Abstract
Interest in carbon materials has soared immensely, not only as a fundamental building block of life, but because its importance has been critical to the advancement of many diverse fields, from medicine to electrochemistry, which has provided much deeper appreciation of carbon functionality in forming unprecedented structures. Since functional group chemistry is intrinsic to the molecular properties, understanding the underlying chemistry of carbon is crucial to broadening its applicability. An area of economic importance associated with carbon materials has been directed towards engineering protective surface coatings that have utility as anticorrosive materials that insulate and provide defense against chemical attack and microbial colonization of surfaces. The chemical organization of nanoscale properties can be tuned to provide reliance of materials in carbon-based coating formulations with tunable features to enhance structural and physical properties. The transition of carbon orbitals across different levels of hybridization characterized by sp1, sp2, and sp3 orientations lead to key properties embodied by high chemical resistance to microbes, gas impermeability, enhanced mechanical properties, and hydrophobicity, among other chemical and physical attributes. The surface chemistry of epoxy, hydroxyl, and carboxyl group functionalities can form networks that aid the dispersibility of coatings, which serves as an important factor to its protective nature. A review of the current state of carbon-based materials as protective coating materials are presented in the face of the main challenges affecting its potential as a future protective coating material. The review aims to explore and discuss the developmental importance to numerous areas that connects their chemical functionality to the broader range of applications
1. Introduction
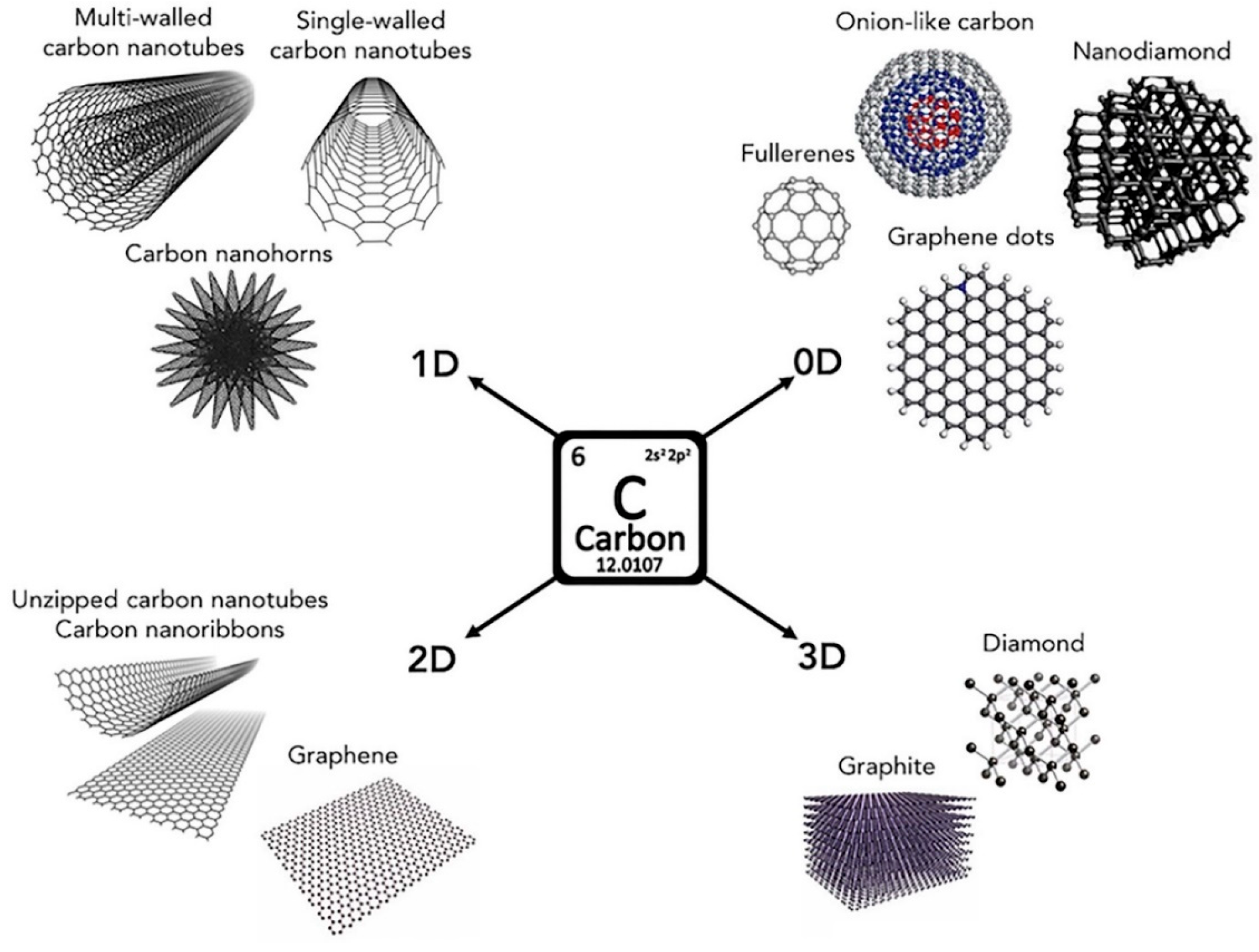
The elemental importance of carbon is signified by its position as the seventeenth most resourceful element that exists in the earth's outer layer, the fifth most abundant in the solar system, and the sixth most plentiful element in space [1]. According to the assessment, carbon’s relative abundance ranges between 180 and 270 parts per million (ppm) [2,3,4]. Carbon has intriguing and unique properties, and the properties of some carbon allotropes take the form of graphite [5], graphene oxide (GO) [6,7], graphene(G) [8], reduced graphene oxide(rGO) [9], fullerenes (C60) [10], carbon nanotubes (CNTs) [11], noncrystalline carbon [12], diamond morphology [13], and lonsdaleite [14]. As a result of its peculiar electronic structure, carbon can transition between different mixed orbital hybridizations, including sp1, sp2, and sp3 forms. Variation in orbital hybridization has led to different structures with several other distinctive properties [15]. The carbon allotropes possess distinctive characteristics, including impenetrability to gases [16], chemical (acid, base, and salt) endurance [17,18], antibacterial potential [19,20], thermal stability [21,22], environmental friendliness, and, more critically, high specific surface area [23]. It has been found that carbon allotropes can also provide a fertile research ground for enhancing protective surface coatings because of their flexible surface chemistry and useful property diversity, making them excellent research targets for advanced material development.
In general, coatings are materials or multilayers that are applied to the surface of bulk materials to preserve the primary or undercoat material. Coatings that are synthetic or engineered can be used either individually or in combination as composites that result in altered functional properties. Individual or property combinations are represented by hierarchical patterns that are morphologically and functionally different and are tunable to deliver material properties with desirable outcomes [24]. Biological damage, corrosion, fouling, and environmental damage are some of the main reasons why functional coatings are used [25]. Besides medical instruments, electronics, and everyday household appliances, a wide range of military and marine applications are available [26]. It should be noted that, in addition to the applications previously mentioned, functional coatings have also been developed to help remove contaminants such as heavy metals and dyes from different natural and fresh waters [27,28]. Due to this, protective coatings go beyond their usual functions of adsorbing toxic substances, killing bacteria, and protecting against frost, fire, and irradiation to also address corrosion, fouling, and mechanical wear as well.
It is well known that coatings are widely used for protecting materials. Nevertheless, some metal coating technologies can negatively impact the environment they are connected with. Since they possess both carcinogenic and biocidal properties, hexavalent chromium (Cr (VI)) and tributyltin (TBT) have been completely banned from use in protective coatings [29,30]. For example, Cr (VI) has been limited to a maximum of 0.1 wt% in corrosion-protective coatings [31]. TBT has also been banned from use in antifouling coatings since 2003 to safeguard against damage to marine environments [32]. Metal coating ingredients used in the form of cadmium (Cd), cobalt (Co), and copper (Cu) have also been determined to be detrimental to the environment and have shown to be carcinogenic to humans [33,34,35]. Cadmium has been identified to cause hazardous effects on the environment and is associated with toxicity and its use as a material has been prohibited in electronic devices and equipment since 2006 [36]. The search for ecofriendly alternatives to replace these materials and fulfill the key industrial and regulatory requirements applied to the coating industry has been challenging. However, diamond-like carbon (DLC) is an interesting material for protective coatings, with useful applications for material toughness and wear resistance, bearing low friction coefficients and thermal conductivity. Further, DLCs offer considerable chemical stability, gas-barrier capability, and high infrared-permeable properties. What is most important is that DLC coatings are biocompatible in nature [37]. Therefore, their applications are diverse, including electronics (e.g., hard disks, video tapes, and integrated circuits), cutting tools (e.g., drills, end mills, and razors), molds (e.g., optical parts and injection molding), automotive parts (e.g., piston rings, cams, clutch plates, pumps, and injectors), optical components (e.g., lenses), plastic bottle oxygen-barrier films, sanitary equipment, windows, bathtub mirrors, and decorative uses [38]. The material properties associated with DLCs have gained much recognition in recent years, increasing their potential use as protective coatings. An extensive account of DLC-based materials as protective coatings is out of the scope of this review due to its rather broad nature; a separate review dedicated to DLCs is anticipated in greater detail.
A carbon-based technology that relies on polymer-based composites is currently the forefront for driving functionalized nanomaterials with carbon as one of its core materials.
The economic impact of combing costly materials with polymers is considerable, as polymers can reduce the cost burden by lowering the requirement of expensive materials in forming composites while maintaining material performance. This strategy has paved the way for fabricating novel protective coating products, particularly graphene-based composites, comprising paints and other useful formulations. With the availability of property-based materials, a broad range of applications is possible by tuning the compositional nature at the microstructure level of the final coating to enhance the level of protection provided by functionalized carbon. Such composites provide the freedom to modify and regulate the characteristics of the final product by altering the components in isolation. Hence, a functionalized carbon coating can deliver superior protective attributes with few shortcomings, depending on the application using (1) pure carbon coating, (2) a carbon-like coating, or (3) a functionalized carbon-enhanced composite-type coating. In this regard, remarkable progress in the advancement of innovative protective coatings has been achieved, aided by such technologies through the customization of functionalized carbon.
This review aims to consider recent developments related to the application of multipurpose carbon-based coatings. With reference to preventing corrosion, fouling, thermal or radiation damage, and contamination, the distinctive physical and chemical assets of carbon allotropes exhibited by different types of coatings will be discussed. The intrinsic protective mechanisms utilized by graphene, graphene oxide (GO), and/or reduced graphene oxide (rGO) are discussed as a prelude to their applicability, which further warrants an in-depth discussion of the merits of their technological importance and potential as engineered materials in design and function. The review also brings into view an analytical perspective on key developmental challenges and highlights future prospects of carbon-based materials in the evolution of protective-coating technologies.
2. Functionalized Carbon-Based Systems
All nanometric forms of carbon discovered to date, including graphite, diamonds, graphene, monohorns of carbon, fullerene, and carbon nanotubes (CNTs), are known as carbon nanoallotropes. A pictorial representation of carbon-based nanoallotropes is shown in Figure 1. Solubility limits the use of carbon allotropes in industrial and biological applications [39]. The solubility of carbon allotrope molecules may be enhanced through functionalization, achieved by introducing polar substituents into the molecular framework as an effective method of improving the solubility of carbon allotropes. The aromatic nature of carbon allotropes in their oxidized form (rGO) is more suppressed while GO exhibits nonaromatic composition, with carbon atoms hybridized with a sp3 configuration. Several deviations to the planarity of the structure are attributed to the presence of sp3 carbon atoms that are bonded to oxygen moieties. A variety of covalent and noncovalent techniques can be used for functionalizing carbon allotropes [15,40]. In the case of covalent functionalization, carbon allotropes and polar substituents are covalently coupled, while weak intermolecular forces (van der Waals forces) occur during noncovalent functionalization.
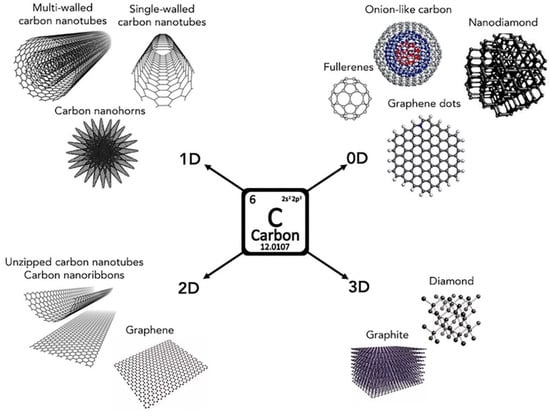
Figure 1.
Carbon allotropes that are commonly found in nature. Reprinted with permission from [2] 2020 Elsevier.
2.1. Non-covalent Approaches for Functionalization
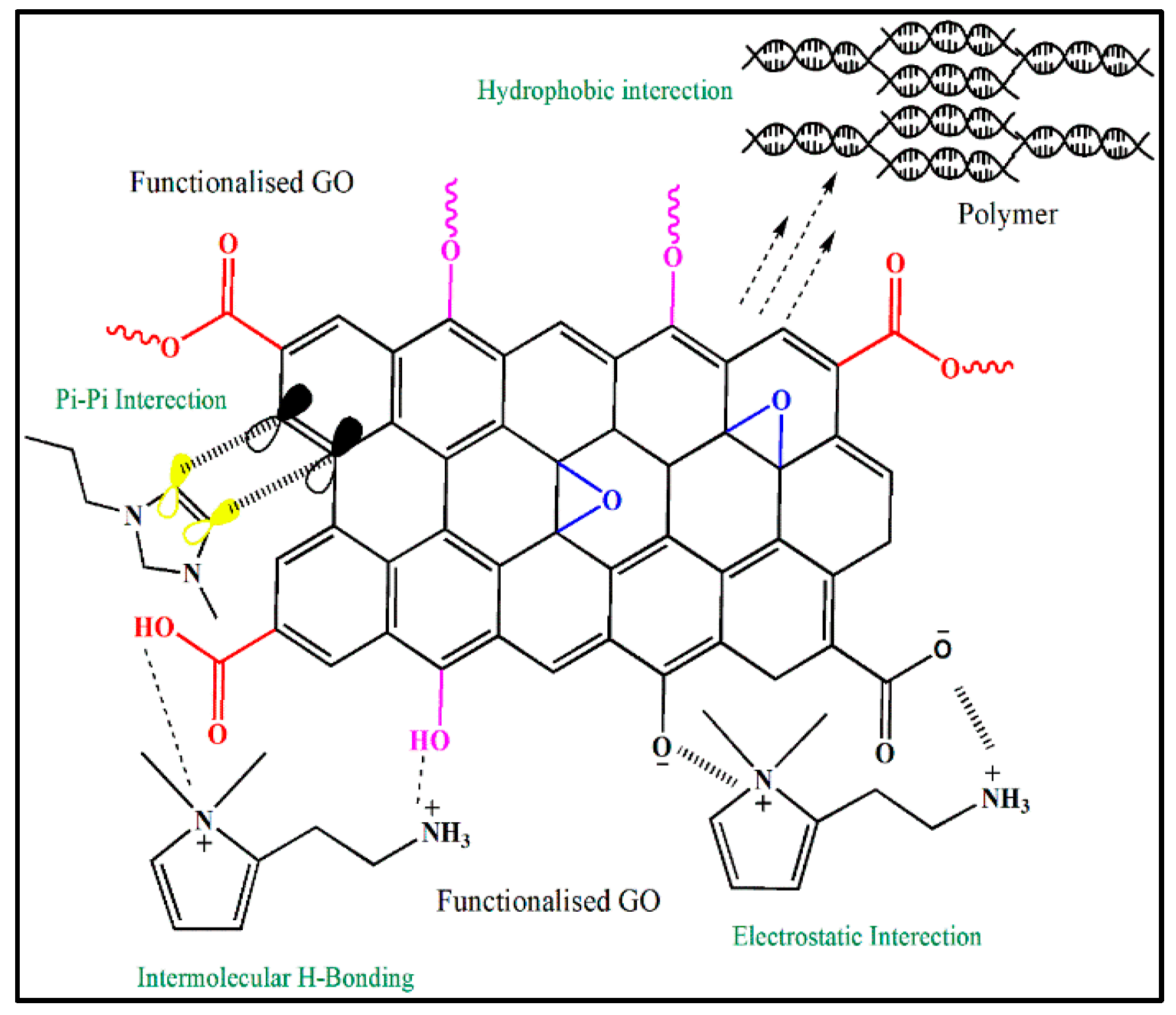
There are several non-covalent functionalization methods using several approaches. Hydrophobic and electrostatic interactions, van der Waals forces of attraction between molecules, and a bundled assemblage conforming to a condensed state feature strongly in the commonly used non-covalent functionalization approaches that are applied to carbon allotropes [41,42]. Non-covalent functionalization provides useful routes to alter surfaces, thus permitting surface functionalization without chemical disruption of the surface structural framework of the carbon allotrope and resulting in changes to the physical and electrical properties following functionalization. Graphene analogues, such as single-walled carbon nanotubes (SWCNTs) and multiwalled carbon nanotubes (MWCNTs) have, in recent years, undergone non-covalent functionalization. It is generally observed that in this mode of interaction, the πe− density of the benzene ring(s) (e.g., pyrene and perylene) to some degree overlaps with the πe- region of the carbon allotrope. Several non-covalent functionalization routes of GO can be seen in Figure 2. The aromatic regions of carbon allotropes are often directed to undergo π-π stacking associations. Interfaces between carbon allotropes (with supermolecules, surface-active agents, ionic liquids, etc.) can be categorized as hydrophobic. The π–π cooperativity has been reported to occur in polymers such as Kevlar [43], polyvinyl chloride, sulfonated polyaniline [44], and polyethylene glycol [45]. To investigate the electron–donor–acceptor interactions in aromatic compounds with graphene nanosheets, pyrene and perylene diimide derivatives were used. Carbon allotropes (G, GO, CNTs, etc.) work together hydrophobically with supermolecules, ionic liquids, and surfactant molecules to form stacked configurations on carbon allotropes and differ from the stacked orientation used by aromatic compounds on carbon allotropes. Both aqueous and organic solvents disperse carbon allotropes by virtue of their dispersion strength arising from chemical interactions between the carbon allotrope and the solvent environment. Electrostatic interactions play a large role in the interaction between ionic liquids and GO (conceivably with different allotrope types), in part because GO contains a variety of ionic groups, such as hydroxylates and carboxylates, which can engage in electrostatic interactions. Hydrogen bonding is not only capable of stacking and hydrophobic and electrostatic interactions but can also occur between allotropes of carbon in addition to stacking.
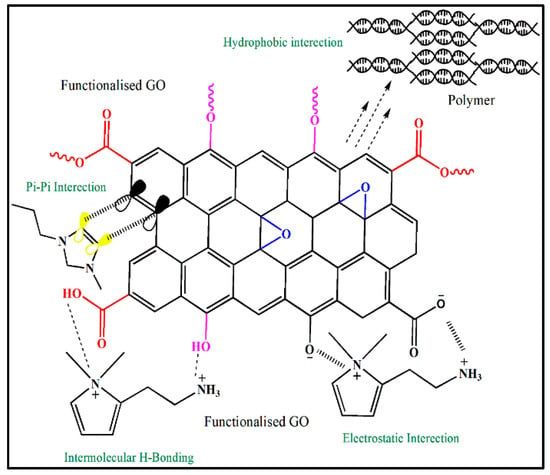
Figure 2.
Non-covalent functionalization of GO.
2.2. Covalent Approaches for Functionalization
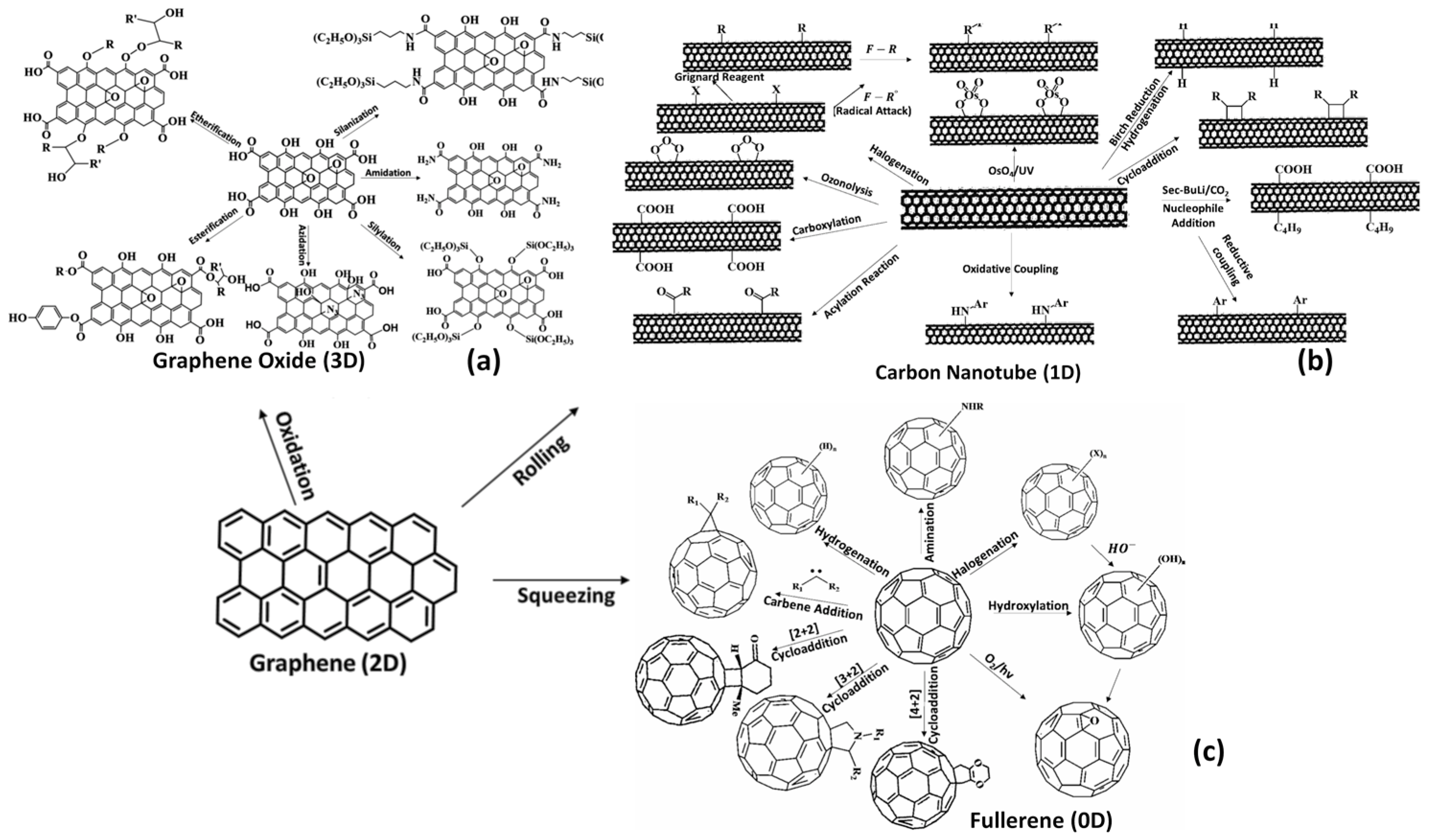
Two kinds of target sites are available on the surface of carbon-based materials such as graphene oxide (GO) and its analogues, which are amenable to undergoing covalent functionalization and can be described by the following events: (i) oxygen-containing groups such as −OH as well as −C-O-C on the basal plane, as well as −COOH at the edges, where new species might be introduced via condensation and esterification; and (ii) −C=C functionality, which requires the attacks by free radicals or dienophile species [46,47]. Commonly used strategies for functionalization of GO include: (i) acylation; (ii) amidation; (iii) silylation; (iv) esterification; (v) cycloaddition; (vi) etherification; and (vii) azidation. The −COOH groups at the edges are possibly modified by amidation or esterification. There have been several amines used in the past in order to covalently functionalize graphene oxide (GO) and, more specifically, C60 fullerenes, porphyrins, polythiophenes, and phthalocyanines. It is possible to activate hydroxyl groups in several ways, namely through salinization, silylation, and etherification.
There are numerous chemical processes that can be used to modify the GO, including ring-opening actions with epoxides, amine (R-NH2), and alcohol (R-OH). A wide range of poly(allyl amine) and poly(vinyl alcohol) moieties can be attached to ringed structures using these reactions. Functionalization of > C=C < may be performed by free-radical-catalyzed cycloaddition reaction (Diels–Alder type reaction). Carbons undergoing sp2 hybridization can transition to sp3 during cycloaddition, which reduces GO’s ability to conduct electrons. This is shown in Figure 3a.
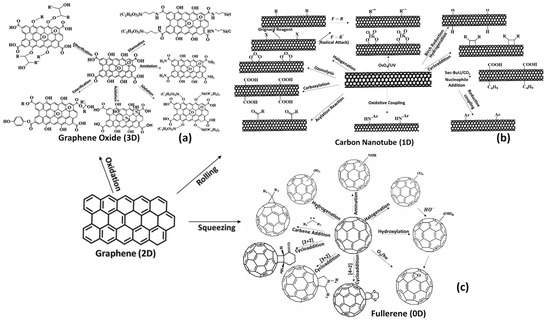
Figure 3.
(a) A scheme showing the modification of graphene and GO using commonly applied functionalization chemical/physical strategies. (b) Surface functionalization of carbon nanotubes. (c) Surface functionalization of fullerene.
A carbon nanotube (CNT) is a cylinder of carbon with a diameter measured in nanometers (nm) and a length also measured in nanometers (nm). Carbon nanotubes are divided into single-walled carbon nanotubes (SWCNs) and multiwall carbon nanotubes (MWCNs) [41]. Van der Waals interactions can provide an avenue for multiple CNT ring connections to form MWCNTs. In material science, CNTs and their derivatives (functionalized CNTs) are used for a variety of applications due to their exceptional mechanical properties and physical properties due to their diminished size. It is important to note that CNTs exhibit exceptional electrical conductivity and are prone to corrosion in aqueous media, as they enhance electron transfer. Despite their various advantages, CNTs are thus limited in their application as corrosion inhibitors, especially in aqueous phases. The desirable properties of anticorrosion can, however, be achieved by covalently functionalizing them. Surface modification of SWCNTs and MWCNTs covalent means can be accomplished using chemical strategies (Figure 3b). Such changes allow the adaptation of CNTs (SWCNTs and MWCNTs), allowing them to undergo a number of physiochemical changes as a result of covalent functionalization, which can be described by: (i) An enhancement in overall dispersion properties; (ii) interfacial compatibility assessed by adhesion and diminished hydrophobicity; (iii) a reduction in aggregation; (iv) a reduction in mechanical properties; and (vi) a reduction in electrical conductance. The functionalized state of GO and CNTs (SWCNTs and MWCNTs) retains the capacity to undergo further chemical refinement by targeting peripheral moieties, which remain exposed for further functionalization [43,44]. The biological and industrial applications of other allotropes of carbon can also be enhanced through covalent functionalization using different chemical species, as can GO and CNTs.
The chemistry of fullerene has been thoroughly studied and investigated to date. The functionalization chemistry of fullerene is more or less similar to the approaches used to functionalize carbon nanotubes and graphene oxide, as shown in Figure 3c.
3. Adaptation of Carbon as a Protective Coating via Surface Functionalization
3.1. Functionalized Coated Carbon for Anticorrosion Applications
Recent investigations have established that corrosion barrier coatings that have been fabricated from functionalized carbon, graphene, and graphene oxide and their composites, are effective anticorrosion layers, which also take the form of alloys used in the range of electrolytes. The reported use of chemically modified GO, however, has been rather limited, with relatively fewer examples of anticorrosive properties compared to its metallized counterparts. This may partly be explained by lower levels of polar electrolytes than in nonpolar electrolytes. Only a few reports have been published about the use of carbon support that has either been functionalized or modified with suitable organic compounds as effective corrosion inhibitors in an aqueous solution. Anticorrosion behavior of different organic functional groups, which can be used to functionalize carbon supports for designing an effective corrosion inhibitor system in an aqueous solution, are summarized in Table 1. Due to its inherent binding tendency and high surface area, chemically modified GO exhibits a relatively high protection capacity.

Table 1.
Different chemically functionalized carbon-based anticorrosion coatings in aqueous phases.
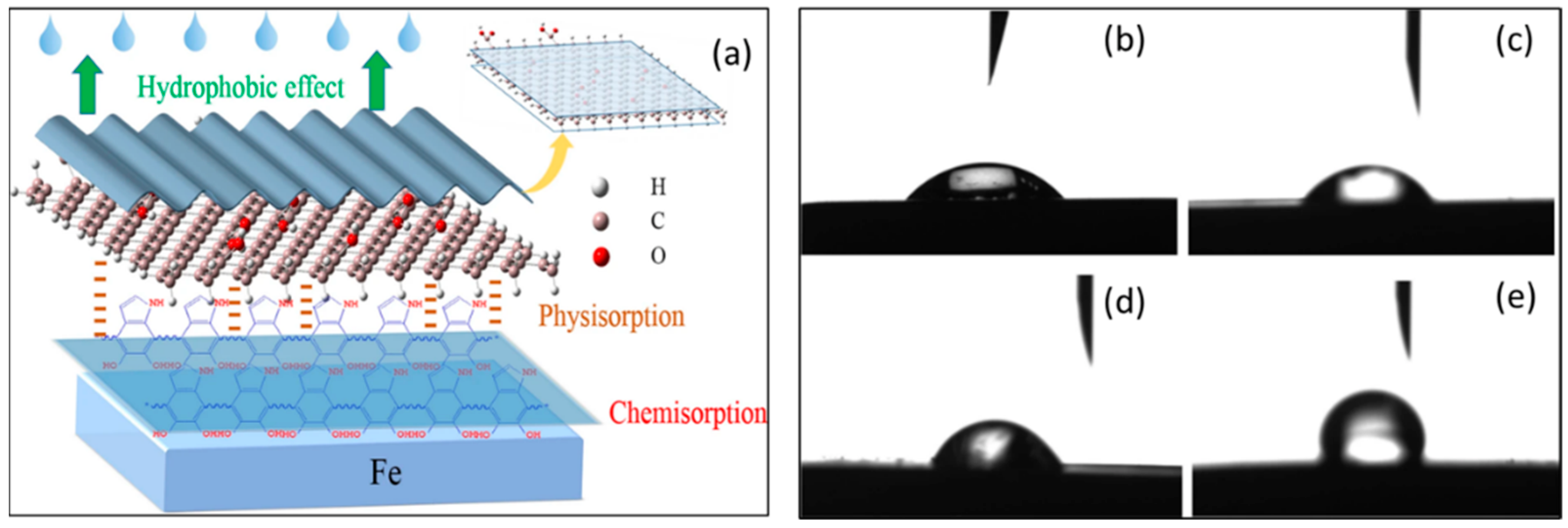
Recently, covalent modifications and self-polymerization have been used to functionalize graphene oxide (GO) with polydopamine (PDA), which was then tested as a corrosion inhibitor in HCl solutions against carbon steel. At 100 mg/L, GO-PDA achieved almost 90% inhibition efficiency, which is a high level of corrosion protection for carbon steel. An investigation of the interaction between immersion time and concentration was conducted. A hydrophobic film was formed in micro–nano structures by lamellar functionalized GO adsorbed onto the surface of carbon steel [48]. The composite carbon material of this dense protective film was extremely hydrophobic, inhibiting the penetration of corrosive agents, as shown in Figure 4a. In this study, a transition from a GO-surface that had a negatively charged surface to a positively charged GO-PDA that significantly enhanced the adsorption at the material intersection. The contact angle measurement study, as shown in Figure 4b–e, evidently showed that the GO-PDA cover layer was hydrophobic in nature, which was corroborated by contact angle measurements at the interface.
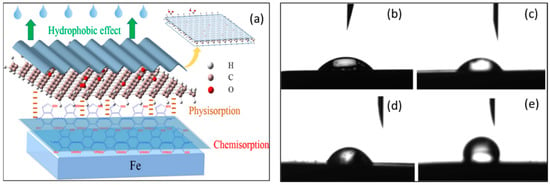
Figure 4.
(a) The figure shows a mechanism showing how GO-PDA is inhibited by metal surfaces when it is in the solution. Contact angle measurements on the surface of carbon steel were measured for four different scenarios, including (b) blank, (c) 60 mg/L GO, (d) 60 mg/L PDA, and (e) 60 mg/L GO-PDA (b–e). Reprinted with permission from [48] 2022 J Mater Sci.
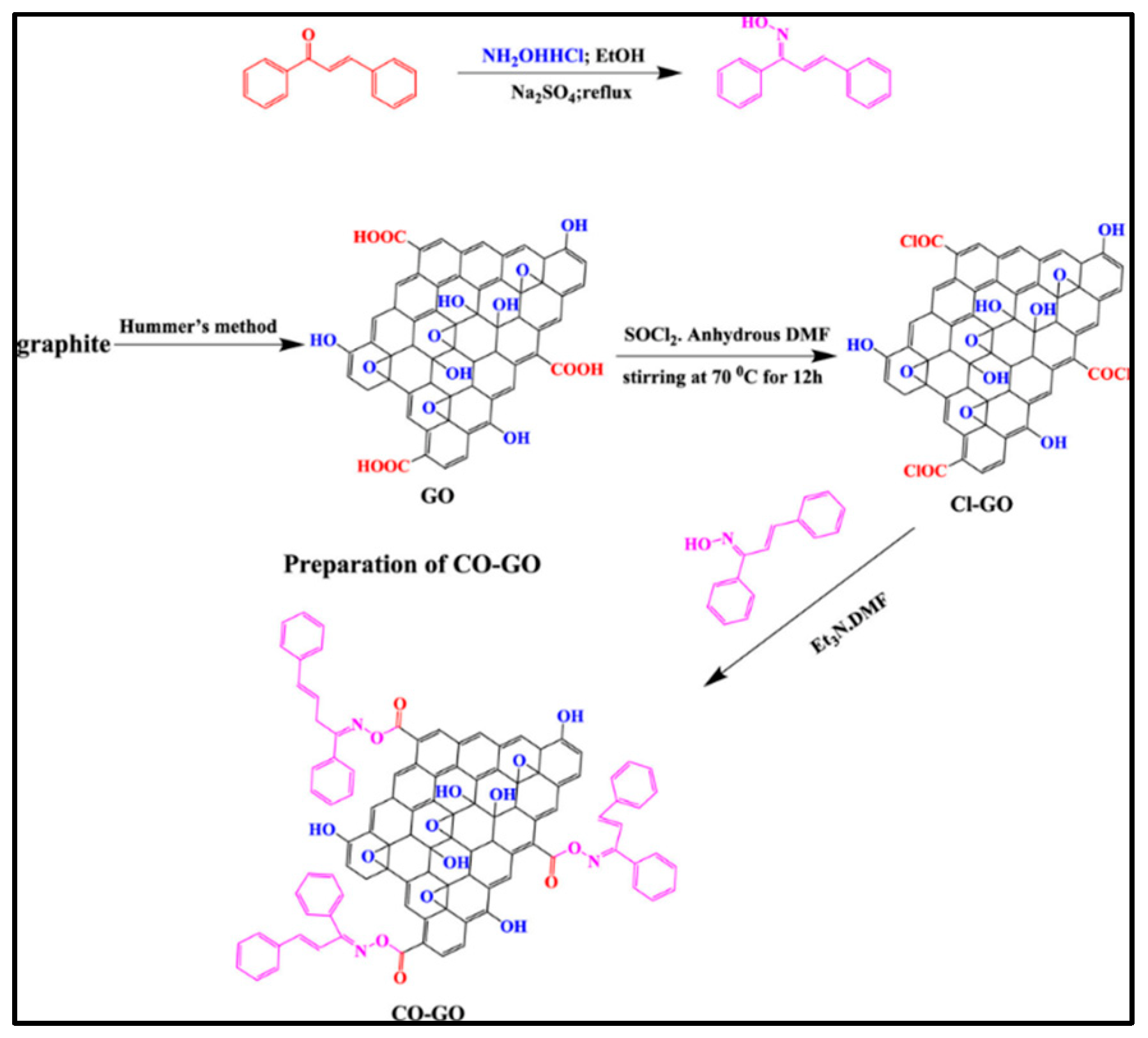
It has been recently demonstrated that 1,3-diphenylprop-2-en-1-one oxime–graphene oxide (CO-GO) can be produced from chalcone, hydroxylamine, and a Cl-modified graphene. For 12 h, the mixture was continuously stirred at 80 °C at a constant speed [72]. The process was completed by subjecting CO-GO to a spinning cycle, followed by a washing process in water and ethanol, and finally drying at 60 °C, as shown in Figure 5. Further, potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) were utilized to investigate corrosion inhibition effectiveness. In addition to its outstanding corrosion inhibition performance, CO-GO exhibits a primarily anodic mechanism with a mixed-type inhibitor function of up to 94%.
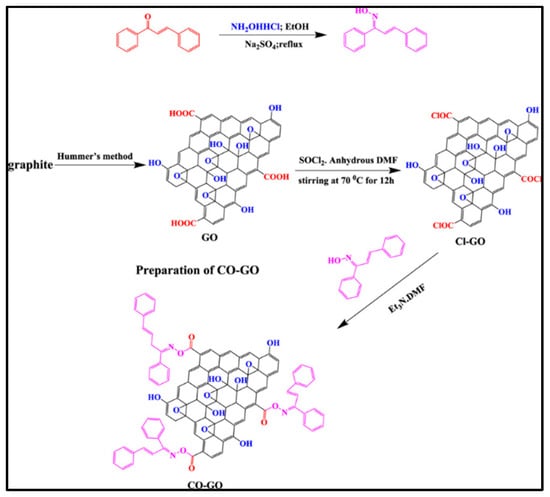
Figure 5.
Scheme for the synthesis of chalcone oxime (CO)-functionalized graphene oxide. Reprinted with permission from [72] 2022 ACS Publ.
A variety of techniques have been employed to confirm inhibitor adsorption onto the carbon steel surface, including scanning electron microscopy and ultraviolet-visible spectroscopy.
The presence of polar substituents on functionalized carbon not only enhance the solubility of carbon-based materials in aqueous electrolytes, but also act as an adsorption center during metal–inhibitor interaction. Surface corrosion protection is obtained by the transfer of nonbonding electrons from the functionalized polar group and/or from the electrons on the aromatic or heteroaromatic rings to the metal center. In general, functionalized carbon-based systems adsorb onto metallic surfaces in two steps. The functionalized carbon-based system is transferred from the bulk solution onto the metal surface in the initial step. Adsorption occurs through coordination bonding in the second step. Electrons transferred from a functionalized carbon-based system to a metal surface without forming bonds are known as donations. Due to the interelectronic repulsion, even metals that are already electron-rich may become thermodynamically unstable as reactions result in electron transfer. Consequently, electrons may be transferred back into heteroatoms' empty p-orbitals during the interaction of carbon-based materials. The process is known as retro (back)-donation. As a result of a phenomenon known as synergism, donations and retro (back)-donations strengthen one another.
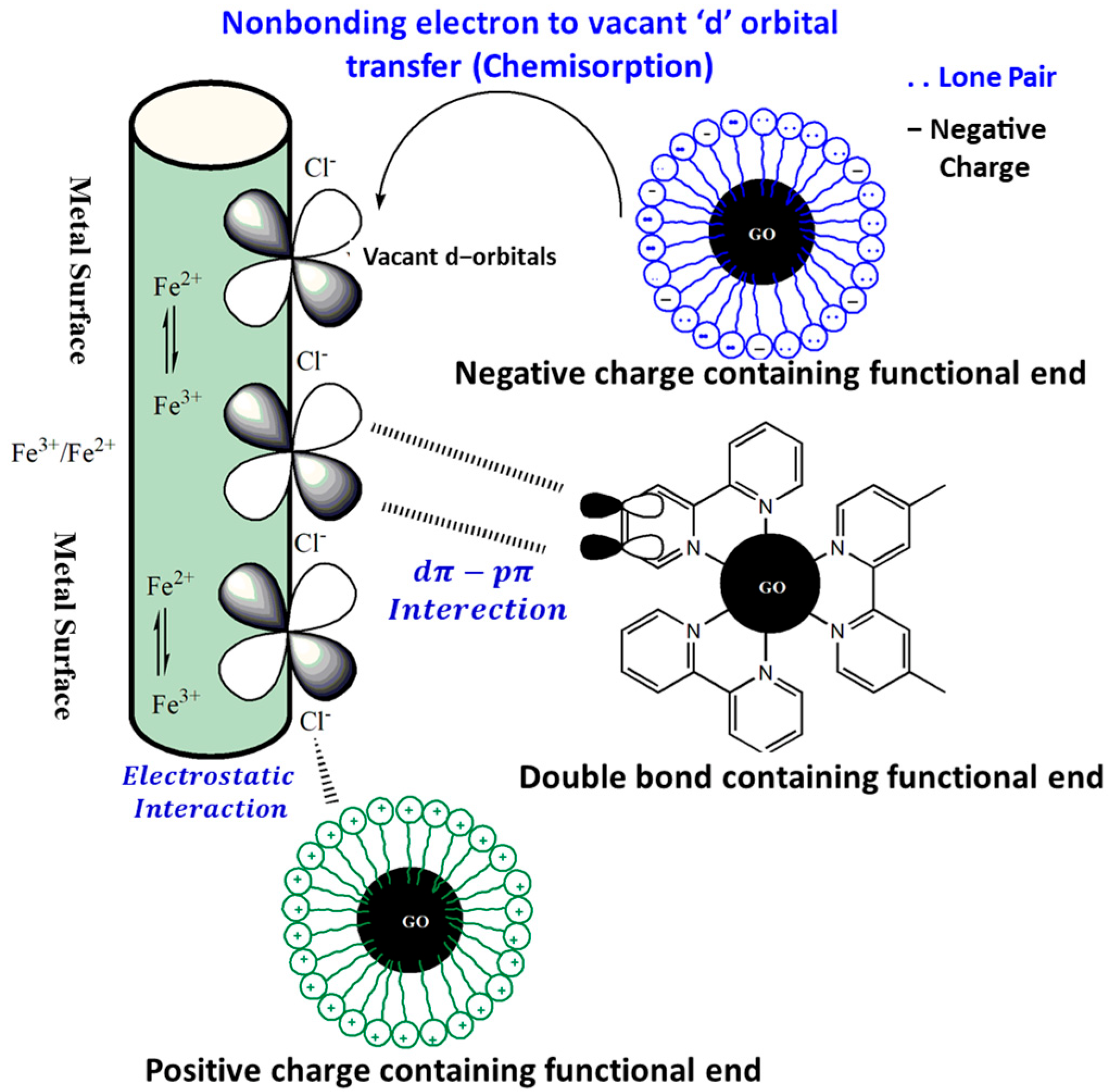
Electrostatic force of attraction is another means of interfacing functionalized nanomaterials with metallic surfaces besides chemical (coordination) bonding by means of physisorption. The adsorption of counter ions on positively charged metallic surfaces in aqueous electrolytes incite a negative charge to the metallic surface. In a separate event, protonation of a functionalized nanomaterial inhibitor group induces a positive charge and drives an electrostatic force of attraction to the negatively charged metallic site, resulting in the induction of physisorption. Figure 6 depicts chemisorption and physisorption in a pictorial manner. The most common mechanism for adsorption of organic corrosion inhibitors is the mixed mode, also known as physiochemisorption. GO and its derivatives are described in this article for their corrosion-inhibition properties.
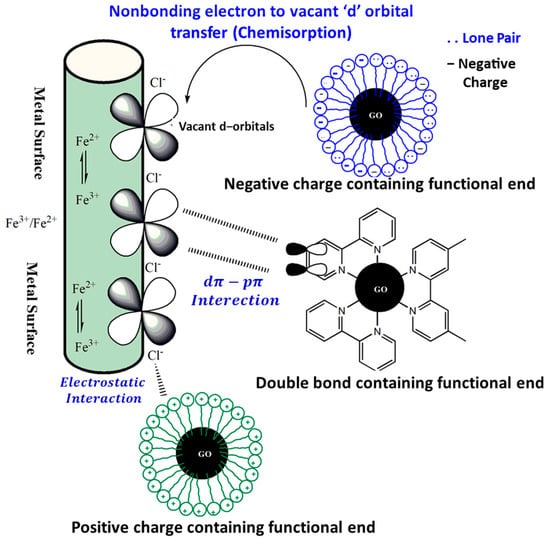
Figure 6.
The interaction of metal surfaces with functionalized graphene oxide is illustrated in a series of pictures.
3.2. Functionalized Carbon for Flame-Retardant Coatings
The boundary region and basal planes of GO forming the graphene precursor constitute an abundance of epoxy, hydroxyl, carboxyl, and -C=C- groups which are freely available for functionalization [73]. Surface conversion of hydrophilic GO to its congruent hydrophobic state facilitates the dispersion of graphene sheets in the polymer matrix, as shown in Figure 7 [74]. The functionalization-based approach has been successfully applied in order to prepare organic flame retardants with functionalized graphene. With the help of this strategy, it is generally possible to covalently link organic flame retardants to graphene or GO in two steps: (i) Providing organic flame retardants with functionalities to enable their reaction with graphene or GO and (ii) reducing functionalized graphene to functionalized graphene using reducing agents. Modifiers that also serve as reducing agents can occasionally functionalize and reduce GO at the same time. A few examples of organic flame retardants functionalized with graphene, as well as the polymer composites that contain these materials, are summarized in Table 2.
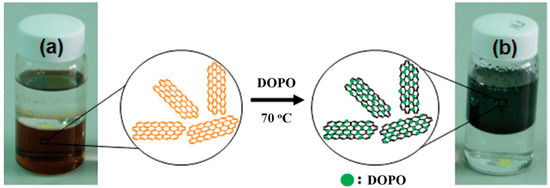
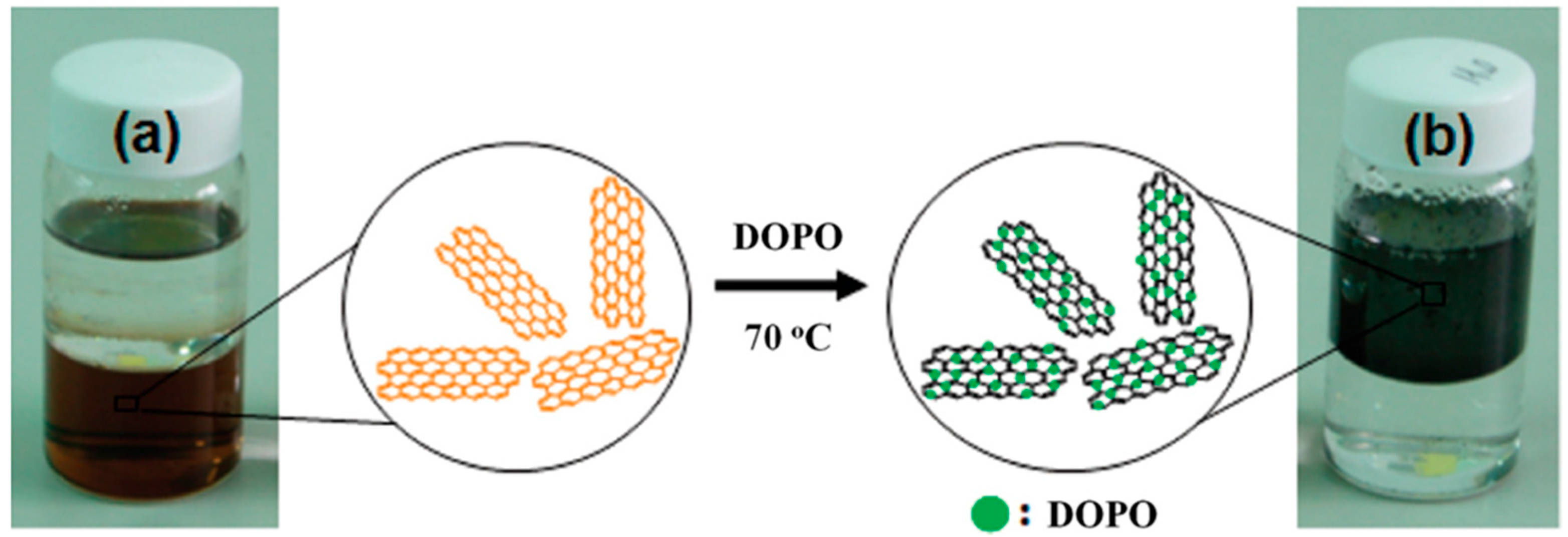
Figure 7.
Photographs of (a) GO and (b) DOPO-reduced GO dispersions in water (bottom) and toluene (top) for 1 month. Reprinted with permission from [74]. 2012, Ind. Eng. Chem. Res.

Table 2.
Summary of flammability of polymer nanocomposites based on functionalized graphene oxide.
burn-out limits of GO have been overcome in the past few decades by grafting several synthetic compounds onto it, including 9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) [84,85], 2-(diphenylphosphino) ethyltriethoxy silane (DPPES) [78], hyper-branched flame retardants [86], polyphosphamide (PPA) [80], and poly(piperazine spirocyclic pentaerythritol bisphosphate) (PPSPB) [82]. Data on the limiting oxygen index (LOI), the UL-94 flame retardancy rating, and the peak heat-release rate (PHRR) for functionalized graphene sheets with organic flame retardants were compared; pristine graphene sheets and functionalized graphene sheets were found to exhibit better flame-retardant properties. The test results indicated that epoxy composites containing a mixture of DPPES-graphene at a loading of 10 wt% were capable of passing the UL-94 V-0 rating—in contrast to their counterparts containing equal amounts of either graphene or DPPES.
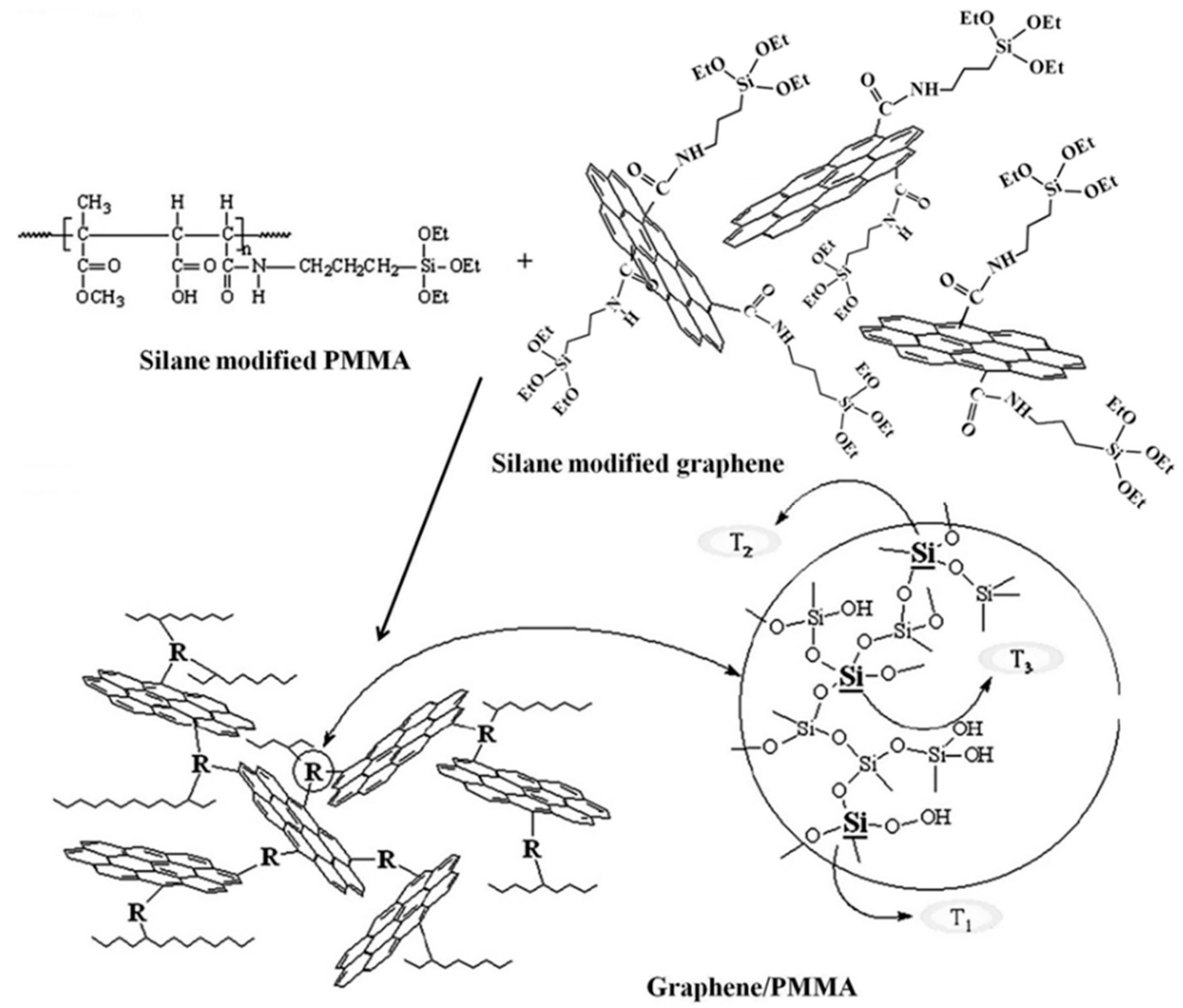
It has also been found that graphene can be functionalized with flame retardants in addition to sol–gel chemistry. Flame retardants modified with siloxane can facilitate reactions with silane-modified GO or silane-modified graphene, permitting a chemically modified interface between the retardant and graphene/GO surface formed via the silane-assisted hydrolysis of siloxane, resulting in a condensed structural composite. Figure 8 describes the step-by-step process for the functionalization of GO/PMMA composites. Several polymers, such as epoxy resins, polyuria (PU), polyvinyl alcohol (PVA), and polymethyl methacrylate (PMMA), can be made more flame-resistant by adding graphene or graphene oxide.
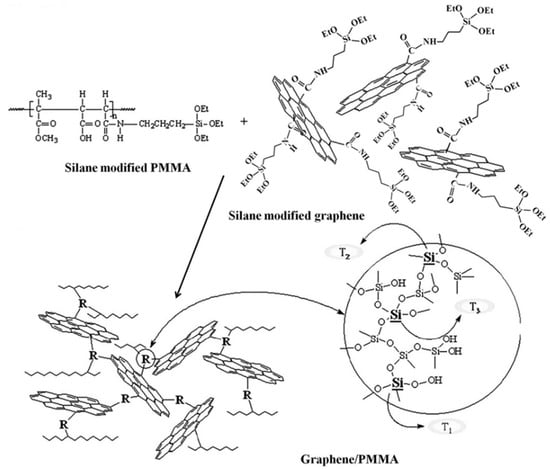
Figure 8.
Schematic showing the use of the sol–gel method to prepare functionalized graphene/PMMA composites. Reprinted with permission from [86]. 2012, Mater. Chem. Phys.
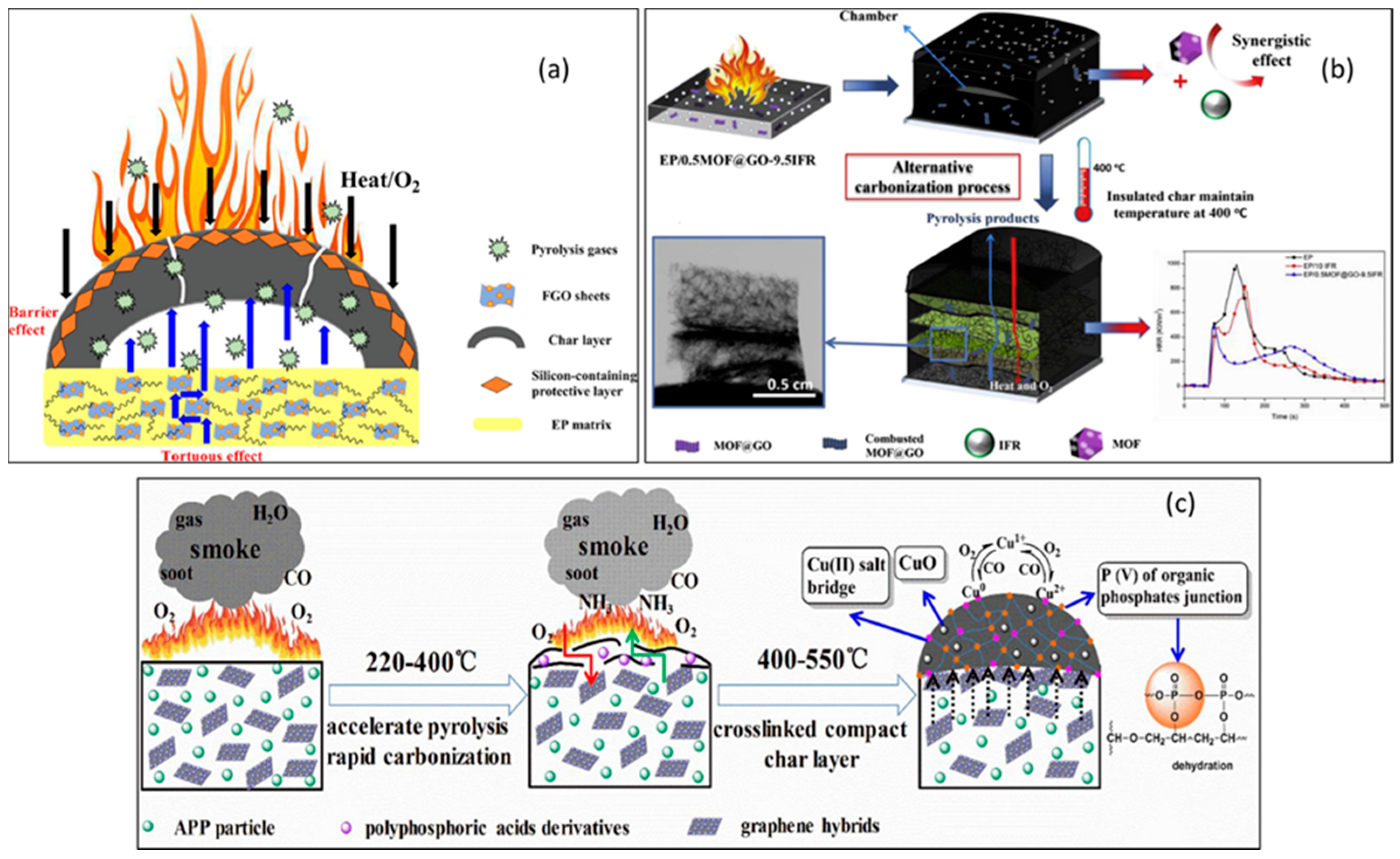
Flame retardants made from functionalized carbon can be effective for polymer materials because of its excellent thermal conductivity, thermal stability, and physical barrier properties. As a result of intensive research on graphene and its derivatives in epoxy resins [78], investigations have concluded that functionalized carbon is flame-retardant in three important areas. Layered structures of sp2-hybridized carbon backbones that prevail in graphene and its derivatives can serve as physical barriers, slowing down heat transfer and preventing the initial stages of matrix decomposition. An investigation was conducted by Qu et al. to understand the mechanisms of flame retardancy of epoxy resin with the addition of POSS (octa glycidyl ether) groups to GO, as shown in Figure 9a [87]. The decomposition of the matrix is inhibited by a tumultuous path effect during the dispersion of the FGO in the epoxy resin matrix as a result of a tortuous path effect that inhibits the diffusion of heat and the degradation of combustible gases. A further benefit is that, when the OGPOSS groups contain Si, the char layer can be strengthened in such a way that heat transfer and oxygen mixing with pyrolysis gas can be prevented, thereby enhancing the flame-retardant properties of epoxy resin as well as its capability to suppress smoke emission. There is also evidence that graphene can form harmless gases (e.g., NH3) when functionalized with organic elements (e.g., compounds containing nitrogen). This gas dilution hinders the combustion of the epoxy resin matrix material. Phytic acid (PA) and piperazine (PiP) were added to graphene oxide by Fang et al. in order to produce PiP-functionalized graphene oxide (PPGO), as shown in Figure 9b [88]. Finally, the benefit of graphene that has been functionalized by inorganic functional groups is that it leads to the formation of a dense, stable, and heat-resistant char layer and reduces the heat loss and smoke emissions from combustion processes. The combination of graphene compound (Cu2+-GO) and ammonium polyphosphate (APP) can enhance flame retardation in epoxy resins, as described by Ye et al. (Figure 9c) [89].
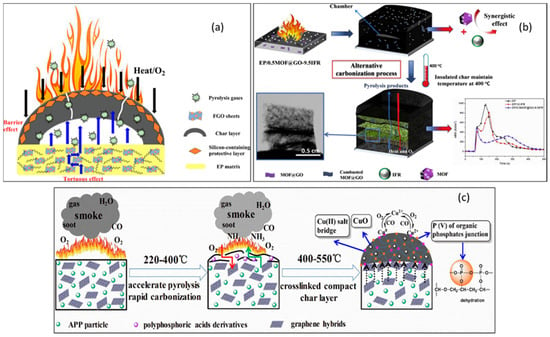
Figure 9.
(a) Flame-retardant mechanisms in FGO/EP. Reprinted with permission from [87] 2020, Eur. Polym. J. (b) an EP-composite flame-retardant-mechanism model. Reprinted with permission from [88]. 2019 Carbon and (c) graphene-based EP/APP composites with flame-retardant mechanisms. Reprinted with permission from [89]. 2019, Compos. Part B Eng.
3.3. Functionalized Carbon for Enhanced Antifouling Coatings
There are many problems associated with fouling on marine vessels and structures. Fouling is caused by the deposition of organic and inorganic materials, which can cause a wide variety of problems, including fouling of cargo ships, naval vessels, recreational vessels, heat exchangers, and sensors for oceanographic investigations, as well as offshore platforms such as oil rigs, desalination processes, and aquaculture systems. For the U.S. navy fleet alone, there are estimated to be maintenance costs from USD 180 million$/year to 260 million$/year due to fouling and surface setting and hardening of hard-to-remove deposits that attach to ships. This includes fuel costs, powering penalties, and maintenance expenses due to particulate fouling. TBT is currently banned in antifouling coatings, which introduces new challenges for developing antifouling technologies. Cost reduction is a major objective in this sector and the development of cost-effective materials with antifouling properties is predicted to reduce the economic burden substantially.
A variety of strategies have been developed as a means of preventing successive fouling of surfaces as well as fighting both inorganic as well as organic foulants. There are several strategies to maintain cleanliness on surfaces, which include surfaces with self-cleaning properties, biomimetic structural design, and the use of biocidal chemical mediators to deter fouling. Amphiphilic modified surfaces are also a recent development [90,91]. An antistick coating agent based on carbon is a promising option. Graphene surfaces can be tailored to achieve the surface polarity and wettability desired for antifouling coatings. In the presence of superhydrophilic and superhydrophobic graphene coatings, particle fouling can be prevented. Coated membranes composed of graphene can eliminate salt more effectively because of its impermeable structure. The use of carbon materials in antifouling coatings is not a novel finding since their properties are known to be associated with antifouling behavior discouraging the settlement of marine organisms and have been extensively studied in the field. A recent study of allotropic carbons as ecofriendly coatings has inspired work to harmonize the use of graphene into antifouling applications as a result of its foul-releasing properties, surface flexibility, selectivity towards protein adhesion, and covalent polymer attachment which is tunable by the degree and nature of surface functionalization.
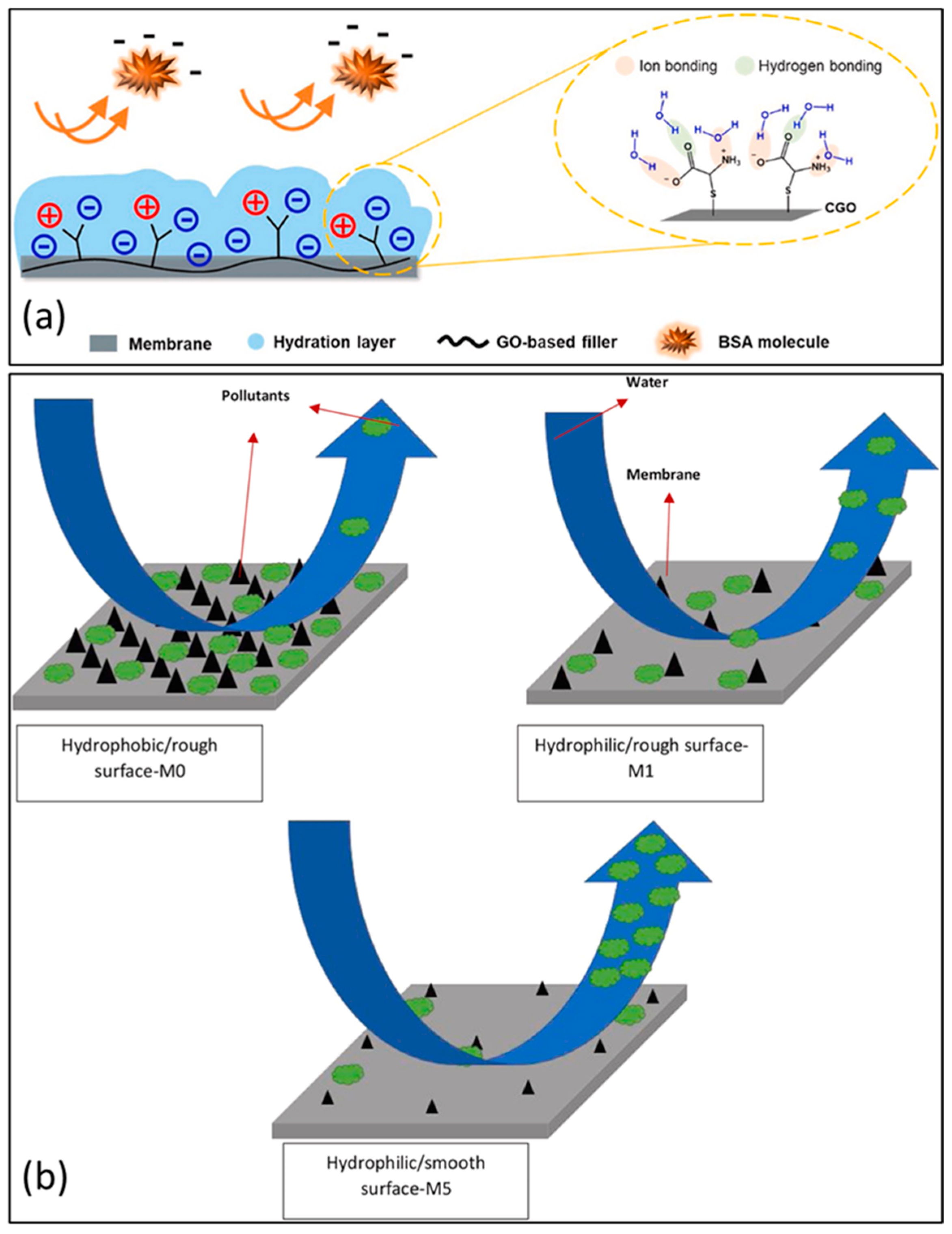
Common organic matter that act as foulants in membrane-fouling-resistance studies are bovine serum albumin (BSA) and humic acid (HA), as shown in Figure 10a. The hydrophobic properties of these organic materials, in addition to their negatively charged surfaces, are their distinguishing characteristics. Cysteine-functionalized graphene oxide (CGO) composites can impose surface densities that are negatively charged in PES nanocomposite membranes. Increase in the CGO concentration from 0 to 0.4 wt% in the PES membrane led to improvements from −17.7 mV and −28.7 mV for surface zeta potential. Negative-charge density is attributed cysteine’s high ionic charge density that resides in CGO-modified membranes, irrespective of the change in pH [1].
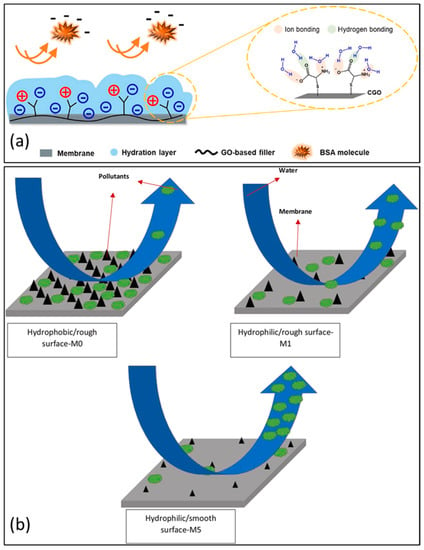
Figure 10.
(a) Scheme showing that hydrogen and ionic bonding sites can be provided by cysteine-functionalized graphene oxide to the PES membrane to inhibit protein adsorption. Reprinted with permission from [1]. 2020, J. Ind. Eng. Chem. (b) the mechanism by which protein fouls the water during the cleaning process. (GO: 1 weight percent; Cs-GO: 1 weight percent, neat PES membrane). Reprinted with permission from [92]. 2018, J. Ind. Eng. Chem.
It has also been established that the roughness of GO-modified membranes may also play a role in the antifouling capabilities of the membrane and its ability to be hydrophilic. Contrary to the hydrophobic nature of the surface of the unmodified PES membrane and its rough surface, the smooth surface of the composite PES membrane allows foulants to be easily discarded. In Figure 10b, the schematic shows how surface cleaning is mechanistically independent of membrane hydrophilicity and surface roughness. Owing to the enhancement of surface hydrophilicity allied to the 1 wt% Cs-graphene-oxide-modified PES membrane and low surface roughness of the membrane, protein foulants on the membrane can be effortlessly removed via surface scrubbing.
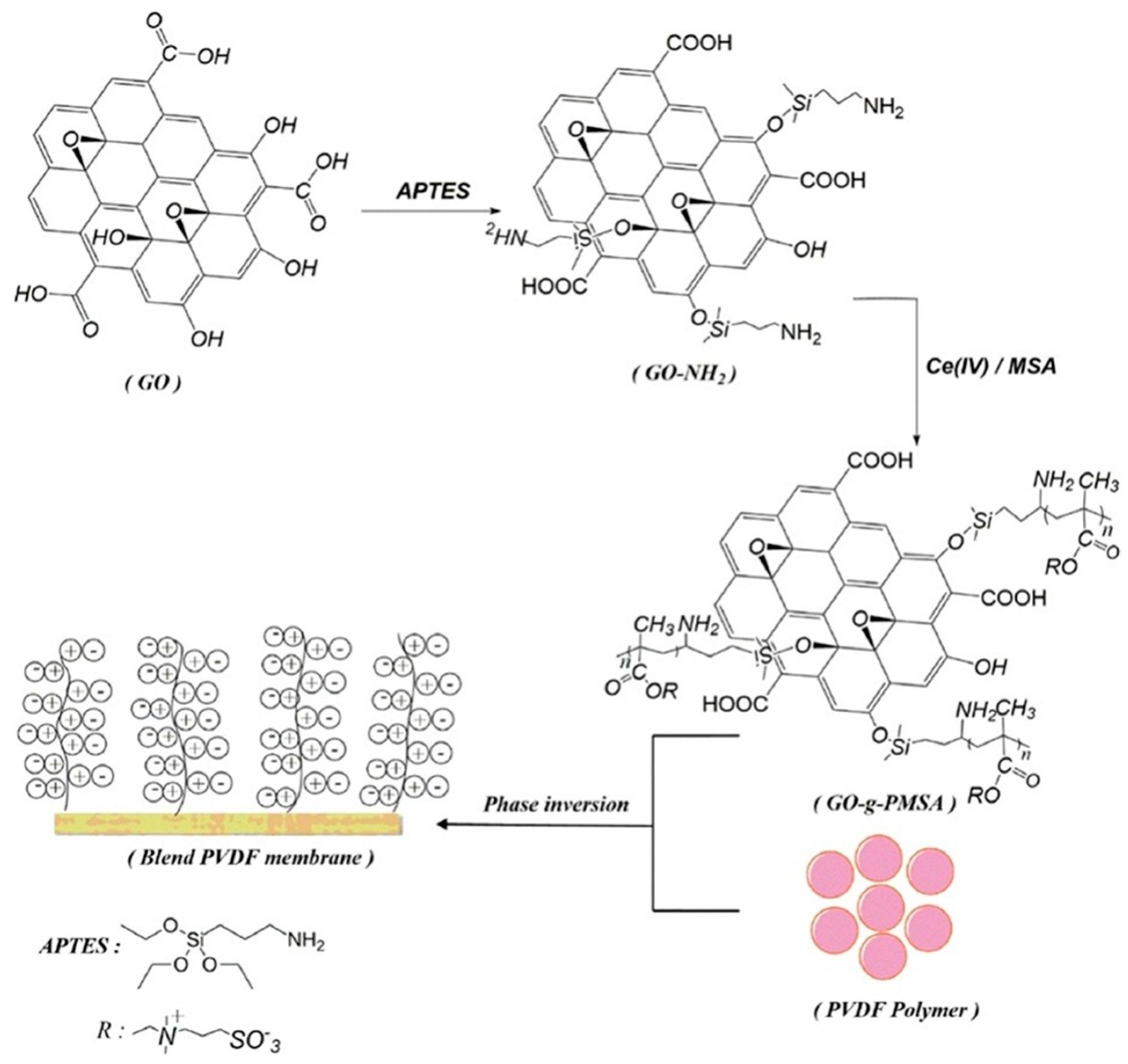
The antifouling properties of zwitter ionic surfaces are effective in protecting surfaces against foulants. The modification of graphene oxide with zwitter ionic groups (GO-g-PMSA) and its incorporation into polyvinylidene fluoride (PVDF), as shown in Figure 11, generates two advantages: it increases the graphene oxide dispersibility in the membrane matrix and supplements GO with an increased antifouling capability. Water empathy and surface hydrophilicity of PVDF hybrid membranes were significantly improved by incorporating the zwitterionic additive. GO-g-PMSA/polyvinylidene fluoride membranes showed improved antifouling properties in comparison to pure polyvinylidene fluoride and graphene oxide–polyvinylidene fluoride membranes as it made use of softer and more hydrophilic properties. In the experiment, 1 wt% wt% of GO-g-PMSA/PVDF membrane showed superior antifouling performance. The study employed buffer solutions composed of phosphates in 1 g/L BSA and FRR was 95.3%, Rr was 32.3%, and Rir was 4.7% at 0.7 MPa. Furthermore, high salt-ion refusal was noted in the salt-rejection experiments. The findings confirmed that zwitter-ionic-polymer-modified GO can potentially be used as an antifouling additive in nanocomposite membrane fabrication [93]. CNT coatings have been shown to prevent and control marine biofouling in several studies so far. These coatings consist primarily of MWCNTs, which have shown efficacy in marine environments, according to Table 3.
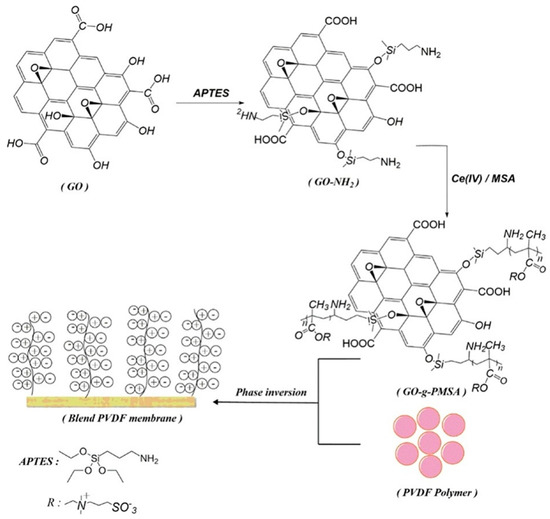
Figure 11.
Scheme showing surface-instigated redox polymerization of 2−(Methacryloyloxy) ethyl dimethyl−(3−sulfopropyl) ammonium hydroxide from graphene oxide surface. Reprinted with permission from [94]. 2019, J. Water Process Eng.

Table 3.
A summary of the fouling resistance of polymeric membranes using GO-based composites, is provided below.
Marine coatings made from pristine MWCNTs (p-MWCNTs) produce differing results. Some studies have shown that carbon nanomaterials, when incorporated into PDMS, can improve antifouling performance by lowering the number of eukaryotic microorganisms [110] and barnacle adhesion strengths [106,107]. This contrasts with other studies which showed that MWCNT-centered coatings have no impact on the settlement of microfoulers and macrofoulers [104].
Furthermore, carboxylate- and hydroxyl-functionalized MWCNTs have been evaluated to determine their contribution to marine coating AF performance. Sun and Zhang performed an extensive two-month field trial, in which MWCNTs were added to PDMS coatings with variations in the hydroxyl and carboxyl content (w/w), the diameter, and the length to examine the effects of introducing the changes [110]. The type of MWCNT applied to the coating, as well as the pioneer eukaryotic communities, had a significant impact on the AF behavior of the coating. The findings of Sun and Zhang demonstrate that AF behavior varies as a function of the bacteria strain type associated with the pioneer biofilm community and also shows dependency on the carboxyl- or hydroxyl-modified coating used. In a different investigation, however, Ji et al. observed that the majority of carboxyl- and hydroxyl-modified carbon coatings had weak modulatory impacts on the biofilm community [109]. It has also been demonstrated that fluorinated MWCNT-based coatings can reduce E. coli [109] to 98% and can prevent pseudobarnacles from adhering [105].
Additionally, results from a combination of silicone oil and lubricants in polymeric matrices showed that MWCNT-based coatings could be improved by the addition of lubricants [108]. Using this method, superhydrophobic fouling-release surfaces were developed by lubricant leaching from the surface over time. This resulted in the formation of a layer of oil serving as a shield against corrosion, originating from frictional forces progressing over time, as well as an enhancement in the antifouling capabilities [117].
The findings of this study suggest that when developing new antifouling coatings for marine environments, CNT-based coatings can be considered as an option.
3.4. Functionalized Carbon Coatings for Pollutant Adsorption
There are many reasons why carbon-based materials are ideal platforms for developing new adsorbents with enhanced functionality, including their noncorrosive nature, the ability to tune the surface chemistry, large surface areas, and the occurrence of oxygen-containing functional groups. In order to manufacture carbon-based materials for environmental applications, most of the processes that are used are chemical processes, such as chemical oxidation and deposition (chemical oxidation), as well as extended chemical processes (for example electrochemical, sol–gel, microemulsion, hydrothermal, and electrochemical).
Water treatment could greatly benefit from the use of these materials if it is designed to obtain suitable properties and structures. Therefore, carbon-based materials are generally functionalized deliberately with a view to obtain synergistic multifunctionalities or for solving specific problems in order to achieve synergistic advantages over conventional materials. Whenever functional groups are present on the surface of a carbon backbone, adsorption is always made easier. Several functional groups have been grafted onto the top of the surface of carbon nanomaterials (such as -NH2, -SH, and -COOH), as well as to enhanced functional groups such as -COOH and -OH, which are present on the surface. It is important to coat the carbon backbone with other nanoparticles (NPs) in order to improve the performance of these materials [118,119]. A significant advantage of the carbon backbone stems from its strong adsorption behavior and large specific surface area [120,121].
Core–shell structure chemistry has received considerable attention in recent years due to the wide range of applications in areas such as biomedicine, catalysis, electronics, pharmaceuticals, and environmental pollution control. In carbon nanomaterials, the core and shell are usually composed of different materials. Material combinations are few, with a handful of examples that include Fe3O4@C, RGO@Al2O3, C@Si, and CNT@C. Nanoscale carbon-based core–shell materials have largely been utilized as high-capacity electrodes to isolate and remove solvated metals; their application in aqueous removal of heavy metals has yet to be established. However, complex nanocomposites have been used to remove heavy metals from aqueous solutions. In this direction, the properties of CNTs and graphene nanomaterials have been drastically altered to induce changes at the metal site, with magnetization facilitating their isolation and removal towards the adsorbing material. A heavy metal is considered toxic if it is nonbiodegradable, bioaccumulative, or extremely hazardous. There are several heavy metals, including Hg, Pb, Cr, and Cd, which are more commonly found and problematic. Plants, animals, and people are at risk for serious diseases due to the presence of heavy metals in the environment and trace quantities are often associated with heightened risk (Table 4). A variety of functionalized CNTs as well as graphene nanomaterials have been successfully applied for the purpose of aqueous heavy metal removal by adsorption.
Magnetized carbon materials have also been developed for the capture of other functionalized nanomaterials, particularly heavy metals. Contact among metal ions and oxygen-rich materials is a major factor in the processing of heavy metal adsorption on CNTs and graphene-based nanomaterials. Pretreatments are usually required to enhance the functional-group abundance of oxygen-associated elements on the surface of raw CNTs. In contrast, the oxygen-rich surface chemistry of graphene requires no acid or oxidation treatment. In the case of Cr(VI), a reduction process must be carried out before either adsorption or precipitation can occur. For iron oxide, carbonization of cellulose to aid the reduction of Fe3O4 nanoparticles can provide a route to the fabrication of magnetic carbon–iron nanoadsorbents. The functionalized nanoadsorbent surface can facilitate the removal of Cr(VI) through the reduction of cellulose and Fe(NO3)3 precipitation. Furthermore, easy separation of MC-N and MC-O was accomplished using a permanent magnet after the reaction. There have been a number of studies on magnetic nanocomposites in recent years. This is largely due to their low cost, environmentally friendly composition, and large surface area of mesoporous nanostructure that permits rapid adsorption and ease of retrieval using an externally applied magnetic field. Magnetic separation of heavy metals allows environmental risks to be minimized as shown in Figure 12a that heavy metal nanoparticles adsorb efficiently by magnetic nanoparticles. Further, magnetically functionalized nanomaterials, while recycling magnetic particles, offer an ecofriendly method to reapply the separation process with minimal to no damage to the environment compared to other methods (Figure 12b). Figure 13b illustrates a typical example of heavy metal removal. Here, surfactant (tween-20)-functionalized Au NPs have been applied for the removal of aqueous Hg (II) in a high-salt matrix (artificial seawater) by reducing Hg2+ to Hg0 through the deposition of Hg0 on the NP surface. The separation process was completed by using magnetic and reduced graphene oxide-Fe3O4 NPs.
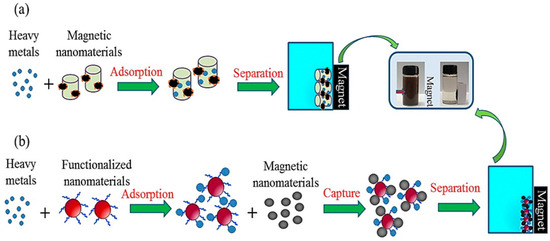
Figure 12.
A schematic diagram showing heavy metal absorption and separation using (a) magnetic nanoparticles (b) magnetic carbon-based nanomaterials used in heavy metal separation and nanoparticle retrieval. Reprinted with permission from [122]. 2018, Chemosphere.
Figure 12.
A schematic diagram showing heavy metal absorption and separation using (a) magnetic nanoparticles (b) magnetic carbon-based nanomaterials used in heavy metal separation and nanoparticle retrieval. Reprinted with permission from [122]. 2018, Chemosphere.
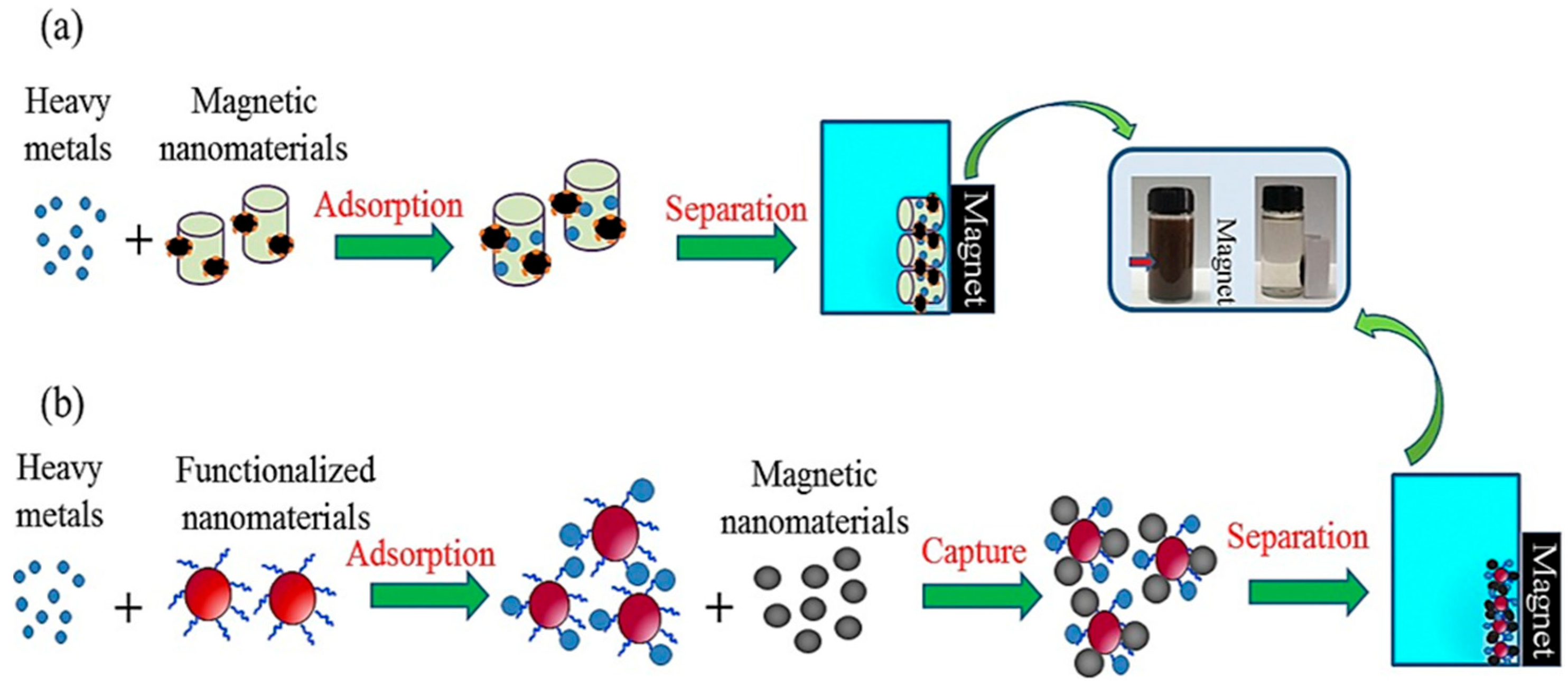

Table 4.
Aqueous toxic heavy metal adsorption by functionalized carbon-based nanomaterials.
Table 4.
Aqueous toxic heavy metal adsorption by functionalized carbon-based nanomaterials.
| S.N. | Carbon-Based nanomaterials | Surface Area (m2 g−1) | pH | Adsorption Capacity (mg g−1) | |
|---|---|---|---|---|---|
| Exp. | Cal. | ||||
| 1. | Pb (II) | ||||
| [123] | Multiwalled Carbon Nanotube | - | 6.0 | - | 15.9 |
| [124] | Acidified MWCNTs | 237.3 | 5.0 | 85.0 | 49.7 |
| [123] | Multiwalled Carbon Nanotube/VP | - | 6.0 | - | 37.0 |
| [125] | Multiwalled Carbon Nanotube/TiO2 | - | 6.0 | 4.6 | 137.0 |
| [126] | Carbon Nanotube/MnO2 | 275.0 | 5.0 | - | 78.7 |
| [127] | Single-walled Carbon Nanotube/COOH | 400.0 | 5.0 | - | 94.7 |
| [128] | Carbon Nanotube/Fe3O4-NH2 | 90.7 | 5.3 | 37.6 | 75.0 |
| [129] | Carbon Nanotube/Fe3O4-MPTS | 97.2 | 6.5 | 42.1 | 65.4 |
| [130] | Graphene Oxide | 430.0 | 6.8 | 328.0 | 367.0 |
| [130] | EDTA/Graphene Oxide | 623.0 | 6.8 | 479.0 | 525.0 |
| [130] | EDTA/Reduced graphene Oxide | 730.0 | 6.8 | 204.0 | 228.0 |
| [131] | Graphene Oxide/MnFe2O4 | 196.0 | 5.0 | - | 673.0 |
| [132] | Reduced graphene oxide/nZVI | - | 5.0 | 550.0 | 585.5 |
| [133] | CoFe2O4/Graphene Oxide | 212.7 | - | 81.3 | 82.3 |
| [134] | M-CHAP/Graphene Oxide | 106.0 | 4.5 | 244.5 | 246.1 |
| [135] | HMO/Graphene Oxide | 383.9 | 5.0 | 553.6 | 512.6 |
| [136] | Smart Magnetic Graphene | 165.0 | 6.5 | - | 6.0 |
| 2. | As (III) | ||||
| [137] | Iron oxide/Multiwalled Carbon Nanotube | - | 8.0 | 1.8 | 4.0 |
| [138] | Fe3O4/Multiwalled Carbon Nanotube | 70.1 | - | - | 53.2 |
| [139] | Fe/Multiwalled Carbon Nanotube | - | 7.0 | 210.0 | 200.0 |
| [140] | EDA/Multiwalled Carbon Nanotube/Fe2+ | 198.5 | 8.0 | 0.7 | N/A |
| [141] | MIO/Multiwalled Carbon Nanotube | 209.8 | 7.0 | 17.2 | 20.2 |
| [131] | Graphene Oxide/MnFe2O4 | 196.0 | 6.5 | - | 146.0 |
| [142] | Fe3O4/Reduced Graphene Oxide | 148 | 7.0 | - | 13.1 |
| [143] | HA/Reduced Graphene Oxide/Fe3O4 | 0.9 | 7.0 | 7.5 | 8.7 |
| [144] | HEG/electrodes | 442.9 | 6.1 | - | 138.8 |
| [145] | GAC/ZrO2 | 903.0 | 7.6 | - | 12.2 |
| [146] | Multiwalled Carbon Nanotube/ZrO2 | 152.0 | 6.0 | 9.8 × 10−2 | 2.0 |
| [147] | Fe3O4/Multiwalled Carbon Nanotube | 153.0 | 4.0 | 9.7 × 10−2 | 1.7 |
| 3. | As (V) | ||||
| [148] | Fe3O4/Multiwalled Carbon Nanotube | 70.1 | - | - | 39.1 |
| [149] | MIO/Multiwalled Carbon Nanotube | 209.8 | 7.0 | 36.3 | 40.8 |
| [139] | Fe/Multiwalled Carbon Nanotube | - | 7.0 | 220.0 | 200.0 |
| [131] | Graphene Oxide/MnFe2O4 | 196.0 | 4.0 | - | 207.0 |
| [147] | Graphene oxide/Fe(OH)3 | - | 4.0 | 23.8 | N/A |
| [142] | Fe3O4/Reduced graphene oxide | 148 | 7.0 | - | 5.83 |
| [143] | HA- Reduced graphene oxide/Fe3O4 | 0.9 | 7.0 | 16.0 | 61.7 |
| [144] | HEG/electrodes | 442.9 | 6.9 | - | 141.9 |
| [148] | Mg-Al LDHs/Graphene Oxide | 35.4 | 5.0 | 183.1 | 180.3 |
| [146] | Reduced graphene oxide/ZrO2 | 152.0 | 6.0 | 0.1 | 5.0 |
| [140] | EDA/Reduced graphene oxide/Fe2+ | 198.5 | 4.0 | 1.0 | 18.1 |
| [149] | Fe3O4/Reduced graphene oxide | 153.0 | 4.0 | 0.1 | 0.2 |
| [136] | Smart Magnetic Graphene | 165.0 | 6.5 | - | 3.3 |
| 4. | Hg (II) | ||||
| [150] | Oxidized Multiwalled Carbon Nanotube | - | 7.0 | - | 3.8 |
| [151] | Multiwalled Carbon Nanotube/COOH | - | 4.3 | 81.6 | 127.6 |
| [151] | Multiwalled Carbon Nanotube/OH | - | 4.3 | 89.4 | 120.1 |
| [152] | Multiwalled Carbon Nanotube/SH | - | 5.0 | 74.2 | 131.6 |
| [153] | Multiwalled Carbon Nanotube/iodide | 153.0 | 6.0 | 100.0 | 123.5 |
| [153] | Multiwalled Carbon Nanotube/S | 155.3 | 6.0 | 100.0 | 151.5 |
| [154] | Carbon Nanotube/MnO2 | 110.4 | 6.0 | 14.3 | 58.8 |
| [155] | KMnO4-DES/Carbon Nanotube | 199.4 | 5.5 | 187.0 | 250.5 |
| [156] | CS/Multiwalled Carbon Nanotube/COOH | - | 4.0 | 183.2 | 181.8 |
| [156] | CS/Multiwalled Carbon Nanotube | - | 4.0 | 167.5 | 169.4 |
| [129] | Multiwalled Carbon Nanotube/Fe3O4-SH | 97.2 | 6.5 | 63.7 | 65.5 |
| [157] | Tween 20-Au/graphite-Fe3O4 | - | - | - | 47.6 |
| 5. | Cr (VI) | ||||
| [158] | IL-oxi-Multiwalled Carbon Nanotube | 87.4 | 3.0 | 2.6 | 85.8 |
| [159] | Carbon Nanotube/CeO2 | - | 7.0 | 30.2 | 31.6 |
| [160] | DBSA-PANI/Multiwalled Carbon Nanotube | - | 2.0 | 49.5 | 55.6 |
| [161] | AC/Carbon Nanotube | 755.8 | 2.0 | 9.0 | 8.6 |
| [162] | AA/Carbon Nanotube | 203.0 | 2.0 | 142.8 | 264.5 |
| [163] | NH2-Fe3O4/Graphene Oxide | 43.6 | 4.5 | 27.3 | 32.3 |
| [164] | MC-N | 136.3 | 1.0 | 327.5 | N/A |
| [136] | Smart Magnetic Graphene | 165.0 | 6.5 | - | 4.9 |
| 6. | Cd (II) | ||||
| [165] | Raw Multiwalled Carbon Nanotube | 187.6 | 8.0 | 1.29 | 1.3 |
| [165] | Oxidized Multiwalled Carbon Nanotube | 78.5 | 8.0 | 22.32 | 22.4 |
| [165] | EDA/Multiwalled Carbon Nanotube | 101.2 | 8.0 | 21.23 | 21.7 |
| [166] | Al2O3 Multiwalled Carbon Nanotube | 109.8 | 7.0 | 0.948 | 27.2 |
| [167] | Single-walled Carbon Nanotube | 400.0 | 5.0 | - | 21.2 |
| [167] | COOH/Single-walled Carbon Nanotube | 400.0 | 5.0 | - | 55.4 |
| [168] | Oxidized Carbon Nanotube | 128.0 | 5.5 | - | 11.0 |
| [162] | AA/Carbon Nanotube | 203.0 | 7.5 | 200.0 | 229.9 |
| 6. | Cu (II) | ||||
| [169] | Oxidized Carbon Nanotube | - | 7.0 | 50.4 | 64.9 |
| [170] | Purified Multiwalled Carbon Nanotube | 169.7 | 5.0 | 37.5 | 36.8 |
| [168] | Oxidized Multiwalled Carbon Nanotube | - | 5.0 | 29 | 28.5 |
| [170] | Sulfonated Multiwalled Carbon Nanotube | 28.7 | 5.0 | 59.6 | 43.2 |
| [171] | OH/Multiwalled Carbon Nanotube | 111.4 | 4.9 | 7.0 | 10.1 |
| [171] | COOH/Multiwalled Carbon Nanotube | 135.2 | 4.9 | 5.5 | 8.1 |
| [167] | Single-walled Carbon Nanotube | 400.0 | 5.0 | - | 22.9 |
| [167] | COOH/Single-walled Carbon Nanotube | 400.0 | 5.0 | - | 72.3 |
| [172] | OH/Single-walled Carbon Nanotube/RGO | - | 6.8 | - | 256.0 |
| [172] | COOH/Single-walled Carbon Nanotube/RGO | - | 6.8 | - | 63.0 |
| [173] | Multiwalled Carbon Nanotube/Fe3O4 | 138.7 | 5.5 | 19.0 | 38.9 |
| 7. | Zn (II) | ||||
| [169] | Oxidized Carbon Nanotube | - | 7.0 | 58.0 | 74.6 |
| [174] | Purified Single-walled Carbon Nanotube | 423.0 | - | 15.4 | 41.8 |
| [175] | Oxidized Multiwalled Carbon Nanotube | 250.0 | 6.5 | 2.0 | 1.1 |
| 8. | Ni (II) | ||||
| [176] | Multiwalled Carbon Nanotube | 197.0 | 5.4 | 2.9 | 3.7 |
| [176] | PAA/Multiwalled Carbon Nanotube | - | 5.4 | - | 3.9 |
| [177] | Oxidized Multiwalled Carbon Nanotube | 102.0 | 6.5 | 12.5 | 17.9 |
| 9. | Co (II) | ||||
| [169] | Oxidized Carbon Nanotube | - | 7.0 | 69.6 | 85.7 |
| [178] | Multiwalled Carbon Nanotube/iron oxide | - | 6.4 | 2.9 | 10.6 |
| 10 | U (VI) | ||||
| [179] | CB/Graphene Oxide/Fe3O4 | - | 5.0 | - | 122.5 |
| [180] | Fe3O4/Graphene Oxide | - | 5.5 | - | 69.5 |
| [181] | Amidoxime Fe3O4/Graphene Oxide | - | 5.0 | 76.88 | 92.4 |
| [182] | CMPEI/CMK | 1350.0 | 4.0 | - | 151.5 |
Electrostatic attraction and surface complexation are major strategies by which heavy metals adsorb onto functionalized carbon-based systems [28]. Figure 13a demonstrates the utility of surface complexation and electrostatic attraction for the removal of heavy metals [183]. As an example, Madadrang et al. used modified graphene oxide complexed with hexadentate ligands using ethylenediaminetetraacetic acid (EDTA). The method has been utilized to treat mercury (II)-, lead(II)-, nickel(II)-, cadmium(II)-, and copper(II)-contaminated samples by two prospective mechanisms [184]. The electrostatic interaction of heavy metal ions (Mx+) with oxygenic functional groups complexed on GO after functionalization via hydroxyl and carboxyl moieties provided easy access to metal uptake. In addition, the nature of EDTA as a hexadentate chelating agent with the ability to form coordination complexes with heavy metal ions (Mx+) enhances the coordination interactions of functionalized GO (Figure 13b).
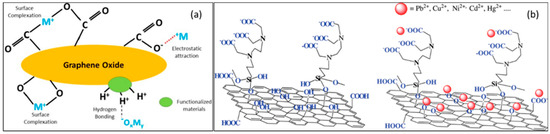
Figure 13.
(a) Probable toxic heavy metal removal mechanism on graphene oxide as an adsorbent from water. Reprinted with permission from [183]. 2020, Chemosphere. (b) Scheme shows the chemical structure of ethylenediaminetetraacetic-acid-functionalized graphene oxide before (left) and after it has been exposed to heavy metal cations (right) Reprinted with permission from [130]. 2012, ACS Appl. Mater. Interfaces.
Figure 13.
(a) Probable toxic heavy metal removal mechanism on graphene oxide as an adsorbent from water. Reprinted with permission from [183]. 2020, Chemosphere. (b) Scheme shows the chemical structure of ethylenediaminetetraacetic-acid-functionalized graphene oxide before (left) and after it has been exposed to heavy metal cations (right) Reprinted with permission from [130]. 2012, ACS Appl. Mater. Interfaces.
3.5. Bioinspired Functional Carbon Coatings
Carbon-based functionalized coatings play a pivotal role in achieving various functions of living organisms. Exoskeleton architectures are one of the better examples of functionalized carbon coatings that exist in nature. Animal exoskeletons, commonly known as cuticles, are covered with various coatings that may comprise a network of hydrocarbons, including phenolamine, which are coproduced with molecules that originate from oenocytes. These are specialized cells that offer protection from water loss, damage, and bacterial degradation. An interesting example of the role of functionalized coatings can be observed in mosquitoes that employ hydrocarbon coatings as defense barriers against insecticides and changes due to climate [184,185,186]. Another interesting study has suggested that the wings of particular fly species contain structural materials; therefore, instead of repelling contaminants, they are capable of killing contaminants and preventing bacterial colonization [187] on the wing surface. The cave barklice, Psyllipsocus Yucatan, from dolomit cave “Toca da Tiquara” in Brazil, illustrates an example of the unique coating of their wing membranes. This species has typically transparent light-brown wings, but some females have been found to have opaque black wings, with their surface covered with a coating. Crystallographic and material analysis of the wing surface has revealed an ultrathin layered-type morphology (1.5 µm) of catalyzed minerals containing iron, oxygen, and sulfur. It appears that the supersaturated climatic conditions and the presence of an iron-excreting bat species residing in caves could be the origin of the film formation. However, the confinement of deposits to only the wing surface, and their exclusion from other parts and hairs, indicate that the high-frequency vibration of the wings could aid the condensation of the thin layer. Detailed knowledge of this type has encouraged multidisciplinary efforts to mimic the natural composition and to advance material design with specific functional properties.
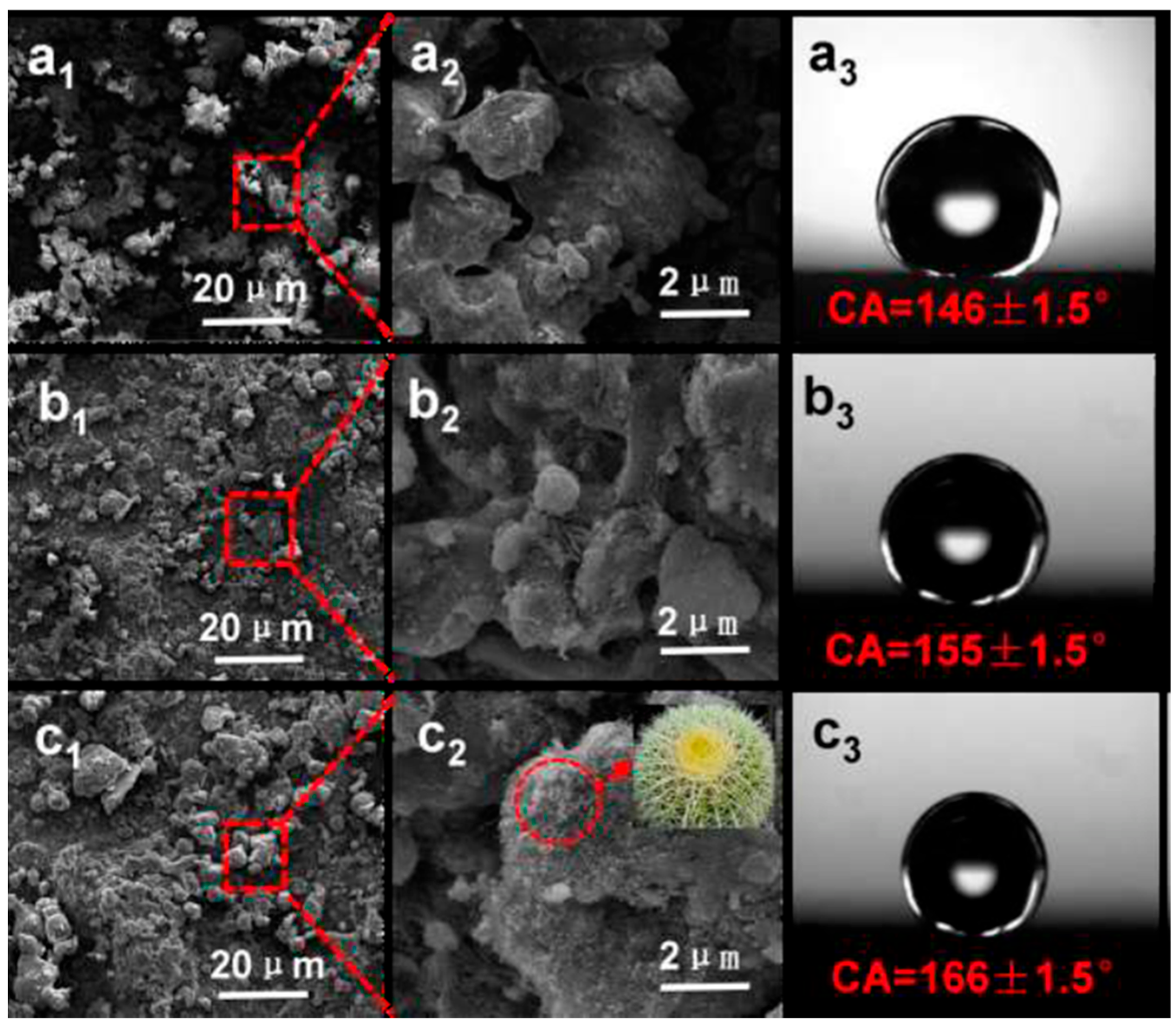
Moreover, in recent investigations, concepts embedded in biomimetics have been increasingly adopted and applied in coating technologies. Several applications aligned to biomimetics have been demonstrated to achieve superhydrophobic coatings by making use of petroleum pipelines, corrosion protection, and biomedical engineering [188,189,190]. These approaches have been largely inspired by features found in natural lotus leaves and have inspired a number of research directions [191,192]. These coatings offer excellent performance in the above-mentioned applications because of their antifouling, self-cleaning, and anticorrosion properties. In a recent study, Zhu et al. developed polysulfone–carbon nanotube–fluorinated ethylene propylene nanocomposite (PCFn) coatings and electrostatic powder spraying was employed to fabricate nanocomposite coatings with biomimetic golden spherical cactus surface structures (with microspheres and nanothorns). PCFn coatings combine the advantages of PSU, CNTs, and FEP to produce coatings that resemble the golden cactus structure and mimic the hydrophobic characteristics, enabling its water-repelling nature to present a durable, self-cleaning, and corrosion-resistant surface (SEM image in Figure 14a–c). Some other examples of applications of the biomimetic approach to achieve functional coatings are summarized in Table 5.
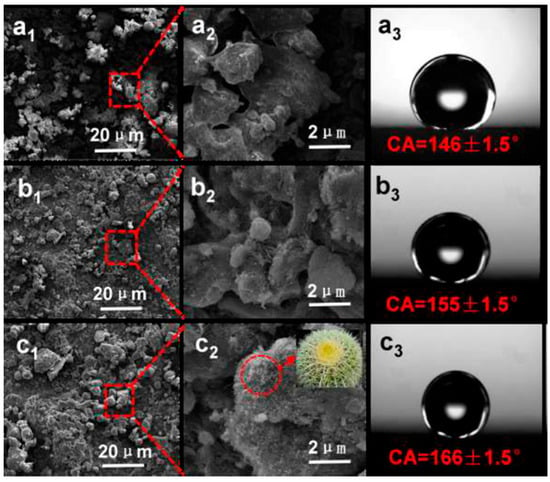
Figure 14.
SEM imaging of PCFn surfaces showing GC-like structured coatings reminiscent of biomimetic features with variable content of (a1–a3) 0 wt%, (b1–b3) 1 wt%, and (c1–c3) 5 wt% CNTs. Insert (c2): An optical image of a golden spherical cactus. Reprinted with permission from [188]. 2018, Chem. Eng.

Table 5.
Biomimicry in functionalized carbon-based coating technology.
4. Conclusions and Future Perspective
It is clear from the scope and the quality of the research discussed in this review article that functionalized carbon-based materials have the potential to be useful candidates for protective coating applications. In the literature, there are some discrepancies that relate to functionalized carbons and their ability to prevent corrosion. Wet corrosion has been found to be more extensive on CVD-grown carbon-based material coatings than uncoated metal surfaces, despite showing a good corrosion barrier for a short period of time. The literature search supports O2 and H2O diffusion through graphene-based material defects and the subsequent oxidization of encapsulated metals. The vulnerability of the surface is also compromised by coating discontinuities in graphene layers. The literature indicates that graphene-like material can protect against corrosion; however, it should also be noted that there is also a need for improved coating technologies and methodologies to enhance the effectiveness of the carbon-family-based system. A few surprising discoveries have been made regarding hybridized or functional groups composed of sp2 hybridized carbon coatings systems that are anticorrosive by nature. The use of sp2 hybridized carbon coatings systems as an anticorrosion barrier is counterintuitive since graphite promotes the corrosion of metals when it is in contact with them.
A new level of fire resistance has been achieved using functionalized carbon-based materials as fire-retardant additives. It is possible to enhance the thermal and barrier properties of thermoset polymers by dispersing functionalized carbon-based materials into them. Functionalized carbon-based material composites are also able to withstand the transfer of noxious and flammable gases during a fire, thanks to their intrinsic impermeability to gases, with high surface area in combination with tumescent flame-retardant behavior. A functionalized carbon surface can reduce melt dripping and delay structural collapse at high temperatures by reinforcing and maintaining its integrity. The development of flame-retardant materials and coatings with optimized formulations is expected in the future.
Depending on the surface chemistry and wettability of functionalized carbon-based materials, the interface can alter the physisorption of different molecules, allowing materials such as antifouling coatings, carriers, and adsorbents to be passively controlled during adsorption and desorption. In addition to being pertinent to both dry and marine atmospheres, functionalized carbon-based materials are also very effective as an antifouling mediator in surface coatings, which can be applied to surfaces such as domestic and construction surfaces, salt removal systems, bioreactors, and aquatic structures. There is a serious problem with the price and the large-scale applicability of coating technology in the antifouling field, since most applications require large quantities of functionalized carbon-based materials. The application of bioinspired or biomimetic coatings to functionalized carbon-based materials can potentially be valuable for antifouling applications if an economical coating approach can be established. It is possible to reduce surface colonization by microorganisms by exploiting graphene surfaces with amphiphilic behaviour. In order to optimize coatings to address the specific and unique antifouling problems associated with specific industries and overcome them, more research is needed. In terms of surface chemistry, functionalized carbon-based materials possess a tunable surface chemistry, which allows it to be used as a highly surface-active material, capable of selectively adsorbing a large quantity of pollutants. It is possible to target a wide range of ingredients, involving hydrocarbons, heavy metal ions, and dyes, among others. Material made from Gr can adsorb and expel 35,000 times its own weight.
Applied research is increasingly reaching the point of being applied to a commercial scale and research efforts around the world are intensifying; however, there remain significant challenges to overcome, particularly in terms of low-cost and scalable Gr manufacturing and handling. The safety of Gr materials must be proven through in-depth studies in medical and environmental applications. Graphene will bring a technological revolution when it is realized, according to the research community. This versatile material will be widely adopted by the coatings industry as an additive and as a single material to improve coating performance in the present era as a genuine versatile tool of the 21st century for a multitude of coating applications.
Finally, the biomimetic significance of carbon-based coatings offers a considerably viable option to incorporate features that better align with the environment, particularly if there is structural and functional resemblance to naturally evolved scaffolds. Such composites are representative of 3D structures, exhibiting physical characteristics such as porosity, polymer morphology, adsorption, and mechanical properties, which can collectively impact both the structural and functional workability of the materials, which may be further tuned as a composite. The desirability of mimicking nature will likely be an important imperative to the design of future materials as synthetics or biosynthetic hybrids, improving the multidimensionality of the coating. The growth of such materials in this direction will likely be important. Therefore, in the future, understanding the functional behavior of coating living organisms and applying them for synthetic coating would be an effective pathway to produce improved carbon-based functional coatings.
Funding
This work acknowledges support from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (No. 2022R1A2C1006090, 2017R1A2B4008801), and NRF Basic Research Program in Science and Engineering by the Ministry of Education (No. 2017R1D1A1B03036226). P.G.J. and V.K. also thankfully acknowledge the support from the National Convergence Research of Scientific Challenges through the National Research Foundation of Korea (NRF), funded by Ministry of Science and ICT (No. 2021M3F7A1017476).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kong, S.; Lim, M.; Shin, H.; Baik, J.-H.; Lee, J.-C. High-flux and antifouling polyethersulfone nanocomposite membranes incorporated with zwitterion-functionalized graphene oxide for ultrafiltration applications. J. Ind. Eng. Chem. 2020, 84, 131–140. [Google Scholar] [CrossRef]
- Fuertes-Espinosa, C.; Pujals, M.; Ribas, X. Supramolecular Purification and Regioselective Functionalization of Fullerenes and Endohedral Metallofullerenes. Chem 2020, 6, 3219–3262. [Google Scholar] [CrossRef]
- Lara, P.; Fitzgerald, R.M.; Karle, N.N.; Talamantes, J.; Miranda, M.; Baumgardner, D.; Stockwell, W.R. Winter and Wildfire Season Optical Characterization of Black and Brown Carbon in the El Paso-Ciudad Juárez Airshed. Atmosphere 2022, 13, 1201. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, B.; Ma, X.; Han, G.; Yang, Q.; Zhang, Y.; Shi, T.; Yuan, J.; Zhong, W.; Peng, Y.; et al. Carbon dioxide cover: Carbon dioxide column concentration seamlessly distributed globally during 2009–2020. Earth Syst. Sci. Data Discuss. 2022, 2022, 1–34. [Google Scholar] [CrossRef]
- Kumar, A.; Hong, J.; Yun, Y.; Jung, H.; Lee, K.-S.; Han, J.W.; Song, S.-J. A stable and active three-dimensional carbon based trimetallic electrocatalyst for efficient overall wastewater splitting. Int. J. Hydrogen Energy 2021, 46, 30762–30779. [Google Scholar] [CrossRef]
- Achary, L.S.K.; Kumar, A.; Rout, L.; Kunapuli, S.V.S.; Dhaka, R.S.; Dash, P. Phosphate functionalized graphene oxide with enhanced catalytic activity for Biginelli type reaction under microwave condition. Chem. Eng. J. 2018, 331, 300–310. [Google Scholar] [CrossRef]
- Kumar, A.; Rout, L.; Achary, L.K.; Mohanty, S.K.; Nayak, P.S.; Barik, B.; Dash, P. Solvent free synthesis of chalcones over graphene oxide-supported MnO2 catalysts synthesized via combustion route. Mater. Chem. Phys. 2021, 259, 124019. [Google Scholar] [CrossRef]
- Rout, L.; Kumar, A.; Dhaka, R.S.; Reddy, G.N.; Giri, S.; Dash, P. Bimetallic Au-Cu alloy nanoparticles on reduced graphene oxide support: Synthesis, catalytic activity and investigation of synergistic effect by DFT analysis. Appl. Catal. A Gen. 2017, 538, 107–122. [Google Scholar] [CrossRef]
- Achary, L.S.K.; Maji, B.; Kumar, A.; Ghosh, S.P.; Kar, J.P.; Dash, P. Efficient room temperature detection of H2 gas by novel ZnFe2O4–Pd decorated rGO nanocomposite. Int. J. Hydrogen Energy 2020, 45, 5073–5085. [Google Scholar] [CrossRef]
- Samadianfard, R.; Seifzadeh, D.; Habibi-Yangjeh, A.; Jafari-Tarzanagh, Y. Oxidized fullerene/sol-gel nanocomposite for corrosion protection of AM60B magnesium alloy. Surf. Coat. Technol. 2020, 385, 125400. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Abadchi, M.R.; Mirzaee, M.; Tabar, F.A.; Ramezanzadeh, B. Recent advances and future perspectives for carbon nanostructures reinforced organic coating for anti-corrosion application. Surf. Interfaces 2021, 23, 100994. [Google Scholar] [CrossRef]
- Ma, C.; Liu, J.; Zhu, X.; Xue, W.; Yan, Z.; Cheng, D.; Fu, J.; Ma, S. Anticorrosive non-crystalline coating prepared by plasma electrolytic oxidation for ship low carbon steel pipes. Sci. Rep. 2020, 10, 15675. [Google Scholar] [CrossRef] [PubMed]
- Catledge, S.A.; Thomas, V.; Vohra, Y.K. 5—Nanostructured diamond coatings for orthopaedic applications. In Diamond-Based Materials for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2013; pp. 105–150. [Google Scholar] [CrossRef]
- Thapliyal, V.; Alabdulkarim, M.E.; Whelan, D.R.; Mainali, B.; Maxwell, J.L. A concise review of the Raman spectra of carbon allotropes. Diam. Relat. Mater. 2022, 127, 109180. [Google Scholar] [CrossRef]
- Speranza, G. The Role of Functionalization in the Applications of Carbon Materials: An Overview. C 2019, 5, 84. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Kravets, V.G.; Wong, S.L.; Waters, J.; Geim, A.K.; Nair, R.R. Impermeable barrier films and protective coatings based on reduced graphene oxide. Nat. Commun. 2014, 5, 4843. [Google Scholar] [CrossRef]
- Nine, M.J.; Cole, M.A.; Tran, D.N.H.; Losic, D. Graphene: A multipurpose material for protective coatings. J. Mater. Chem. A 2015, 3, 12580–12602. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Raul, P.K.; Thakuria, A.; Das, B.; Devi, R.R.; Tiwari, G.; Yellappa, C.; Kamboj, D.V. Carbon Nanostructures As Antibacterials and Active Food-Packaging Materials: A Review. ACS Omega 2022, 7, 11555–11559. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, S.; Cai, W.; Zhang, Y.; Zhang, X. A review of carbon-based thermal interface materials: Mechanism, thermal measurements and thermal properties. Mater. Des. 2021, 209, 109936. [Google Scholar] [CrossRef]
- Babu, K.; Rendén, G.; Mensah, R.A.; Kim, N.K.; Jiang, L.; Xu, Q.; Restás, Á.; Neisiany, R.E.; Hedenqvist, M.S.; Försth, M.; et al. A Review on the Flammability Properties of Carbon-Based Polymeric Composites: State-of-the-Art and Future Trends. Polymers 2020, 12, 1518. [Google Scholar] [CrossRef] [PubMed]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, L.; Mou, J.; Wu, D.; Xu, M.; Zhou, P.; Ren, Y. Research Strategies to Develop Environmentally Friendly Marine Antifouling Coatings. Mar. Drugs 2020, 18, 371. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chu, X.; Lan, H.; Huang, R.; Huang, J.; Xie, Y.; Huang, J.; Huang, G. Current Implementation Status of Cold Spray Technology: A Short Review. J. Therm. Spray Technol. 2022, 31, 848–865. [Google Scholar] [CrossRef]
- Barea, E.; Montoro, C.; Navarro, J.A.R. Toxic gas removal—Metal–organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef]
- Barik, B.; Kumar, A.; Nayak, P.S.; Achary, L.S.K.; Rout, L.; Dash, P. Ionic liquid assisted mesoporous silica-graphene oxide nanocomposite synthesis and its application for removal of heavy metal ions from water. Mater. Chem. Phys. 2020, 239, 122028. [Google Scholar] [CrossRef]
- den Braver-Sewradj, S.P.; van Benthem, J.; Staal, Y.C.M.; Ezendam, J.; Piersma, A.H.; Hessel, E.V.S. Occupational exposure to hexavalent chromium. Part II. Hazard assessment of carcinogenic effects. Regul. Toxicol. Pharmacol. 2021, 126, 105045. [Google Scholar] [CrossRef]
- Kotrikla, A. Environmental management aspects for TBT antifouling wastes from the shipyards. J. Environ. Manag. 2009, 90, S77–S85. [Google Scholar] [CrossRef]
- Kordas, G. Corrosion Barrier Coatings: Progress and Perspectives of the Chemical Route. Corros. Mater. Degrad. 2022, 3, 376–413. [Google Scholar] [CrossRef]
- Uc-Peraza, R.G.; Castro, B.; Fillmann, G. An absurd scenario in 2021: Banned TBT-based antifouling products still available on the market. Sci. Total Environ. 2022, 805, 150377. [Google Scholar] [CrossRef]
- L’Azou, B.; Passagne, I.; Mounicou, S.; Tréguer-Delapierre, M.; Puljalté, I.; Szpunar, J.; Lobinski, R.; Ohayon-Courtès, C. Comparative cytotoxicity of cadmium forms (CdCl2, CdO, CdS micro- and nanoparticles) in renal cells. Toxicol. Res. 2014, 3, 32–41. [Google Scholar] [CrossRef]
- Bal, W.; Kasprzak, K.S. Induction of oxidative DNA damage by carcinogenic metals. Toxicol. Lett. 2002, 127, 55–62. [Google Scholar] [CrossRef]
- Templeton, D.M.; Liu, Y. Multiple roles of cadmium in cell death and survival. Chem. -Biol. Interact. 2010, 188, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ankit; Saha, L.; Kumar, V.; Tiwari, J.; Sweta; Rawat, S.; Singh, J.; Bauddh, K. Electronic waste and their leachates impact on human health and environment: Global ecological threat and management. Environ. Technol. Innov. 2021, 24, 102049. [Google Scholar] [CrossRef]
- Rajak, D.K.; Kumar, A.; Behera, A.; Menezes, P.L. Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications. Appl. Sci. 2021, 11, 4445. [Google Scholar] [CrossRef]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and Classification of Diamond-Like Carbon Films. Materials 2021, 14, 315. [Google Scholar] [CrossRef]
- Nakashima, N. Soluble Carbon Nanotubes: Fundamentals and Applications. Int. J. Nanosci. 2005, 4, 119–137. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Fan, J.; Shi, Z.; Li, H.; Yin, J. Kevlar®-functionalized graphene nanoribbon for polymer reinforcement. Polymer 2014, 55, 2578–2587. [Google Scholar] [CrossRef]
- Bai, H.; Xu, Y.; Zhao, L.; Li, C.; Shi, G. Non-covalent functionalization of graphene sheets by sulfonated polyaniline. Chem. Commun. 2009, 13, 1667–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-L.; Zhang, S.-B.; Zhang, Y.-P.; Kitajima, K. Effects of phylogeny and climate on seed oil fatty acid composition across 747 plant species in China, Ind. Crops Prod. 2015, 63, 1–8. [Google Scholar] [CrossRef]
- Chen, X.; Fan, K.; Liu, Y.; Li, Y.; Liu, X.; Feng, W.; Wang, X. Recent Advances in Fluorinated Graphene from Synthesis to Applications: Critical Review on Functional Chemistry and Structure Engineering. Adv. Mater. 2022, 34, 2101665. [Google Scholar] [CrossRef]
- Gong, X.; Liu, G.; Li, Y.; Yu, D.Y.W.; Teoh, W.Y. Functionalized-Graphene Composites: Fabrication and Applications in Sustainable Energy and Environment. Chem. Mater. 2016, 28, 8082–8118. [Google Scholar] [CrossRef]
- Cen, H.; Wu, C.; Chen, Z. Polydopamine functionalized graphene oxide as an effective corrosion inhibitor of carbon steel in HCl solution. J Mater Sci. 2022, 57, 1810–1832. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Rodríguez-Gómez, F.J.; Martínez-Cruz, I.K.; Ángeles-Beltrán, D.; Negrón-Silva, G.E.; Palomar-Pardavé, M.; Romero, L.L.; Pérez-Martínez, D.; Navarrete-López, A.M. Adsorption and corrosion inhibition behaviour of new theophylline–triazole-based derivatives for steel in acidic medium. R. Soc. Open Sci. 2019, 6, 181738. [Google Scholar] [CrossRef]
- Khan, G.; Basirun, W.J.; Kazi, S.N.; Ahmed, P.; Magaji, L.; Ahmed, S.M.; Khan, G.M.; Rehman, M.A.; Badry, A.B.B.M. Electrochemical investigation on the corrosion inhibition of mild steel by Quinazoline Schiff base compounds in hydrochloric acid solution. J. Colloid Interface Sci. 2017, 502, 134–145. [Google Scholar] [CrossRef]
- Richards, C.A.J.; McMurray, H.N.; Williams, G. Smart-release inhibition of corrosion driven organic coating failure on zinc by cationic benzotriazole based pigments. Corros. Sci. 2019, 154, 101–110. [Google Scholar] [CrossRef]
- Qiu, P.; Yang, H.F.; Yang, L.J.; Chen, Z.S.; Lv, L.J.; Song, Y.; Chen, C.F. Enhanced inhibition of steel corrosion by L-cysteine under visible-light illumination. Mater. Corros. 2017, 68, 1004–1012. [Google Scholar] [CrossRef]
- Alrashed, M.M.; Jana, S.; Soucek, M.D. Corrosion performance of polyurethane hybrid coatings with encapsulated inhibitor. Prog. Org. Coat. 2019, 130, 235–243. [Google Scholar] [CrossRef]
- Li, Y.Z.; Xu, N.; Guo, X.P.; Zhang, G.A. Inhibition effect of imidazoline inhibitor on the crevice corrosion of N80 carbon steel in the CO2-saturated NaCl solution containing acetic acid. Corros. Sci. 2017, 126, 127–141. [Google Scholar] [CrossRef]
- Erami, R.S.; Amirnasr, M.; Meghdadi, S.; Talebian, M.; Farrokhpour, H.; Raeissi, K. Carboxamide derivatives as new corrosion inhibitors for mild steel protection in hydrochloric acid solution. Corros. Sci. 2019, 151, 190–197. [Google Scholar] [CrossRef]
- Zhang, H.H.; Qin, C.K.; Chen, Y.; Zhang, Z. Inhibition behaviour of mild steel by three new benzaldehyde thiosemicarbazone derivatives in 0.5 M H2SO4: Experimental and computational study. R. Soc. Open Sci. 2019, 6, 190192. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Wang, Z.; Gong, Y.; Huang, H.; Ma, Y.; Xie, H.; Li, H.; Zhang, S.; Gao, F. Photo and thermally stable branched corrosion inhibitors containing two benzotriazole groups for copper in 3.5 wt% sodium chloride solution. Corros. Sci. 2018, 138, 353–371. [Google Scholar] [CrossRef]
- Dutta, A.; Saha, S.K.; Adhikari, U.; Banerjee, P.; Sukul, D. Effect of substitution on corrosion inhibition properties of 2-(substituted phenyl) benzimidazole derivatives on mild steel in 1M HCl solution: A combined experimental and theoretical approach. Corros. Sci. 2017, 123, 256–266. [Google Scholar] [CrossRef]
- El-Sayed, A.-R.; Shaker, A.M.; El-Lateef, H.M.A. Corrosion inhibition of tin, indium and tin–indium alloys by adenine or adenosine in hydrochloric acid solution. Corros. Sci. 2010, 52, 72–81. [Google Scholar] [CrossRef]
- Verma, C.; Sorour, A.A.; Ebenso, E.E.; Quraishi, M.A. Inhibition performance of three naphthyridine derivatives for mild steel corrosion in 1M HCl: Computation and experimental analyses. Results Phys. 2018, 10, 504–511. [Google Scholar] [CrossRef]
- Baux, J.; Caussé, N.; Esvan, J.; Delaunay, S.; Tireau, J.; Roy, M.; You, D.; Pébère, N. Impedance analysis of film-forming amines for the corrosion protection of a carbon steel. Electrochim. Acta 2018, 283, 699–707. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Gong, Y.; Gao, F.; Luo, Z.; Zhang, S.; Li, H. Water soluble corrosion inhibitors for copper in 3.5 wt% sodium chloride solution. Corros. Sci. 2017, 123, 339–350. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Yan, S.; Zou, X.; Chen, S. Three indazole derivatives as corrosion inhibitors of copper in a neutral chloride solution. Corros. Sci. 2017, 126, 295–304. [Google Scholar] [CrossRef]
- Ahmed, M.H.O.; Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.H.; Mohamad, A.B.; Gaaz, T.S. Synthesis and characterization of a novel organic corrosion inhibitor for mild steel in 1 M hydrochloric acid. Results Phys. 2018, 8, 728–733. [Google Scholar] [CrossRef]
- Kovačević, N.; Milošev, I.; Kokalj, A. How relevant is the adsorption bonding of imidazoles and triazoles for their corrosion inhibition of copper? Corros. Sci. 2017, 124, 25–34. [Google Scholar] [CrossRef]
- Liao, L.L.; Mo, S.; Luo, H.Q.; Feng, Y.J.; Yin, H.Y.; Li, N.B. Relationship between inhibition performance of melamine derivatives and molecular structure for mild steel in acid solution. Corros. Sci. 2017, 124, 167–177. [Google Scholar] [CrossRef]
- Likhanova, N.V.; Arellanes-Lozada, P.; Olivares-Xometl, O.; Hernández-Cocoletzi, H.; Lijanova, I.V.; Arriola-Morales, J.; Castellanos-Aguila, J. Effect of organic anions on ionic liquids as corrosion inhibitors of steel in sulfuric acid solution. J. Mol. Liq. 2019, 279, 267–278. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Alvarez, L.X.; Santos, N.E.d.; Barrios, A.C.M.; Ponzio, E.A. Green synthesis of 1-benzyl-4-phenyl-1H-1,2,3-triazole, its application as corrosion inhibitor for mild steel in acidic medium and new approach of classical electrochemical analyses. Corros. Sci. 2019, 149, 185–194. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Singh, A. 2-Amino-5-nitro-4,6-diarylcyclohex-1-ene-1,3,3-tricarbonitriles as new and effective corrosion inhibitors for mild steel in 1M HCl: Experimental and theoretical studies. J. Mol. Liq. 2015, 212, 804–812. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Qi, S.; Dong, D.; Cang, H.; Lu, G. The inhibition performance of long-chain alkyl-substituted benzimidazole derivatives for corrosion of mild steel in HCl. Corros. Sci. 2016, 102, 517–522. [Google Scholar] [CrossRef]
- Nahlé, A.; Salim, R.; El Hajjaji, F.; Aouad, M.R.; Messali, M.; Ech-Chihbi, E.; Hammouti, B.; Taleb, M. Novel triazole derivatives as ecological corrosion inhibitors for mild steel in 1.0 M HCl: Experimental & theoretical approach. RSC Adv. 2021, 11, 4147–4162. [Google Scholar] [CrossRef]
- Thoume, A.; Left, D.B.; Elmakssoudi, A.; Achagar, R.; Dakir, M.; Azzi, M.; Zertoubi, M. Performance Evaluation of New Chalcone Oxime Functionalized Graphene Oxide as a Corrosion Inhibitor for Carbon Steel in a Hydrochloric Acid Solution. Langmuir 2022, 38, 7472–7483. [Google Scholar] [CrossRef]
- Guo, S.; Garaj, S.; Bianco, A.; Ménard-Moyon, C. Controlling covalent chemistry on graphene oxide. Nat. Rev. Phys. 2022, 4, 247–262. [Google Scholar] [CrossRef]
- Liao, S.-H.; Liu, P.-L.; Hsiao, M.-C.; Teng, C.-C.; Wang, C.-A.; Ger, M.-D.; Chiang, C.-L. One-Step Reduction and Functionalization of Graphene Oxide with Phosphorus-Based Compound to Produce Flame-Retardant Epoxy Nanocomposite. Ind. Eng. Chem. Res. 2012, 51, 4573–4581. [Google Scholar] [CrossRef]
- Li, K.-Y.; Kuan, C.-F.; Kuan, H.-C.; Chen, C.-H.; Shen, M.-Y.; Yang, J.-M.; Chiang, C.-L. Preparation and properties of novel epoxy/graphene oxide nanosheets (GON) composites functionalized with flame retardant containing phosphorus and silicon. Mater. Chem. Phys. 2014, 146, 354–362. [Google Scholar] [CrossRef]
- Hu, W.; Zhan, J.; Wang, X.; Hong, N.; Wang, B.; Song, L.; Stec, A.A.; Hull, T.R.; Wang, J.; Hu, Y. Effect of Functionalized Graphene Oxide with Hyper-Branched Flame Retardant on Flammability and Thermal Stability of Cross-Linked Polyethylene. Ind. Eng. Chem. Res. 2014, 53, 3073–3083. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Qian, X.; Xing, W.; Yang, H.; Ma, L.; Lin, Y.; Jiang, S.; Song, L.; Hu, Y.; et al. Functionalized graphene oxide/phosphoramide oligomer hybrids flame retardant prepared via in situ polymerization for improving the fire safety of polypropylene. RSC Adv. 2014, 4, 31782–31794. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Hu, Y. Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: Synthesis, flammability and mechanism. Polym. Chem. 2014, 5, 1145–1154. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Tang, S.; Gao, J. A novel intumescent flame retardant-functionalized graphene: Nanocomposite synthesis, characterization, and flammability properties. Mater. Chem. Phys. 2012, 135, 938–947. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, P.; Qian, Y.; Liu, J. The synthesis of a novel graphene-based inorganic–organic hybrid flame retardant and its application in epoxy resin. Compos. Part B Eng. 2014, 60, 341–349. [Google Scholar] [CrossRef]
- Zhang, T.; Du, Z.; Zou, W.; Li, H.; Zhang, C. Hydroxyl-phosphazene-wrapped carbon nanotubes and its application in ethylene-vinyl acetate copolymer. J. Appl. Polym. Sci. 2013, 130, 4245–4254. [Google Scholar] [CrossRef]
- Tang, Y.; Gou, J.; Hu, Y. Covalent functionalization of carbon nanotubes with polyhedral oligomeric silsequioxane for superhydrophobicity and flame retardancy. Polym. Eng. Sci. 2013, 53, 1021–1030. [Google Scholar] [CrossRef]
- Pal, K.; Kang, D.J.; Zhang, Z.X.; Kim, J.K. Synergistic Effects of Zirconia-Coated Carbon Nanotube on Crystalline Structure of Polyvinylidene Fluoride Nanocomposites: Electrical Properties and Flame-Retardant Behavior. Langmuir 2010, 26, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, A.; Varganici, C.; Rosu, L.; Mustata, F.; Rosu, D.; Gaan, S. Recent advances in flame retardant epoxy systems containing non-reactive DOPO based phosphorus additives. Polym. Degrad. Stab. 2022, 200, 109962. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Cai, W.; Hu, Y. 3—Graphene-based polymer composites for flame-retardant application. In Innovations in Graphene-Based Polymer Composites; Woodhead Publishing: Sawston, UK, 2022; pp. 61–89. [Google Scholar] [CrossRef]
- Li, Y.-L.; Kuan, C.-F.; Chen, C.-H.; Kuan, H.-C.; Yip, M.-C.; Chiu, S.-L.; Chiang, C.-L. Preparation, thermal stability and electrical properties of PMMA/functionalized graphene oxide nanosheets composites. Mater. Chem. Phys. 2012, 134, 677–685. [Google Scholar] [CrossRef]
- Qu, L.; Sui, Y.; Zhang, C.; Li, P.; Dai, X.; Xu, B.; Fang, D. POSS-functionalized graphene oxide hybrids with improved dispersive and smoke-suppressive properties for epoxy flame-retardant application. Eur. Polym. J. 2020, 122, 109383. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zhang, L.; Molleja, J.G.; Wang, D.-Y. Bimetallic metal-organic frameworks and graphene oxide nano-hybrids for enhanced fire retardant epoxy composites: A novel carbonization mechanism. Carbon 2019, 153, 407–416. [Google Scholar] [CrossRef]
- Ye, T.-P.; Liao, S.-F.; Zhang, Y.; Chen, M.-J.; Xiao, Y.; Liu, X.-Y.; Liu, Z.-G.; Wang, D.-Y. Cu(0) and Cu(II) decorated graphene hybrid on improving fireproof efficiency of intumescent flame-retardant epoxy resins. Compos. Part B Eng. 2019, 175, 107189. [Google Scholar] [CrossRef]
- Mirfarsi, S.H.; Parnian, M.J.; Rowshanzamir, S.; Kjeang, E. Current status of cross-linking and blending approaches for durability improvement of hydrocarbon-based fuel cell membranes. Int. J. Hydrogen Energy 2022, 47, 13460–13489. [Google Scholar] [CrossRef]
- Mirfarsi, S.H.; Parnian, M.J.; Rowshanzamir, S. Self-Humidifying Proton Exchange Membranes for Fuel Cell Applications: Advances and Challenges. Processes 2020, 8, 1069. [Google Scholar] [CrossRef]
- Bagheripour, E.; Moghadassi, A.R.; Hosseini, S.M.; van der Bruggen, B.; Parvizian, F. Novel composite graphene oxide/chitosan nanoplates incorporated into PES based nanofiltration membrane: Chromium removal and antifouling enhancement. J. Ind. Eng. Chem. 2018, 62, 311–320. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Wang, F.; Liang, W.; Yang, H.; Wu, D. Fabrication of acrylic acid modified graphene oxide (AGO)/acrylate composites and their synergistic mechanisms of anticorrosion and antifouling properties. Prog. Org. Coat. 2022, 168, 106910. [Google Scholar] [CrossRef]
- Rahimi, A.; Mahdavi, H. Zwitterionic-functionalized GO/PVDF nanocomposite membranes with improved anti-fouling properties. J. Water Process Eng. 2019, 32, 100960. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Jeyasubramanian, K.; Premanathan, M.; Subbiah, G.; Shin, H.S.; Kim, S.J. Graphene oxide nanopaint. Carbon 2014, 72, 328–337. [Google Scholar] [CrossRef]
- Jiang, T.; Qi, L.; Qin, W. Improving the Environmental Compatibility of Marine Sensors by Surface Functionalization with Graphene Oxide. Anal. Chem. 2019, 91, 13268–13274. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Bing, W.; Tian, L.; Wang, P.; Zhao, J. Combined Effects of Color and Elastic Modulus on Antifouling Performance: A Study of Graphene Oxide/Silicone Rubber Composite Membranes. Materials 2019, 12, 2608. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, T.; Bing, W.; Dong, S.; Tian, L. Antifouling performance and mechanism of elastic graphene–silicone rubber composite membranes. J. Mater. Chem. B 2019, 7, 488–497. [Google Scholar] [CrossRef]
- Parra, C.; Dorta, F.; Jimenez, E.; Henríquez, R.; Ramírez, C.; Rojas, R.; Villalobos, P. A nanomolecular approach to decrease adhesion of biofouling-producing bacteria to graphene-coated material. J. Nanobiotechnology 2015, 13, 82. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, R.; Song, D.; Yu, J.; Sun, G.; Liu, Q.; Han, S.; Liu, J.; Zhang, H.; Wang, J. Guanidine-functionalized graphene to improve the antifouling performance of boron acrylate polymer. Prog. Org. Coat. 2021, 159, 106396. [Google Scholar] [CrossRef]
- Fazli-Shokouhi, S.; Nasirpouri, F.; Khatamian, M. Epoxy-matrix polyaniline/p-phenylenediamine-functionalised graphene oxide coatings with dual anti-corrosion and anti-fouling performance. RSC Adv. 2021, 11, 11627–11641. [Google Scholar] [CrossRef]
- Xie, M.; Zhao, W.; Wu, Y. Preventing algae biofilm formation via designing long-term oil storage surfaces for excellent antifouling performance. Appl. Surf. Sci. 2021, 554, 149612. [Google Scholar] [CrossRef]
- Sun, Y.; Lang, Y.; Sun, T.; Liu, Q.; Pan, Y.; Qi, Z.; Ling, N.; Feng, Y.; Yu, M.; Ji, Y.; et al. Antifouling potential of multi-walled carbon nanotubes-modified chlorinated rubber-based composites on the colonization dynamics of pioneer biofilm-forming eukaryotic microbes. Int. Biodeterior. Biodegrad. 2020, 149, 104921. [Google Scholar] [CrossRef]
- Carl, C.; Poole, A.J.; Vucko, M.J.; Williams, M.R.; Whalan, S.; de Nys, R. Enhancing the efficacy of fouling-release coatings against fouling by Mytilus galloprovincialis using nanofillers. Biofouling 2012, 28, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Suffredini, M.; Galli, G.; Glisenti, A.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Williams, D.; Lyall, G. Amphiphilic block copolymer/poly(dimethylsiloxane) (PDMS) blends and nanocomposites for improved fouling-release. Biofouling 2011, 27, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Beigbeder, A.; Degee, P.; Conlan, S.L.; Mutton, R.J.; Clare, A.S.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Dubois, P. Preparation and characterisation of silicone-based coatings filled with carbon nanotubes and natural sepiolite and their application as marine fouling-release coatings. Biofouling 2008, 24, 291–302. [Google Scholar] [CrossRef]
- Yang, J.-L.; Li, Y.-F.; Guo, X.-P.; Liang, X.; Xu, Y.-F.; Ding, D.-W.; Bao, W.-Y.; Dobretsov, S. The effect of carbon nanotubes and titanium dioxide incorporated in PDMS on biofilm community composition and subsequent mussel plantigrade settlement. Biofouling 2016, 32, 763–777. [Google Scholar] [CrossRef]
- Ba, M.; Zhang, Z.; Qi, Y. The influence of MWCNTs-OH on the properties of the fouling release coatings based on polydimethylsiloxane with the incorporation of phenylmethylsilicone oil. Prog. Org. Coat. 2019, 130, 132–143. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z. New anti-biofouling carbon nanotubes-filled polydimethylsiloxane composites against colonization by pioneer eukaryotic microbes. Int. Biodeterior. Biodegrad. 2016, 110, 147–154. [Google Scholar] [CrossRef]
- Ji, Y.; Sun, Y.; Lang, Y.; Wang, L.; Liu, B.; Zhang, Z. Effect of CNT/PDMS Nanocomposites on the Dynamics of Pioneer Bacterial Communities in the Natural Biofilms of Seawater. Materials 2018, 11, 902. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Wu, G.; Yang, Z.; Cui, Y.; Li, H.; Zhang, Y. Fluorinated Carbon Nanotube Superamphiphobic Coating for High-Efficiency and Long-Lasting Underwater Antibiofouling Surfaces. ACS Appl. Bio Mater. 2021, 4, 6351–6360. [Google Scholar] [CrossRef]
- Irani, F.; Jannesari, A.; Bastani, S. Effect of fluorination of multiwalled carbon nanotubes (MWCNTs) on the surface properties of fouling-release silicone/MWCNTs coatings. Prog. Org. Coat. 2013, 76, 375–383. [Google Scholar] [CrossRef]
- Sun, Y.; Lang, Y.; Yan, Z.; Wang, L.; Zhang, Z. High-throughput sequencing analysis of marine pioneer surface-biofilm bacteria communities on different PDMS-based coatings. Colloids Surf. B Biointerfaces 2020, 185, 110538. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Aguilar, S.; Rao, E.; Mak, W.H.; Huang, X.; He, N.; Chen, D.; Jun, D.; Curson, P.A.; McVerry, B.T.; et al. Direct grafting of tetraaniline via perfluorophenylazide photochemistry to create antifouling, low bio-adhesion surfaces. Chem. Sci. 2019, 10, 4445–4457. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-I.; Kim, D.-A.; Patel, K.D.; Shin, U.S.; Kim, H.-W.; Lee, J.-H.; Lee, H.-H. Carbon nanotube incorporation in PMMA to prevent microbial adhesion. Sci. Rep. 2019, 9, 4921. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Sahle-Demessie, E.; Sorial, G.A. Inhibition of biofilm growth on polymer-MWCNTs composites and metal surfaces. Sci. Total Environ. 2018, 633, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Zheng, Y.; Ba, M.; Wang, Y.; Kong, J.; Liu, J.; Wu, Q. Long time super-hydrophobic fouling release coating with the incorporation of lubricant. Prog. Org. Coat. 2021, 152, 106136. [Google Scholar] [CrossRef]
- Kumar, A.; Rout, L.; Dhaka, R.S.; Samal, S.L.; Dash, P. Design of a graphene oxide-SnO2 nanocomposite with superior catalytic efficiency for the synthesis of β-enaminones and β-enaminoesters. RSC Adv. 2015, 5, 39193–39204. [Google Scholar] [CrossRef]
- Achary, L.S.K.; Nayak, P.S.; Barik, B.; Kumar, A.; Dash, P. Ultrasonic-assisted green synthesis of β-amino carbonyl compounds by copper oxide nanoparticles decorated phosphate functionalized graphene oxide via Mannich reaction. Catal. Today 2020, 348, 137–147. [Google Scholar] [CrossRef]
- Kumar, A.; Rout, L.; Achary, L.S.K.; Mohanty, S.K.; Dash, P. A combustion synthesis route for magnetically separable graphene oxide-CuFe2O4-ZnO nanocomposites with enhanced solar light-mediated photocatalytic activity. New J. Chem. 2017, 41, 10568–10583. [Google Scholar] [CrossRef]
- Kumar, A.; Rout, L.; Achary, L.S.K.; Mohanty, A.; Dhaka, R.S.; Dash, P. An investigation into the solar light-driven enhanced photocatalytic properties of a graphene oxide-SnO2-TiO2 ternary nanocomposite. RSC Adv. 2016, 6, 32074–32088. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Ren, X.; Shao, D.; Zhao, G.; Sheng, G.; Hu, J.; Yang, S.; Wang, X. Plasma Induced Multiwalled Carbon Nanotube Grafted with 2-Vinylpyridine for Preconcentration of Pb(II) from Aqueous Solutions. Plasma Process. Polym. 2011, 8, 589–598. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhou, A.L.; Peng, F.; Yu, H.; Chen, L.F. Adsorption characteristic of acidified carbon nanotubes for heavy metal Pb(II) in aqueous solution. Mater. Sci. Eng. A 2007, 466, 201–206. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, Q.; Song, N.; Zhou, W.; Li, Y. Adsorption of Pb(II) from an Aqueous Solution by Titanium Dioxide/Carbon Nanotube Nanocomposites: Kinetics, Thermodynamics, and Isotherms. J. Chem. Eng. Data 2010, 55, 4428–4433. [Google Scholar] [CrossRef]
- Wang, S.-G.; Gong, W.-X.; Liu, X.-W.; Yao, Y.-W.; Gao, B.-Y.; Yue, Q.-Y. Removal of lead(II) from aqueous solution by adsorption onto manganese oxide-coated carbon nanotubes. Sep. Purif. Technol. 2007, 58, 17–23. [Google Scholar] [CrossRef]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Ji, L.; Zhou, L.; Bai, X.; Shao, Y.; Zhao, G.; Qu, Y.; Wang, C.; Li, Y. Facile synthesis of multiwall carbon nanotubes/iron oxides for removal of tetrabromobisphenol A and Pb(ii). J. Mater. Chem. 2012, 22, 15853–15862. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, J.; Li, J.; Tang, Y.; Cai, W. Efficient removal of heavy metal ions by thiol-functionalized superparamagnetic carbon nanotubes. Chem. Eng. J. 2012, 210, 45–52. [Google Scholar] [CrossRef]
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S. Adsorption Behavior of EDTA-Graphene Oxide for Pb (II) Removal. ACS Appl. Mater. Interfaces 2012, 4, 1186–1193. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, R.R.; Pillai, P.B.; Gupta, S.N.; Iyengar, M.A.R.; Sood, A.K. Graphene Oxide–MnFe2O4 Magnetic Nanohybrids for Efficient Removal of Lead and Arsenic from Water. ACS Appl. Mater. Interfaces 2014, 6, 17426–17436. [Google Scholar] [CrossRef]
- Jabeen, H.; Kemp, K.C.; Chandra, V. Synthesis of nano zerovalent iron nanoparticles—Graphene composite for the treatment of lead contaminated water. J. Environ. Manag. 2013, 130, 429–435. [Google Scholar] [CrossRef]
- Ma, S.; Zhan, S.; Jia, Y.; Zhou, Q. Highly Efficient Antibacterial and Pb(II) Removal Effects of Ag-CoFe2O4-GO Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 10576–10586. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, Y.; Hu, L.; Gao, L.; Du, B.; Wei, Q. Mechanism of Pb(ii) and methylene blue adsorption onto magnetic carbonate hydroxyapatite/graphene oxide. RSC Adv. 2015, 5, 9759–9770. [Google Scholar] [CrossRef]
- Wan, S.; He, F.; Wu, J.; Wan, W.; Gu, Y.; Gao, B. Rapid and highly selective removal of lead from water using graphene oxide-hydrated manganese oxide nanocomposites. J. Hazard. Mater. 2016, 314, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Gollavelli, G.; Chang, C.-C.; Ling, Y.-C. Facile Synthesis of Smart Magnetic Graphene for Safe Drinking Water: Heavy Metal Removal and Disinfection Control. ACS Sustain. Chem. Eng. 2013, 1, 462–472. [Google Scholar] [CrossRef]
- Tawabini, B.S.; Al-Khaldi, S.F.; Khaled, M.M.; Atieh, M.A. Removal of arsenic from water by iron oxide nanoparticles impregnated on carbon nanotubes. J. Environ. Sci. Health Part A 2011, 46, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Ramaprabhu, S. Magnetite Decorated Multiwalled Carbon Nanotube Based Supercapacitor for Arsenic Removal and Desalination of Seawater. J. Phys. Chem. C 2010, 114, 2583–2590. [Google Scholar] [CrossRef]
- Alijani, H.; Shariatinia, Z. Effective aqueous arsenic removal using zero valent iron doped MWCNT synthesized by in situ CVD method using natural α-Fe2O3 as a precursor. Chemosphere 2017, 171, 502–511. [Google Scholar] [CrossRef]
- Veličković, Z.; Vuković, G.D.; Marinković, A.D.; Moldovan, M.-S.; Perić-Grujić, A.A.; Uskoković, P.S.; Ristić, M.Đ. Adsorption of arsenate on iron(III) oxide coated ethylenediamine functionalized multiwall carbon nanotubes. Chem. Eng. J. 2012, 181–182, 174–181. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, Z.; Ma, J.; Yang, M.; Hong, J.; Hu, X.; Qiu, Y.; Chen, J. One-pot, solid-phase synthesis of magnetic multiwalled carbon nanotube/iron oxide composites and their application in arsenic removal. J. Colloid Interface Sci. 2014, 434, 9–17. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.-C.; Kim, K.S. Water-Dispersible Magnetite-Reduced Graphene Oxide Composites for Arsenic Removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef]
- Paul, B.; Parashar, V.; Mishra, A. Graphene in the Fe3O4 nano-composite switching the negative influence of humic acid coating into an enhancing effect in the removal of arsenic from water. Environ. Sci. Water Res. Technol. 2015, 1, 77–83. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Functionalized graphene sheets for arsenic removal and desalination of sea water. Desalination 2011, 282, 39–45. [Google Scholar] [CrossRef]
- Sandoval, R.; Cooper, A.M.; Aymar, K.; Jain, A.; Hristovski, K. Removal of arsenic and methylene blue from water by granular activated carbon media impregnated with zirconium dioxide nanoparticles. J. Hazard. Mater. 2011, 193, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Ntim, S.A.; Mitra, S. Adsorption of arsenic on multiwall carbon nanotube–zirconia nanohybrid for potential drinking water purification. J. Colloid Interface Sci. 2012, 375, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dwivedi, V.; Chi, C.; Wu, J. Graphene oxide/ferric hydroxide composites for efficient arsenate removal from drinking water. J. Hazard. Mater. 2010, 182, 162–168. [Google Scholar] [CrossRef]
- Wen, T.; Wu, X.; Tan, X.; Wang, X.; Xu, A. One-Pot Synthesis of Water-Swellable Mg–Al Layered Double Hydroxides and Graphene Oxide Nanocomposites for Efficient Removal of As(V) from Aqueous Solutions. ACS Appl. Mater. Interfaces 2013, 5, 3304–3311. [Google Scholar] [CrossRef]
- Ntim, S.A.; Mitra, S. Removal of Trace Arsenic To Meet Drinking Water Standards Using Iron Oxide Coated Multiwall Carbon Nanotubes. J. Chem. Eng. Data 2011, 56, 2077–2083. [Google Scholar] [CrossRef]
- El-Sheikh, A.H.; Al-Degs, Y.S.; Al-As’ad, R.M.; Sweileh, J.A. Effect of oxidation and geometrical dimensions of carbon nanotubes on Hg(II) sorption and preconcentration from real waters. Desalination 2011, 270, 214–220. [Google Scholar] [CrossRef]
- Chen, P.H.; Hsu, C.-F.; Tsai, D.D.-W.; Lu, Y.-M.; Huang, W.-J. Adsorption of mercury from water by modified multi-walled carbon nanotubes: Adsorption behaviour and interference resistance by coexisting anions. Environ. Technol. 2014, 35, 1935–1944. [Google Scholar] [CrossRef]
- Bandaru, N.M.; Reta, N.; Dalal, H.; Ellis, A.V.; Shapter, J.; Voelcker, N.H. Enhanced adsorption of mercury ions on thiol derivatized single wall carbon nanotubes. J. Hazard. Mater. 2013, 261, 534–541. [Google Scholar] [CrossRef]
- Gupta, A.; Vidyarthi, S.R.; Sankararamakrishnan, N. Enhanced sorption of mercury from compact fluorescent bulbs and contaminated water streams using functionalized multiwalled carbon nanotubes. J. Hazard. Mater. 2014, 274, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, H.K.; Pakizeh, M. Experimental study on mercury ions removal from aqueous solution by MnO2/CNTs nanocomposite adsorbent. J. Ind. Eng. Chem. 2015, 21, 221–229. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Hayyan, M.; Akib, S.; Ibrahim, M.; Hashim, M.A. Allyl triphenyl phosphonium bromide based DES-functionalized carbon nanotubes for the removal of mercury from water. Chemosphere 2017, 167, 44–52. [Google Scholar] [CrossRef]
- Shawky, H.A.; El-Aassar, A.H.M.; Abo-Zeid, D.E. Chitosan/carbon nanotube composite beads: Preparation, characterization, and cost evaluation for mercury removal from wastewater of some industrial cities in Egypt. J. Appl. Polym. Sci. 2012, 125, E93–E101. [Google Scholar] [CrossRef]
- Shih, Y.-C.; Ke, C.-Y.; Yu, C.-J.; Lu, C.-Y.; Tseng, W.-L. Combined Tween 20-Stabilized Gold Nanoparticles and Reduced Graphite Oxide–Fe3O4 Nanoparticle Composites for Rapid and Efficient Removal of Mercury Species from a Complex Matrix. ACS Appl. Mater. Interfaces 2014, 6, 17437–17445. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.K.; Jiang, S.-J.; Tseng, W.-L. Effective adsorption of chromium(vi)/Cr(iii) from aqueous solution using ionic liquid functionalized multiwalled carbon nanotubes as a super sorbent. J. Mater. Chem. A 2015, 3, 7044–7057. [Google Scholar] [CrossRef]
- Di, Z.-C.; Ding, J.; Peng, X.-J.; Li, Y.-H.; Luan, Z.-K.; Liang, J. Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere 2006, 62, 861–865. [Google Scholar] [CrossRef]
- Kumar, R.; Ansari, M.O.; Barakat, M.A. DBSA doped polyaniline/multi-walled carbon nanotubes composite for high efficiency removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2013, 228, 748–755. [Google Scholar] [CrossRef]
- Atieh, M.A. Removal of Chromium (VI) from polluted water using carbon nanotubes supported with activated carbon. Procedia Environ. Sci. 2011, 4, 281–293. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Jaiswal, M.; Verma, N. Composite nanofloral clusters of carbon nanotubes and activated alumina: An efficient sorbent for heavy metal removal. Chem. Eng. J. 2014, 235, 1–9. [Google Scholar] [CrossRef]
- Liu, M.; Wen, T.; Wu, X.; Chen, C.; Hu, J.; Li, J.; Wang, X. Synthesis of porous Fe3O4 hollow microspheres/graphene oxide composite for Cr(vi) removal. Dalton Trans. 2013, 42, 14710–14717. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Gu, H.; Yan, X.; Guo, J.; Wang, Y.; Sun, D.; Wang, Q.; Khan, M.; Zhang, X.; Weeks, B.L.; et al. Cellulose derived magnetic mesoporous carbon nanocomposites with enhanced hexavalent chromium removal. J. Mater. Chem. A 2014, 2, 17454–17462. [Google Scholar] [CrossRef]
- Vuković, G.D.; Marinković, A.D.; Čolić, M.; Ristić, M.Đ.; Aleksić, R.; Perić-Grujić, A.A.; Uskoković, P.S. Removal of cadmium from aqueous solutions by oxidized and ethylenediamine-functionalized multi-walled carbon nanotubes. Chem. Eng. J. 2010, 157, 238–248. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Yuan, X.; Dong, H.; Zeng, G.; Wu, H.; Wang, H.; Liu, J.; Hua, S.; Zhang, S.; et al. Facile synthesis of alumina-decorated multi-walled carbon nanotubes for simultaneous adsorption of cadmium ion and trichloroethylene. Chem. Eng. J. 2015, 273, 101–110. [Google Scholar] [CrossRef]
- Moradi, O. The Removal of Ions by Functionalized Carbon Nanotube: Equilibrium, Isotherms and Thermodynamic Studies. Chem. Biochem. Eng. Q. 2011, 25, 229–240. Available online: https://www.researchgate.net/publication/268360458 (accessed on 20 August 2022).
- Li, Y.-H.; Ding, J.; Luan, Z.; Di, Z.; Zhu, Y.; Xu, C.; Wu, D.; Wei, B. Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 2003, 41, 2787–2792. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard. Mater. 2011, 185, 140–147. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z.; Xiao, D.; Xiong, P.; Ye, N. Sulfonated multi-walled carbon nanotubes for the removal of copper (II) from aqueous solutions. J. Ind. Eng. Chem. 2014, 20, 1765–1771. [Google Scholar] [CrossRef]
- Rosenzweig, S.; Sorial, G.A.; Sahle-Demessie, E.; Mack, J. Effect of acid and alcohol network forces within functionalized multiwall carbon nanotubes bundles on adsorption of copper (II) species. Chemosphere 2013, 90, 395–402. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Webber, M.R.; Gorman, W.R.; Rogers, R.E. Removal of Copper Ions from Aqueous Solutions via Adsorption on Carbon Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 15674–15680. [Google Scholar] [CrossRef]
- Tang, W.-W.; Zeng, G.-M.; Gong, J.-L.; Liu, Y.; Wang, X.-Y.; Liu, Y.-Y.; Liu, Z.-F.; Chen, L.; Zhang, X.-R.; Tu, D.-Z. Simultaneous adsorption of atrazine and Cu (II) from wastewater by magnetic multi-walled carbon nanotube. Chem. Eng. J. 2012, 211–212, 470–478. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H.; Liu, C. Removal of Zinc(II) from Aqueous Solution by Purified Carbon Nanotubes: Kinetics and Equilibrium Studies. Ind. Eng. Chem. Res. 2006, 45, 2850–2855. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Alicia, R.F.; Abdullah, E.C.; Sahu, J.N.; Haslija, A.B.A.; Tan, J. Statistical optimization and kinetic studies on removal of Zn2+ using functionalized carbon nanotubes and magnetic biochar. J. Environ. Chem. Eng. 2013, 1, 486–495. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Shao, D.; Hu, J.; Wang, X. Adsorption of Ni(II) on oxidized multi-walled carbon nanotubes: Effect of contact time, pH, foreign ions and PAA. J. Hazard. Mater. 2009, 166, 109–116. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Ebrahimi, M. Removal of divalent nickel cations from aqueous solution by multi-walled carbon nano tubes: Equilibrium and kinetic processes. Res. Chem. Intermed. 2012, 38, 2205–2222. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Chen, C.; Ren, X.; Hu, J.; Wang, X. Removal of cobalt from aqueous solution by magnetic multiwalled carbon nanotube/iron oxide composites. Chem. Eng. J. 2011, 174, 126–133. [Google Scholar] [CrossRef]
- Shao, L.; Wang, X.; Ren, Y.; Wang, S.; Zhong, J.; Chu, M.; Tang, H.; Luo, L.; Xie, D. Facile fabrication of magnetic cucurbit[6]uril/graphene oxide composite and application for uranium removal. Chem. Eng. J. 2016, 286, 311–319. [Google Scholar] [CrossRef]
- Zong, P.; Wang, S.; Zhao, Y.; Wang, H.; Pan, H.; He, C. Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(VI) from aqueous solutions. Chem. Eng. J. 2013, 220, 45–52. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Zhang, S.; Chen, H.; Shao, D. Efficient enrichment of uranium(vi) on amidoximated magnetite/graphene oxide composites. RSC Adv. 2013, 3, 18952–18959. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, S.; Park, S.-J.; Kim, J.M. Preparation of functionalized nanoporous carbons for uranium loading. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 292–295. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Salleh, W.N.W.; Ismail, A.F.; Yusof, N.; Yusop, M.Z.M.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Benedetti, A. Superhydrophobic surfaces for applications in seawater. Adv. Colloid Interface Sci. 2015, 222, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Grigoraki, L.; Grau-Bové, X.; Yates, H.C.; Lycett, G.J.; Ranson, H. Isolation and transcriptomic analysis of Anopheles gambiae oenocytes enables the delineation of hydrocarbon biosynthesis. eLife 2020, 9, e58019. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, G.J.; Bagnères, A.-G. (Eds.) Insect Hydrocarbons; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, F.; Qian, H.; Wang, H.; Mu, L.; Zhu, J. A biomimetic spherical cactus superhydrophobic coating with durable and multiple anti-corrosion effects. Chem. Eng. J. 2018, 338, 670–679. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, X.; Du, A.; Wu, S.; Shen, J.; Zhou, B. Timing of polyethylene glycol addition for the control of SiO2 sol structure and sol–gel coating properties. J. Coat. Technol. Res. 2017, 14, 447–454. [Google Scholar] [CrossRef]
- Tran, N.G.; Chun, D.-M. Ultrafast and Eco-Friendly Fabrication Process for Robust, Repairable Superhydrophobic Metallic Surfaces with Tunable Water Adhesion. ACS Appl. Mater. Interfaces 2022, 14, 28348–28358. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Ye, Q.; Gao, T.; Zhou, F.; Xue, Q. Superamphiphobic coatings with coralline-like structure enabled by one-step spray of polyurethane/carbon nanotube composites. J. Mater. Chem. 2012, 22, 9624–9631. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Ouyang, Q.; Zha, F.; Jing, Z.; Li, X.; Lei, Z. Facile fabrication of translucent superamphiphobic coating on paper to prevent liquid pollution. Chem. Eng. J. 2014, 246, 238–243. [Google Scholar] [CrossRef]
- Zachariah, S.; Chuo, T.-W.; Liu, Y.-L. Crosslinked polybenzoxazine coatings with hierarchical surface structures from a biomimicking process exhibiting high robustness and anticorrosion performance. Polymer 2018, 155, 168–176. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Nguyen, S.H.; Guo, Y.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Wandiyanto, J.V.; Garvey, C.J.; Mahon, P.J.; Mainwaring, D.E.; et al. Bactericidal activity of self-assembled palmitic and stearic fatty acid crystals on highly ordered pyrolytic graphite. Acta Biomater. 2017, 59, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, Y. Enhanced biomimic bactericidal surfaces by coating with positively-charged ZIF nano-dagger arrays. Nanomedicine 2017, 13, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, P.; Wang, J.; Tu, Q.; Bai, L.; Xiong, K.; Qiu, H.; Zhao, X.; Maitz, M.F.; Wang, H.; et al. Endothelium-Mimicking Multifunctional Coating Modified Cardiovascular Stents via a Stepwise Metal-Catechol-(Amine) Surface Engineering Strategy. Research 2020, 2020, 9203906. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, A.; Parbat, D.; Manna, U. Catalyst-Free and Rapid Chemical Approach for in Situ Growth of “Chemically Reactive” and Porous Polymeric Coating. ACS Appl. Mater. Interfaces 2019, 11, 34316–34329. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, C.; Jing, J.; Zhang, X.; Wang, C.; Liu, F.; Jiang, M.; Wang, H. Bristle worm inspired ultra-durable superhydrophobic coating with repairable microstructures and anti-corrosion/scaling properties. Chem. Eng. J. 2022, 436, 135273. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, Q.; Deng, X.; Cui, J. Earthworm-Inspired Rough Polymer Coatings with Self-Replenishing Lubrication for Adaptive Friction-Reduction and Antifouling Surfaces. Adv. Mater. 2018, 30, 1802141. [Google Scholar] [CrossRef]
- Pillai, K.; Arzate, F.N.; Zhang, W.; Renneckar, S. Towards Biomimicking Wood: Fabricated Free-standing Films of Nanocellulose, Lignin, and a Synthetic Polycation. J. Vis. Exp. 2014, 88, e51257. [Google Scholar] [CrossRef]
- Zhou, P.; Lui, G.N.; Zhang, X. An experimental investigation of the effect of owl-inspired velvety coating on trailing edge noise. In Proceedings of the 25th AIAA/CEAS Aeroacoustics Conference, Delft, The Netherlands, 20–23 May 2019. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Z.; Wang, W.; Liu, W.; Xue, Q. Various curing conditions for controlling PTFE micro/nano-fiber texture of a bionic superhydrophobic coating surface. Mater. Chem. Phys. 2010, 119, 40–47. [Google Scholar] [CrossRef]
- Lackner, J.M.; Waldhauser, W.; Major, R.; Major, L.; Hartmann, P. Biomimetics in thin film design—Wrinkling and fracture of pulsed laser deposited films in comparison to human skin. Surf. Coat. Technol. 2013, 215, 192–198. [Google Scholar] [CrossRef]
- Oyane, A.; Kakehata, M.; Sakamaki, I.; Pyatenko, A.; Yashiro, H.; Ito, A.; Torizuka, K. Reprint of “Biomimetic apatite coating on yttria-stabilized tetragonal zirconia utilizing femtosecond laser surface processing”. Surf. Coat. Technol. 2016, 307, 1144–1151. [Google Scholar] [CrossRef]
- Lienhard, C.; Ferreira, R.L.; Gnos, E.; Hollier, J.; Eggenberger, U.; Piuz, A. Microcrystals coating the wing membranes of a living insect (Psocoptera: Psyllipsocidae) from a Brazilian cave. Sci. Rep. 2012, 2, 408. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Lee, D.; Lee, H.; Hong, S. A nature-inspired protective coating on soft/wet biomaterials for SEM by aerobic oxidation of polyphenols. Mater. Horiz. 2020, 7, 1387–1396. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).