Electrodeposition of Cu-Reinforced Polyaniline Coating for Protection of AH36 Steel in Natural Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
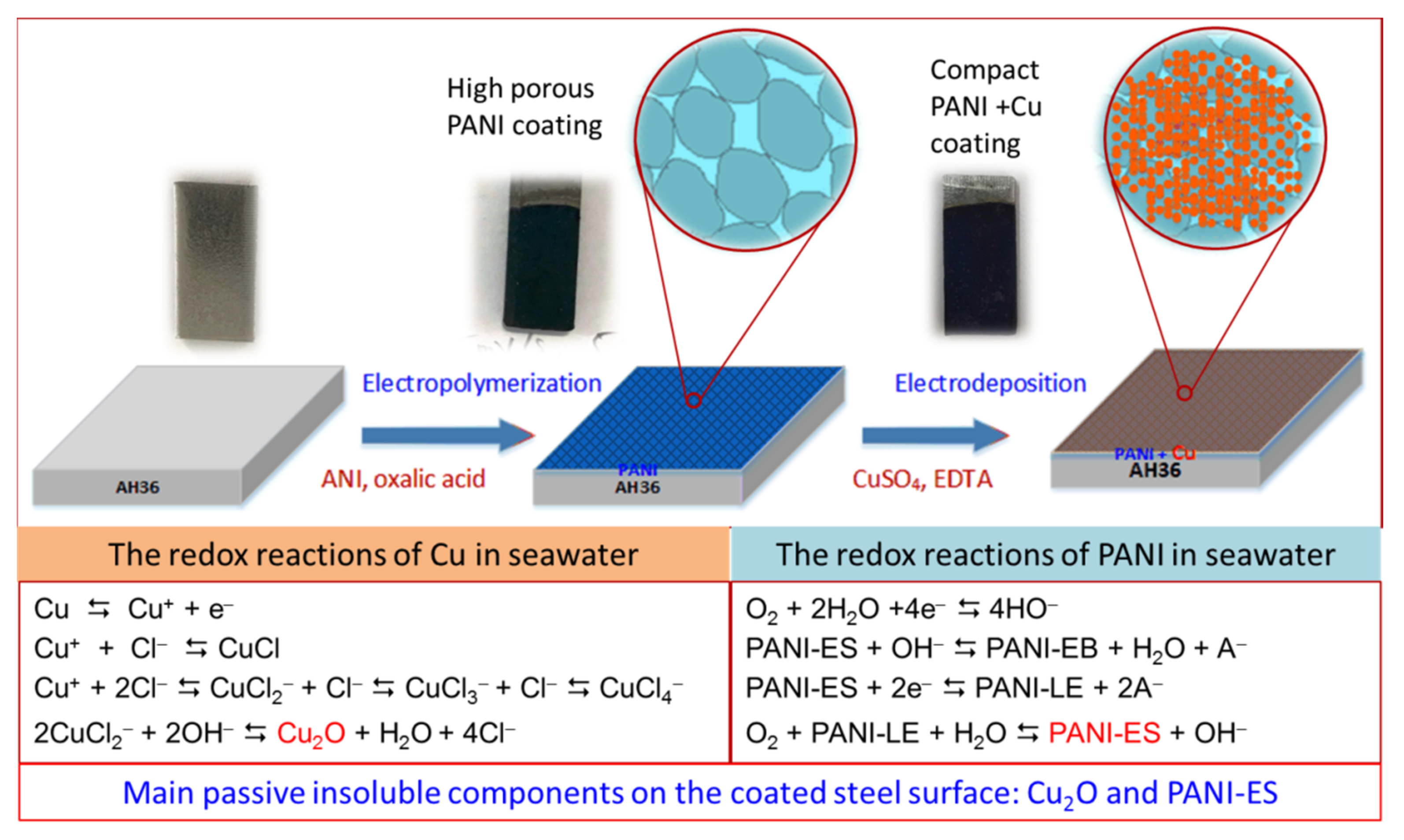
2.2. Electrodeposition of PANI and Cu on the AH36 Steel
2.3. Characterization and Electrochemical Corrosion Test
3. Results and Discussions
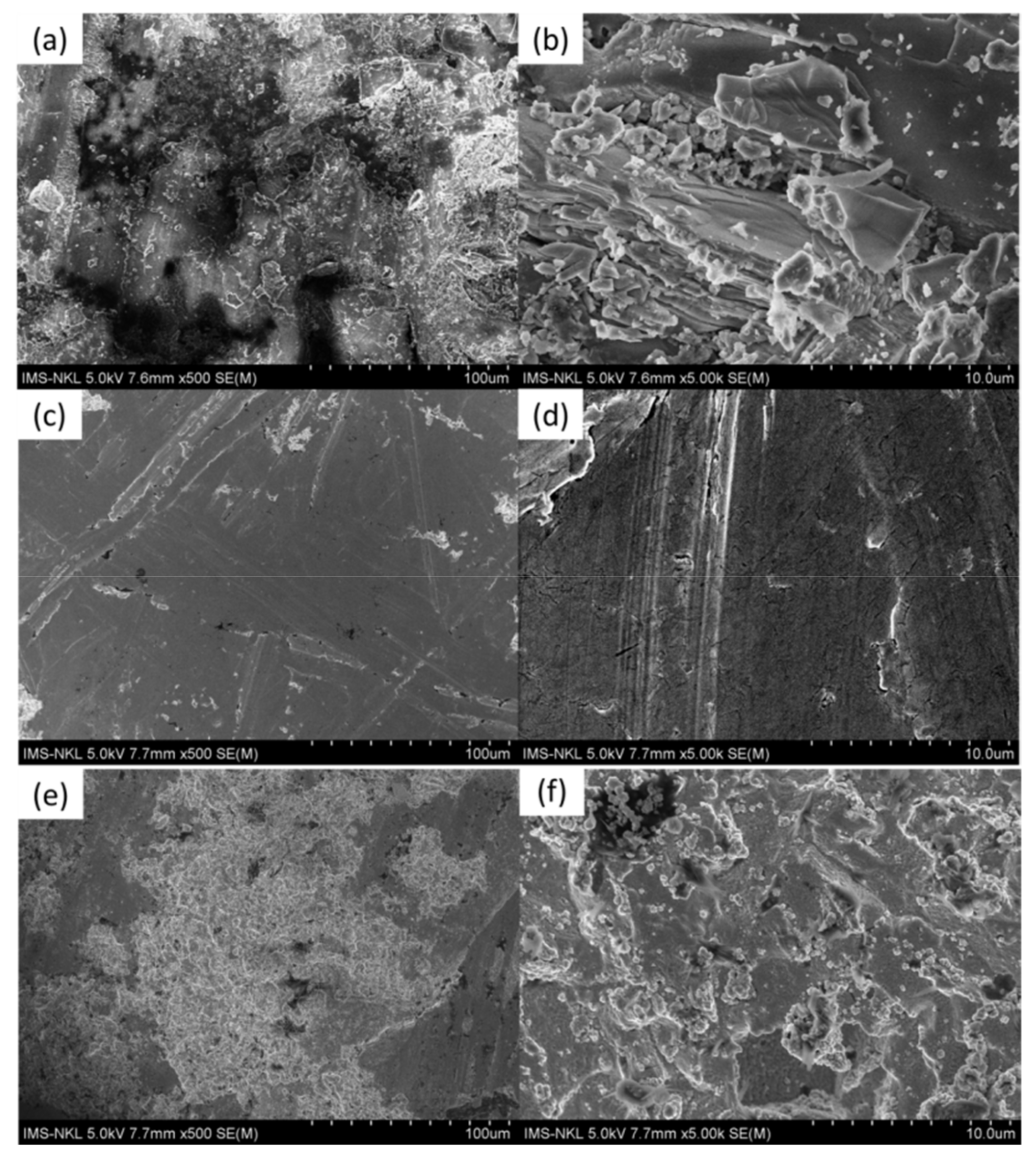
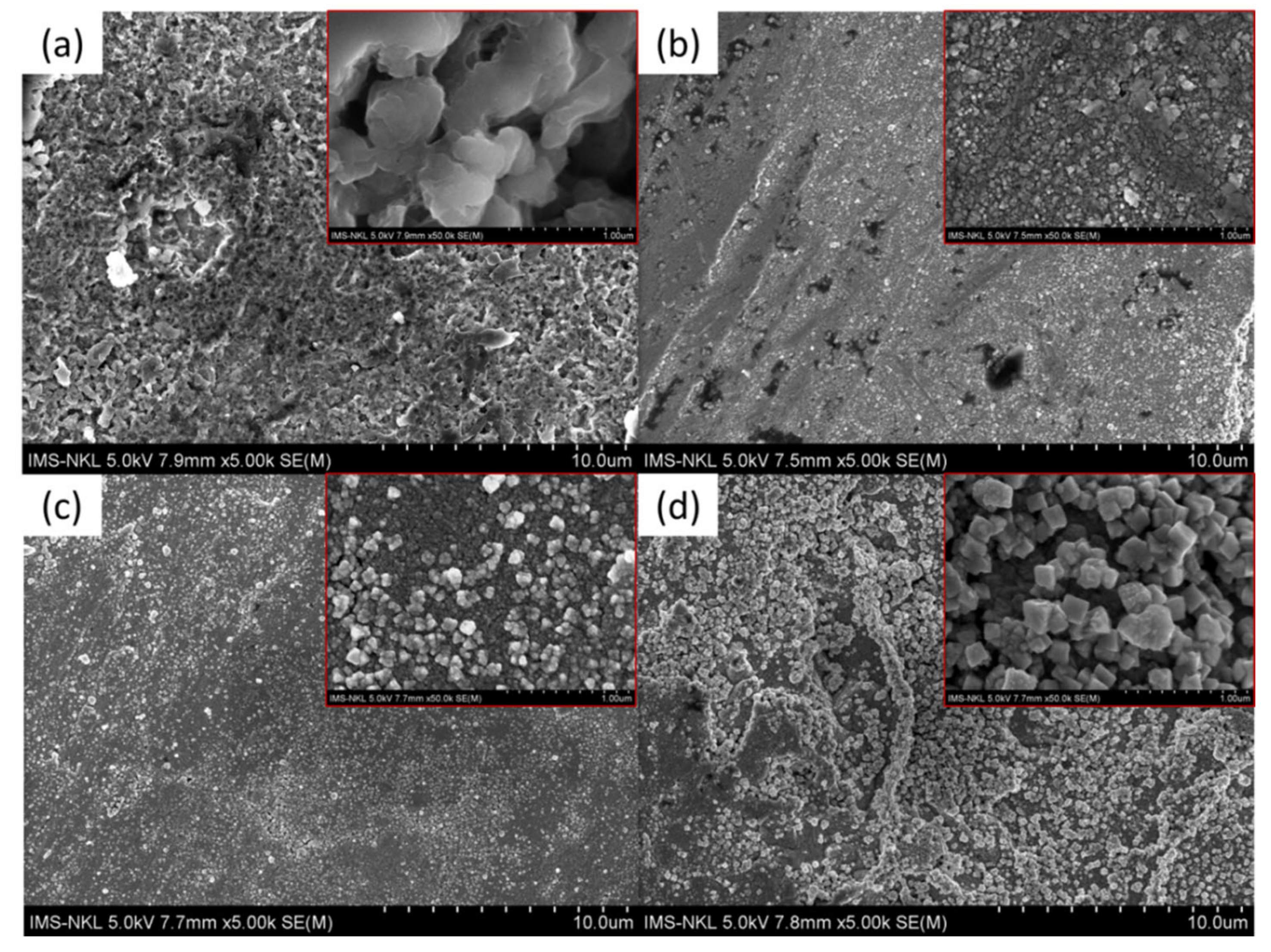
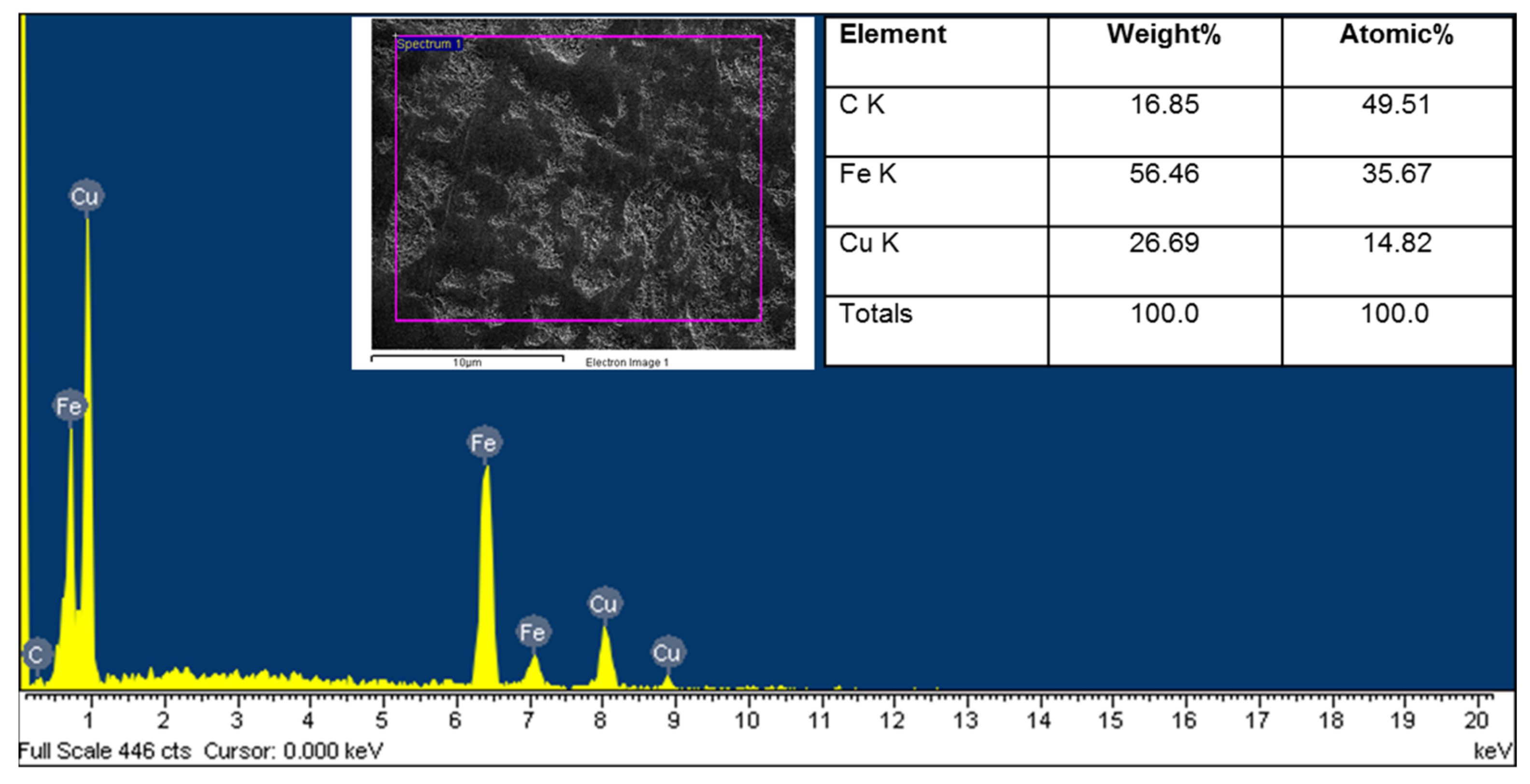
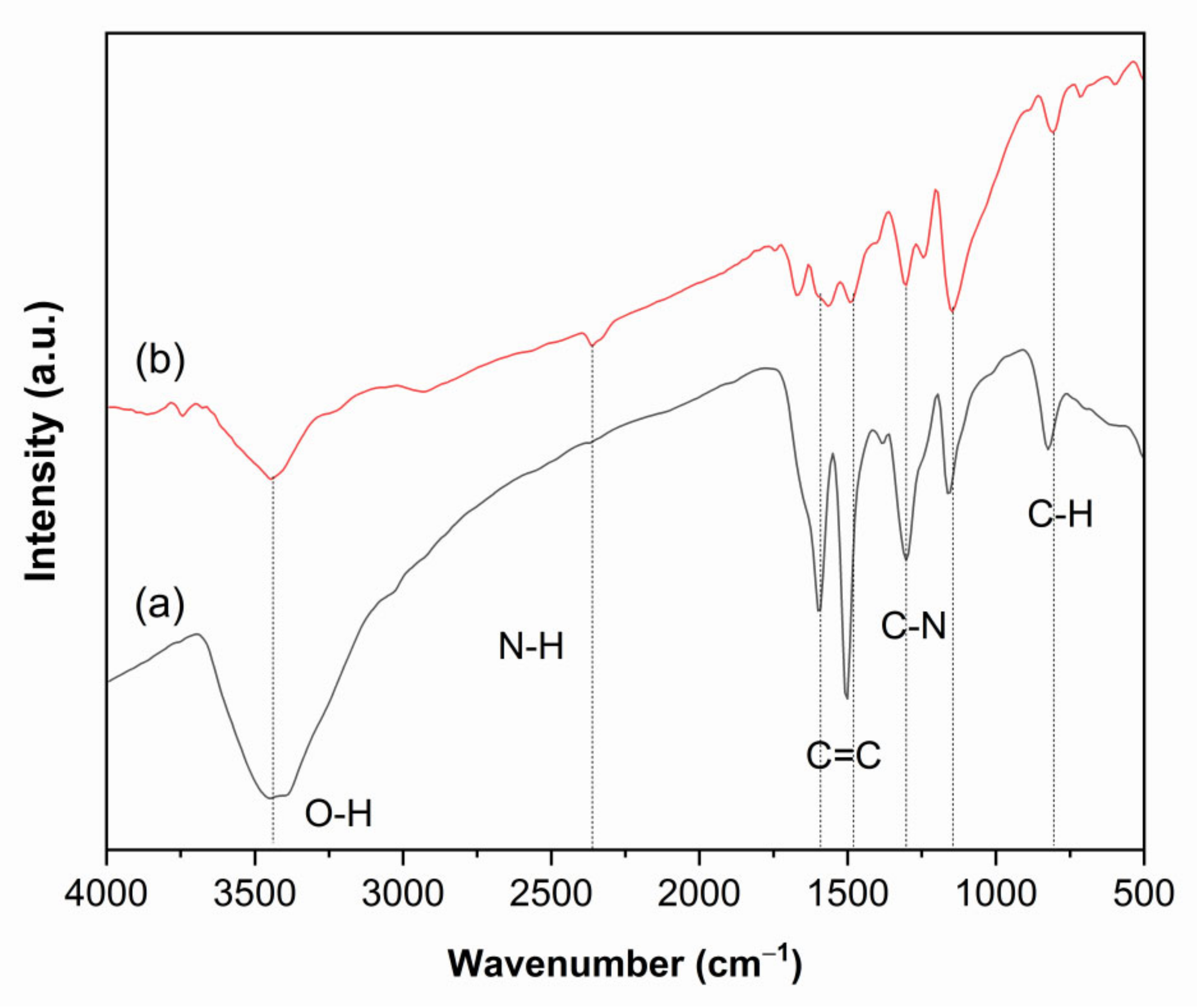
3.1. Characteristics of the Coated Samples
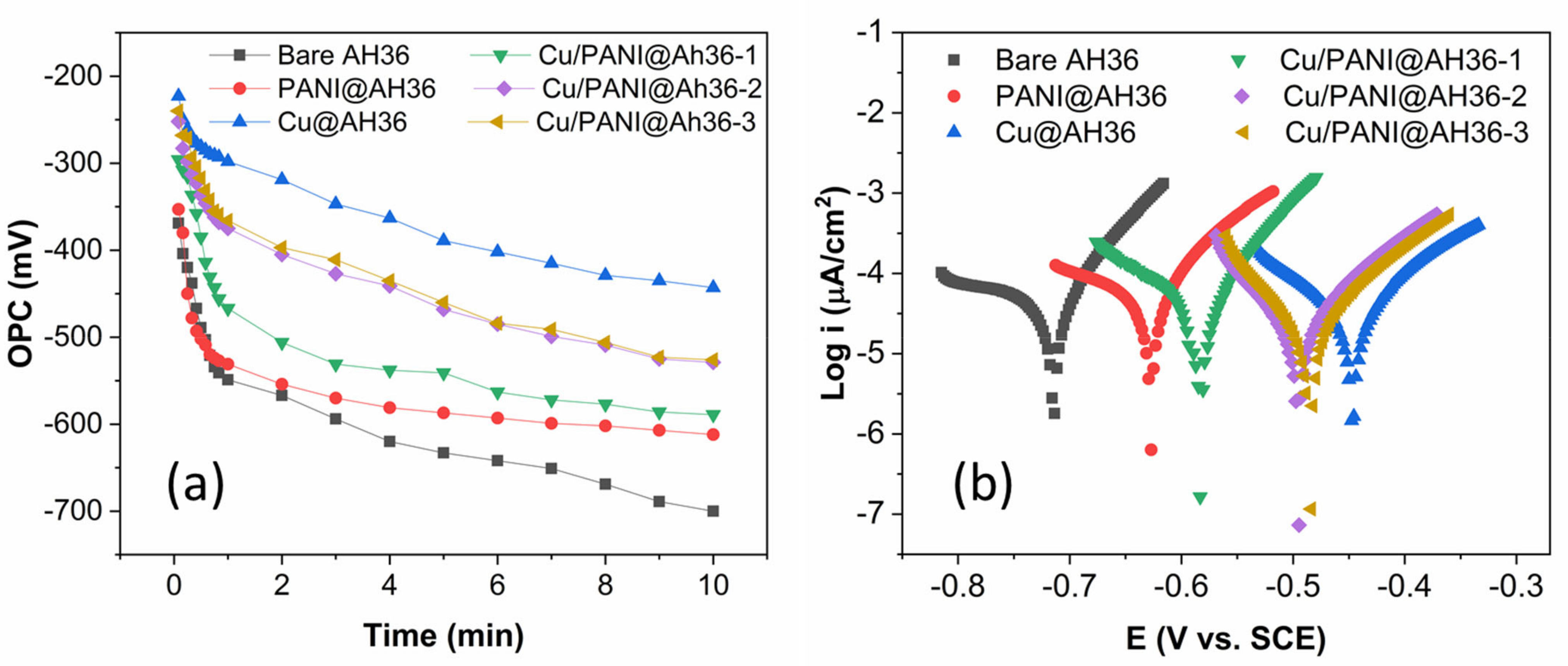
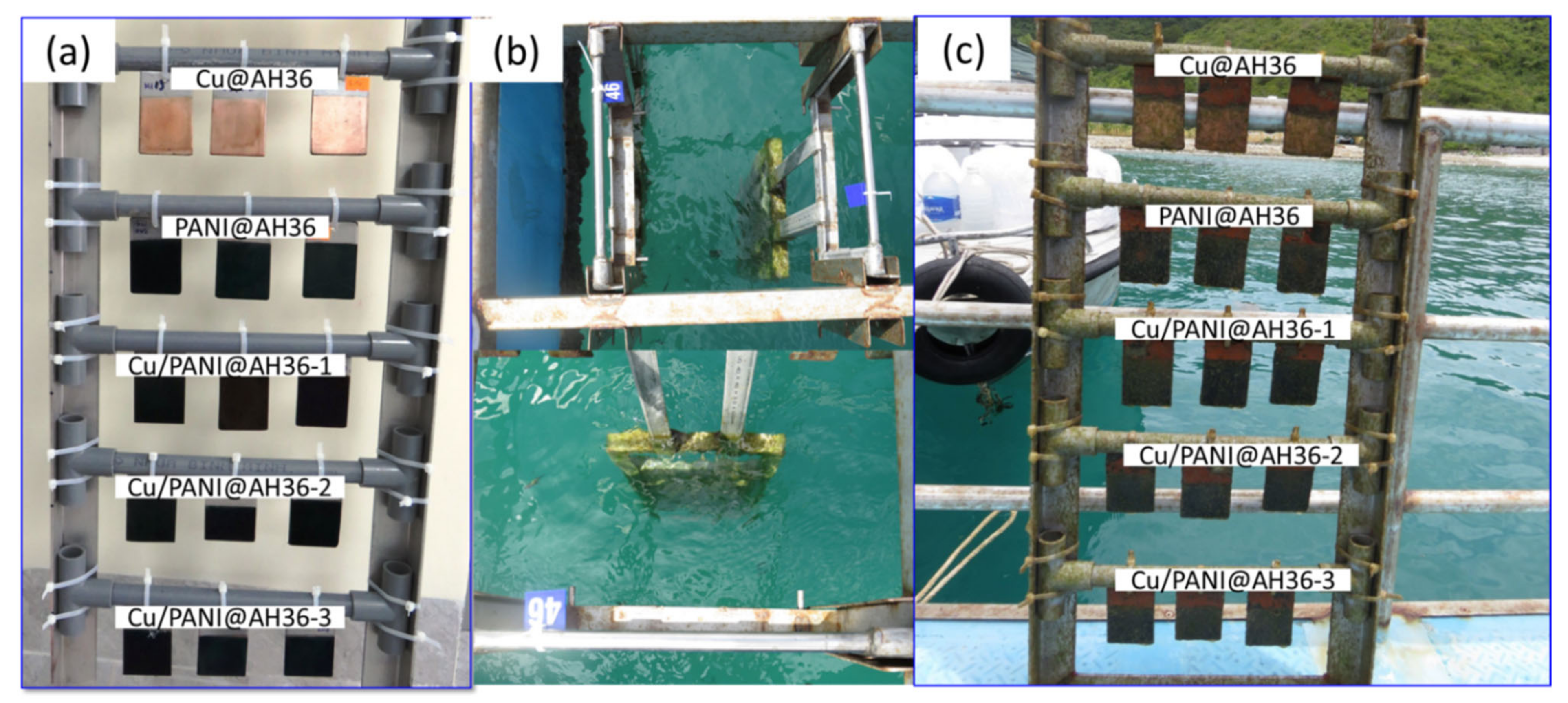
3.2. Electrochemical Performance
3.3. Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthieu, G.; Ratova, M.; Kelly, P. Design and Optimisation of A Low-Cost Titanium Dioxide-Coated Stainless Steel Mesh Photocatalytic Water Treatment Reactor. J. Clean. Prod. 2021, 297, 126641–126652. [Google Scholar]
- Lu, Y.; Ding, Y.; Wang, M.; Yang, L.; Wang, Y. An Environmentally Friendly Laser Cleaning Method to Remove Oceanic Micro-Biofoulings from AH36 Steel Substrate and Corrosion Protection. J. Clean. Prod. 2021, 314, 127961–127972. [Google Scholar] [CrossRef]
- Wanga, Y.; Wharton, J.A.; Shenoi, R.A. Ultimate Strength Analysis of Aged Steel-Plated Structures Exposed to Marine Corrosion Damage: A Review. Corros. Sci. 2014, 86, 42–60. [Google Scholar] [CrossRef]
- Melchers, R.E. Corrosion Uncertainty Modelling for Steel Structures. J. Constr. Steel Res. 1999, 52, 3–19. [Google Scholar] [CrossRef]
- Refait, P.; Grolleau, A.M.; Jeannin, M.; Rémazeilles, C.; Sabot, R. Corrosion of Carbon Steel in Marine Environments: Role of the Corrosion Product Layer. Corros. Mater. Degrad. 2020, 1, 198–218. [Google Scholar] [CrossRef]
- Procópio, L. The role of biofilms in the corrosion of steel in marine environments. World J. Microbiol. Biotechnol. 2019, 35, 73–81. [Google Scholar] [CrossRef]
- Jiang, L.; Syed, J.A.; Gao, Y.; Lu, H.; Meng, X. Electrodeposition of Ni(OH)2 Reinforced Polyaniline Coating for Corrosion Protection of 304 Stainless Steel. Appl. Surf. Sci. 2018, 440, 1011–1021. [Google Scholar] [CrossRef]
- Chen, S.; Li, B.; Xiao, R.; Luo, H.; Yu, S.; He, J.; Liao, X. Design an Epoxy Coating with TiO2/GO/PANI Nanocomposites for Enhancing Corrosion Resistance of Q235 Carbon Steel. Materials 2021, 14, 2629–2643. [Google Scholar] [CrossRef]
- Yang, G.; Liu, F.; Hou, N.; Peng, S.; He, C.; Fang, P. Preparation of One-Dimensional Polyaniline Nanotubes as Anticorrosion Coatings. Materials 2022, 15, 3192–3202. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Syed, J.A.; Lu, H.B.; Meng, X.K. Anti-corrosive performance of electropolymerized phosphomolybdic acid doped PANI coating on 304SS. Appl. Surf. Sci. A 2016, 360, 389–397. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting Polymers for Corrosion Protection: A Review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Camalet, J.L.; Lacroix, J.C.; Aeiyach, S.; Chane-Ching, K.; Lacaze, P.C. Electrodeposition of Protective Polyaniline Films on Mild Steel. J. Electroanal. Chem. 1996, 416, 179–182. [Google Scholar] [CrossRef]
- Nagashree, K.L.; Ahmed, M.F. Electrocatalytic Oxidation of Methanol on Cu Modified Polyaniline Electrode in Alkaline Medium. J. Appl. Electrochem. 2009, 39, 403–410. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Liu, C.; Cai, Z.; Wang, J.; Fu, X. Cathodic Deposition of Copper on Polyaniline-Coated Textiles from a Citrate Bath: Effects of Electroplating Conditions. J. Mater. Sci.-Mater. Electron. 2015, 26, 3621–3628. [Google Scholar] [CrossRef]
- Momeni, M.M.; Nazari, Z. Pt/PANI-MWCNTs Nanocomposite Coating Prepared by Electropolymerisation-Electrodeposition for Glycerol Electro-Oxidation. Surf. Eng. 2013, 31, 472–479. [Google Scholar] [CrossRef]
- Gao, L.; Lv, S.; Xing, S. Facile Route to Achieve Silver@Polyaniline Nanofibers. Synth. Met. 2012, 162, 948–952. [Google Scholar] [CrossRef]
- Tolstopyatova, E.G.; Pogulyaichenko, N.A.; Kondratiev, V.V. Synthesis and Electrochemical Properties of Composite Films Based on Poly-3,4-ethylenedioxythiophene with Inclusions of Silver Particles. Russ. J. Electrochem. 2014, 50, 510–516. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical Corrosion of Unalloyed Copper in Chloride Media—A Critical Review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Mansfeld, F.; Liu, G.; Xiao, H.; Tsai, C.H.; Little, B.J. The Corrosion Behavior of Copper Alloys, Stainless Steels and Titanium in Seawater. Corros. Sci. 1994, 36, 2063–2095. [Google Scholar] [CrossRef]
- Mathiyarasu, J.; Palaniswamy, N.; Muralidharan, V.S. Electrochemical behaviour of copper-nickel alloy in chloride solution. J. Chem. Sci. 1999, 111, 377–386. [Google Scholar] [CrossRef]
- Elguindy, J.; Moffitt, S.; Hasman, H.; Andrade, C.; Raghavan, S.; Rensing, C. Metallic Copper Corrosion Rates, Moisture Content, and Growth Medium Influence Survival of Copper Ion-Resistant Bacteria. Appl. Microbiol. Biotechnol. 2011, 89, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, Y.; Kamboj, A.; Rekha, M.Y.; Rao, N.P.N.; Srivastava, C. Copper-Graphene Oxide Composite Coatings for Corrosion Protection of Mild Steel in 3.5% NaCl. Thin Solid Films 2017, 636, 107–115. [Google Scholar] [CrossRef]
- Qiu, C.; Liu, D.; Jin, K.; Fang, L.; Xie, G.; Robertson, J. Electrochemical functionalization of 316 stainless steel with polyaniline-graphene oxide: Corrosion resistance study. Mater. Chem. Phys. 2017, 198, 90–98. [Google Scholar] [CrossRef]
- Yağan, A.; Pekmez, N.Ö.; Yıldız, A. Inhibition of corrosion of mild steel by homopolymer and bilayer coatings of polyaniline and polypyrrole. Prog. Org. Coat. 2007, 59, 297–303. [Google Scholar] [CrossRef]
- Iverson, W.P. Microbial Corrosion of Metals. Adv. Corros. Met. 1987, 32, 1–36. [Google Scholar]
- Bianchi, G.; Longhi, P. Copper in Seawater, Potential-pH Diagrams. Corros. Sci. 1973, 13, 853–864. [Google Scholar] [CrossRef]
- Arjmand, F.; Adriaens, A. Influence of pH and Chloride Concentration on the Corrosion Behavior of Unalloyed Copper in NaCl Solution: A Comparative Study Between the Micro and Macro Scales. Materials 2012, 5, 2439–2464. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.B.; Zhou, Y.Z.; Vongehr, S.; Hu, K.; Meng, X.K. Electropolymerization of PANI Coating in Nitric Acid for Corrosion Protection of 430 SS. Synth. Met. 2011, 161, 1368–1376. [Google Scholar] [CrossRef]


| Sample | Thickness (µm) | Adhesion (MPa) |
|---|---|---|
| PANI@AH36 | 47.2 ± 1.5 | 3.89 ± 0.01 |
| Cu@AH36 | 22.8 ± 0.7 | 5.03 ± 0.01 |
| Cu@PANI-1 | 52.6 ± 1.1 | 4.04 ± 0.01 |
| Cu@PANI-2 | 52.9 ± 1.6 | 4.79 ± 0.01 |
| Cu@PANI-3 | 53.4 ± 0.5 | 4.93 ± 0.01 |
| Parameters | Bare AH36 | PANI@AH36 | Cu@AH36 | Cu/PANI@AH36–1 | Cu/PANI@AH36-2 | Cu/PANI@AH36–3 |
|---|---|---|---|---|---|---|
| Ecorr (mV vs. Ag/AgCl) | −713 | −620 | −447 | −583 | −494 | −492 |
| icorr (μA cm−2) | 17.2 | 11.6 | 5.7 | 5.5 | 4.4 | 4.3 |
| Rp (Ω) | 362 | 551 | 697 | 726 | 905 | 912 |
| βa (mV/dec) | 393 | 199 | 115 | 75 | 64 | 63 |
| βc (mV/dec) | −66 | −68 | −62 | −69 | −67 | −65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, N.V.; Quan, L.H.; Linh, C.N.; Quang, N.Q.; Anh, N.D.; Kien, D.V.; Hoa, N.V. Electrodeposition of Cu-Reinforced Polyaniline Coating for Protection of AH36 Steel in Natural Seawater. Coatings 2022, 12, 1680. https://doi.org/10.3390/coatings12111680
Chi NV, Quan LH, Linh CN, Quang NQ, Anh ND, Kien DV, Hoa NV. Electrodeposition of Cu-Reinforced Polyaniline Coating for Protection of AH36 Steel in Natural Seawater. Coatings. 2022; 12(11):1680. https://doi.org/10.3390/coatings12111680
Chicago/Turabian StyleChi, Nguyen Van, Le Hong Quan, Cao Nhat Linh, Nong Quoc Quang, Nguyen Duc Anh, Dong Van Kien, and Nguyen Van Hoa. 2022. "Electrodeposition of Cu-Reinforced Polyaniline Coating for Protection of AH36 Steel in Natural Seawater" Coatings 12, no. 11: 1680. https://doi.org/10.3390/coatings12111680
APA StyleChi, N. V., Quan, L. H., Linh, C. N., Quang, N. Q., Anh, N. D., Kien, D. V., & Hoa, N. V. (2022). Electrodeposition of Cu-Reinforced Polyaniline Coating for Protection of AH36 Steel in Natural Seawater. Coatings, 12(11), 1680. https://doi.org/10.3390/coatings12111680







