Role of Crystallographic Orientation of β-Sn Grain on Electromigration Failures in Lead-Free Solder Joint: An Overview
Abstract
:1. Introduction
2. Influence of Crystallographic Orientation of β-Sn Grain on EM
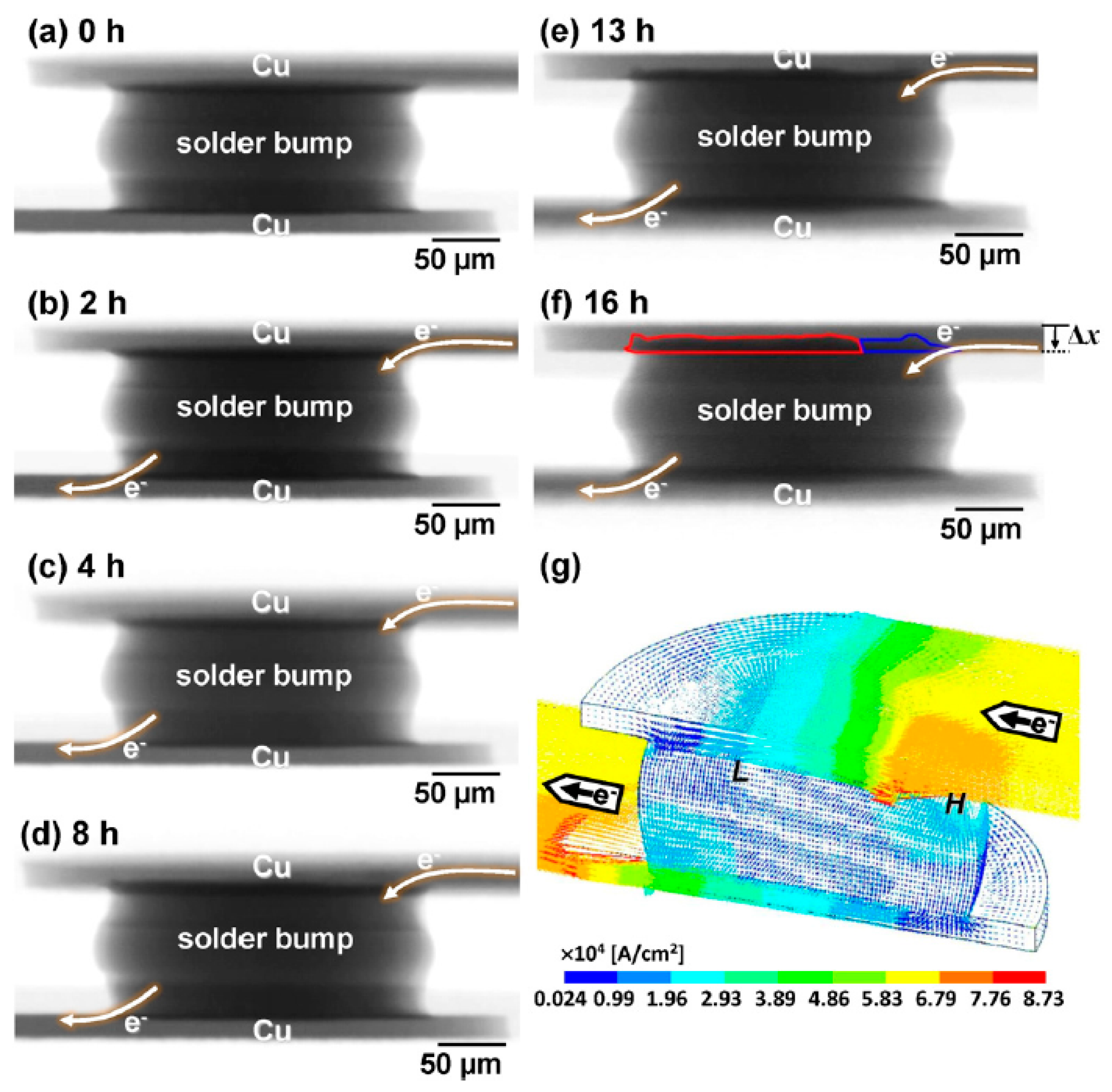
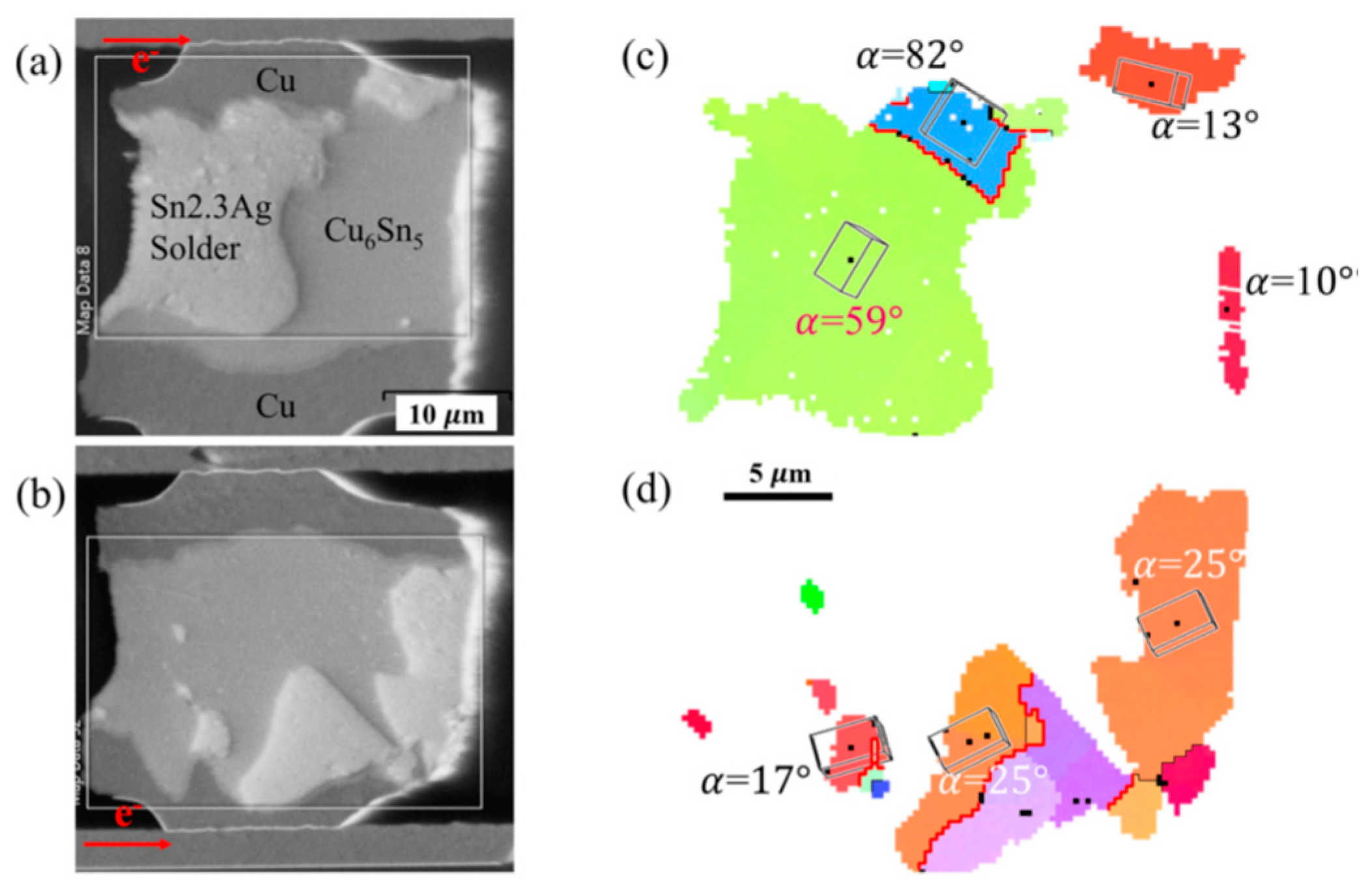
2.1. Influence on Cu Migration
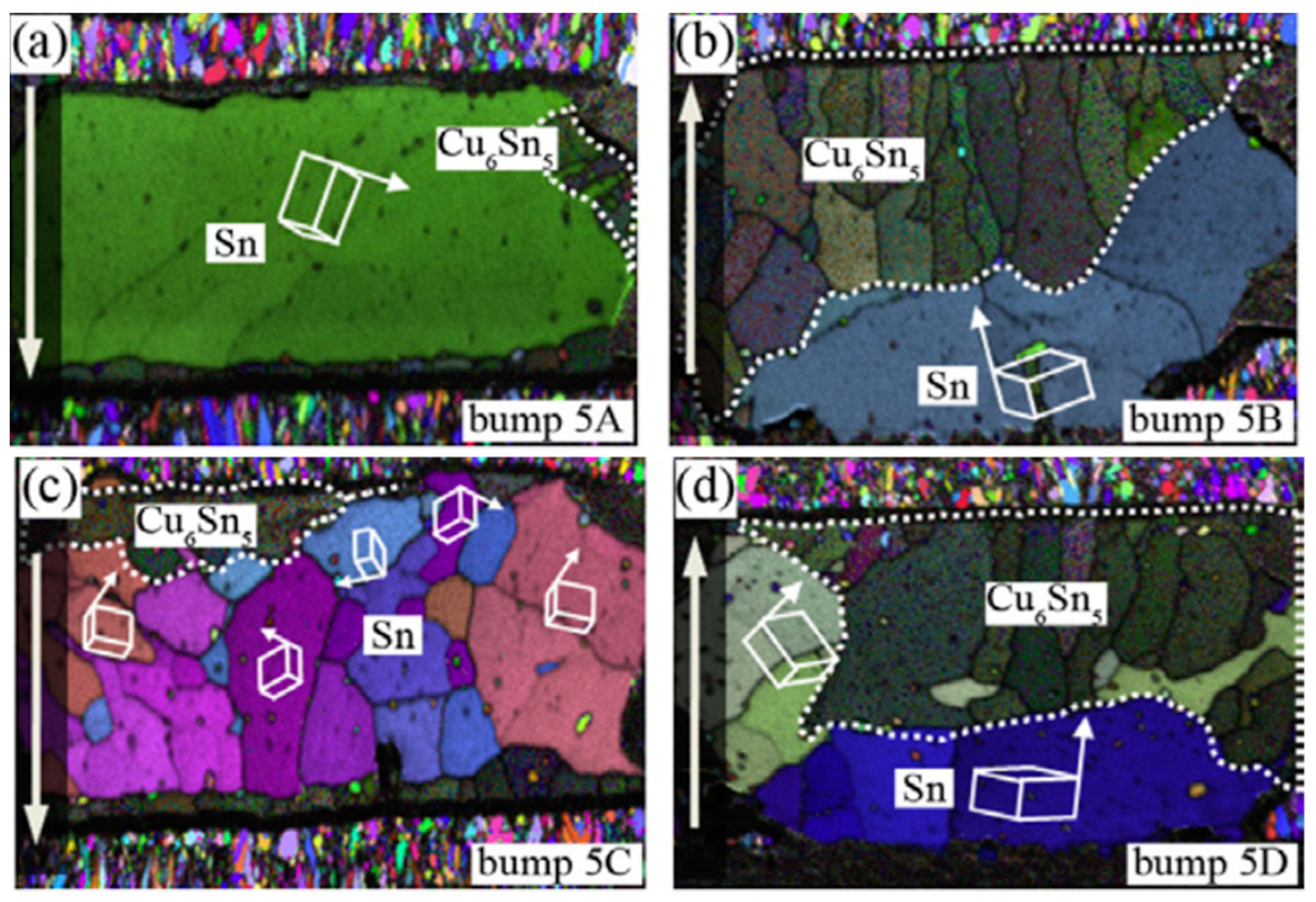
2.2. Influence on the Microstructure of the Solder Bump
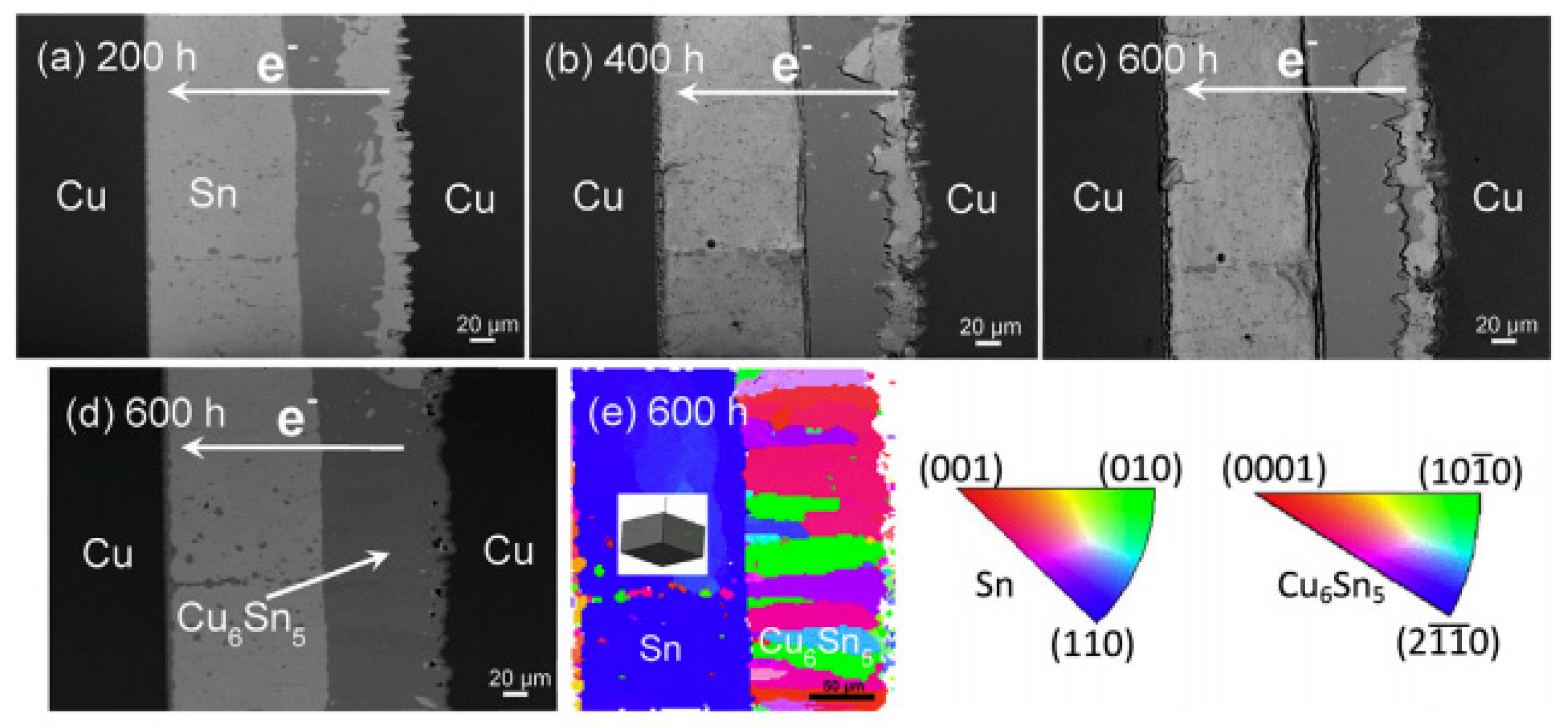
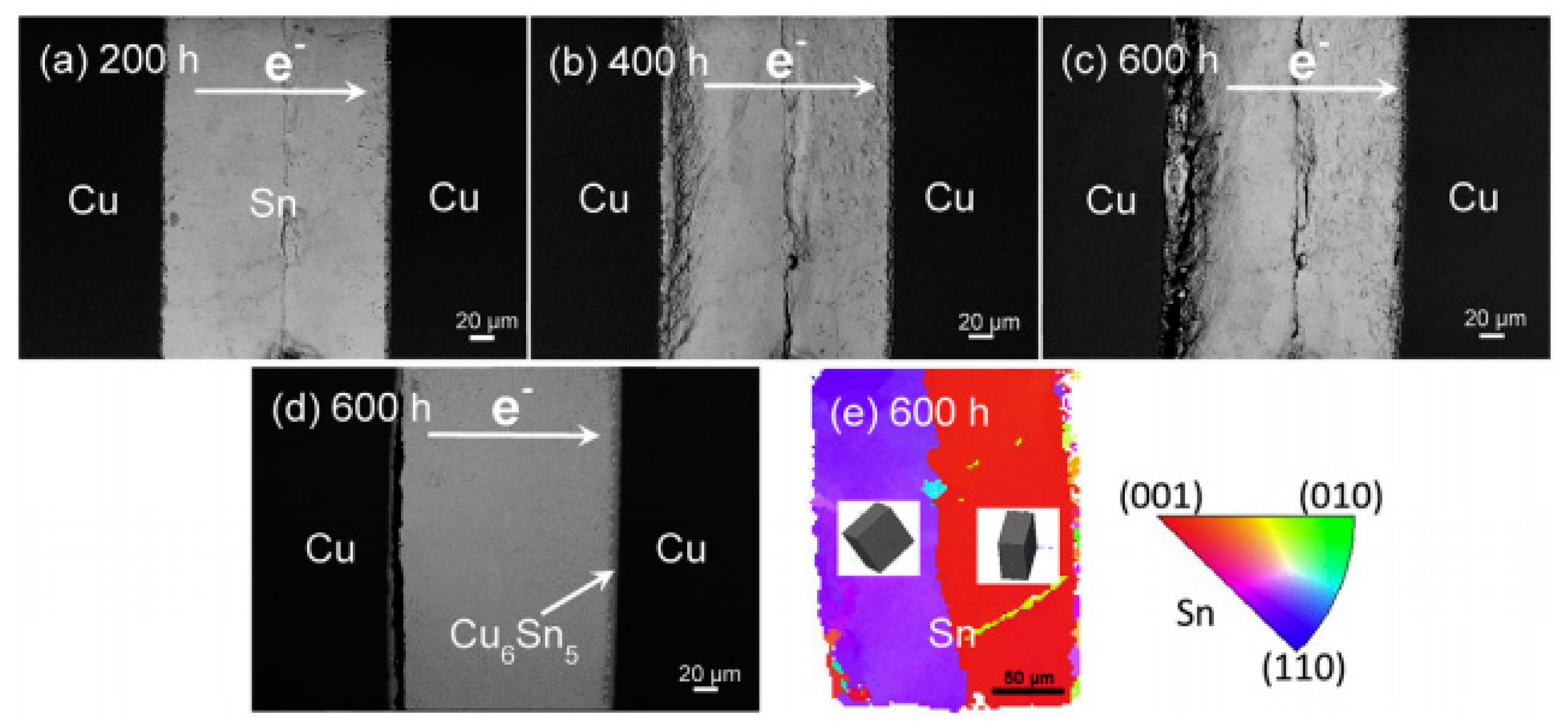
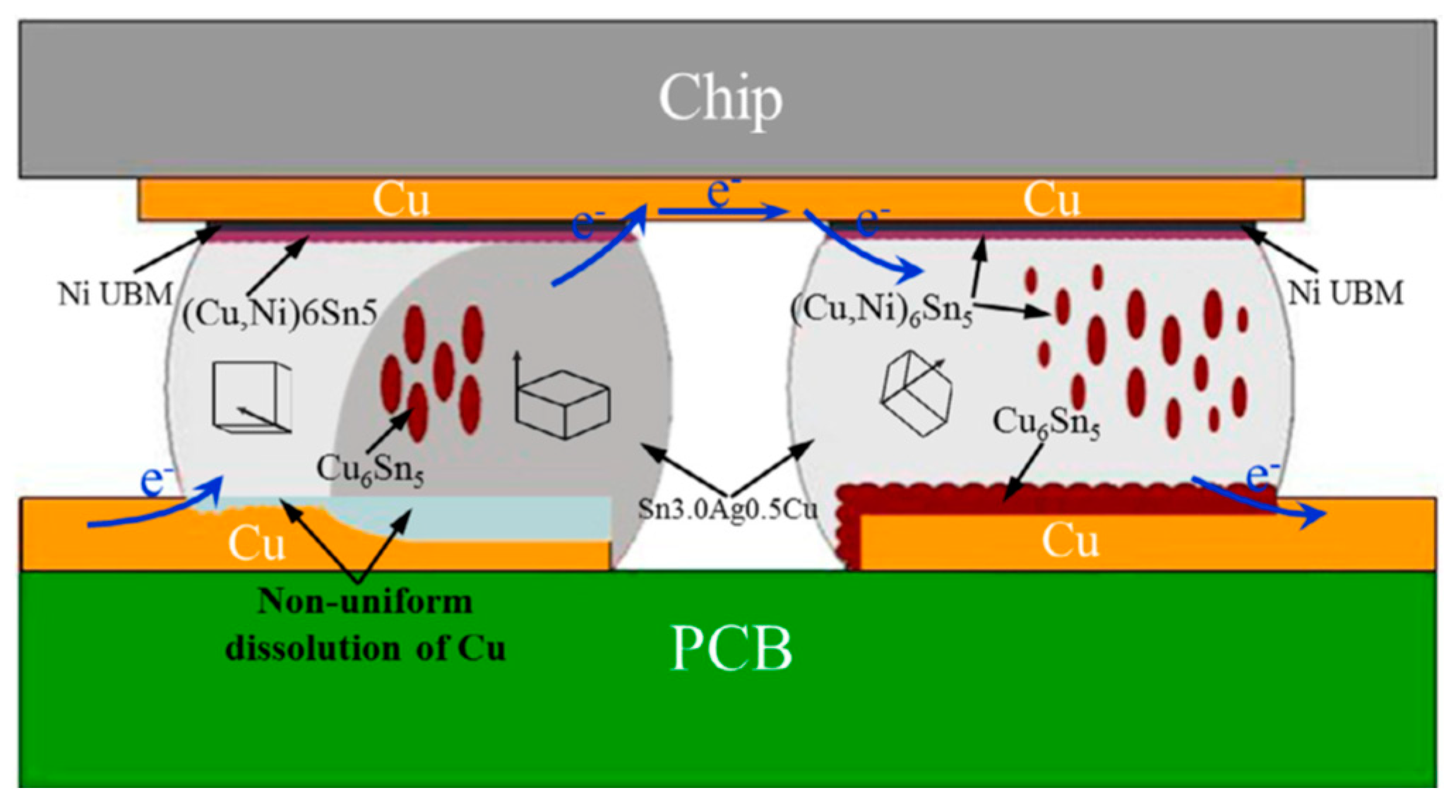
2.2.1. Serrated Dissolution at the Cathode Interface
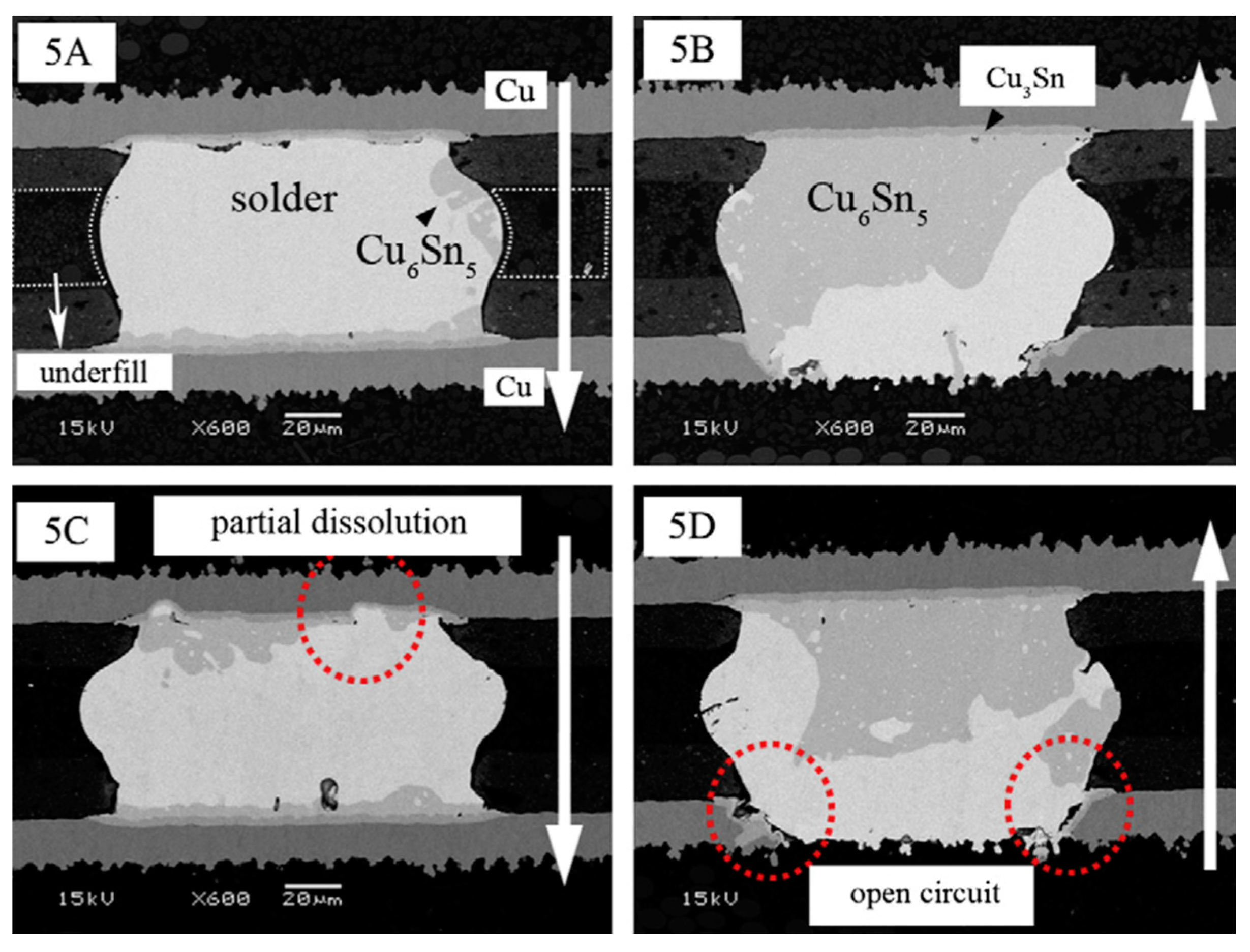
2.2.2. Voids and Cracks at the Cathode Side
2.2.3. Influence on the Growth of Cu6Sn5 IMC
2.3. Influence on Ni migration
3. Discussion
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasan, A.A.; Ahmed Alkahtani, A.; Shahahmadi, S.A.; Nur, E.; Alam, M.; Islam, M.A.; Amin, N. Delamination-and electromigration-related failures in solar panels—A review. Sustainability 2021, 13, 6882. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, S.; Wang, J. New challenges of miniaturization of electronic devices: Electromigration and thermomigration in lead-free solder joints. Mater. Des. 2020, 192, 108726. [Google Scholar] [CrossRef]
- Wang, J.; Xue, S.; Zhang, P.; Zhai, P.; Tao, Y. The reliability of lead-free solder joint subjected to special environment: A review. J. Mater. Sci. Mater. Electron. 2019, 30, 9065–9086. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, L.; Liu, Z.-Q.; Sun, L.; Long, W.-M.; He, P.; Xiong, M.-Y.; Zhao, M. Reliability issues of lead-free solder joints in electronic devices. Sci. Technol. Adv. Mater. 2019, 20, 876–901. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Yu, X.; Li, K.; Pecht, M. Defect inspection of flip chip solder joints based on non-destructive methods: A review. Microelectron. Reliab. 2020, 110, 113657. [Google Scholar] [CrossRef]
- Li, M.-l.; Zhang, L.; Jiang, N.; Zhang, L.; Zhong, S.-j. Materials modification of the lead-free solders incorporated with micro/nano-sized particles: A review. Mater. Des. 2021, 197, 109224. [Google Scholar] [CrossRef]
- Nam, H.; Gam, S.; Gangnam, H. A Review on Growth Behavior of Intermetallic Compounds in Various Solder Joints by Electromigtation. Korean Weld. Join. Soc. 2020, 11, 201. [Google Scholar]
- Zhang, P.; Xue, S.; Wang, J.; Xue, P.; Zhong, S.; Long, W. Effect of nanoparticles addition on the microstructure and properties of lead-free solders: A review. Appl. Sci. 2019, 9, 2044. [Google Scholar] [CrossRef] [Green Version]
- Nasir Bashir, M.; Saad, H.M.; Rizwan, M.; Bingöl, S.; Channa, I.A.; Gul, M.; Haseeb, A.; Naher, S. Effect of cobalt nanoparticles on mechanical properties of Sn–58Bi solder joint. J. Mater. Sci. Mater. Electron. 2022, 33, 22573–22579. [Google Scholar] [CrossRef]
- Bashir, M.N.; Saad, H.M.; Rizwan, M.; Quazi, M.; Ali, M.M.; Ahmed, A.; Zaidi, A.A.; Soudagar, M.E.M.; Haseeb, A.; Naher, S. Effects of tin particles addition on structural and mechanical properties of eutectic Sn–58Bi solder joint. J. Mater. Sci. Mater. Electron. 2022, 33, 22499–22507. [Google Scholar] [CrossRef]
- Bashir, M.N.; Haseeb, A.; Wakeel, S.; Khan, M.A.; Quazi, M.; Khan, N.B.; Ahmed, A.; Soudagar, M.E.M. Effect of Ni and Co nanoparticle-doped flux on microstructure of SAC305 solder matrix. J. Mater. Sci. Mater. Electron. 2022, 33, 20106–20120. [Google Scholar] [CrossRef]
- Bashir, M.N.; Haseeb, A. Grain size stability of interfacial intermetallic compound in Ni and Co nanoparticle-doped SAC305 solder joints under electromigration. J. Mater. Sci. Mater. Electron. 2022, 33, 14240–14248. [Google Scholar] [CrossRef]
- Liu, P.; Wang, S.; Li, D.; Li, Y.; Chen, X.-Q. Fast and Huge Anisotropic Diffusion of Cu (Ag) and Its Resistance on the Sn Self-diffusivity in Solid β–Sn. J. Mater. Sci. Technol. 2015, 32, 121–128. [Google Scholar] [CrossRef]
- Ren, F.; Nah, J.-W.; Tu, K.; Xiong, B.; Xu, L.; Pang, J.H. Electromigration induced ductile-to-brittle transition in lead-free solder joints. Appl. Phys. Lett. 2006, 89, 141914. [Google Scholar] [CrossRef]
- Kumar, A.; Yang, Y.; Wong, C.C.; Kripesh, V.; Chen, Z. Effect of electromigration on the mechanical performance of Sn-3.5 Ag solder joints with Ni and Ni-P metallizations. J. Electron. Mater. 2009, 38, 78–87. [Google Scholar] [CrossRef]
- Xu, S.; Chan, Y.C.; Zhang, K.; Yung, K. Interfacial intermetallic growth and mechanical properties of carbon nanotubes reinforced Sn3.5Ag0.5Cu solder joint under current stressing. J. Alloys Compd. 2014, 595, 92–102. [Google Scholar] [CrossRef]
- Zhang, J.; Chan, Y.C.; Wu, Y.; Xi, H.; Wu, F. Electromigration of Pb-free solder under a low level of current density. J. Alloys Compd. 2008, 458, 492–499. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, L.; Zuo, Y.; Liu, S.; Guo, F. Retarding Electromigration in Lead-Free Solder Joints by Alloying and Composite Approaches. J. Electron. Mater. 2013, 42, 280–287. [Google Scholar] [CrossRef]
- Ma, L.; Xu, G.; Sun, J.; Guo, F.; Wang, X. Effects of Co additions on electromigration behaviors in Sn–3.0 Ag–0.5 Cu-based solder joint. J. Mater. Sci. 2011, 46, 4896–4905. [Google Scholar] [CrossRef]
- Zhang, L.; Ou, S.; Huang, J.; Tu, K.; Gee, S.; Nguyen, L. Effect of current crowding on void propagation at the interface between intermetallic compound and solder in flip chip solder joints. Appl. Phys. Lett. 2006, 88, 012106. [Google Scholar] [CrossRef]
- Chen, C.; Tong, H.; Tu, K. Electromigration and thermomigration in Pb-free flip-chip solder joints. Annu. Rev. Mater. Res. 2010, 40, 531–555. [Google Scholar] [CrossRef]
- Guo, R.; Gao, L.; Li, M.; Mao, D.; Qian, K.; Chiu, H. Microstructure evolution of Ag–8Au–3Pd alloy wire during electromigration. Mater. Charact. 2015, 110, 44–51. [Google Scholar] [CrossRef]
- Chao, B.; Chae, S.-H.; Zhang, X.; Lu, K.-H.; Im, J.; Ho, P.S. Investigation of diffusion and electromigration parameters for Cu–Sn intermetallic compounds in Pb-free solders using simulated annealing. Acta Mater. 2007, 55, 2805–2814. [Google Scholar] [CrossRef]
- Zeng, K.; Stierman, R.; Chiu, T.-C.; Edwards, D.; Ano, K.; Tu, K. Kirkendall void formation in eutectic SnPb solder joints on bare Cu and its effect on joint reliability. J. Appl. Phys. 2005, 97, 024508. [Google Scholar] [CrossRef]
- Ding, M.; Wang, G.; Chao, B.; Ho, P.S.; Su, P.; Uehling, T. Effect of contact metallization on electromigration reliability of Pb-free solder joints. J. Appl. Phys. 2006, 99, 094906. [Google Scholar] [CrossRef]
- Ebersberger, B.; Bauer, R.; Alexa, L. Reliability of lead-free SnAg solder bumps: Influence of electromigration and temperature. In Proceedings of the 2005 Electronic Components and Technology Conference, Lake Buena Vista, FL, USA, 31 May–3 June 2005; pp. 1407–1415. [Google Scholar]
- Pecht, M.; Fukuda, Y.; Rajagopal, S. The impact of lead-free legislation exemptions on the electronics industry. Electron. Packag. Manuf. IEEE Trans. 2004, 27, 221–232. [Google Scholar] [CrossRef]
- Rungyusiri, V.; Sa-ngiamsak, C.; Harnsoongnoen, S.; Intarakul, P. Comparison of electromigration for lead-free solder joints of Cu vs. Ni UBM flip chip structure. In Proceedings of the 2009 6th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology, Chonburi, Thailand, 6–9 May 2009; Volume 1, pp. 469–472. [Google Scholar]
- Tu, K. Recent advances on electromigration in very-large-scale-integration of interconnects. J. Appl. Phys. 2003, 94, 5451–5473. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, S.-B.; Gao, L.-L.; Sheng, Z.; Ye, H.; Xiao, Z.-X.; Zeng, G.; Chen, Y.; Yu, S.-L. Development of Sn–Zn lead-free solders bearing alloying elements. J. Mater. Sci. Mater. Electron. 2010, 21, 1–15. [Google Scholar] [CrossRef]
- Gan, H.; Tu, K. Polarity effect of electromigration on kinetics of intermetallic compound formation in Pb-free solder V-groove samples. J. Appl. Phys. 2005, 97, 063514. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Q.; Zhu, Q.; Wang, Z.; Shang, J. Effects of current stressing on shear properties of Sn-3.8 Ag-0.7 Cu solder joints. J. Mater. Sci. Technol. 2010, 26, 737–742. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Shang, J. Current-induced weakening of Sn3.5Ag0.7Cu Pb-free solder joints. Scr. Mater. 2007, 56, 381–384. [Google Scholar] [CrossRef]
- Ho, C.; Yang, C.; Chen, C.; Chen, B. Abnormal depletion of Cu metallization pads in line–bump–line solder joints under electron current stressing. Thin Solid Films 2015, 596, 216–221. [Google Scholar] [CrossRef]
- Basaran, C.; Lin, M.H.; Ye, H. A thermodynamic model for electrical current induced damage. Int. J. Solids Struct. 2003, 40, 7315–7327. [Google Scholar] [CrossRef]
- Selvaraj, M.K. An Experimental Study of Electromigration in Flip Chip Packages; ProQuest: Binghamton, NY, USA, 2007. [Google Scholar]
- Dusek, M.; Okoro, C.; Hunt, C. Establishing the stress/strain behaviour of solder alloys under multiple constant strain cycles with isothermal conditions. In Proceedings of the ESTC 2006: 1st Electronics Systemintegration Technology Conference, Vols 1 and 2, Dresden, Germany, 5–7 September 2006; pp. 942–946. [Google Scholar]
- Bauer, R.; Fischer, A.H.; Birzer, C.; Alexa, L. Electromigration Behavior of Interconnects between Chip and Board for Embedded Wafer Level Ball Grid Array (eWLB). In Proceedings of the 2011 IEEE 61st Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 31 May–3 June 2011; pp. 317–325. [Google Scholar]
- Huang, M.L.; Zhang, F.; Yang, F.; Zhao, N. Size effect on tensile properties of Cu/Sn-9Zn/Cu solder interconnects under aging and current stressing. J. Mater. Sci. Mater. Electron. 2015, 26, 2278–2285. [Google Scholar] [CrossRef]
- Jang, J.W.; Ramanathan, L.N.; Tang, J.; Frear, D.R. Secondary current crowding effect during electromigration of flip-chip solder joints. J. Electron. Mater. 2008, 37, 185–188. [Google Scholar] [CrossRef]
- Yang, T.; Yu, J.; Li, C.; Lin, Y.; Kao, C. Dominant effects of Sn orientation on serrated cathode dissolution and resulting failure in actual solder joints under electromigration. J. Alloys Compd. 2015, 627, 281–286. [Google Scholar] [CrossRef]
- Ho, C.-E.; Yang, C.-H.; Lee, P.-T.; Chen, C.-T. Real-time X-ray microscopy study of electromigration in microelectronic solder joints. Scr. Mater. 2016, 114, 79–83. [Google Scholar] [CrossRef]
- Chae, S.H.; Zhang, X.F.; Lu, K.H.; Chao, H.L.; Ho, P.S.; Ding, M.; Su, P.; Uehling, T.; Ramanathan, L.N. Electromigration statistics and damage evolution for Pb-free solder joints with Cu and NiUBM in plastic flip-chip packages. J. Mater. Sci. Mater. Electron. 2007, 18, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Chae, S.H.; Zhang, X.F.; Chao, H.L.; Lu, K.H.; Ho, P.S.; Ding, M.; Su, P.; Uehling, T.; Ramanathan, L.N. Electromigration lifetime statistics for Pb-free solder joints with Cu and NiUBM in plastic flip-chip packages. In Proceedings of the 56th Electronic Components and Technology Conference 2006, San Diego, CA, USA, 30 May–2 June 2006; pp. 650–656. [Google Scholar]
- Huang, T.; Yang, T.; Ke, J.; Hsueh, C.; Kao, C. Effects of Sn grain orientation on substrate dissolution and intermetallic precipitation in solder joints under electron current stressing. Scr. Mater. 2014, 80, 37–40. [Google Scholar] [CrossRef]
- Lee, K.; Kim, K.-S.; Tsukada, Y.; Suganuma, K.; Yamanaka, K.; Kuritani, S.; Ueshima, M. Influence of crystallographic orientation of Sn–Ag–Cu on electromigration in flip-chip joint. Microelectron. Reliab. 2011, 51, 2290–2297. [Google Scholar] [CrossRef]
- Dyson, B.F. Diffusion of gold and silver in tin single crystals. J. Appl. Phys. 1966, 37, 2375–2377. [Google Scholar] [CrossRef]
- Huang, M.; Zhao, J.; Zhang, Z.; Zhao, N. Dominant effect of high anisotropy in β-Sn grain on electromigration-induced failure mechanism in Sn-3.0 Ag-0.5 Cu interconnect. J. Alloys Compd. 2016, 678, 370–374. [Google Scholar] [CrossRef]
- Dyson, B.F.; Anthony, T.R.; Turnbull, D. Interstitial diffusion of copper in tin. J. Appl. Phys. 1967, 38, 3408. [Google Scholar] [CrossRef]
- Ho, C.-E.; Lee, P.-T.; Chen, C.-N.; Yang, C.-H. Electromigration in 3D-IC scale Cu/Sn/Cu solder joints. J. Alloys Compd. 2016, 676, 361–368. [Google Scholar] [CrossRef]
- Ho, C.; Yang, C.; Hsu, L. Electromigration in thin-film solder joints. Surf. Coat. Technol. 2014, 259, 257–261. [Google Scholar] [CrossRef]
- Mertens, J.; Kirubanandham, A.; Chawla, N. Electromigration mechanisms in Sn-0.7 Cu/Cu couples by four dimensional (4D) X-ray microtomography and electron backscatter diffraction (EBSD). Acta Mater. 2016, 102, 220–230. [Google Scholar] [CrossRef]
- Yeh, D.C.; Huntington, H.B. Extreme fast-diffusion system: Nickel in single-crystal tin. Phys. Rev. Lett. 1984, 53, 1469. [Google Scholar] [CrossRef]
- Huang, F.H.; Huntington, H.B. Diffusion of Sb124, Cd109, Sn113, and Zn65 in tin. Phys. Rev. B 1974, 9, 1479. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Liu, K.-L.; Guo, J.-D.; Ma, H.-C.; Wei, S.; Shang, J.-K. Electromigration anisotropy introduced by tin orientation in solder joints. J. Alloys Compd. 2017, 703, 264–271. [Google Scholar] [CrossRef]
- Shen, Y.-A.; Chen, C. Effect of Sn grain orientation on formation of Cu6Sn5 intermetallic compounds during electromigration. Scr. Mater. 2017, 128, 6–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, H.; Chen, H. Formation mechanism of a cathodic serrated interface and voids under high current density. Mater. Lett. 2018, 211, 191–194. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, H.; Xiao, Y.; Cao, Y.; Li, M.; Yu, Y. Electromigration-induced growth mode transition of anodic Cu6Sn5 grains in Cu|SnAg3.0Cu0.5|Cu lap-type interconnects. J. Alloys Compd. 2017, 703, 1–9. [Google Scholar] [CrossRef]
- Feng, J.; Hang, C.; Tian, Y.; Wang, C.; Liu, B. Effect of electric current on grain orientation and mechanical properties of Cu-Sn intermetallic compounds joints. J. Alloys Compd. 2018, 753, 203–211. [Google Scholar] [CrossRef]
- Huang, M.; Zhao, J.; Zhang, Z.; Zhao, N. Role of diffusion anisotropy in β-Sn in microstructural evolution of Sn-3.0 Ag-0.5 Cu flip chip bumps undergoing electromigration. Acta Mater. 2015, 100, 98–106. [Google Scholar] [CrossRef]
- Huang, T.; Yang, T.; Ke, J.; Li, C.; Kao, C. Precipitation induced by diffusivity anisotropy in Sn grains under electron current stressing. J. Alloys Compd. 2013, 555, 237–240. [Google Scholar] [CrossRef]
- Wei, S.; Ma, H.C.; Chen, J.Q.; Guo, J.D. Extreme anisotropy of electromigration: Nickel in single-crystal tin. J. Alloys Compd. 2016, 687, 999–1003. [Google Scholar] [CrossRef]
- Bashir, M.N.; Haseeb, A.; Rahman, A.Z.M.S.; Fazal, M.; Kao, C. Reduction of electromigration damage in SAC305 solder joints by adding Ni nanoparticles through flux doping. J. Mater. Sci. 2015, 50, 6748–6756. [Google Scholar] [CrossRef]
- Bashir, M.N.; Haseeb, A.; Rahman, A.Z.M.S.; Fazal, M. Effect of cobalt doping on the microstructure and tensile properties of lead free solder joint subjected to electromigration. J. Mater. Sci. Technol. 2016, 32, 1129–1136. [Google Scholar] [CrossRef]
- Tu, K.; Lee, T.; Jang, J.; Li, L.; Frear, D.; Zeng, K.; Kivilahti, J. Wetting reaction versus solid state aging of eutectic SnPb on Cu. J. Appl. Phys. 2001, 89, 4843–4849. [Google Scholar] [CrossRef]
- Chang, Y.; Liang, S.; Chen, C. Study of void formation due to electromigration in flip-chip solder joints using Kelvin bump probes. Appl. Phys. Lett. 2006, 89, 032103. [Google Scholar] [CrossRef]
- Hsu, C.-m.; Chen, S.-w. Interfacial reactions with and without current stressing at Sn–Co/Ag and Sn–Co/Cu solder joints. J. Mater. Sci. 2013, 48, 6640–6646. [Google Scholar] [CrossRef]
- Xie, H.; Friedman, D.; Mirpuri, K.; Chawla, N. Electromigration Damage Characterization in Sn-3.9 Ag-0.7 Cu and Sn-3.9 Ag-0.7 Cu-0.5 Ce Solder Joints by Three-Dimensional X-ray Tomography and Scanning Electron Microscopy. J. Electron. Mater. 2014, 43, 33–42. [Google Scholar] [CrossRef]
- Sun, J.; Xu, G.; Guo, F.; Xia, Z.; Lei, Y.; Shi, Y.; Li, X.; Wang, X. Effects of electromigration on resistance changes in eutectic SnBi solder joints. J. Mater. Sci. 2011, 46, 3544–3549. [Google Scholar] [CrossRef]
- He, H.; Xu, G.; Guo, F. Electromigration-enhanced intermetallic growth and phase evolution in Cu/Sn–58Bi/Cu solder joints. J. Mater. Sci. 2010, 45, 929–935. [Google Scholar] [CrossRef]
- Westbrook, J. Segregation at grain boundaries. Metall. Rev. 1964, 9, 415–471. [Google Scholar] [CrossRef]
- Hu, S.; Nozawa, J.; Koizumi, H.; Fujiwara, K.; Uda, S. Grain Boundary Segregation of Impurities During Polycrystalline Colloidal Crystallization. Cryst. Growth Des. 2015, 15, 5685–5692. [Google Scholar] [CrossRef]
- Bashir, M.N.; Haseeb, A. Improving mechanical and electrical properties of Cu/SAC305/Cu solder joints under electromigration by using Ni nanoparticles doped flux. J. Mater. Sci. Mater. Electron. 2018, 29, 3182–3188. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, K.H.; Gupta, V.; Stiborek, L.; Shirley, D.; Chae, S.-H.; Im, J.; Ho, P.S. Effects of Sn grain structure on the electromigration of Sn–Ag solder joints. J. Mater. Res. 2012, 27, 1131–1141. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, M.N.; Butt, S.U.; Mansoor, M.A.; Khan, N.B.; Bashir, S.; Wong, Y.H.; Alamro, T.; Eldin, S.M.; Jameel, M. Role of Crystallographic Orientation of β-Sn Grain on Electromigration Failures in Lead-Free Solder Joint: An Overview. Coatings 2022, 12, 1752. https://doi.org/10.3390/coatings12111752
Bashir MN, Butt SU, Mansoor MA, Khan NB, Bashir S, Wong YH, Alamro T, Eldin SM, Jameel M. Role of Crystallographic Orientation of β-Sn Grain on Electromigration Failures in Lead-Free Solder Joint: An Overview. Coatings. 2022; 12(11):1752. https://doi.org/10.3390/coatings12111752
Chicago/Turabian StyleBashir, Muhammad Nasir, Sajid Ullah Butt, Muhammad Adil Mansoor, Niaz Bahadur Khan, Shahid Bashir, Yew Hoong Wong, Turki Alamro, Sayed Mohamed Eldin, and Mohammed Jameel. 2022. "Role of Crystallographic Orientation of β-Sn Grain on Electromigration Failures in Lead-Free Solder Joint: An Overview" Coatings 12, no. 11: 1752. https://doi.org/10.3390/coatings12111752





