Visual Inspection of the Heavy-Duty Paint Systems Used in Steel Bridges for Assessing the Level of Fire Damage
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of Painted Steel Specimens
2.2. Electric Furnace Heating Test Conditions
2.3. Electric Furnace Heating Test
2.4. Evaluation of Coated Surface Conditions and Anticorrosion Performance
3. Evaluation of the Coated Surface
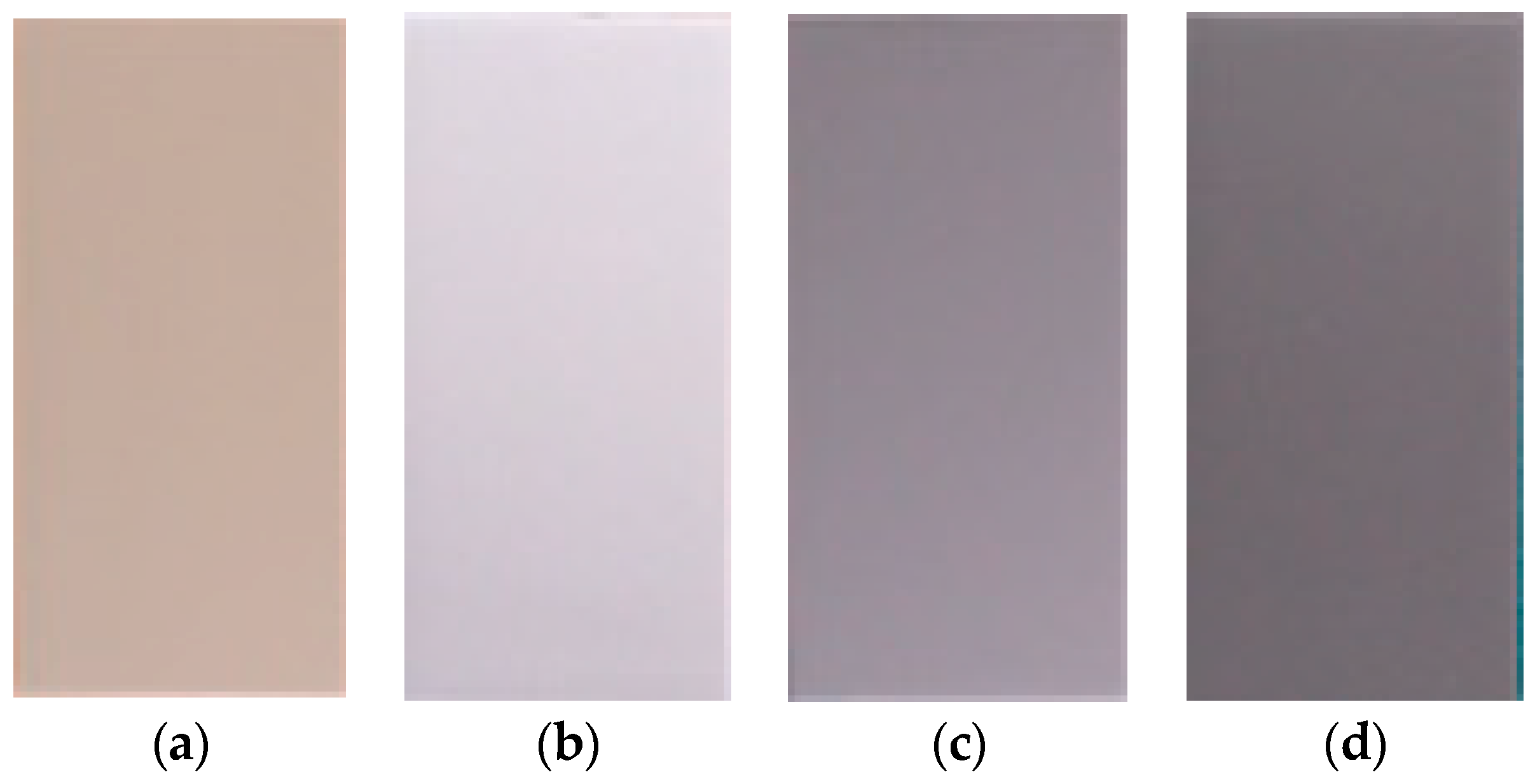
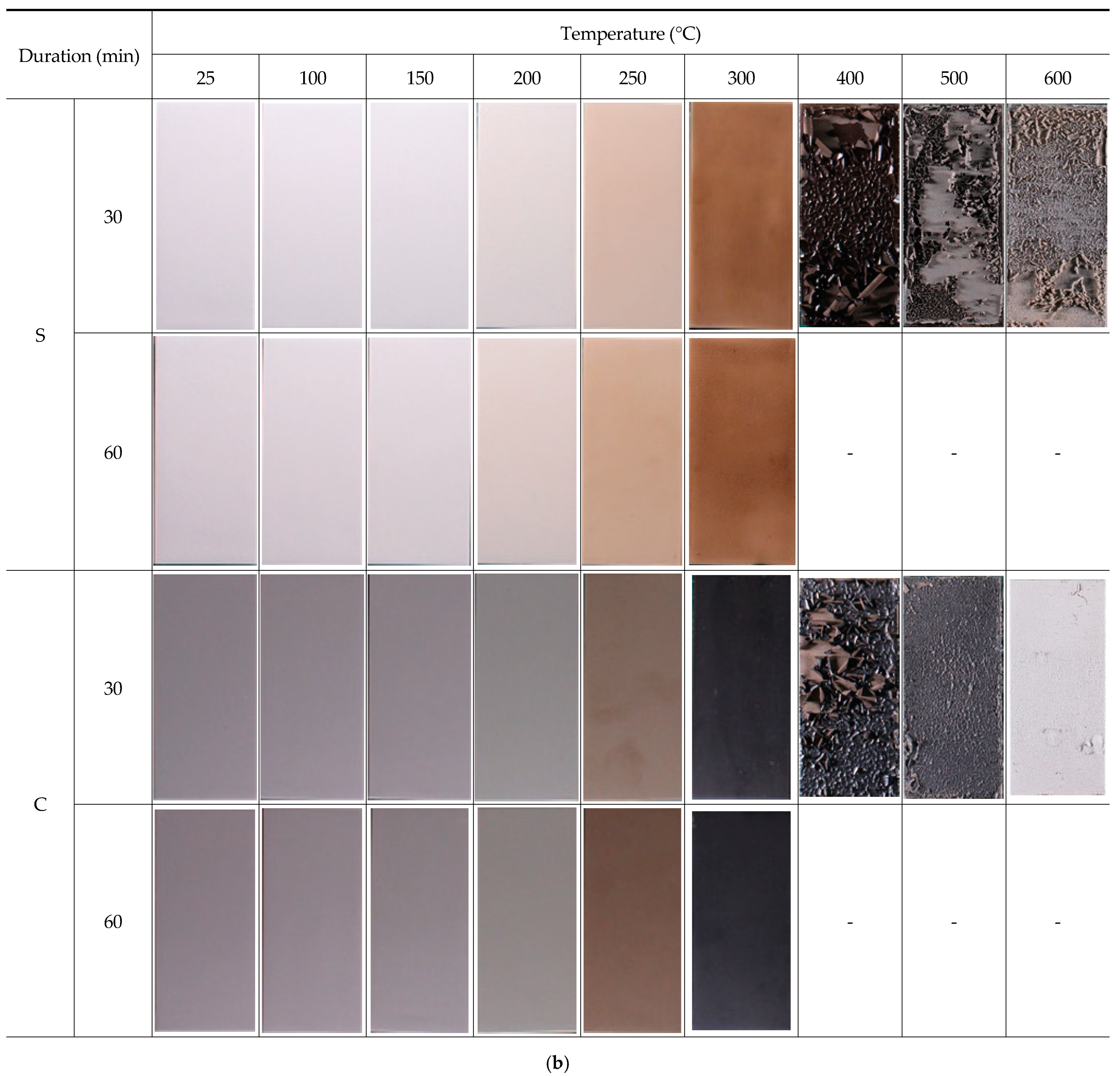
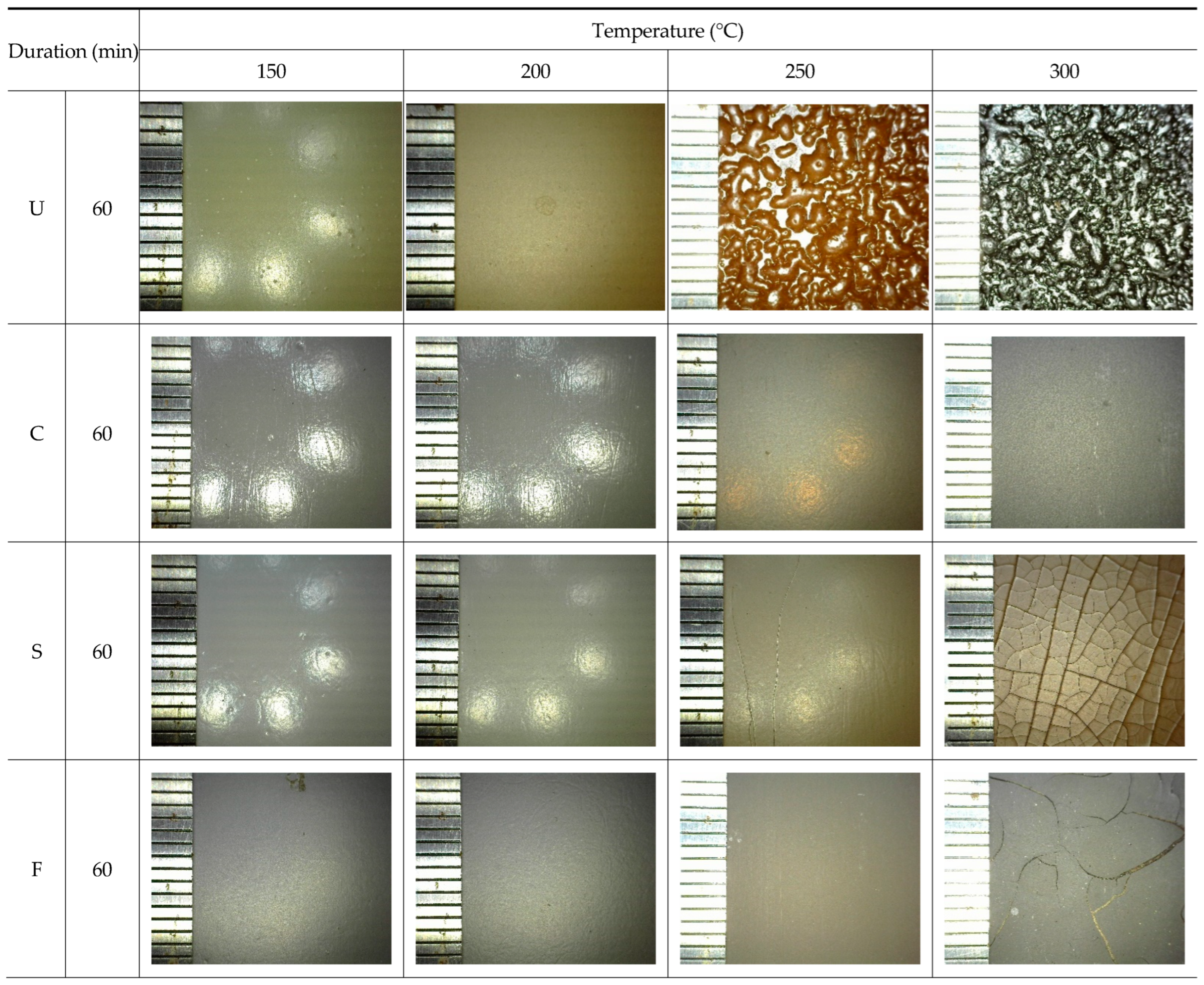
3.1. Visual Inspection (Discoloration, Delamination, Blistering, and Cracking)
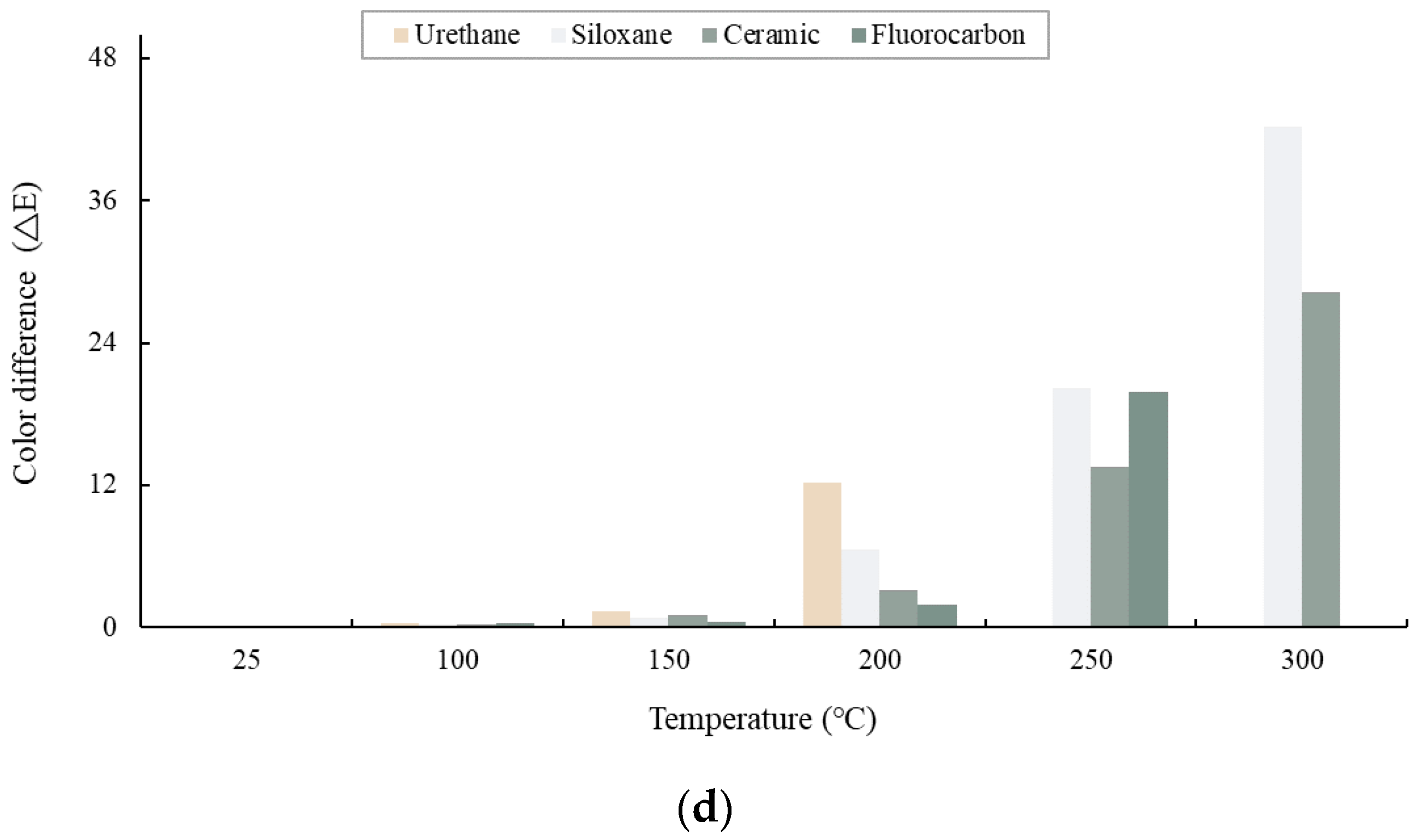
3.2. Color Differences of Paint Films
3.3. Visual Inspection of the Coated Surface and Estimation of the Temperature
3.4. Evaluation for the Reuse of Coating Affected by Fire
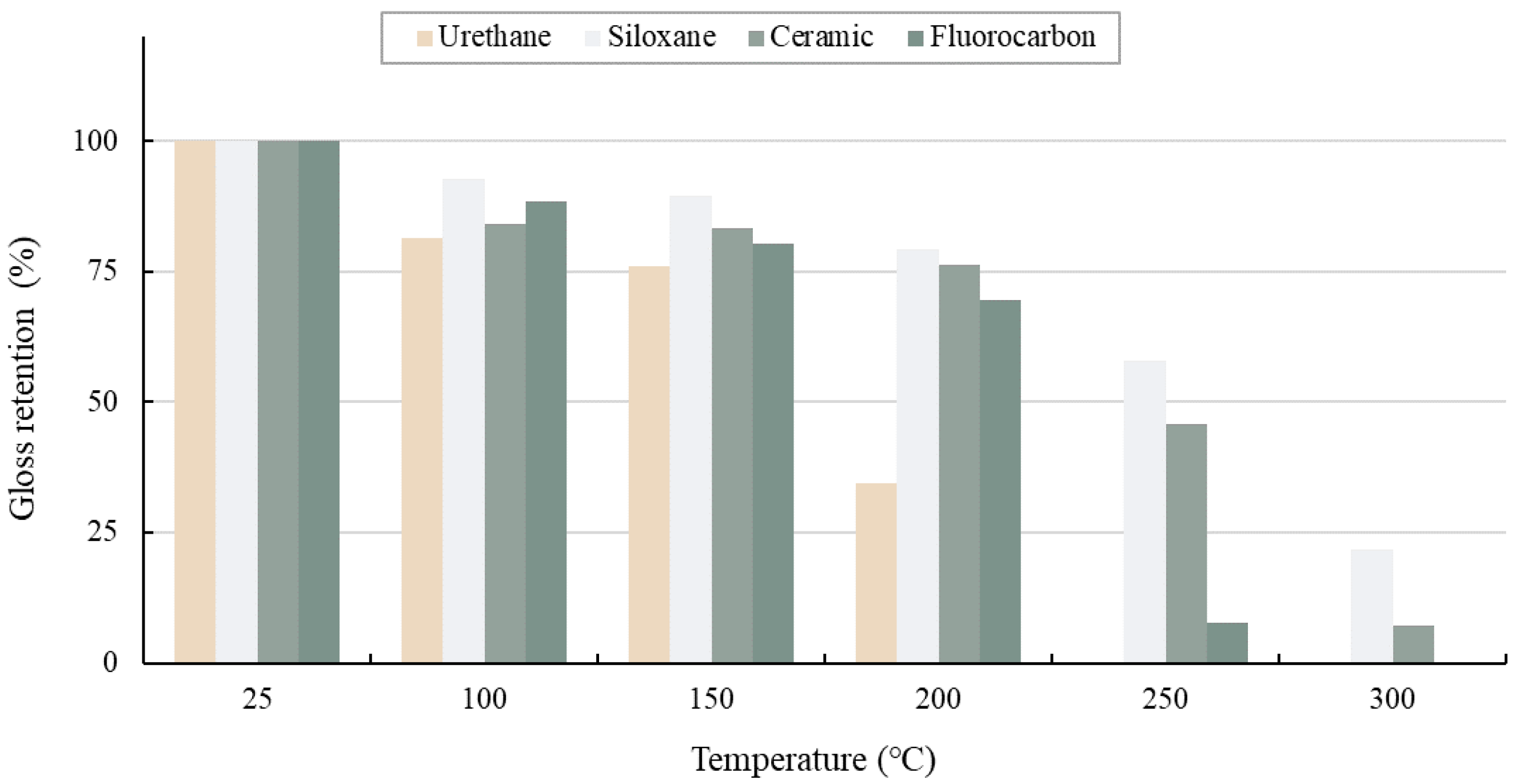
3.4.1. Gloss Retention
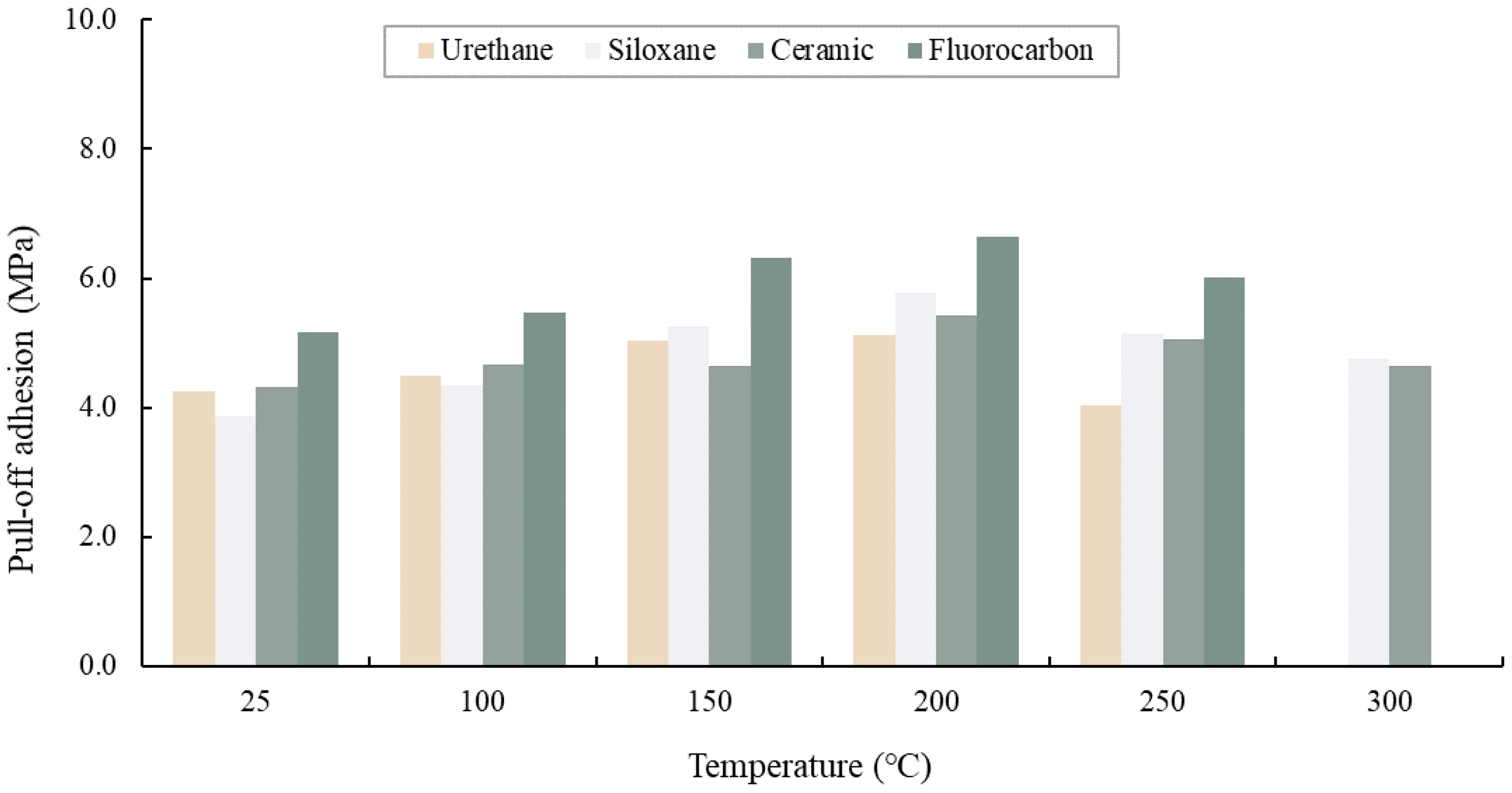
3.4.2. Pull-Off Adhesion
3.4.3. Electrochemical Impedance Spectroscopy (EIS)
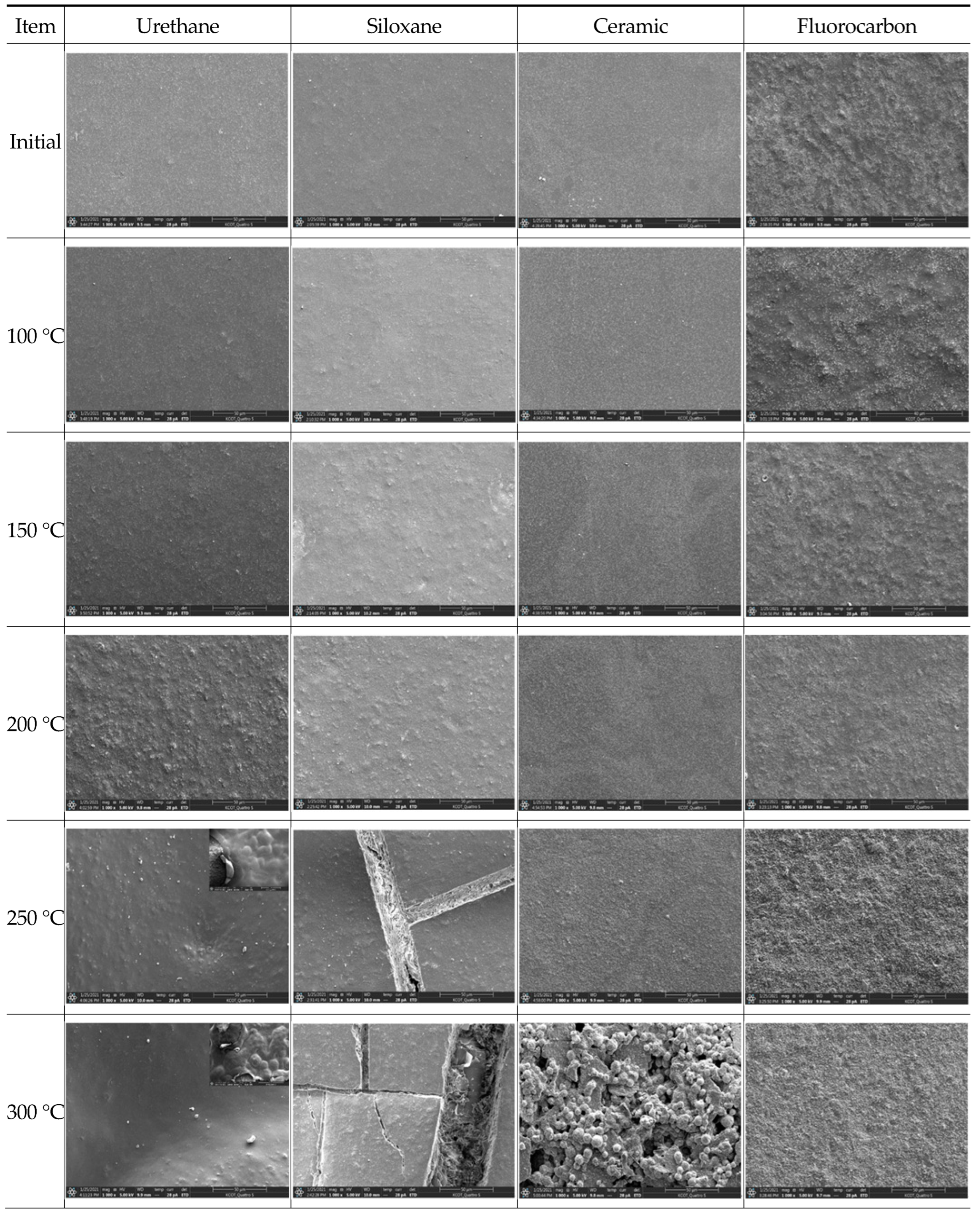
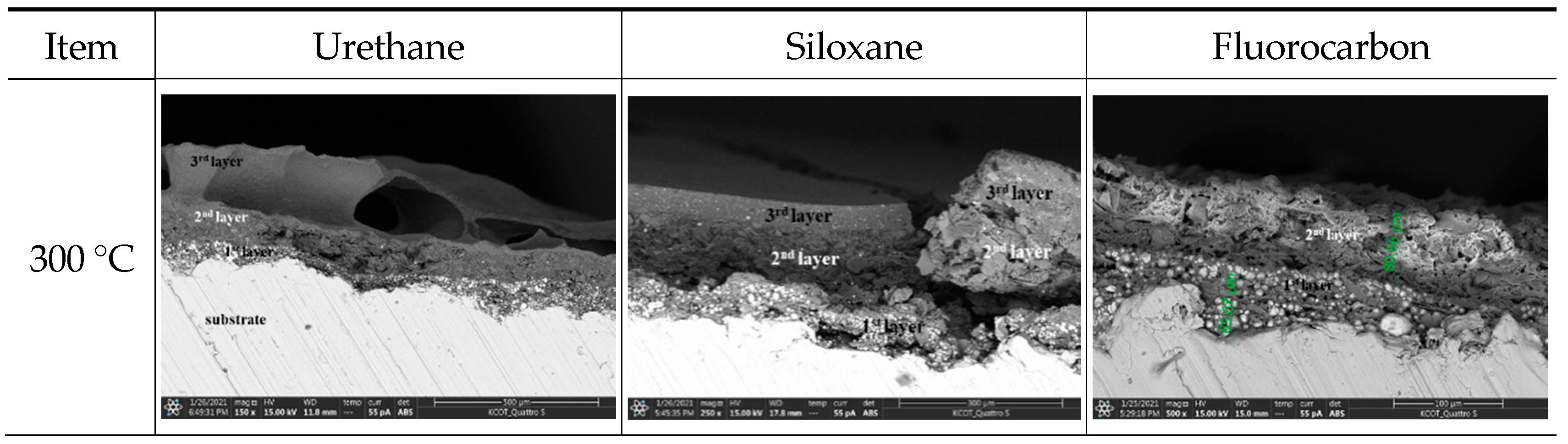
3.4.4. Surface Morphology Analysis through Scanning Electron Microscopy (E-SEM)
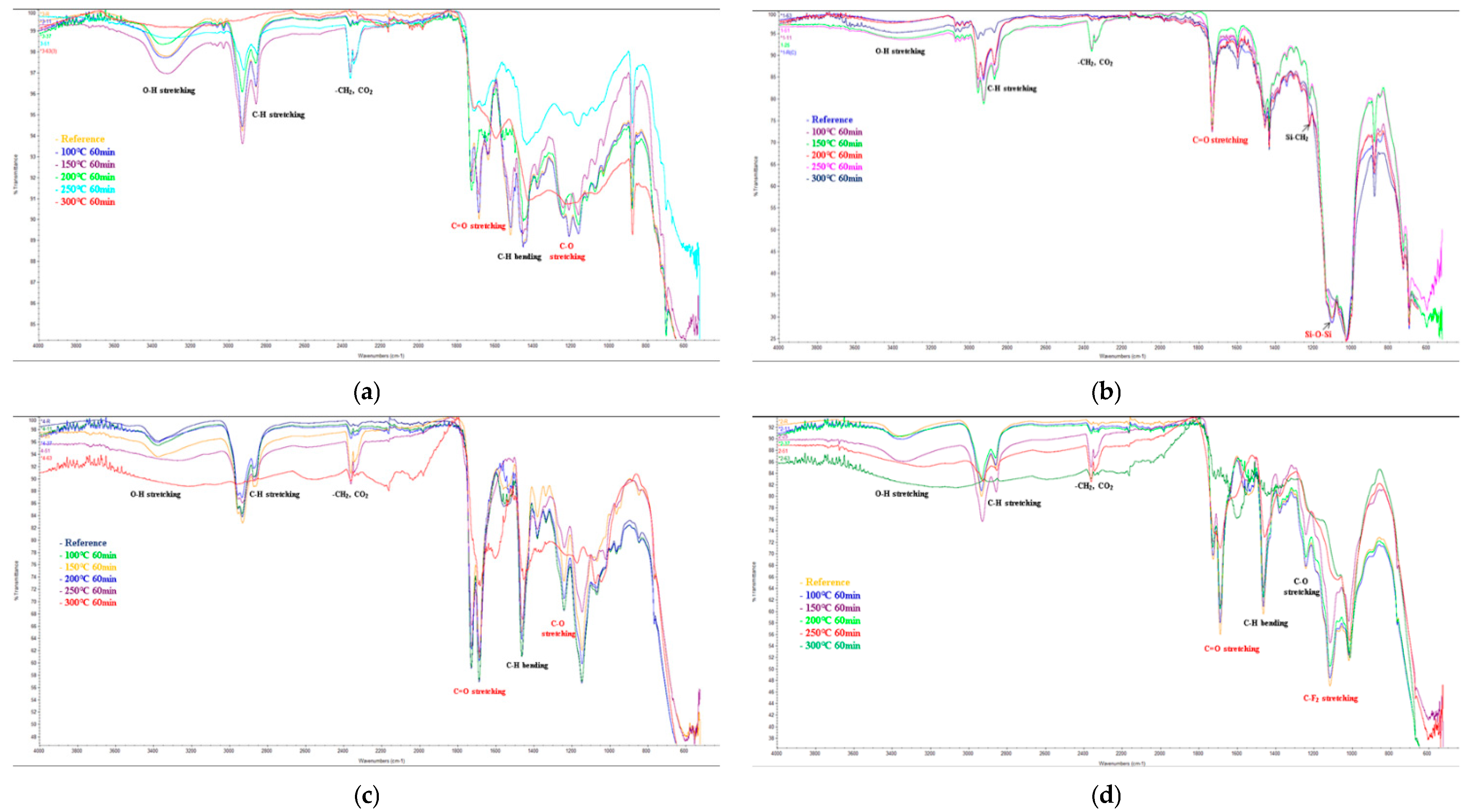
3.4.5. Organic Structural Analysis
4. Potential Applications of the Test Results
5. Conclusions
- (1)
- As the heating temperature increased from 100 to 600 °C, a noticeable discoloration occurred at 200–300 °C, followed by blackening at 300–400 °C and whitening at 400–600 °C. At 200–300 °C, localized paint film cracking and delamination were observed, while all the topcoats showed delamination at 300–600 °C.
- (2)
- As the heating temperature increased from 100 to 200 °C, a trend of an increase in the pull-off adhesion of the paint film by 20%–30% was followed by a slight drop at 200–250 °C. In addition, a notable decrease in pull-off adhesion did not occur when the paint film showed no blistering or delamination.
- (3)
- The EIS, E-SEM, and FT-IR analyses to assess the anticorrosion performance of the four painting systems showed that the temperature at which fire damage occurred was 200 °C for urethane and 250 °C for siloxane, ceramic, and fluorocarbon. The anticorrosion performance was retained until the heating temperature of 200 °C.
- (4)
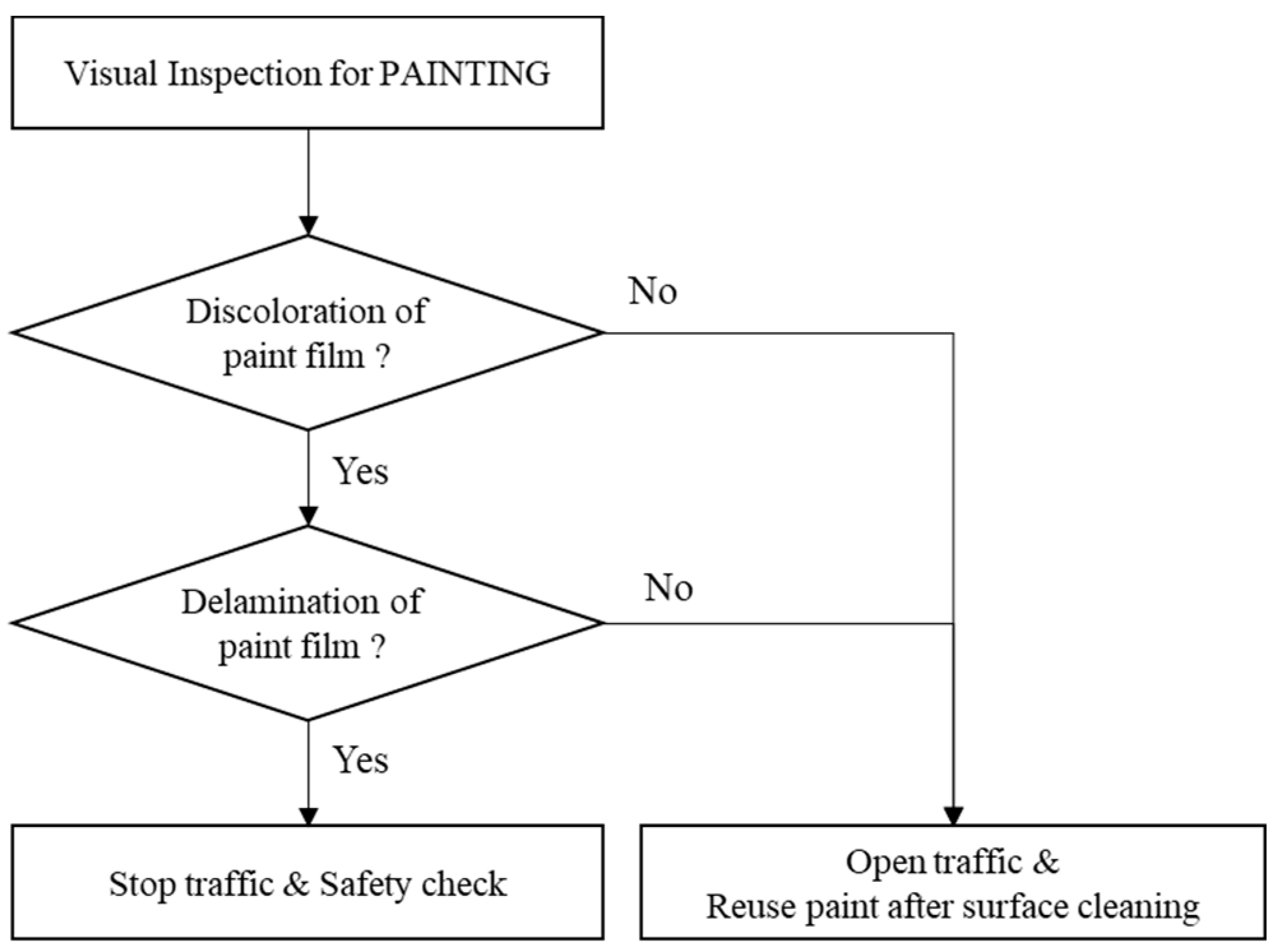
- The temperature-dependent changes in the surface conditions for the paint systems under study were confirmed to constitute a useful indicator in the visual inspection used to estimate the fire temperature and its range for a steel bridge. The visual inspection method (Figure 3) and the levels of discoloration and delamination of each system (Table 9) can allow for rapid responses to steel bridge fires with respect to deciding whether to stop or re-open the bridge to traffic after the fire’s control and determining the scope and methods of the repair and maintenance of the steel bridge.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kodur, V.; Gu, L.; Garlock, M.E. Review and assessment of fire hazard in bridge. J. Transport. Res. Board 2010, 2172, 23–29. [Google Scholar] [CrossRef]
- Garlock, M.; Paya-Zaforteza, I.; Kodur, V.; Gu, L. Fire hazard in bridges: Review, assessment and repair strategies. Eng. Struct. 2012, 35, 89–98. [Google Scholar] [CrossRef]
- Shim, J.W.; Shin, Y.H. Fire Damage Evaluation on Fire Source under the Bridge. J. Korean Soc. Steel Construct. 2010, 22, 75–78. (In Korean) [Google Scholar]
- Ohyama, O.; Imagawa, Y.; Kurita, A. Damage examples of bridge caused by fire. Bridge Found. Eng. 2008, 42, 35–39. (In Japanese) [Google Scholar]
- Hong, S.Y.; Jeong, S.Y.; Baek, S.G.; Choi, Y.H. The social cost of a fire under Bucheon Viaduct of Seoul ring expressway. Mag. Transp. Technol. Policy 2011, 8, 83–87. (In Korean) [Google Scholar]
- Kim, S.H.; Chung, K.S.; Choi, S.M. Residual strength of steel and composite structures damaged by fire. Mag. Korean Soc. Steel Construct. 2010, 26, 34–39. (In Korean) [Google Scholar]
- Kim, Y.S.; Choi, B.J. Experimental study on the fire resistance of steel-reinforced concrete column in fire according to load ratio. J. Korean Soc. Steel Construct. 2019, 31, 459–470. (In Korean) [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.S.; Lee, H.D.; Shin, K.J. Collapse behavior of PEB building through fire test. J. Korean Soc. Steel Construct. 2020, 32, 1–10. (In Korean) [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, S.D.; Choi, S.K.; Kim, M.H.; Kim, S.D. Experimental study on the fire resistance of the iTECH composite beam. J. Korean Soc. Steel Construct. 2006, 18, 643–653. (In Korean) [Google Scholar]
- Short, N.R.; Purkiss, J.A.; Guise, S.E. Assessment of fire damaged concrete using colour image analysis. Construct. Build. Mater. 2001, 16, 9–15. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, K.H.; Hong, K.P. Color and material property changes in concrete exposed to high temperatures. J. Asian Archit. Build. Eng. Build. Mater. 2009, 8, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Lempereur, C.; Andral, R.; Prudhomme, J.Y. Surface temperature measurement on engine components by means of irreversible thermal coating. Meas. Sci. Technol. 2008, 19, 105501–105512. [Google Scholar] [CrossRef]
- Pelvich, C.W.; Foulk, D.L.; Polec, T.W. Method of Sensing High Surface Temperature in an Aircraft. Patent EP1959246 A2, 19 June 2008. [Google Scholar]
- Belykh, A.V.; Efremov, A.M.; Mikhailov, M.D. Thermochromic Material. European Patent EP1405890 B1, 17 October 2012. [Google Scholar]
- Lataste, E.; Demourgues, A.; Salmi, J.; Naporea, C.; Gaudon, M. Thermochromic behaviour (400 < T °C < 1200 °C) of barium carbonate/binary metal oxide mixtures. Dyes Pigm. 2011, 91, 396–403. [Google Scholar]
- KCS 14 31 40:2019; Korean Construction Specification. Paint. Ministry of Land, Infrastructure and Transport: Sejong, Korea, 2019. (In Korean)
- KS M ISO 8501-1; Preparation of Steel Substrates before Application of Paints and Related Products—Visual Assessment of Surface Cleanliness. Korean Agency for Technology and Standards: Chungcheongbuk-do, Korea, 2021. (In Korean)
- ANSI/AISC 360-16; Specification for Structural Steel Buildings. American Institute of Steel Construction: Chicago, IL, USA, 2016; pp. 222–226.
- Korea Expressway Corporation. Exploring of Fire Resistance Design of Bridge Substructure and Underneath Structure; Korea Expressway Corporation: Gimcheon, Korea, 2013. [Google Scholar]
- National Fire Agency. Fire Statistical Yearbook; National Fire Agency: Sejong, Korea, 2019. (In Korean)
- Ministry of Land, Infrastructure and Transport, and Korea Authority of Land & Infrastructure Safety. Detailed Guideline for Facility Safety Management and Maintenance (Performance Assessment); Korea Authority of Land & Infrastructure Safety: Jinju, Korea, 2018.
- Mohammed, Q.A.Q.; Salman, S.B. Color change of direct resin-based composites after bleaching: An in vitro study. King Saud Univ. J. Dent. Sci. 2011, 2, 23–27. [Google Scholar]
- KS M ISO 4624:2016; Paint and Varnishes-Pull off Test for Adhesion. Korean Agency for Technology and Standards: Chungcheongbuk-do, Korea, 2016. (In Korean)
- Lee, L.H. Adhesive Bonding; Springer: New York, NY, USA, 1991; p. 260. [Google Scholar]
- Knudsen, O.O.; Forsgren, A. Corrosion Control through Organic Coatings; CRC Press: Boca Raton, FL, USA, 2017; p. 141. [Google Scholar]
- Amirudin, A.; Thierry, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lee, S.H.; Park, J.H. Evaluation of deterioration of epoxy primer for steel bridge coating using image processing and electrochemical impedance spectroscopy. J. Corros. Sci. Technol. 2009, 8, 53–61. [Google Scholar]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Liu, J.; Tang, J.; Wang, X.; Wu, D. Synthesis, characterization and curing properties of a novel cyclolinear phosphazene-based epoxy resin for halogen-free flame retardancy and high performance. RSC Adv. 2012, 2, 5789–5799. [Google Scholar] [CrossRef]
- Awaja, F.; Zhang, S.; Tripathi, M.; Nikiforov, A.; Pugno, N. Cracks, microcracks and fracture in polymer structures. Prog. Mater. Sci. 2016, 83, 536–573. [Google Scholar] [CrossRef]
- Sigma Aldrich, IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/KR/ko/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 20 May 2022).
- Doblies, A.; Boll, B.; Fiedler, B. Prediction of thermal exposure and mechanical behavior of epoxy resin using artificial neural networks and fourier transform infrared spectroscopy. Polymers 2019, 11, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S. Thermal decomposition of fluoropolymers and firing characteristics of priming compositions with fluoropolymers. J. Appl. Polym. Sci. 2005, 97, 2054–2059. [Google Scholar] [CrossRef]
- Architectural Institute of Japan. Recommendation for Fire Resistant Design of Steel Structures; Maruzen: Hiroshima, Japan, 2008. (In Japanese) [Google Scholar]



| Range of Temperature (°C) | Color | Appearance | Condition |
|---|---|---|---|
| T < 300 | Normal | Normal | Normal |
| 300 ≤ T < 600 | Pink or red | Surface crazing, cracking, and aggregate pop outs | Sound, but strength may be reduced |
| 600 ≤ T < 900 | Whitish grey | Spalling, exposed of steel reinforcement and powdered existence | Weak |
| T ≥ 1000 | Buff | Extreme spalling | Extreme/severe |
| Range of Temperature (°C) | Color | Appearance | Condition |
|---|---|---|---|
| T < 600 | Little change | No visible damage or distortion may occur | Weak |
| T > 600 | Little change | Large deflection or localized distortion | Extreme/severe |
| Material | Yield Strength [MPa] | Tensile Strength [MPa] | Elongation at Failure [%] |
|---|---|---|---|
| SS275 | 342 | 416 | 40 |
| Material | C | Si | Mn | P | S |
|---|---|---|---|---|---|
| SS275 | 0.150 | 0.100 | 0.390 | 0.014 | 0.007 |
| Painting System | Symbol | Color | Coating Layer (µm) | Paint Film Thickness (µm) | ||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | ||||
| Urethane | U | Bone | Inorganic Zinc (75) | High-solid epoxy (80) | Urethane (60) | 215 |
| Siloxane | S | Anti-flash White | Inorganic Zinc (75) | High-solid epoxy (80) | Polysiloxane (60) | 215 |
| Ceramic | C | Spanish Gray | Inorganic Zinc (75) | Ceramic protective paint (75) | Ceramic urethane (75) | 225 |
| Fluorocarbon | F | Dolphin Gray | Inorganic Zinc (75) | High-solid epoxy (100) | Fluorocarbon (50) | 225 |
| Temperature (°C) | Duration Time (min) | |||||||
|---|---|---|---|---|---|---|---|---|
| 30 | 60 | |||||||
| U | S | C | F | U | S | C | F | |
| Initial | 2 | 2 | 2 | 2 | - | |||
| 100 | 6 | 6 | 6 | 6 | 7 | 7 | 7 | 7 |
| 150 | 6 | 6 | 6 | 6 | 7 | 7 | 7 | 7 |
| 200 | 6 | 6 | 6 | 6 | 7 | 7 | 7 | 7 |
| 250 | 6 | 6 | 6 | 6 | 7 | 7 | 7 | 7 |
| 300 | 6 | 6 | 6 | 6 | 7 | 7 | 7 | 7 |
| 400 | 1 | 1 | 1 | 1 | - | |||
| 500 | 1 | 1 | 1 | 1 | - | |||
| 600 | 1 | 1 | 1 | 1 | - | |||
| N.B.S Unit | Sensitive Expression of Color Difference |
|---|---|
| 0.0–0.5 | Trace (Extremely slight change) |
| 0.5–1.5 | Slight (Slight change) |
| 1.5–3.0 | Noticeable (Perceivable change) |
| 3.0–6.0 | Appreciable (Marked change) |
| 6.0–12.0 | Much (Extremely marked change) |
| >12.0 | Very much (Change to another color) |
| Temperature (°C) | Discoloration | Paint Film Condition |
|---|---|---|
| ~150 | Extremely slight change | Extremely slight change |
| 150–200 | Slight change | |
| 200–300 | Marked change | Blistering, Partial delamination |
| 300–400 | Blackening (Changes to black) | Full delamination |
| 400–600 | Whitening (Changes to white) |
| Item | Impedance (Ω cm2, 0.01 Hz) | |||
|---|---|---|---|---|
| Urethane | Siloxane | Ceramic | Fluorocarbon | |
| 100 °C | 2.00 × 1010 | 1.23 × 1010 | 2.73 × 1010 | 2.36 × 1010 |
| 150 °C | 2.79 × 1010 | 2.29 × 1010 | 3.26 × 1010 | 2.49 × 1010 |
| 200 °C | 2.41 × 1010 | 2.63 × 1010 | 3.17 × 1010 | 3.42 × 1010 |
| 250 °C | - | - | 3.01 × 1010 | 2.25 × 1010 |
| 300 °C | - | - | 2.34 × 1010 | - |
| Appearance | Temperature, T (°C) | Steel Member | Painting Systems |
|---|---|---|---|
| Extremely slight change | T ≤ 150 | OK as is | OK as is |
| Slight change (discoloration) | 150 < T ≤ 200 | OK as is | OK as is |
| Blistering Partial delamination | 200 < T ≤ 300 | OK as is | Repair |
| Blackening/Whitening Full delamination | 300 < T ≤ 600 | Reinforcement with new members or heating straightening required | Repair |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.-T.; Cha, K.-H.; Jeong, Y.-S.; Shin, A.-S. Visual Inspection of the Heavy-Duty Paint Systems Used in Steel Bridges for Assessing the Level of Fire Damage. Coatings 2022, 12, 1697. https://doi.org/10.3390/coatings12111697
Kim I-T, Cha K-H, Jeong Y-S, Shin A-S. Visual Inspection of the Heavy-Duty Paint Systems Used in Steel Bridges for Assessing the Level of Fire Damage. Coatings. 2022; 12(11):1697. https://doi.org/10.3390/coatings12111697
Chicago/Turabian StyleKim, In-Tae, Ki-Hyuk Cha, Young-Soo Jeong, and An-Seob Shin. 2022. "Visual Inspection of the Heavy-Duty Paint Systems Used in Steel Bridges for Assessing the Level of Fire Damage" Coatings 12, no. 11: 1697. https://doi.org/10.3390/coatings12111697





