1. Introduction
Numerous applications in nanomedicine, electronics, and catalysis utilize metallic materials in the form of nanowires, nanorods, or nanotubes. The physical characteristics of nanostructured materials are substantially influenced by the particle size and geometry, and are distinct from those of the same materials in bulk or single-crystal form [
1,
2,
3,
4,
5,
6,
7,
8]. The increased surface/volume ratio [
9,
10], the particular shapes of the nanostructured materials [
11], their aspect ratio [
12], and the preparation conditions are all responsible for the change in physical and chemical characteristics [
13,
14].
The surface chemistry, the geometry of the nanoparticles, and the nature of the magnetic material may greatly affect the magnetic behavior of the nanoparticles. Magnetic nanoparticles have found a series of biomedical applications including hyperthermia, drug delivery systems, tissue engineering, biomaterial/device coatings, theranostic platforms, lab on a chip, and magnetic separation due to their specific chemical and physical properties [
15,
16]. Moreover, tumor cell destruction by magneto-mechanical actuation of magnetic particles has been proven as a new promising physical approach in the field. This technique utilizes mechanical forces to kill the cancer cells through vibrations/rotations of magnetic particles, including magnetic nanowires (MNWs) [
17]. Due to their increased potential in biomedical areas, such as live cell manipulation, cancer treatment, nanowarming, and/or contrast agents, noble metal and transition-metal nanowires are among the most investigated nanowires [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29].
According to the World Health Organization, cancer is the cause of death for 8.2 million people each year, representing approximately 13% of all deaths, and the diagnosis rate increased by 4.5% from 2014 to 2018 [
30]. In order to improve cancer outcome and survival, early cancer detection remains the priority for both patients and clinicians. In this context, the development of new useful materials for cancer detection in the earliest stages is imperative. Moreover, the combination of the diagnosis and therapy provided by a single-nanomaterial-based platform comes with a series of advantages, such as improved diagnosis, targeted delivery of antitumor drugs, lower cytotoxic effects on healthy tissues, etc. [
31].
However, a major drawback of uncoated nanoparticles/nanowires in biomedical applications arises from their limited stability over time due to their propensity to clump together. By using 1D nanostructures, such as nanowires, this drawback may be greatly overcome since the agglomeration phenomena are less likely to occur in one-dimensional structures [
32]. The cytotoxicity of uncoated magnetic nanoparticles and the fact that these materials might quickly deteriorate in the human body are other specific drawbacks. To avoid or diminish the toxicity and to prevent rapid degradation, the surface of the nanomaterials is often covered with organic molecules, such as chitosan, polyethylene glycol, or dextran [
33,
34]. This encapsulation of magnetic nanoparticles in a layer of organic compounds increases the duration of circulation in the body and affords good biocompatibility. However, due to the high reactivity of organic compounds in the blood, the coating can still be degraded by the human body. In order to avoid the rapid biodegradation of nanomaterials and to reduce their toxicity, a new method has been developed which involves coating magnetic nanoparticles with noble metals, because they are known to be nontoxic and stable in the human body [
35]. The new smart materials prepared following this route are multifunctional, meaning they can be used both for diagnosis and treatment. The advantages of using noble metal (e.g., Au) to coat magnetic 1D nanoparticles arise from their high biocompatibility, high corrosion resistance, high chemical and physical stability, and increased capability to be functionalized via a thin, biocompatible layer of the noble metal, which creates an ideal interface to bind other biofunctional molecules via thiol groups [
36].
A versatile method for metal plating is electroless deposition, representing a spontaneous or in situ reduction of metal ions to their metallic state in the absence of an external power source [
37]. This particular method can be used to prepare core–shell nanostructures, in order to improve the mechanical, physical, and chemical properties of the nanomaterials [
38,
39].
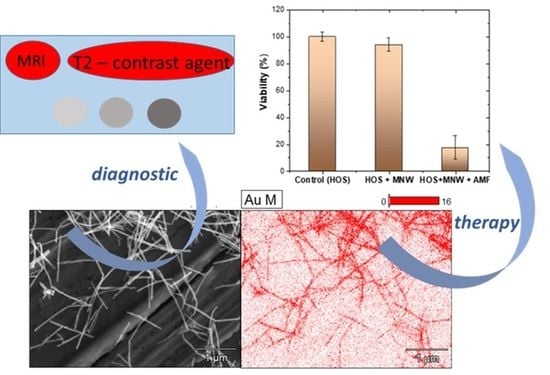
The aim of this work was to prepare a new nanocomposite material, based on core–shell nanowires, to be used in theranostic applications, especially in oncology. To this end, we have developed an easy two-step method based on the electrodeposition of magnetic material into AAO (Anodic Aluminum Oxide) templates, followed by a galvanic displacement reaction to prepare core–shell MNWs as follows: NiFe@Au, CoPt@Au, CoFe@Au. The most biocompatible core–shell nanowires were tested for rapid in vitro destruction of cancer cells by magneto-mechanical actuation as well as for their MRI capabilities. Remarkably, r2 transverse relaxivities up to 10.64 mM−1 s−1 (at 1.5 T), together with a higher biocompatibility, were achieved when CoFe@Au core–shell nanowires were used. This capability allows highly accurate cancer cell detection. At the same time, the as-prepared core–shell nanowires manifested an efficient antitumor effect when the AMF (Alternating Magnetic Fields) was applied. Our study showed for the first time the ability of core–shell nanowires to be used for theranostic applications. More specifically, we prepared magnetic core nanowires based on NiFe, CoPt, and CoFe alloys via electrodeposition inside AAO templates, followed by gold-shell electroplating. The as-prepared core–shell nanowires were analyzed from a morphological, compositional, and structural point of view using scanning electron microscopy, energy-dispersive X-ray spectroscopy, and X-ray diffraction, respectively. Next, the biocompatibility of the gold-shell nanowires was evaluated using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The antitumor effect of the NW-mediated magneto-mechanical actuation was evaluated, and the relaxation times were determined on a Bruker Minispec mq60 NMR analyzer at 1.5 T. The results show that the as-synthetized gold-shell nanowires can be successfully used for theranostic applications.
2. Materials and Methods
The MNW core was synthesized by pulse electrodeposition in a closed three-electrode cell from an aqueous electrochemical bath containing the ions of the core magnetic materials (Ni, Fe, Co, and Pt) into the nanopores of the AAO templates (with pore diameters of 200 nm and a template thickness of 40 µm) provided by Whatman Ltd. (Chalfont St. Giles, England). The applied potential was controlled through a Bipotentiostat/Galvanostat HEKA PG 340 (HEKA, Ludwigshafen/Rhein, Germany). After performing the electrodeposition, the magnetic core nanowires were liberated from the template by dissolution of the alumina in an aqueous 3 M NaOH solution. Finally, a gold shell was plated from a tetra-Chloroauric (III) acid aqueous solution. All reagents for synthesis and analysis were of 99.98% purity and procured from AlfaAesar, (Karlsruhe, Germany). The length and morphology of the as-prepared nanowires were observed by HR-SEM (high-resolution—scanning electron microscopy) using a CrossBeam System Carl Zeiss NEON40EsB (Carl Zeiss, Jena, Germany). The elemental composition was determined by EDX (energy-dispersive X-ray spectroscopy) measurements. The crystalline structure of the electrodeposited nanowires was studied by X-ray diffraction (XRD) by means of a Bruker AXS D8-Advance X-Ray Diffractometer (Bruker, Brno, Czech Republic )with parallel optical geometry using Cu Kα radiation (λ = 1.5406 Å). The relaxation times (longitudinal—T1 and transverse—T2) were measured on a Bruker Minispec mq60 NMR Analyzer (Bruker, Woonsocket, USA) at 1.5 T (60 MHz) at 25 °C using the inversion recovery sequence and the Carr–Purcell–Meiboom–Gill sequence, respectively. Prior to the measurements, the core–shell nanowire surfaces were functionalized using poly(ethylene glycol) 2-mercaptoethyl ether acetic acid, SH-PEG-COOH.
The in vitro biocompatibility of the core–shell NiFe, CoFe, and CoPt nanowires and their core–shell counterparts was tested on human fibroblast cells while the potential antitumor effect of the most biocompatible nanowires, i.e., CoFe@Au, was tested on human osteosarcoma cells. The cell viability was evaluated using the MTT assay, a test that consists of the cell reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to formazan. Briefly, cells were seeded in 96-well plates until confluency, when the nanowires dispersed in cell media were added. For biocompatibility, the MTT assay was performed 24 h after co-incubation of cells with nanowires, while for the magneto-mechanically actuated ones, the samples were first treated in the magnetic field and evaluated after another 24 h.
All the experiments were performed in triplicate. Statistical analysis was performed using Microsoft Excel 2013 to calculate the mean ± SD and Origin 2019 software to test for differences between means through ANOVA analysis with a post hoc Tukey’s test.
3. Results
3.1. Core–Shell Nanowire Preparation
The main steps of preparing nanowires with a magnetic core and Au shell, using electrodeposition inside AAO (anodic aluminum oxide) templates, followed by Au shell coating using electroless plating, are shown in
Figure 1.
The synthesis of the core–shell nanocomposite materials involved several steps. In the first step, the synthesis of MNWs was conducted by electrodeposition of the magnetic material inside the nanopores of AAO template. In the second step, the nanowires were released from AAO by dissolution of the AAO template in an aqueous 3 M NaOH solution. Finally, a thin layer of Au was deposited on the surface of the MNWs by galvanic displacement.
Magnetic NiFe, CoPt, and CoFe nanowires, used as core magnetic materials, were grown by electrodeposition inside the AAO template procured from Whatman International Ltd. AAO templates with diameters of 25 mm, thicknesses of 40 µm, and nominal pore diameters of 200 nm were used. Prior to the alloy electrodeposition, thin layers of Ti (10 nm) and Cu (300 nm) were deposited by sputtering onto one side of the AAO template, covering the pores completely, and serving as a working electrode during the electrodeposition. The role of the 10 nm Ti layer was to increase the adherence of the Cu layer to the AAO. Each magnetic material was electrodeposited from a different electrochemical bath containing the ions of the metals that are found in the composition of the alloy.
The electrodeposition of NiFe alloy nanowires was carried out in a sulfate/chloride plating solution containing 0.4 M H
3BO
3, 0.3 M NH
4Cl, 0.2 M NiSO
4·6H
2O, 0.02 M FeSO
4·7H
2O, 5 mM saccharin as a Na salt, and 0.3 mM sodium lauryl sulfate (Na-LS). The electrolyte solution was adjusted to pH 3.0. These relatively high concentrations of Fe
2+ and Ni
2+ salts were used following recently proposed concentration optimization in the case of NiFe nanowire electrodeposition [
5]. The potential was pulsed between −1.0 V/SCE during a “time-on” period of 2.5 s and a “rest” potential of −0.7 V/SCE during a time-off period of 1 s.
The electrolyte used for the electrodeposition of the CoPt alloy was a stable hexachloroplatinate CoPt solution with pH = 5.5 containing 0.4 M H
3BO
3, 0.3 M NH
4Cl, 0.1 M CoSO
4·7H
2O, and 0.00386 M H
2PtCl
6, with saccharine as an additive. The solution pH was adjusted using an aqueous solution of 0.1 M NaOH [
40]. The CoPt alloys were electrodeposited by pulsing the potential between −0.8 V/SCE during the time-on period of 2.5 s and −0.1 V/SCE during the time-off period of 1 s.
The CoFe nanowires were electrodeposited by pulsing the potential between −1.15 V/SCE during the time-on period of 2.5 s and −0.7 V/SCE during the time-off period of 1 s, using a sulfate/chloride plating solution containing 0.4 M H
3BO
3, 0.3 M NH
4Cl, 0.1 M CoSO
4·7H
2O, 0.2 M FeSO
4·7H
2O, 1.0 mM malonic acid as an organic additive (MA), and 0.3 mM sodium lauryl sulfate (Na-LS) as a wetting agent. The electrolyte solution was adjusted to pH 3.0 [
11].
The pulse electrodeposition was performed in a three-electrode cell with a platinum wire as a counter electrode, an AAO template working electrode, and a saturated calomel electrode (SCE) reference. The cell volume used for the experiments was 100 mL. A schematic representation of the experimental process is presented in
Figure 2.
The crystallinity and the purity of the as-prepared nanowires were checked with the XRD analysis, with the data being presented in the
Supplementary Materials, Figures S1–S3. Our data show that NiFe as well as CoFe presented a cubic structure while CoPt had a hexagonal structure. The diffraction patterns presented only the reflections of the NiFe, CoPt, and CoFe alloys (from the electrodeposited nanowires), with no other peaks being visible on the diffractograms. After the preparation of the magnetic core in the nanopores of the AAO template, the AAO membrane was dissolved in a 3 M NaOH aqueous solution in order to release the nanowires. After template dissolution, the MNWs were rinsed several times with distilled water and magnetically separated. In the next step, a thin coating of Au was chemically deposited by electroless plating on the surface of the MNWs. In order to cover the MNWs’ surface with Au, the washed nanowires were immersed in Au plating solution for 5 min. The plating solution contained 0.5 mM HAuCl
4 and 5M H
3BO
3. The experiments were carried out at room temperature. In order to measure the relaxation times, the surfaces of the CoFe@Au core–shell nanowires were functionalized according to the procedure presented previously by Shore et al. [
41] as follows: 1 mL of the SH-PEG-COOH solution was poured onto the nanowires, mixed, and left overnight to functionalize.
3.2. Morphological and Compositional Characterization
The properties of nanowires are significantly impacted by their shape, aspect ratio, and composition. Hence, it is crucial to evaluate these characteristics. In this context, extremely precise control over the synthesis parameters is required to produce alloys with very low batch-to-batch variations. After electrodeposition, the cross-section of the AAO template with nanowires within was examined by HR-SEM. All samples had an identical distribution of electrodeposited material inside the AAO template as seen in the HR-SEM pictures. The length of the electrodeposited nanowires was 1 ± 0.2 µm.
Figure 3 shows a typical HR-SEM image of an AAO template cross-section filled with electrodeposited MNWs, including NiFe nanowires (
Figure 3a,d), CoPt nanowires (
Figure 3b,e), and CoFe nanowires (
Figure 3c,f). The measured diameter of the nanowire was 200 nm.
The preparation process of core–shell nanowires was characterized, after each synthesis step, by scanning electron microscopy (SEM).
Figure 4a shows an SEM microscopy image of CoFe nanowires prepared and released from the AAO template, connected via the Cu working electrode. In order to remove the Cu electrode, prior to the Au coating process, the nanowires were rinsed with an aqueous 0.1 M HNO
3 solution. In this way, the thin Cu layer was dissolved and the CoFe magnetic core nanowires were detached from the Cu electrode.
Figure 4b (as well as
Figure S4 in the Supplementary Materials) shows the SEM picture of the core–shell nanowires with a magnetic CoFe core and Au coating.
The SEM microscopy images show that the nanowires were completely released from the AAO template during the dissolution step and the fact that the nanowires’ surface was covered with a thin layer of Au. This is also clear from EDX analysis.
Figure 5 shows the SEM images of the magnetic core–shell nanowires of CoPt and NiFe with Au sheaths, respectively, together with the obtained EDX spectra. The amount of Au in the two samples, determined using EDX analysis, was 6%. The compositional analysis indicates that the prepared samples contained the following elements: NiFe and Au (for the NiFe@Au core–shell nanowires), Co, Pt, and Au (for the CoPt@Au core–shell nanowires), and Co, Fe, and Au (for the CoFe@Au core–shell nanowires). These elements are also the ones we expected to find; nickel, cobalt, platinum, and iron came from the electrodeposited magnetic core, while gold came from the coating made of noble metal.
3.3. Evaluation of the Gold-Shell MNWs for Theranostic Applications
In order to determine the plausibility of their use for biomedical applications, we analyzed the “in vitro” biocompatibility of the core–shell NWs and their potential to be used for tumor cell destruction. The ability of artificial materials not to produce a negative reaction from the host’s immune system, especially an inflammatory one, in a specific application, preserving its designed functionality related to a biomedical treatment, is generally known as biocompatibility. Testing a medical device’s biocompatibility is mandatory to guarantee patient security.
The biocompatibility of the as-prepared core–shell nanowires was evaluated using human fibroblast cells after co-incubation for 40 h. Cell viability was determined using the MTT assay (5-dimethylthiazol-2-yl-2, 5-diphenyltetrazolium bromide), according to the supplier’s instructions. Dimethyl sulfoxide was used as the dissolving agent, whereas the absorbance was read at 570 nm (Synergy HTX Multi-Mode Reader—Bioteck, Santa Clara, USA). Cell viability (CV), expressed by optical density (OD), was calculated using the formula CV = 100 × (ODs − ODb)/(ODc − ODb), where ODs = OD of the cells incubated with nanowires; ODb = OD of blank (media only); ODc = OD of untreated (control) cells.
The results are shown in
Figure 6. Bare and gold-coated CoPt nanowires were found to be the most cytotoxic to fibroblast cells. At the opposite pole, CoFe@Au nanowires were found to be highly biocompatible, as were NiFe and NiFe@Au nanowires. Statistical analysis indicated that there was a significant difference between CoFe@Au NWs and CoPt@Au NWs, but not between NiFe@Au NWs and CoPt@Au NWs. Additionally, we did not observe a statistical difference between CoFe@Au NWs and NiFe@Au NWs. However, similar viabilities would have been expected from the coated nanowires regardless of the metallic core. This difference between the coated nanowires may simply be the result of the different quality properties of the gold coatings during electroless plating induced by the different galvanic potentials of the metallic substrates, for an identical reaction time. Therefore, an optimized electroless plating would most likely lead to similar viability results for all gold-coated nanowires, regardless of core composition. However, by corroborating the statistical and the absolute viability results, we considered only CoFe@Au nanowires in testing antitumor capabilities via magneto-mechanical actuation.
In order to increase the efficiency of the magneto-mechanic process, magnetic nanomaterials with improved magnetic susceptibilities, such as nickel, cobalt, or NiFe alloys, coated with a gold layer to ensure biocompatibility, may be required [
17]. A collateral advantage of a gold layer on nanowires is the possibility of grafting organic molecules via self-assembly of thiolates [
17], which are known to quickly adsorb on gold surfaces.
The antitumor effect of the NW-mediated magneto-mechanical actuation was evaluated on a culture of human osteosarcoma cells incubated with NWs in 96-well plates, at a density of 1 × 105 cells/well, and 0.2 mg NWs/mL, in an alternating magnetic field. Each well contained 0.2 mL of culture medium. The nanowire solution was prepared by weighing the 0.2 mg of dried CoFe@Au core–shell nanowires, followed by their release in the culture medium. In order to generate and control the magnetic field, a Helmholtz coil system and proprietary software were used. The well plate was positioned at the center of the system, and the magnetic field (85 Oe) was rotated with a frequency of 2 Hz.
The results show a decrease in the tumor cell viability down to 20% when the AMF was applied for 30 min (
Figure 7). Given the reduced time of AMF application and one-shot treatment, the decrease in the cell viability is very significant. Most probably, serial applications of the AMF for longer time periods induce a more intense cytotoxic effect on such tumor lines. Compared to other reported data [
17], the synthesized CoFe@Au nanowires afforded one of the best results in terms of “in vivo” antitumor efficacy, given the treatment time period. Consequently, we assume that this type of magnetic gold-coated nanowire could be considered for magneto-mechanical therapy of solid tumors. Regarding the magnetic properties of the CoFe nanowires, a detailed analysis can be found in our previous reported work [
11], including data about the easy axis of the magnetization that was found to be parallel to the longitudinal axis of the nanowire.
The relaxation times (longitudinal (T1) and transverse (T2)) of the core–shell MNWs in deionized water were determined on a Bruker Minispec mq60 NMR analyzer at 1.5 T (60 MHz) at 25 °C using the inversion recovery sequence and the Carr–Purcell–Meiboom–Gill sequence, respectively. Prior to the experiments, the CoFe@Au core–shell nanowires were subjected to SH-PEG-COOH functionalization, as previously described. The measurements showed that the r1 and r2 relaxivity values were 0.38 and 10.64 mM−1 s−1, respectively, while the r2/r1 ratio was 28. The r2/r1 ratio value indicates if the compounds can be used as T1-dominated contrast agents (compounds showing r2/r1 < 5) or as T2-dominated contrast agents (compounds showing r2/r1 > 8). Our data clearly showed that the as-prepared CoFe@Au core–shell nanowires can be used as T2-dominated contrast agents.
4. Conclusions
In this work, gold-coated MNWs (with magnetic cores formed by NiFe, CoPt, or CoFe) were successfully prepared by combining electrodeposition inside AAO templates with electroless plating. The microstructure and the chemical composition of the as-prepared magnetic core, as well as the core–shell nanowires, were analyzed by SEM and EDX techniques, which showed nanowires of about 1 µm in length and a uniform Au shell over the magnetic core. The amount of Au determined by EDX was about 6%, regardless of the nature of the magnetic core. The biocompatibility of the as-prepared nanocomposite materials were also evaluated, with the results showing that the CoPt@Au core–shell nanowires manifest the poorest biocompatibility, while the CoFe@Au core–shell nanowires manifest the highest biocompatibility value. Accordingly, the CoFe@Au core–shell nanowires were further tested for a theranostic application, i.e., the capacity for tumor destruction through a magneto-mechanical process, as well as the evaluation of their relaxation times. The obtained data showed a decrease in the tumor cell viability of about 80% after 30 min of magnetic actuation in an alternating magnetic field. At the same time, the relaxation time measurements showed that the r2 value of the CoFe@Au core–shell nanowires was 10.64, while the r2/r1 ratio was 28. The results show that the as-prepared magnetic core–shell nanowires can also be successfully used as T2-dominated contrast agents. In conclusion, in accord with the aim of this work, we have successfully demonstrated that CoFe@Au nanocomposite materials in shape of nanowires can be used for theranostic applications, with the materials manifesting good biocompatibility, tumor destruction capacities, and high relaxation time values. Future work will be carried out in order to evaluate the influence of the core–shell nanowires’ aspect ratio (e.g., ratio between nanowires’ diameter and length) on their theranostic application potential. In this regard, CoFe@Au core–shell nanowires with different lengths (ranging from 0.3 to 1.5 µm) and different diameters (45, 75, and 200 nm) will be prepared, and the biocompatibility, the capacity for tumor destruction, and the relaxation time will be determined.