhang, N.; Xie, C.; He, Q.; Jiang, D.; et al. Bridging the Surface Charge and Catalytic Activity of a Defective Carbon Electrocatalyst. Angew. Chem. Int. Ed. 2019, 58, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Uchida, H.; Kameda, T.; Kumagai, S.; Saito, Y.; Mizushina, K.; Itou, I.; Han, T.; Yoshioka, T. Synthesis of MnO2/Mg-Al layered double hydroxide and evaluation of its NO-removal performance. J. Alloys Compd. 2021, 867, 159038. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Wen, Z.Q.; Zhang, Y.X. Flower-like NiFe layered double hydroxides coated MnO2 for high-performance flexible supercapacitors. J. Energy Storage 2017, 11, 242–248. [Google Scholar] [CrossRef]
- Zhong, M.; Li, X.; Chu, X.; Gui, H.; Zuo, S.; Yao, C.; Li, Z.; Chen, Y. Solar driven catalytic conversion of cellulose biomass into lactic acid over copper reconstructed natural mineral. Appl. Catal. B 2022, 317, 121718. [Google Scholar] [CrossRef]
- Xu, C.; Dong, S.; Chen, T.; Liu, H.; Zou, X.; Ji, M.; Han, Z.; Shu, D.; Wang, C.; Chen, D. Low-temperature catalytic performance of toluene oxidation over Cu-Mn oxide catalysts derived from LDH precursor. Fuel 2023, 347, 128401. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Wang, Z.; Ashraf, G.; Wang, J.; Luo, H.; Chen, X.; Xiao, F.; Liu, H. Hierarchical CNTs@CuMn Layered Double Hydroxide Nanohybrid with Enhanced Electrochemical Performance in H2S Detection from Live Cells. Anal. Chem. 2019, 91, 3912–3920. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, D.; Li, S.; Zhang, L.; Zheng, G.; Guo, L. Layered copper manganese oxide for the efficient catalytic CO and VOCs oxidation. Chem. Eng. J. 2019, 357, 258–268. [Google Scholar] [CrossRef]
- Luo, M.; Cheng, Y.; Peng, X.; Pan, W. Copper modified manganese oxide with tunnel structure as efficient catalyst for low-temperature catalytic combustion of toluene. Chem. Eng. J. 2019, 369, 758–765. [Google Scholar] [CrossRef]
- Hao, M.; Gao, P.; Yang, D.; Chen, X.; Xiao, F.; Yang, S. Highly efficient adsorption behavior and mechanism of Urea-Fe3O4@LDH for triphenyl phosphate. Environ. Pollut. 2020, 267, 114142. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.H.; Kim, H.; Koo Lee, Y.-E.; Shrestha, N.K.; Sung, M.M. UV-enhanced atomic layer deposition of Al2O3 thin films at low temperature for gas-diffusion barriers. RSC Adv. 2017, 7, 5601–5609. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Liang, W.; Chen, L.; Li, Y.; He, X. Non-enzymatic glucose sensor with high sensitivity based on Cu-Al layered double hydroxides. Sens. Actuators B 2018, 273, 41–47. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, M.; Yuan, Y.; Wu, Q.; Wang, H.; He, Y.; Lin, Z.; Zhou, F.; Ling, M.; Qian, C.; et al. Accommodation of Silicon in an Interconnected Copper Network for Robust Li-Ion Storage. Adv. Funct. Mater. 2020, 30, 1910249. [Google Scholar] [CrossRef]
- Lyu, L.; Yan, D.; Yu, G.; Cao, W.; Hu, C. Efficient Destruction of Pollutants in Water by a Dual-Reaction-Center Fenton-like Process over Carbon Nitride Compounds-Complexed Cu(II)-CuAlO2. Environ. Sci. Technol. 2018, 52, 4294–4304. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, W.; Qin, X.; Qu, F.; Lu, L. Self-powered cathodic photoelectrochemical aptasensor based on in situ–synthesized CuO-Cu2O nanowire array for detecting prostate-specific antigen. Microchim. Acta 2020, 187, 325. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Zhang, Z.; Liu, Y.; Chen, J.; Liu, J.; Du, P.; Guo, H.; Lu, X. MnO2 Nanospheres Assisted by Cysteine Combined with MnO2 Nanosheets as a Fluorescence Resonance Energy Transfer System for “Switch-on” Detection of Glutathione. Anal. Chem. 2021, 93, 9621–9627. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently Enhancing Electrocatalytic Activity of α-MnO2 Nanorods/N-Doped Ketjenblack Carbon for Oxygen Reduction Reaction and Oxygen Evolution Reaction Using Facile Regulated Hydrothermal Treatment. Catalysts 2018, 8, 138. [Google Scholar] [CrossRef]
- Fang, R.-C.; Sun, Q.-Q.; Zhou, P.; Yang, W.; Wang, P.-F.; Zhang, D.W. High-performance bilayer flexible resistive random access memory based on low-temperature thermal atomic layer deposition. Nanoscale Res. Lett. 2013, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Capelli, S.; Motta, D.; Evangelisti, C.; Dimitratos, N.; Prati, L.; Pirola, C.; Villa, A. Effect of Carbon Support, Capping Agent Amount, and Pd NPs Size for Bio-Adipic Acid Production from Muconic Acid and Sodium Muconate. Nanomaterials 2020, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; He, L.; Jiang, Z.W.; Li, Y.; Cao, Z.M.; Huang, C.Z.; Li, Y.F. CuO nanoparticles derived from metal-organic gel with excellent electrocatalytic and peroxidase-mimicking activities for glucose and cholesterol detection. Biosens. Bioelectron. 2019, 145, 111704. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, L.; Fei, Z.; Dyson, P.J.; Zhang, L. On the origin of the synergy between the Pt nanoparticles and MnO2 nanosheets in Wonton-like 3D nanozyme oxidase mimics. Biosens. Bioelectron. 2018, 121, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, Z.; Lian, Q.; Liu, H.; Chen, L.; Zhou, L.; Jiang, Y.; Gao, J. Preparation of Flower-like NiMnO3 as Oxidase Mimetics for Colorimetric Detection of Hydroquinone. ACS Sustain. Chem. Eng. 2021, 9, 12766–12778. [Google Scholar] [CrossRef]
- Baye, A.F.; Nguyen, H.T.; Kim, H. Fe0/Fe3C-assisted Fe3O4 redox sites as robust peroxidase mimics for colorimetric detection of H2O2. Sens. Actuators B 2023, 377, 133097. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Liu, D.; Li, X.; Liang, H. Enhanced catalytic performance of Cu-doped MnFe2O4 magnetic ferrites: Tetracycline hydrochloride attacked by superoxide radicals efficiently in a strong alkaline environment. Chemosphere 2022, 297, 134154. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Long, Y.; Chen, J.; Zhao, S.; Li, C.; Qu, F.; Han, B.; Zhang, Z.; Zhang, B.-P. In situ synthesis of Tree-branch-like Copper-manganese oxides nanoarrays supported on copper foam as a superior efficiency Fenton-like catalyst for enhanced degradation of 4-chlorophenol. Appl. Surf. Sci. 2022, 593, 153241. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, L.; Yang, X.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y. Rational design of a stable peroxidase mimic for colorimetric detection of H2O2 and glucose: A synergistic CeO2/Zeolite Y nanocomposite. J. Colloid Interface Sci. 2019, 535, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, J.; Jiao, L.; Xu, W.; Wang, H.; Wei, X.; Gu, W.; Ren, G.; Zhang, N.; Zhang, Q.; et al. Cascade Reaction System Integrating Single-Atom Nanozymes with Abundant Cu Sites for Enhanced Biosensing. Anal. Chem. 2020, 92, 3373–3379. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Xu, W.; Zhang, Y.; Wu, Y.; Gu, W.; Ge, X.; Chen, B.; Zhu, C.; Guo, S. Boron-doped Fe-N-C single-atom nanozymes specifically boost peroxidase-like activity. Nano Today 2020, 35, 100971. [Google Scholar] [CrossRef]
- Xu, W.; Jiao, L.; Yan, H.; Wu, Y.; Chen, L.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Glucose Oxidase-Integrated Metal-Organic Framework Hybrids as Biomimetic Cascade Nanozymes for Ultrasensitive Glucose Biosensing. ACS Appl. Mater. Interfaces 2019, 11, 22096–22101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-p.; Xing, Y.-p.; Liu, L.-h.; Zhou, X.-h.; Shi, H.-c. Fenton reaction-triggered colorimetric detection of phenols in water samples using unmodified gold nanoparticles. Sens. Actuators B 2016, 225, 593–599. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, M.; Cao, W.; Wang, X.; Wang, Q.; Li, S.; Wei, H. Light-Responsive Metal–Organic Framework as an Oxidase Mimic for Cellular Glutathione Detection. Anal. Chem. 2019, 91, 8170–8175. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Wen, S.; Chen, X.; Deng, D.; Yang, X.; Zhang, R. Sweetsop-like α-Fe2O3@CoNi catalyst with superior peroxidase-like activity for sensitive and selective detection of hydroquinone. RSC Adv. 2021, 11, 24065–24071. [Google Scholar] [CrossRef]
- Deng, D.; Wang, Y.; Wen, S.; Kang, Y.; Cui, X.; Tang, R.; Yang, X. Metal-organic framework composite Mn/Fe-MOF@Pd with peroxidase-like activities for sensitive colorimetric detection of hydroquinone. Anal. Chim. Acta 2023, 1279, 341797. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, C.; Yang, Y.; Wang, Y.; Li, C.; Xie, Y.; Zhao, P.; Fei, J. A light-driven photoelectrochemical sensor for highly selective detection of hydroquinone based on type-II heterojunction formed by carbon nanotubes immobilized in 3D honeycomb CdS/SnS2. J. Colloid Interface Sci. 2023, 643, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bao, H.; Liu, C.; Wu, F.; Fu, T. “Turn-on” fluorometric probe for hydroquinone and catechol based on an in situ reaction between protamine sulfate and dihydroxybenzene isomers and the formation of fluorescent polymer nanoparticles. Sens. Actuators B. Chem. 2021, 333, 129565. [Google Scholar] [CrossRef]



| Sample | SBET (m2g−1) | VTotal (cm3g−1) | Dp (nm) |
|---|---|---|---|
| CuAl-LDHs | 167.8 | 0.521 | 3.712 |
| MnO2@CuAl-CLDHs | 245.4 | 0.694 | 2.831 |
| Materials | Km (mM) | Vmax (10−8 M s−1) | Kcat (s−1) | Kcat/Km (s−1·mM−1) | ||||
|---|---|---|---|---|---|---|---|---|
| H2O2 | TMB | H2O2 | TMB | H2O2 | TMB | H2O2 | TMB | |
| HRP | 3.7 | 0.43 | 8.71 | 10 | - | - | - | - |
| MnO2@CuAl-CLDHs | 0.473 | 0.102 | 1.473 | 7.170 | 0.074 | 0.359 | 0.156 | 3.519 |
| Nnozymes | Kcat (s−1) | Kcat (s−1) | Ref. |
|---|---|---|---|
| TMB | H2O2 | ||
| 20CeO2/Y | 0.003 | 0.860 | [42] |
| Cu-N-C | 0.283 | 0.075 | [43] |
| Fe-N-C | 0.075 | 0.073 | [44] |
| Fe3O4-Fe0/Fe3C | 0.680 | 0.071 | [39] |
| Fe-MOF | 0.001 | 0.0005 | [45] |
| MnO2@CuAl-CLDHs | 0.359 | 0.074 | This work |
| Sensors | Methods | LOD | Linear Range | Ref. |
|---|---|---|---|---|
| - | HPLC | 0.05 mg/mL | 0.2–10 mg/L | [7] |
| - | AAS | 0.039 μg/mL | 0.1–25 μg/mL | [5] |
| Au nanoparticles | colorimetry | 0.8 μM | 1–30 μM | [46] |
| NiMnO3 | colorimetry | 0.68 μM | 1–85 μM | [47] |
| α-Fe2O3@CoNi | colorimetry | 0.16 μM | 0.5–30 μM | [48] |
| Mn/Fe-MOF@Pd1.0 | colorimetry | 0.09 μM | 0.3–30 μM | [49] |
| CdS/SnS2 | Electrochemistry | 0.1 μM | 0.2–100 μM | [50] |
| Fluorescent Polymer Nanoparticles s | Fluorescent | 0.21 μM | 0–25 μM | [51] |
| MnO2@CuAl-CLDHs | colorimetry | 0.183 | 1–100 μM | This work |
| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| Tap water | 5 | 4.96 | 99.21 | 1.01 |
| 30 | 29.61 | 98.71 | 2.15 | |
| 60 | 60.15 | 100.25 | 2.07 | |
| 80 | 80.50 | 100.63 | 1.91 | |
| Lake water | 5 | 4.95 | 98.91 | 2.18 |
| 30 | 29.75 | 99.18 | 2.27 | |
| 60 | 60.19 | 100.31 | 1.89 | |
| 80 | 81.01 | 101.26 | 2.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, N.; Yang, L.; Wang, L.; Lu, Y.; Zhang, H.; Sun, X.; Zhao, M.; Tan, W.; Yang, J. Cu/Mn Synergy Catalysis-Based Colorimetric Sensor for Visual Detection of Hydroquinone. Coatings 2024, 14, 453. https://doi.org/10.3390/coatings14040453
Xing N, Yang L, Wang L, Lu Y, Zhang H, Sun X, Zhao M, Tan W, Yang J. Cu/Mn Synergy Catalysis-Based Colorimetric Sensor for Visual Detection of Hydroquinone. Coatings. 2024; 14(4):453. https://doi.org/10.3390/coatings14040453
Chicago/Turabian StyleXing, Ningning, Lilin Yang, Li Wang, Yanxiang Lu, Hongkun Zhang, Xijun Sun, Min Zhao, Wenjie Tan, and Jie Yang. 2024. "Cu/Mn Synergy Catalysis-Based Colorimetric Sensor for Visual Detection of Hydroquinone" Coatings 14, no. 4: 453. https://doi.org/10.3390/coatings14040453





