Tunnel Magnetoresistance-Based Sensor for Biomedical Application: Proof-of-Concept
Abstract
:1. Introduction
2. Materials and Methods
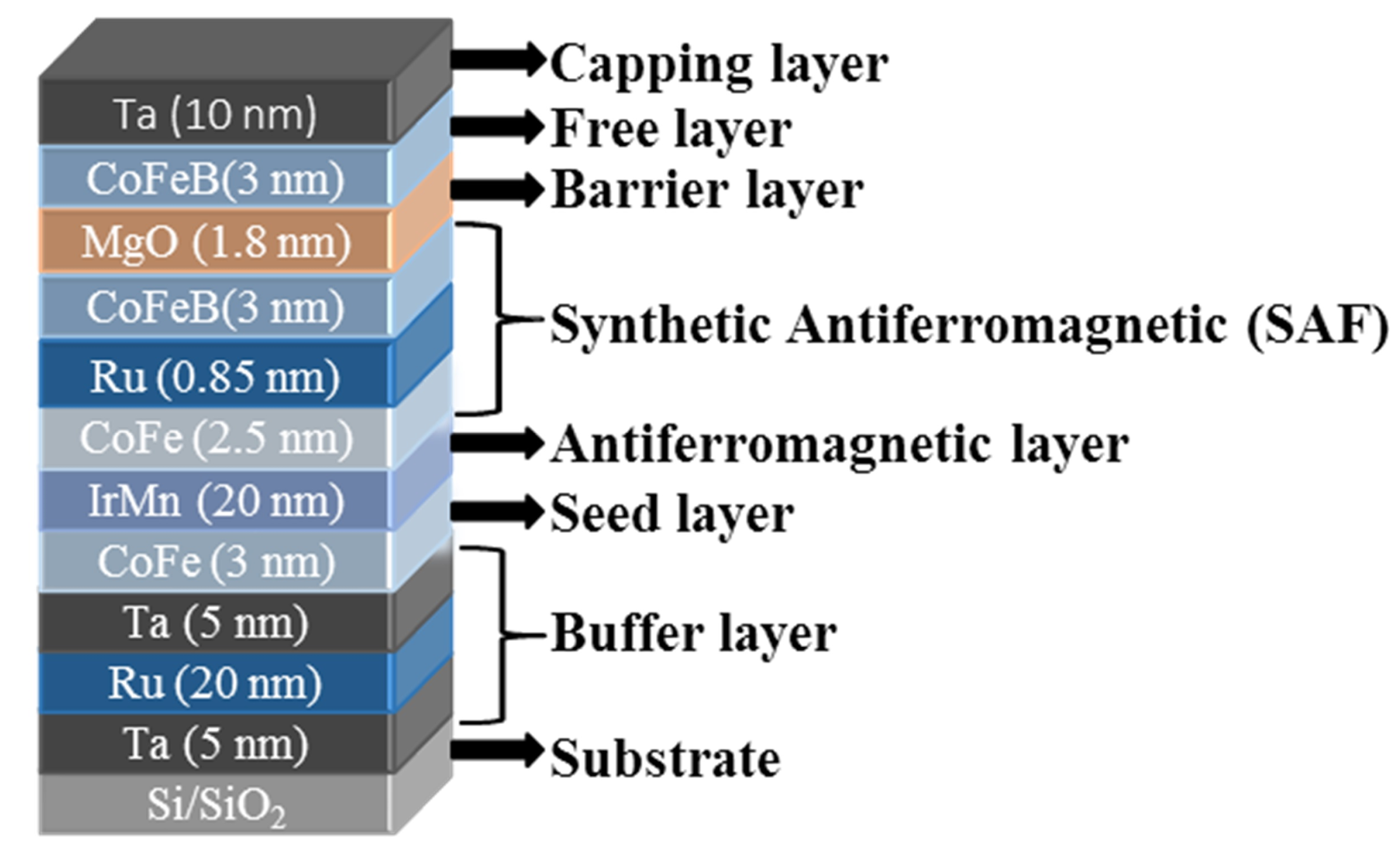
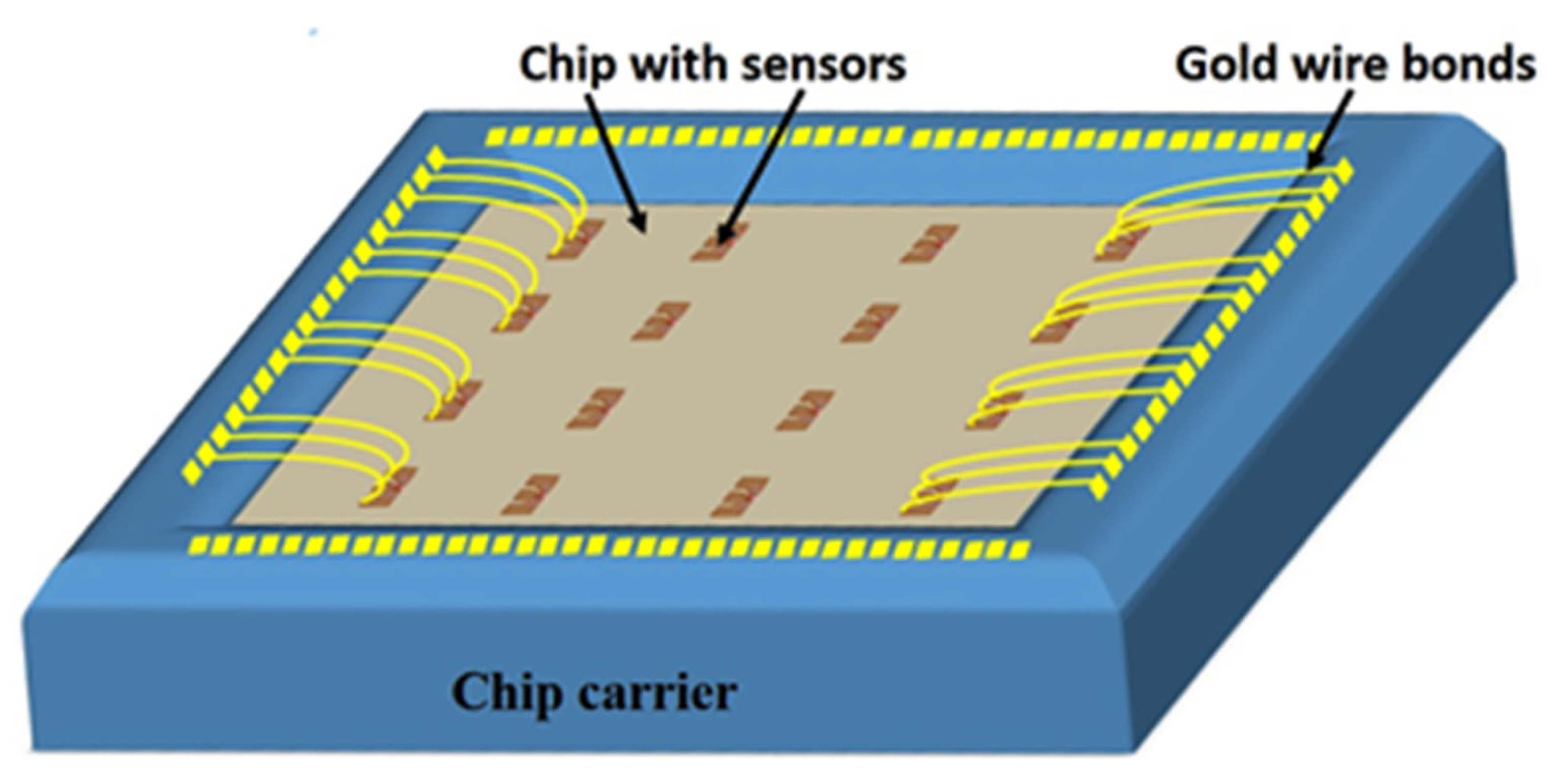
2.1. TMR-Based Sensor Microfabrication
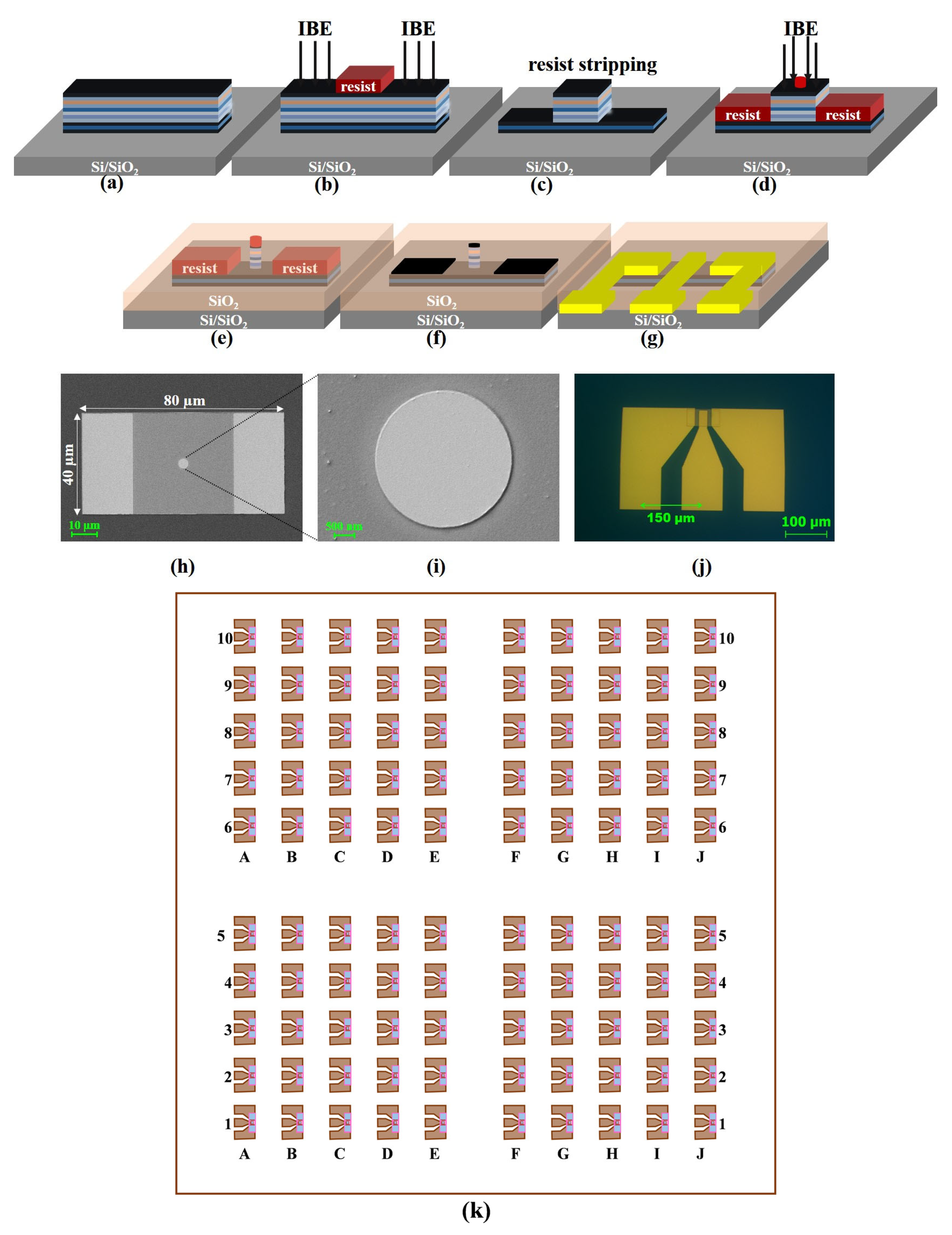
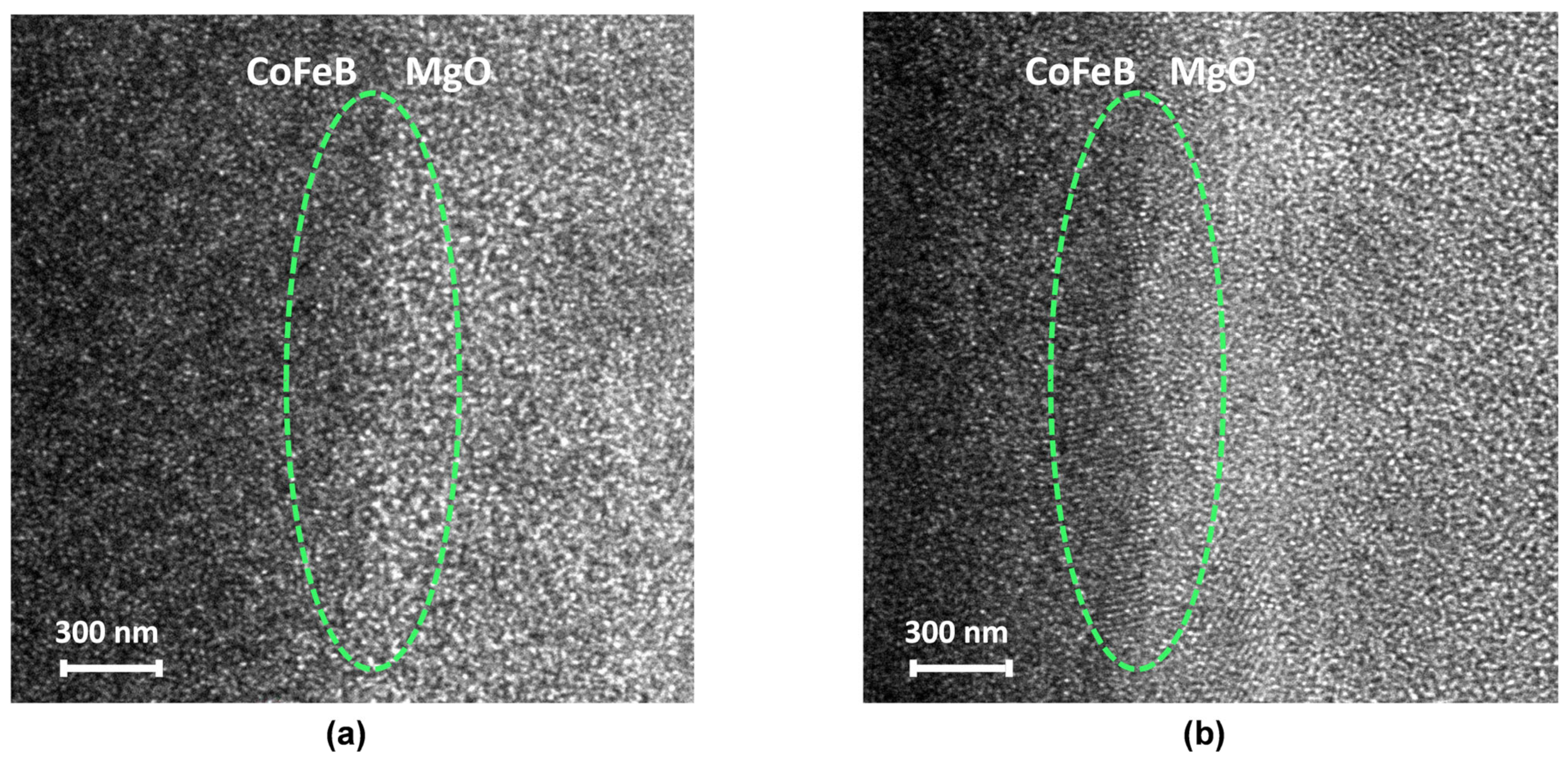
2.2. Magnetic Annealing
2.3. Magnetic Nanoparticles
3. Results
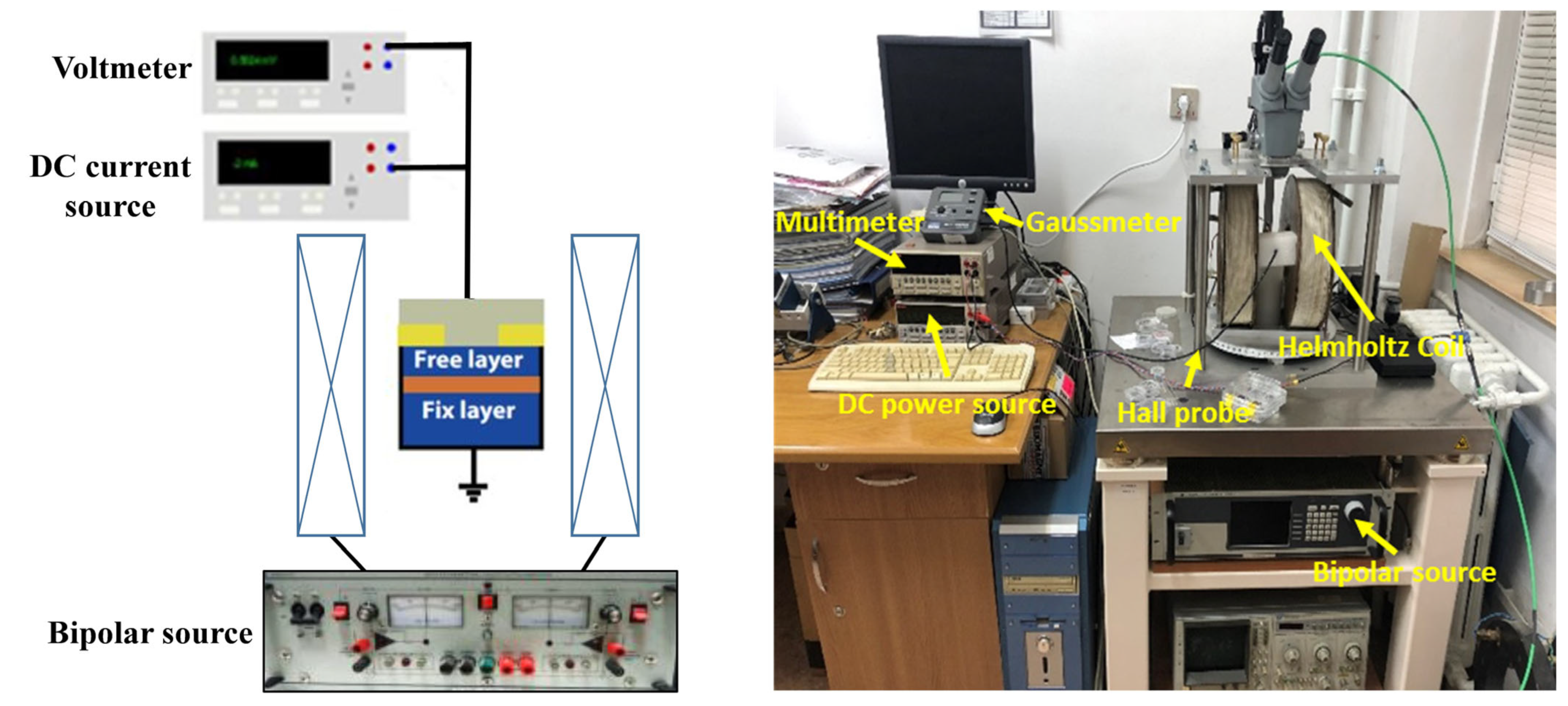
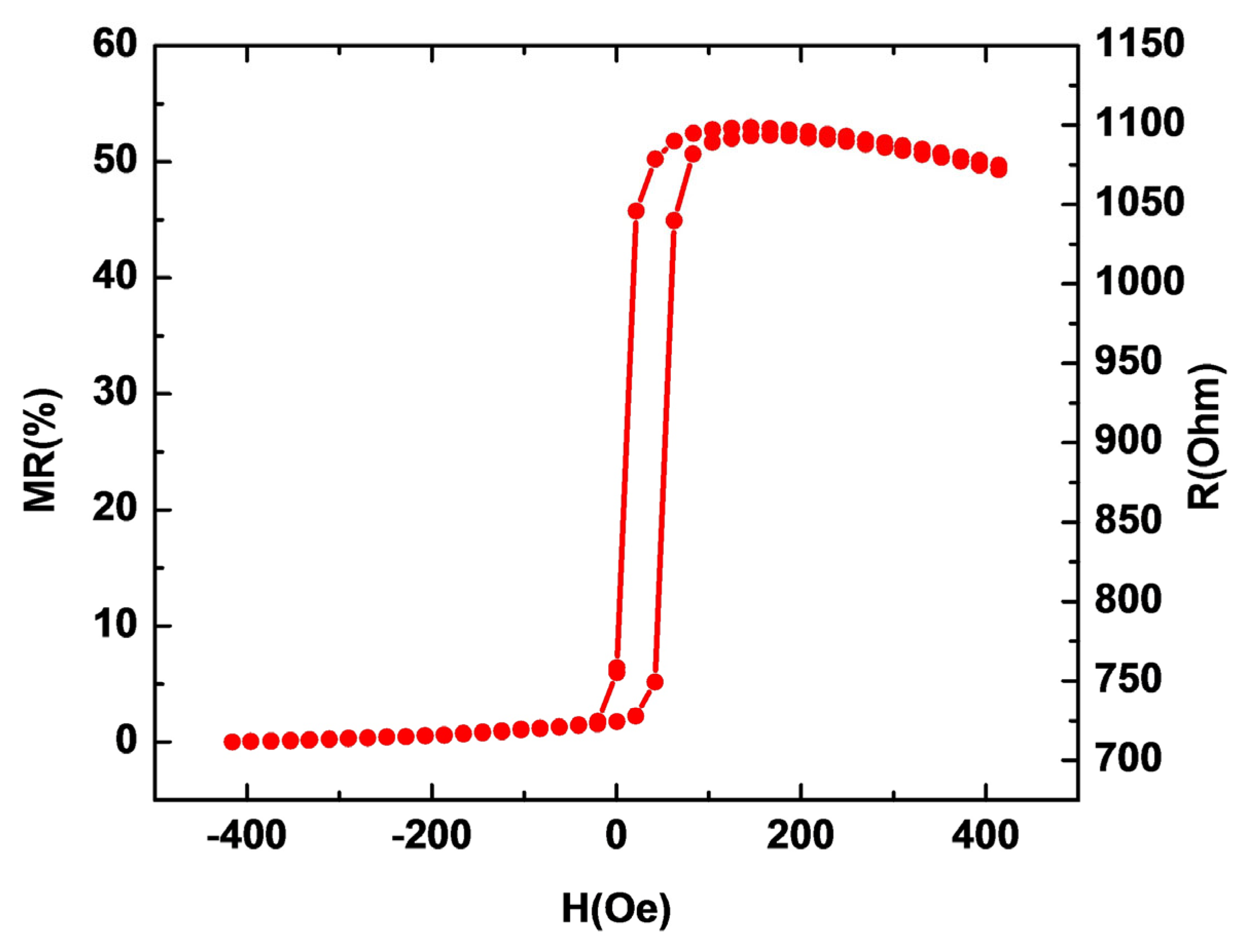
3.1. Sensor Performance
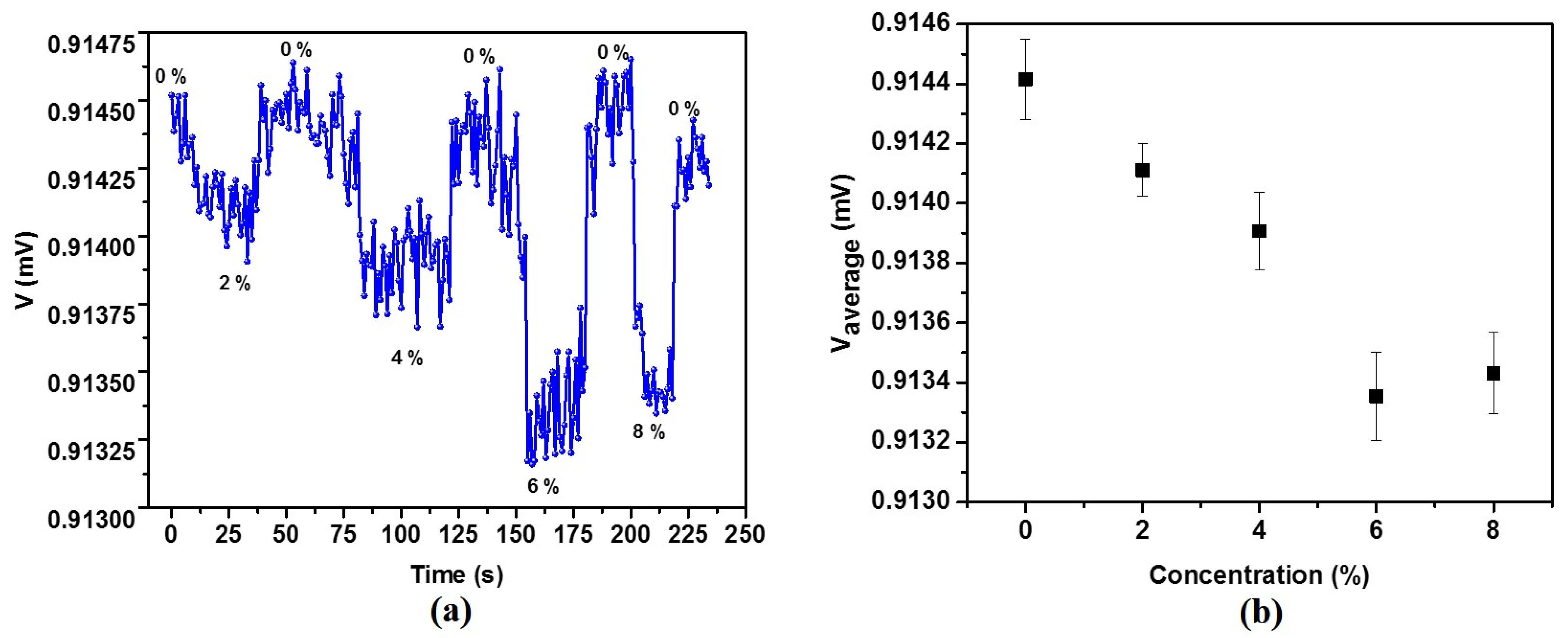
3.2. Detection of Magnetic Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, Z.Q.; Li, L.; Li, G.J.; Leung, C.W.; Shi, J.; Wong, C.M.; Lo, K.C.; Chan, W.K.; Mak, C.S.K.; Chan, S.B.; et al. Liver cancer immunoassay with magnetic nanoparticles and MgO-based magnetic tunnel junction sensors. J. Appl. Phys. 2012, 111, 07E505. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhi, S.; Lei, C.; Zhou, Y. Investigation of contactless detection using a giant magnetoresistance sensor for detecting prostate specific antigen. Biomed Microdevices 2016, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, Y.; Maehara, H.; Tsunekawa, K.; Djayaprawira, D.D.; Watanabe, N.; Yuasa, S.; Ando, K. Ultralow resistance-area product of 0.4 Ω(Μm)2 and high magnetoresistance above 50% in CoFeB/MgO/CoFeB magnetic tunnel junctions. Appl. Phys. Lett. 2006, 89, 162507. [Google Scholar] [CrossRef]
- Cardoso, S.; Leitao, D.C.; Gameiro, L.; Cardoso, F.; Ferreira, R.; Paz, E.; Freitas, P.P. Magnetic tunnel junction sensors with pTesla sensitivity. Microsyst. Technol. 2014, 20, 793–802. [Google Scholar] [CrossRef]
- Zhang, Y.; He, G.; Zhang, X.; Xiao, G. Magnetotransport and electronic noise in superparamagnetic magnetic tunnel junctions. Appl. Phys. Lett. 2019, 115, 022402. [Google Scholar] [CrossRef]
- Franco, F.; Cardoso, S.; Freitas, P.P. Reconfigurable spintronics wheatstone bridge sensors with offset voltage compensation at wafer level. IEEE Trans. Magn. 2019, 55, 4400705. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Weissleder, R.; Cheng, H.C.; Bogdanova, A.; Bogdanov, A., Jr. Magnetically labeled cells can be detected by MR imaging. J. Magn. Reson. Imaging 1997, 7, 258–263. [Google Scholar] [CrossRef]
- Ahmed, M.; Douek, M. The role of magnetic nanoparticles in the localization and treatment of breast cancer. Biomed Res. Int. 2013, 281230. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Liebl, M.; Steinhoff, U.; Wiekhorst, F.; Haueisen, J.; Trahms, L. Quantitative imaging of magnetic nanoparticles by magnetorelaxometry with multiple excitation coil. Phys. Med. Biol. 2014, 59, 6607–6620. [Google Scholar] [CrossRef]
- Gleich, B.; Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.C.; Chomsuwan, K.; Gooneratne, C.P.; Yamada, S. A novel needle-type SV-GMR sensor for biomedical applications. IEEE Sens. J. 2007, 7, 401–408. [Google Scholar] [CrossRef]
- Loureiro, J.; Fermon, C.; Pannetier-Lecoeur, M.; Arrias, G.; Ferreira, R.; Cardoso, S.; Freitas, P.P. Magnetoresistive detection of magnetic beads flowing at high speed in microfluidic channels. IEEE Trans. Magn. 2009, 45, 4873–4876. [Google Scholar] [CrossRef]
- Li, L.; Mak, K.Y.; Leung, C.W.; Ng, S.M.; Lei, Z.Q.; Pong, P.W.T. Detection of 10-nm superparamagnetic iron oxide nanoparticles using exchange-biased GMR sensors in wheatstone bridge. IEEE Trans. Magn. 2013, 49, 4056–4059. [Google Scholar] [CrossRef] [Green Version]
- Kokkinis, G.; Jamalieh, M.; Cardoso, F.; Cardoso, S.; Keplinger, F.; Giouroudi, I. Magnetic-based biomolecule detection using giant magnetoresistance sensors. J. Appl. Phys. 2015, 117, 17B731. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Lien, K.-Y.; Huang, K.-J.; Lei, H.-Y.; Lee, G.-B. Micro flow cytometry utilizing a magnetic bead-based immunoassay for rapid virus detection. Biosens. Bioelectron. 2008, 24, 855–862. [Google Scholar] [CrossRef]
- Herea, D.-D.; Labusca, L.; Radu, E.; Chiriac, H.; Grigoras, M.; Panzaru, O.D.; Lupu, N. Human adipose-derived stem cells loaded with drug-coated magnetic nanoparticles for in-vitro tumor cells targeting. Mater. Sci. Eng. C 2019, 94, 666–676. [Google Scholar] [CrossRef]
- Chiriac, H.; Lupu, N.; Lostun, M.; Ababei, G.; Grigoraş, M.; Dănceanu, C. Low TC Fe-Cr-Nb-B glassy submicron powders for hyperthermia applications. J. Appl. Phys. 2014, 115, 17B520. [Google Scholar] [CrossRef]
- Chiriac, H.; Whitmore, L.; Grigoras, M.; Ababei, G.; Stoian, G.; Lupu, N. Influence of Cr on the nanoclusters formation and superferromagnetic behavior of Fe-Cr-Nb-B glassy alloys. J. Appl. Phys. 2015, 117, 17B522. [Google Scholar] [CrossRef]
- Luz Ventosa, T.L.L. High Performance Magnetic Tunnel Junctions for Magnetic Scanning. Master’s Thesis, Instituto Superi-or Tecnico, Lisboa, Portugal, May 2017. [Google Scholar]
- Edris, A.; Choi, B.; Aguilar, G.; Nelson, J.S. Measurements of laser light attenuation following cryogen spray cooling spurt termination. Laser Surg. Med. 2003, 32, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, D.R.; Martin, R.J.; Darlison, R. Epoxy resin based tissue substitutes. Br. J. Radiol. 1977, 50, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Yemby, H.T.; Arnold, M.P.; Giancarlo, A.V.; Felipe, C.; José, V.R. Construction and characterization of materials equivalent to the tissues and organs of the human body for radiotherapy. Radiat. Phys. Chem. 2019, 159, 70–75. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghemes, C.; Dragos-Pinzaru, O.-G.; Tibu, M.; Lostun, M.; Lupu, N.; Chiriac, H. Tunnel Magnetoresistance-Based Sensor for Biomedical Application: Proof-of-Concept. Coatings 2023, 13, 227. https://doi.org/10.3390/coatings13020227
Ghemes C, Dragos-Pinzaru O-G, Tibu M, Lostun M, Lupu N, Chiriac H. Tunnel Magnetoresistance-Based Sensor for Biomedical Application: Proof-of-Concept. Coatings. 2023; 13(2):227. https://doi.org/10.3390/coatings13020227
Chicago/Turabian StyleGhemes, Crina, Oana-Georgiana Dragos-Pinzaru, Mihai Tibu, Mihaela Lostun, Nicoleta Lupu, and Horia Chiriac. 2023. "Tunnel Magnetoresistance-Based Sensor for Biomedical Application: Proof-of-Concept" Coatings 13, no. 2: 227. https://doi.org/10.3390/coatings13020227
APA StyleGhemes, C., Dragos-Pinzaru, O.-G., Tibu, M., Lostun, M., Lupu, N., & Chiriac, H. (2023). Tunnel Magnetoresistance-Based Sensor for Biomedical Application: Proof-of-Concept. Coatings, 13(2), 227. https://doi.org/10.3390/coatings13020227








