Deposition of Iridium Coating on Pure Tungsten and High-Temperature Oxidation Behavior at 1300 K
Abstract
:1. Introduction
2. Experimental
2.1. Substrate and Pre-Treatment
2.2. Electrolytes and Electrochemical Measurements
2.3. Testing and Characterization
3. Results and Discussion
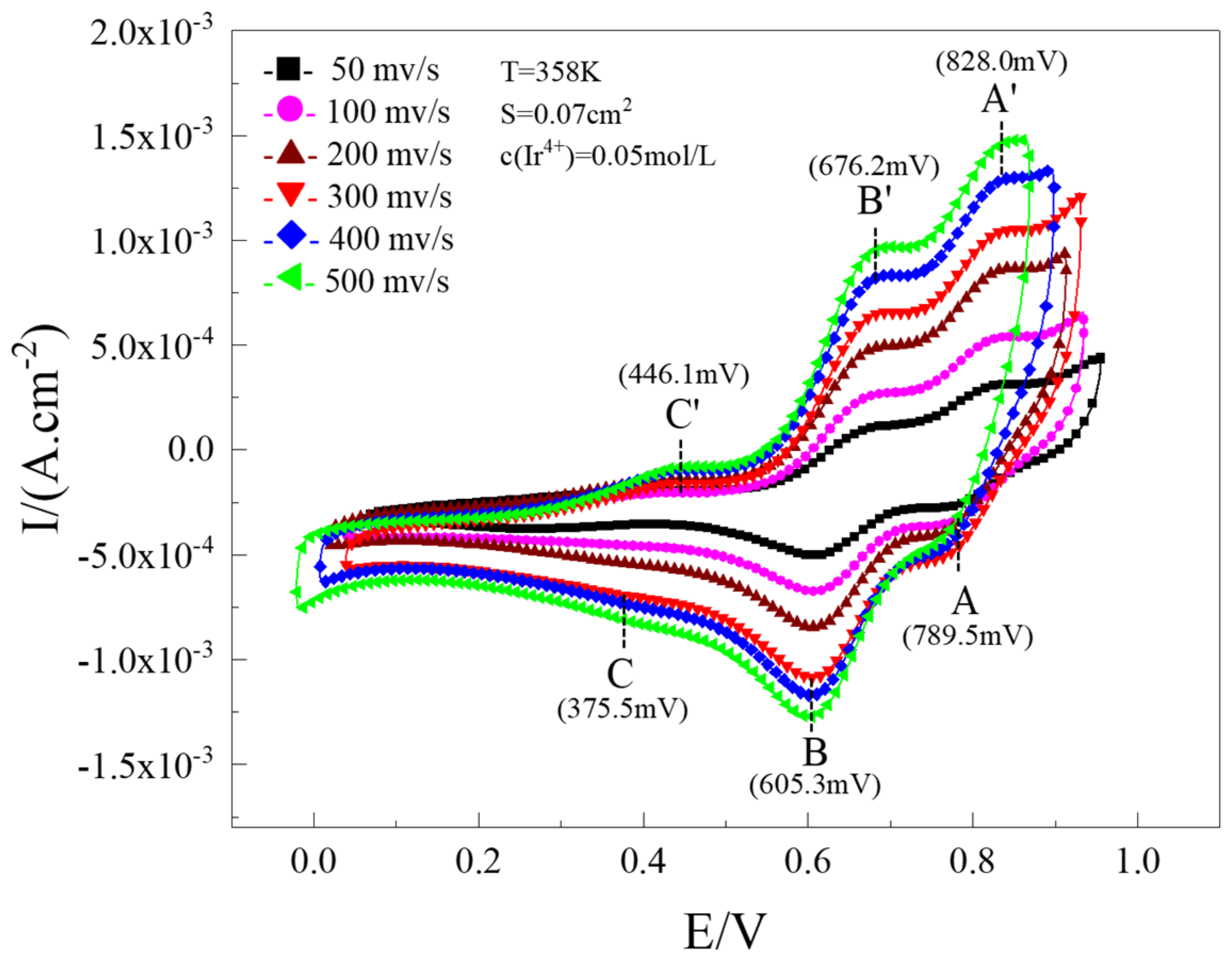
3.1. Electrochemical Reduction of 4-Valent Iridium Ions
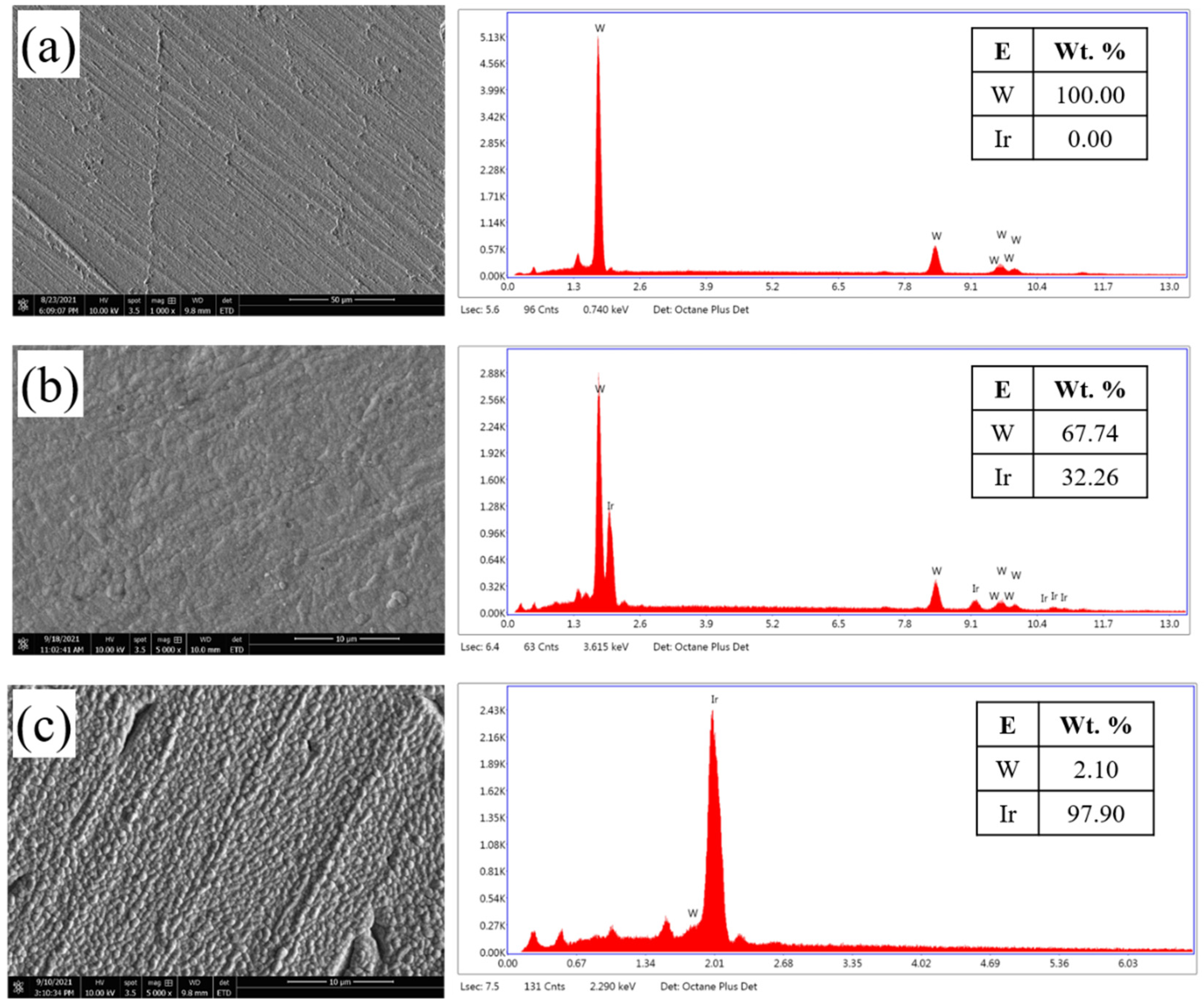
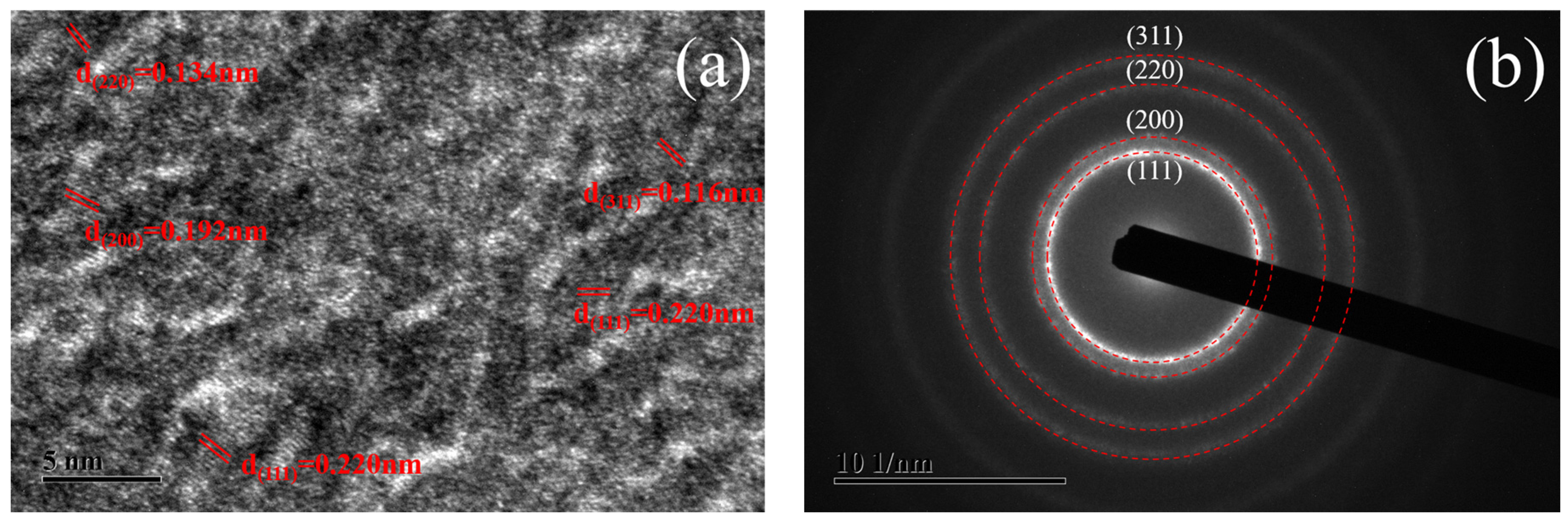
3.2. Electrodeposition of Iridium Coating on Tungsten Substrate
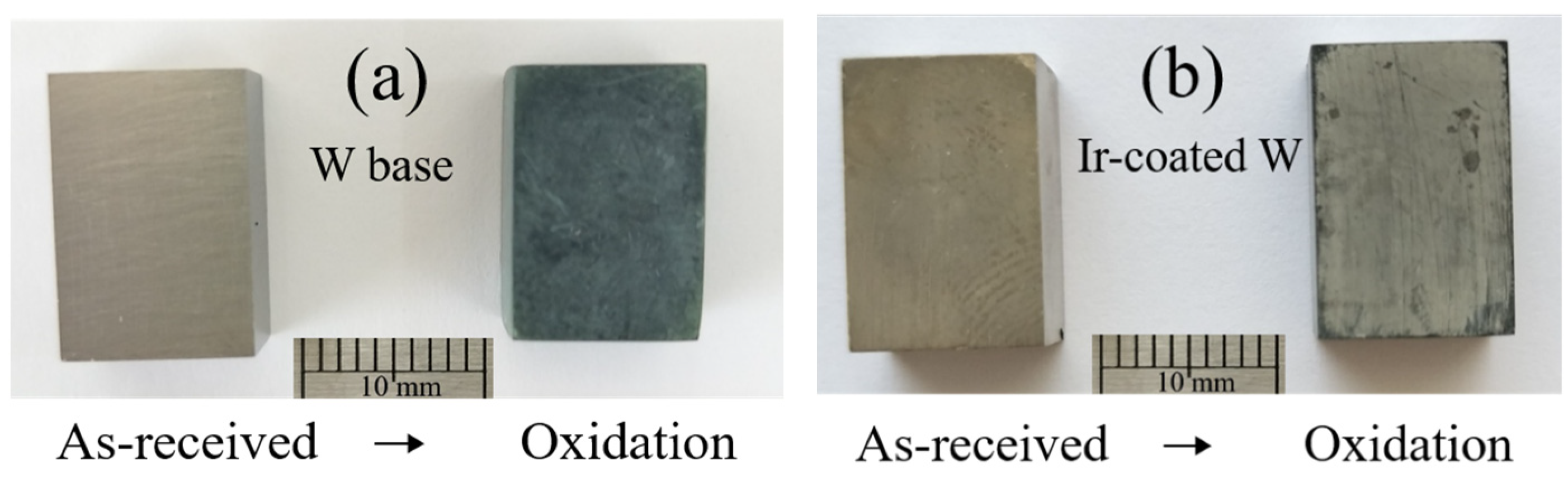
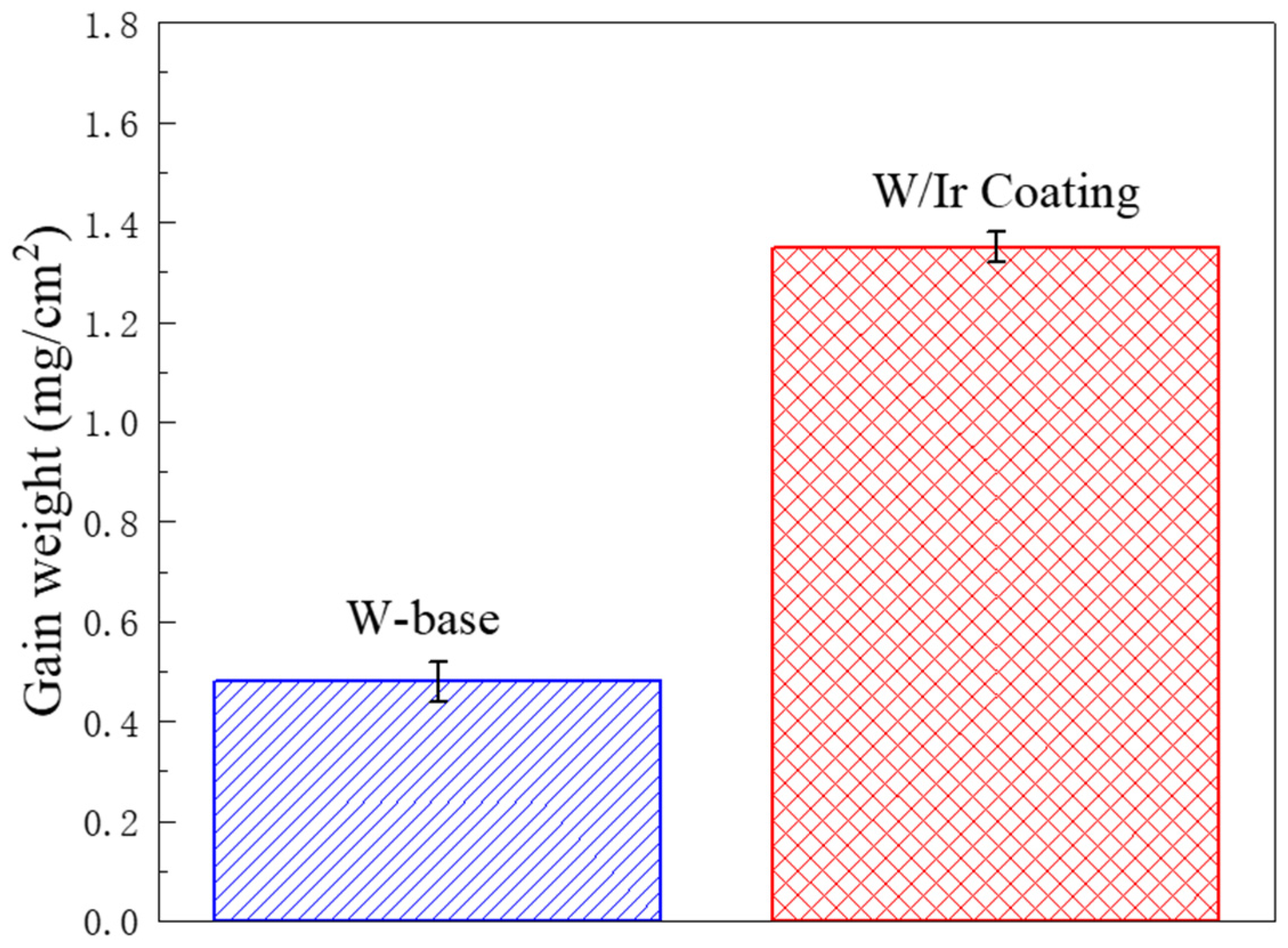
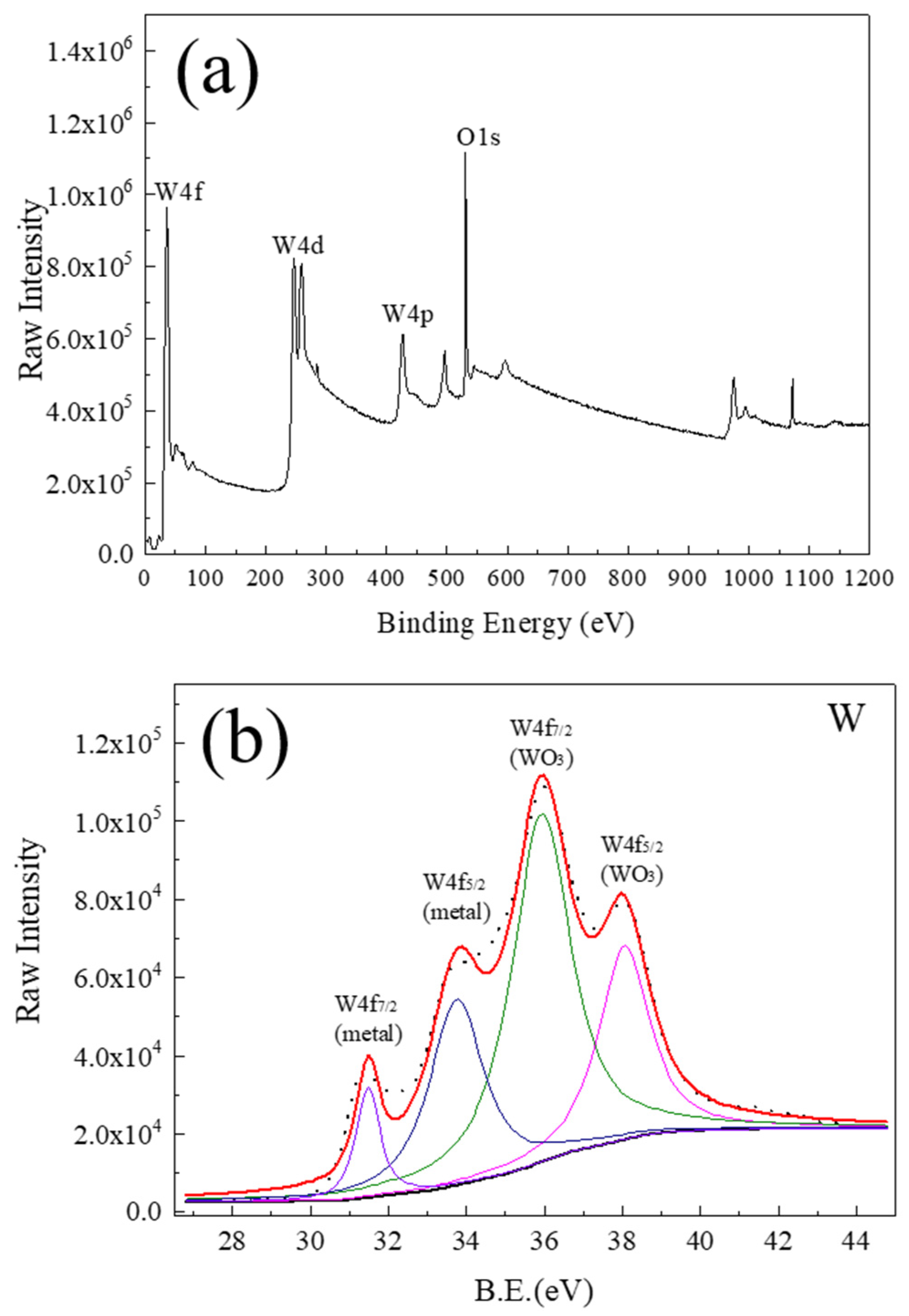
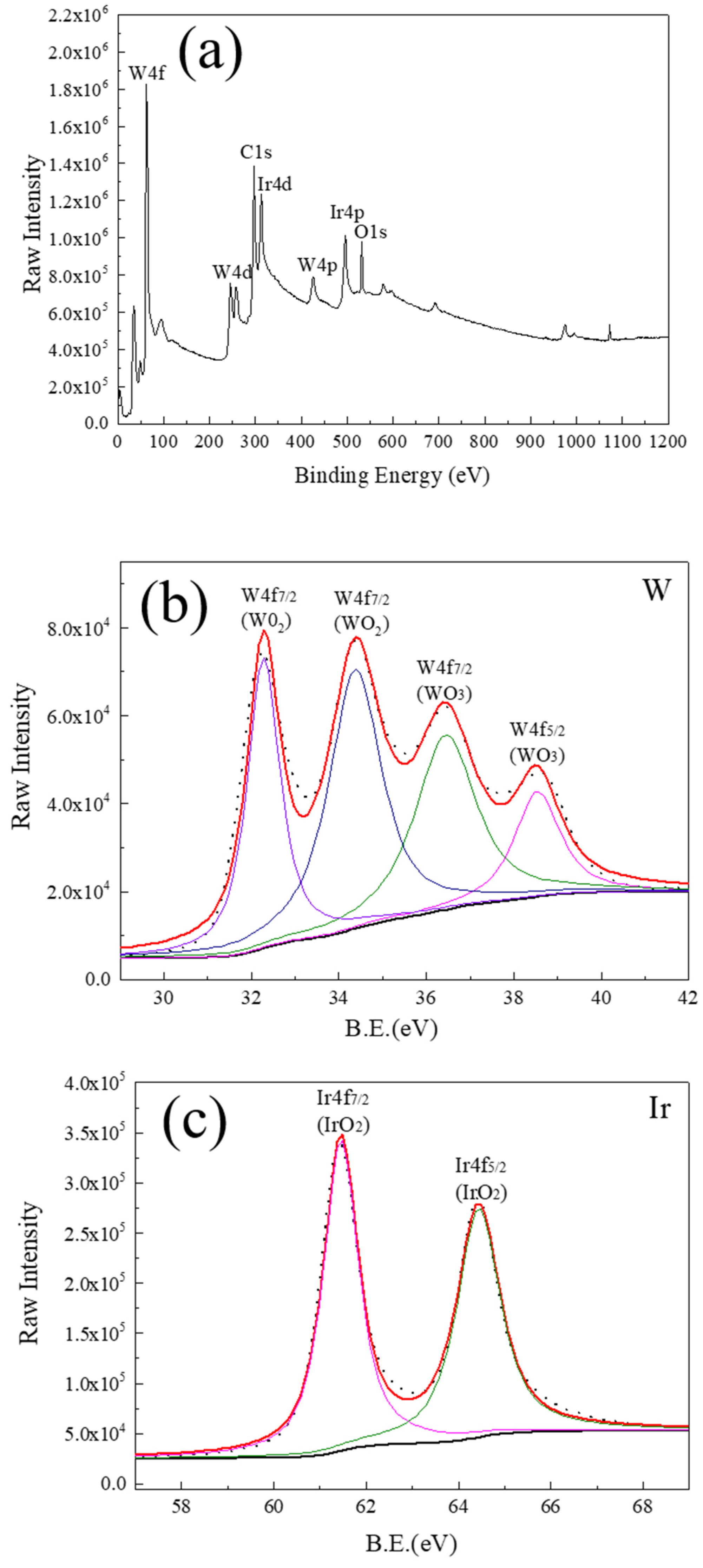
3.3. Oxidation Behavior and Mechanisms of Iridium Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukuda, M.; Hasegawa, A.; Nogami, S. Thermal properties of pure tungsten and its alloys for fusion applications. Fusion Eng. Des. 2018, 132, 1–6. [Google Scholar] [CrossRef]
- Pérez, P.; Monge, M.A.; Muñoz, Á.; Adeva, P. Influence of 1 and 5 wt% TiC additions on the oxidation behaviour of pure tungsten. Nucl. Mater. Energy 2020, 24, 100780. [Google Scholar] [CrossRef]
- Khyzhun, O.; Solonin, Y.; Dobrovolsky, V. Electronic structure of hexagonal tungsten trioxide: XPS, XES, and XAS studies. J. Alloys Compd. 2001, 320, 1–6. [Google Scholar] [CrossRef]
- Baklanova, N.; Morozova, N.; Kriventsov, V.; Titov, A. Synthesis and microstructure of iridium coatings on carbon fibers. Carbon 2013, 56, 243–254. [Google Scholar] [CrossRef]
- Baklanova, N.; Lozanov, V.; Morozova, N.; Titov, A. The effect of heat treatment on the tensile strength of the iridium-coated carbon fiber. Thin Solid Films 2015, 578, 148–155. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Kal’nyi, D.B.; Shubin, Y.V.; Kokovkin, V.V.; Morozova, N.B.; Hassan, A.; Basova, T.V. Metal Ir coatings on endocardial electrode tips, obtained by MOCVD. Appl. Surf. Sci. 2017, 425, 1052–1058. [Google Scholar] [CrossRef]
- Yang, S.; Yu, X.; Tan, C.; Wang, Y.; Ma, H.; Liu, K.; Cai, H. Growth kinetics and microstructure of MOCVD iridium coating from iridium(III) acetylacetonate with hydrogen. Appl. Surf. Sci. 2015, 329, 248–255. [Google Scholar] [CrossRef]
- Näther, J.; Köster, F.; Freudenberger, R.; Schöberl, C.; Lampke, T. Electrochemical deposition of iridium and iridium-nickel-alloys. Mater. Sci. Eng. 2017, 181, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Bai, S.; Zhang, H.; Ye, Y.; Zhu, L. Electrochemical studies of Ir coating deposition from NaCl-KCl-CsCl molten salts. Surf. Coat. Technol. 2017, 322, 76–85. [Google Scholar] [CrossRef]
- Petrossians, A.; Whalen, J.J.; Weiland, J.D.; Mansfeld, F. Electrodeposition and Characterization of Thin-Film Platinum-Iridium Alloys for Biological Interfaces. J. Electrochem. Soc. 2011, 158, 269–276. [Google Scholar] [CrossRef]
- Fang, Y.-Y.; Hsieh, Y.-C.; Lin, C.-W.; Dugas, R.; Tavares, A.C.; Guay, D. Electroplating of Nanostructured Pt, Ir and Pt-Ir at Room Temperature. J. Electrochem. Soc. 2012, 159, 518–520. [Google Scholar] [CrossRef]
- Le Vot, S.; Roué, L.; Bélanger, D. Electrodeposition of iridium onto glassy carbon and platinum electrodes. Electrochim. Acta 2012, 59, 49–56. [Google Scholar] [CrossRef]
- Qian, J.-G.; Yin, Y.; Li, X.; Li, T.-J. Electrodeposition of iridium from composite ionic liquid. Trans. Nonferrous Met. Soc. China 2015, 25, 1685–1691. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, S.; Zhang, H.; Ye, Y. Oxidation of iridium coating on rhenium coated graphite at elevated temperature in stagnated air. Appl. Surf. Sci. 2015, 328, 436–443. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, S.; Zhang, H.; Ye, Y.; Zhu, L. Oxidation of iridium coatings on rhenium substrates at ultrahigh temperature in stagnant air: Its failure mechanism and life model. Surf. Coat. Technol. 2016, 288, 52–61. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, L.; Ye, Y.; Zhang, H.; Bai, S. Iridium coatings with various grain structures prepared by electrodeposition from molten salts: Growth mechanism and high temperature oxidation resistance. Surf. Coat. Technol. 2017, 325, 190–199. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, S.; Zhang, H.; Ye, Y. Growth mechanism and mechanical property of laminar iridium coating by electrodeposition. Int. J. Refract. Met. Hard Mater. 2015, 50, 204–209. [Google Scholar] [CrossRef]
- Zhu, L.; Bai, S.; Zhang, H.; Ye, Y. Effects of cathodic current density and temperature on morphology and microstructure of iridium coating prepared by electrodeposition in molten salt under the air atmosphere. Appl. Surf. Sci. 2013, 265, 537–545. [Google Scholar] [CrossRef]
- Zhu, L.; Bai, S.; Zhang, H.; Ye, Y.; Tong, Y. Comparative Investigation of Iridium Coating Electrodeposited on Molybdenum, Rhenium and C/C Composite Substrates in Molten Salt in the Air Atmosphere. Phys. Procedia 2013, 50, 238–247. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Bai, S.; Zhang, H.; Ye, Y.; Gao, W. Long-term high-temperature oxidation of iridium coated rhenium by electrical resistance heating method. Int. J. Refract. Met. Hard Mater. 2014, 44, 42–48. [Google Scholar] [CrossRef]
- Probst, A.-C.; Stollenwerk, M.; Emmerich, F.; Büttner, A.; Zeising, S.; Stadtmüller, J.; Riethmüller, F.; Stehlíková, V.; Wen, M.; Proserpio, L.; et al. Influence of sputtering pressure on the nanostructure and the X-ray reflectivity of iridium coatings. Surf. Coat. Technol. 2018, 343, 101–107. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Z.; Cheng, X.; Wang, Y. EBSD study of (110) orientation of iridium (Ir) coating on niobium (Nb) substrate by double glow plasma. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2013, 307, 315–319. [Google Scholar] [CrossRef]
- An, D.; Dai, J.; Xiao, P. Study on heating model and heat transfer law of anti-oxidation coating materials in high vacuum environment. Results Phys. 2019, 13, 102267. [Google Scholar] [CrossRef]
- Gao, H.; Xiong, Y.; Zhang, K.; Wang, W.; Cao, S.; Wang, L. A first-principles study of deposition of iridium coating on Mo (1 1 0) surface by atomic layer deposition. Phys. B Condens. Matter 2022, 630, 413601. [Google Scholar] [CrossRef]
- Cui, E.; Wang, C.; Zuo, Y.; Leng, B.; Yu, G.; Zhang, W.; Yan, L.; Li, X. Preparation of iridium-hafnium intermetallic compound coatings in molten salts. Surf. Coat. Technol. 2021, 427, 127821. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, L.; Bai, S.; Ye, Y.; Zhang, H.; Li, S.; Tang, Y.; Zhang, J.; Wang, G. Ablation behavior of an Ir-Hf coating: A novel idea for ultra-high temperature coatings in non-equilibrium conditions. J. Alloys Compd. 2020, 818, 152829. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Brett, A.M.O. Cyclic Voltammetry and linear sweep techniques. Electrochem. Princ. Methods Appl. 1993, 9, 176–185. [Google Scholar]
- Munoz, A.G.; Lewerenz, H.J. Electroplating of Iridium onto Single-Crystal Silicon: Chemical and Electronic Properties of n-Si(111)/Ir Nanojunctions. J. Electrochem. Soc. 2009, 156, 184–187. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Gati, D.; Dzhurinskii, B.F. Simple and coordination compounds. Russ. J. Inorg. Chem. 1975, 20, 2307–2314. [Google Scholar]
- Riga, J.; Tenret-Noël, C.; Pireaux, J.J.; Caudano, R.; Verbist, J.J.; Gobillon, Y. Electronic Structure of Rutile Oxides TiO2, RuO2 and IrO2 Studied by X-ray Photoelectron Spectroscopy. Phys. Scr. 1977, 16, 351–354. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; VCH Verlagsgesellschaft mbH: Weinheim, Germany, 1996. [Google Scholar]
| Categories | Chemical Designation | Formula | Concentration | Operating Condition |
|---|---|---|---|---|
| Activation | Hydrofluoric acid | HF | 0.9 mol/L | T = 298 K T = 2 min Da = 2 A/dm2 |
| Nitric acid | HNO3 | 3.2 mol/L | ||
| Electrolyte | Ammonium hexachloroiridate(IV) | H8Cl6IrN2 | 0.05 mol/L | T = 358 K T = 8 h pH = 0.5~1.5 Dc = 0.2 A/dm2 |
| Boric acid | H3BO3 | 0.5 mol/L | ||
| Sulfamic acid | H3NO3S | 0.4 mol/L | ||
| Sodium malonate | C3H2Na2O4 | 0.02 mol/L |
| Redox Reaction | , mV | , mV | , mV | n | |
|---|---|---|---|---|---|
| A-A’ | 828.0 | 789.5 | 38.5 | 1.84 | ≈2 |
| B-B’ | 676.2 | 605.3 | 70.9 | 1.00 | 1 |
| C-C’ | 446.1 | 375.5 | 70.6 | 1.01 | 1 |
| Substance | (kJ/mol) | |
|---|---|---|
| 664.772 | −26.711 | |
| 468.526 | −18.826 | |
| −18.880 | 0.759 | |
| 187.869 | −7.549 | |
| −513.405 | 20.629 | |
| −221.035 | 8.881 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chen, D.; Zhang, X.; Niu, B.; Wang, B.; Li, R. Deposition of Iridium Coating on Pure Tungsten and High-Temperature Oxidation Behavior at 1300 K. Coatings 2022, 12, 1761. https://doi.org/10.3390/coatings12111761
Zhang J, Chen D, Zhang X, Niu B, Wang B, Li R. Deposition of Iridium Coating on Pure Tungsten and High-Temperature Oxidation Behavior at 1300 K. Coatings. 2022; 12(11):1761. https://doi.org/10.3390/coatings12111761
Chicago/Turabian StyleZhang, Jifu, Dongchu Chen, Xueying Zhang, Ben Niu, Biao Wang, and Runxia Li. 2022. "Deposition of Iridium Coating on Pure Tungsten and High-Temperature Oxidation Behavior at 1300 K" Coatings 12, no. 11: 1761. https://doi.org/10.3390/coatings12111761
APA StyleZhang, J., Chen, D., Zhang, X., Niu, B., Wang, B., & Li, R. (2022). Deposition of Iridium Coating on Pure Tungsten and High-Temperature Oxidation Behavior at 1300 K. Coatings, 12(11), 1761. https://doi.org/10.3390/coatings12111761





