Water-Soluble Holographic Photopolymers for a Sustainable Future—A Review
Abstract
:1. Introduction
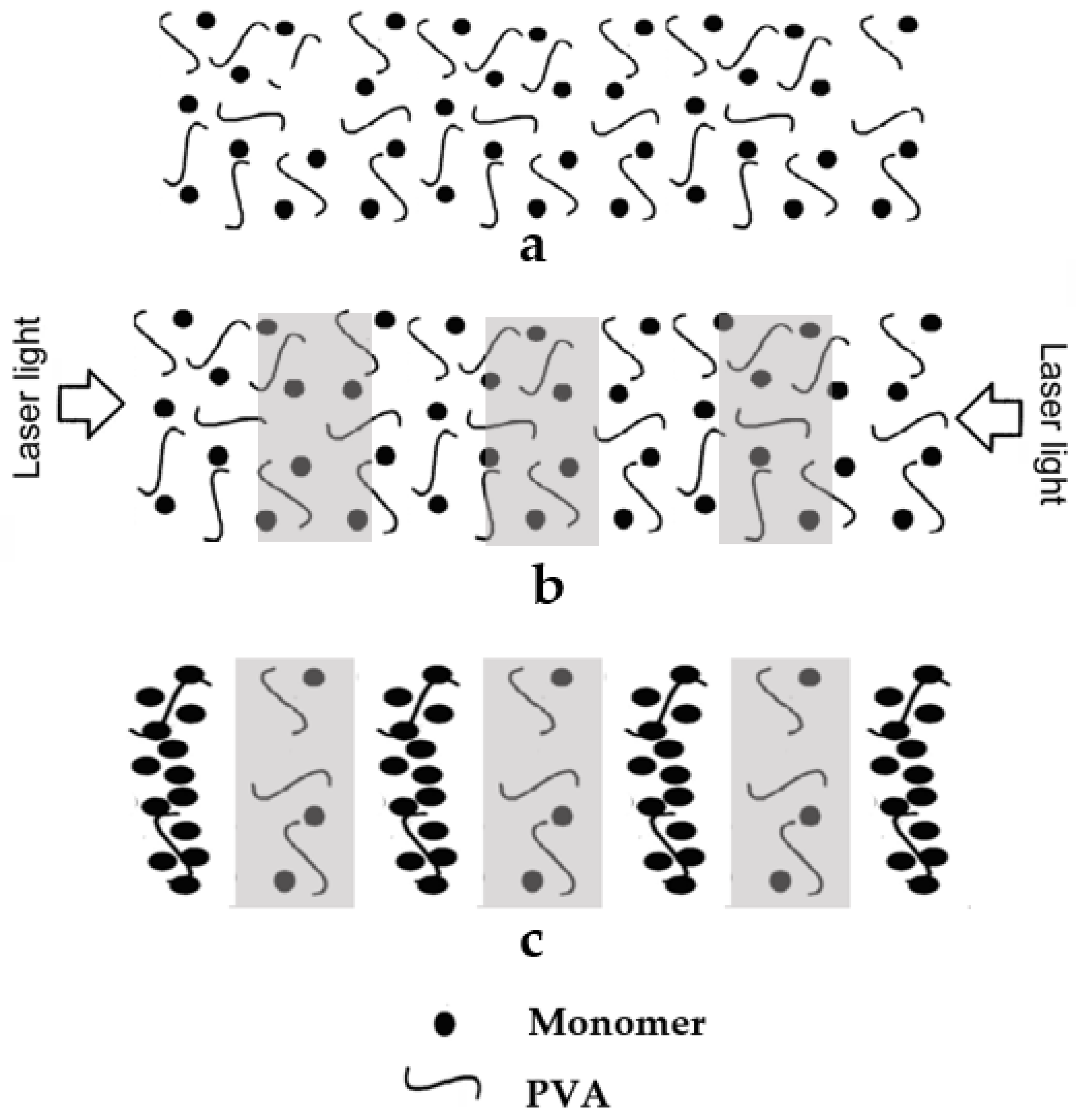
2. Water-Soluble Photopolymer Materials
- Very good optical quality
- Low scattering
- Sufficient thickness to have the required storage density for holographic storage applications
- Photosensitivity to the wavelength of the laser used for recording
- No need for heat treatment or chemical processing
- Long-term thermal stability and humidity within a certain range to achieve sufficient storage life
3. Low-Toxicity Water-Soluble Photopolymers
3.1. Biophotopol Photopolymer
3.2. NIPA-Based Photopolymer
3.3. Diacetone Acrylamide-Based Photopolymer
3.4. Bright Holograms in DA-Based Photopolymer
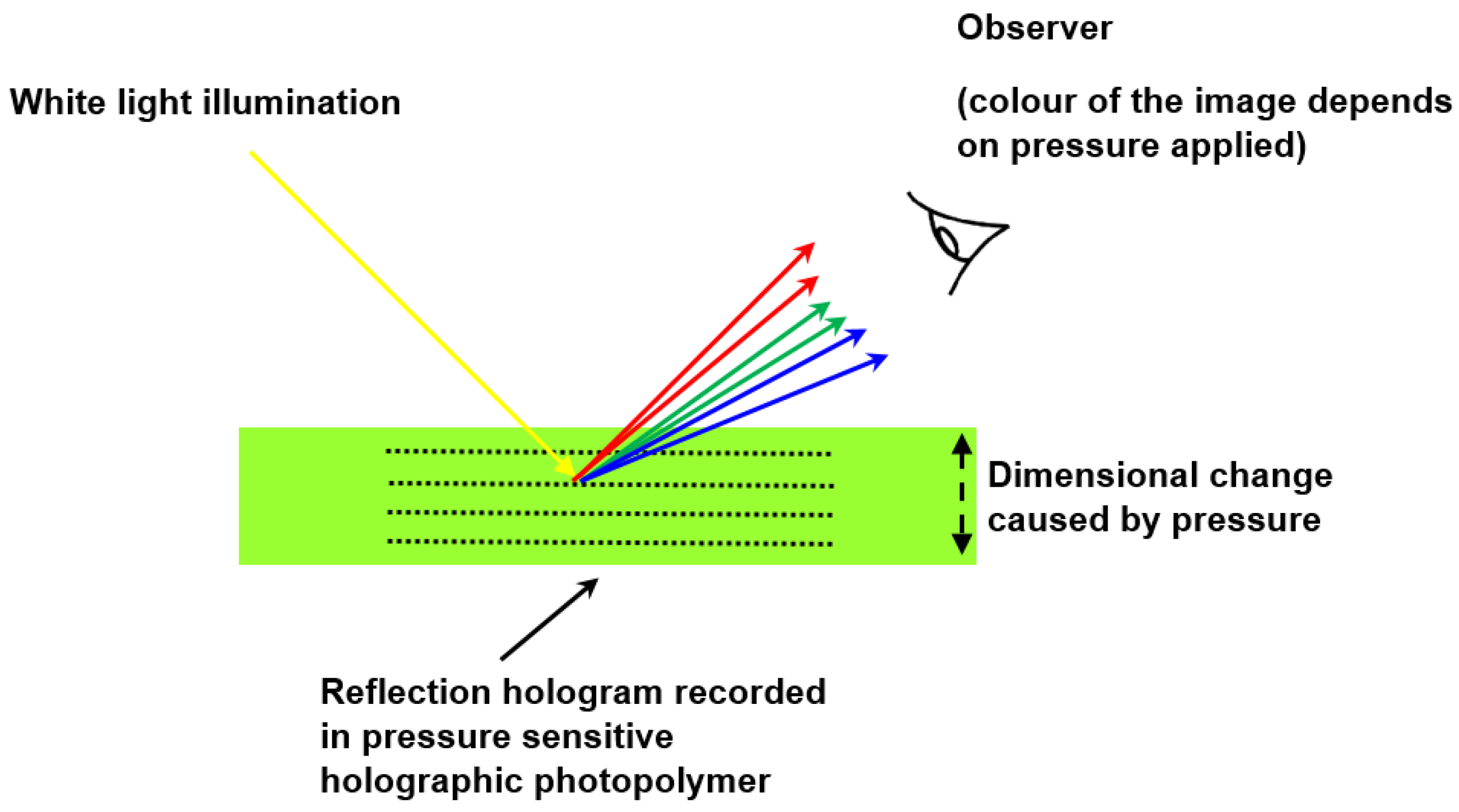
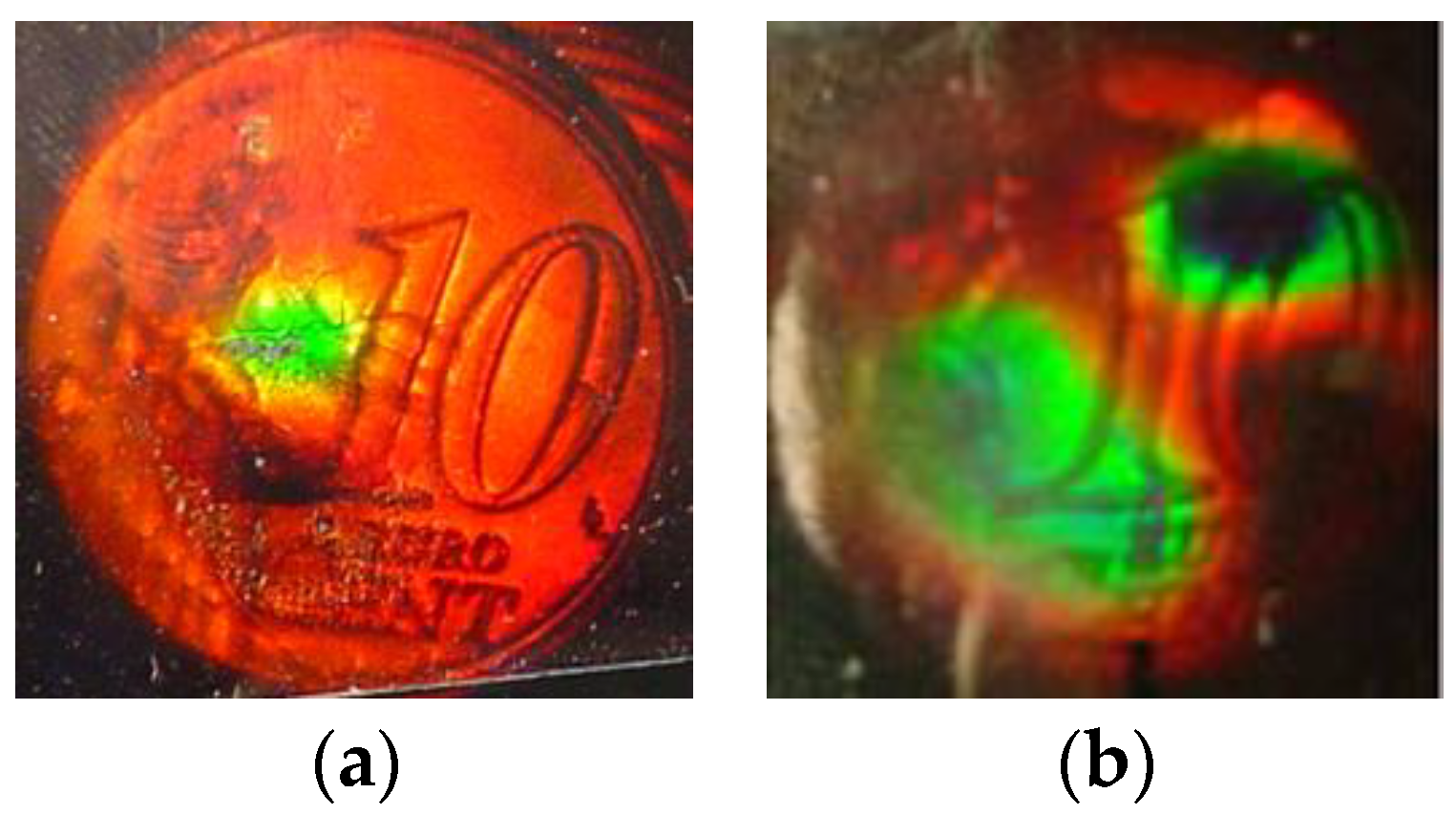
3.5. Holographic Pressure Sensors in DA-Based Photopolymer
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calixto, S. Dry polymer for holographic recording. Appl. Opt. 1987, 26, 3904–3910. [Google Scholar] [CrossRef] [PubMed]
- Fimia, A.; Lopez, N.; Mateos, F.; Sastre, R.; A Pineda, J.; Amat-Guerri, F. Acrylamide photopolymers for use in real-time holography: Improving energetic sensitivity. Proc. SPIE 1992, 1732, 105–110. [Google Scholar] [CrossRef]
- Fimia, A.; Lopez, N.; Mateos, F.; Sastre, R.; Pineda, J.; Amat-Guerri, F. Elimination of Oxygen Inhibition in Photopolymer Systems Used as Holographic Recording Materials. J. Mod. Opt. 1993, 40, 699–706. [Google Scholar] [CrossRef]
- Martin, S.; Leclere, P.; Renotte, Y.; Toal, V.; Lion, Y. Characterisation of an acrylamide-based dry photopolymer holographic recording material. Opt. Eng. 1994, 33, 3942–3946. [Google Scholar]
- Blaya, S.; Mallavia, R.; Carretero, L.; Fimia, A.; Madrigal, R.F. Highly sensitive photopolymerisable dry film for use in real time holography. Appl. Phys. Lett. 1998, 73, 1628–1630. [Google Scholar] [CrossRef]
- Blaya, S.; Carretero, L.; Fimia, A.; Mallavia, R.; Madrigal, R.F.; Sastre, R.; Amat-Guerri, F. Optimal composition of an acrylamide and N,N′-methylenebisacrylamide holographic recording material. J. Mod. Opt. 1998, 45, 2573–2584. [Google Scholar] [CrossRef]
- Shi, D.; Yek, P.N.Y.; Ge, S.; Shi, Y.; Liew, R.K.; Peng, W.; Sonne, C.; Tabatabaei, M.; Aghbashlo, M.; Lam, S.S. Production of highly porous biochar via microwave physiochemical activation for dechlorination in water treatment. Chemosphere 2022, 309, 136624. [Google Scholar] [CrossRef]
- Hossain, M.K.; Rubel, M.; Akbar, A.; Ahmed, M.H.; Haque, N.; Rahman, F.; Hossain, J.; Hossain, K.M. A review on recent applications and future prospects of rare earth oxides in corrosion and thermal barrier coatings, catalysts, tribological, and environmental sectors. Ceram. Int. 2022, 48, 32588–32612. [Google Scholar] [CrossRef]
- Irfan, M.; Martin, S.; Naydenova, I. Temperature-Sensitive Holograms with Switchable Memory. Adv. Photon-Res. 2021, 2, 2100062. [Google Scholar] [CrossRef]
- Ramírez, M.G.; Sirvent, D.; Morales-Vidal, M.; Ortuño, M.; Martínez-Guardiola, F.J.; Francés, J.; Pascual, I. LED-Cured Reflection Gratings Stored in an Acrylate-Based Photopolymer. Polymers 2019, 11, 632. [Google Scholar] [CrossRef] [Green Version]
- Irfan, M.; Martin, S.; Obeidi, M.A.; Miller, S.; Kuster, F.; Brabazon, D.; Naydenova, I. A Magnetic Nanoparticle-Doped Photopolymer for Holographic Recording. Polymers 2022, 14, 1858. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, E.; Cody, D.; Naydenova, I.; Martin, S.; Toal, V. Research on Holographic Sensors and Novel Photopolymers at the Centre for Industrial and Engineering Optics. In Holography—Basic Principles And Contemporary Applications; Intech: Tokyo, Japan, 2013; pp. 89–102. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.; Feely, C.A.; Toal, V. Holographic recording characteristics of an acrylamide-based photopoly-mer. Appl. Opt. 1997, 36, 5757–5768. [Google Scholar] [CrossRef] [PubMed]
- Garcıa, C.; Fimia, A.; Pascual, I. Holographic behavior of a photopolymer at high thicknesses and high monomer concentrations: Mechanism of photopolymerization. Appl. Phys. A 2001, 72, 311–316. [Google Scholar] [CrossRef]
- Neipp, C.; Beléndez, A.; Gallego, S.; Ortuño, M.; Pascual, I.; Sheridan, J.T. Angular responses of the first and second diffracted orders in transmission diffraction grating recorded on photopolymer material. Opt. Express 2003, 11, 1835–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meka, C.; Jallapuram, R.; Naydenova, I.; Martin, S.; Toal, V. Development of a panchromatic acryla-midebased photopolymer for multicolor reflection holography. Appl. Opt. 2010, 49, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liu, H.; Mao, D.; Geng, Y.; Wang, W.; Sun, L.; Lv, J. Enhancement of spectrum strength in holographic sensing in nanozeolites dispersed acrylamide photopolymer. Opt. Express 2015, 23, 29113–29126. [Google Scholar] [CrossRef]
- Fernández, R.; Bleda, S.; Gallego, S.; Neipp, C.; Márquez, A.; Tomita, Y.; Pascual, I.; Beléndez, A. Holographic waveguides in photopolymers. Opt. Express 2019, 27, 827–840. [Google Scholar] [CrossRef] [Green Version]
- Cody, D.; Moothanchery, M.; Mihaylova, E.; Toal, V.; Mintova, S.; Naydenova, I. Compositional Changes for Reduc-tion of Polymerisation-Induced Shrinkage in Holographic Photopolymers. In Advances in Materials Science and Engineering; Hindawi Publishing Corporation: London, UK, 2016; Volume 2016, p. 8020754. [Google Scholar]
- Hashimoto, K.; Aldridge, W. Biochemical studies on acrylamide, a neurotoxic agent. Biochem. Pharmacol. 1970, 19, 2591–2604. [Google Scholar] [CrossRef]
- Mendel, F. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food. Chem. 2003, 51, 4504–4526. [Google Scholar]
- Cody, D.; Casey, A.; Naydenova, I.; Mihaylova, E. A Comparative Cytotoxic Evaluation of Acrylamide and Diacetone Acrylamide to Investigate Their Suitability for Holographic Photopolymer Formulations. Int. J. Polym. Sci. 2013, 2013, 564319. [Google Scholar] [CrossRef] [Green Version]
- Ortuño, M.; Fernández, E.; Gallego, S.; Beléndez, A.; Pascual, I. New photopolymer holographic recording ma-terial with sustainable design. Opt. Express 2007, 15, 12425–12435. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, M.; Gallego, S.; Márquez, A.; Neipp, C.; Pascual, I.; Beléndez, A. Biophotopol: A Sustainable Photo-polymer for Holographic Data Storage Applications. Materials 2012, 5, 772–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Fuster, V.; Ortuño, M.; Gallego, S.; Márquez, A.; Beléndez, A.; Pascual, I. Biophotopol’s energetic sensitivity improved in 300 μm layers by tuning the recording wavelength. Opt. Mater. 2016, 52, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Fuster, V.; Ortuño, M.; Fernández, R.; Gallego, S.; Márquez, A.; Beléndez, A.; Pascual, I. Peristrophic multiplexed holograms recorded in a low toxicity photopolymer. Opt. Mater. Express 2016, 7, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Mikulchyk, T.; Martin, S.; Naydenova, I. N-isopropylacrylamide-based photopolymer for hol-ographic recording of thermosensitive transmission and reflection gratings. Appl. Opt. 2017, 56, 6348–6356. [Google Scholar] [CrossRef]
- Cody, D.; Gul, S.-E.; Mikulchyk, T.; Irfan, M.; Kharchenko, A.; Goldyn, K.; Martin, S.; Mintova, S.; Cassidy, J.; Naydenova, I. Self-processing photopolymer materials for versatile design and fabrication of holographic sensors and interactive holograms. Appl. Opt. 2018, 57, E173–E183. [Google Scholar] [CrossRef]
- Cody, D.; Naydenova, I.; Mihaylova, E. New non-toxic holographic photopolymer material. J. Opt. A Pure Appl. Opt. 2012, 14, 015601. [Google Scholar] [CrossRef] [Green Version]
- Siemiatycki, J.; Richardson, L.; Straif, K.; Latreille, B.; Lakhani, R.; Campbell, S.; Rousseau, M.; Boffetta, P. Listing occupational carcinogens Environ. Health Perspect. 2004, 112, 1447–1459. [Google Scholar] [CrossRef]
- Acrylamide, D.; Sigma-Aldrich. Available online: http://www.sigmaaldrich.com/catalog/DisplayMSDSContent.do (accessed on 30 June 2011).
- Coleman, L.E.; Bork, J.F.; Wyman, D.P.; Hoke, D.I. Synthesis and polymerization of N [2-(2-methyl-4-oxopen-tyl)]-acrylamide–A new reactive vinyl monomer. J. Polym. Sci. 1965, 3, 1601–1608. [Google Scholar]
- Gallego, S.; Ortuno, M.; Neipp, C.; Garcia, C.; Belendez, A.; Pascual, I. Overmodulation effects in volume holograms recorded on photopolymers. Opt. Comm. 2003, 215, 263–269. [Google Scholar] [CrossRef]
- Blaya, S.; Carretero, L.; Mallavia, R.; Fimia, A.; Madrigal, R.F.; Ulibarrena, M.; Levy, D. Optimization of an acrylamide-based dry film used for holographic recording. Opt. Soc. Amer. 1998, 37, 7604–7610. [Google Scholar] [CrossRef] [PubMed]
- Huawen, Y.; Mingju, H.; Zhongyu, C.; Lisong, H.; Fuxi, G. Optimization of two-monomer-based photopolymer used for holographic recording. Mater. Lett. 2001, 56, 3–8. [Google Scholar]
- Hai, L.; Ruo-Ping, L.; Cai-Xia, S.; Yong, X.; Dao-Guang, T.; Ming-ju, H. Holographic property of photopolymers with different amine photoinitiators. Chin. Phys. B 2010, 19, 242121–242127. [Google Scholar] [CrossRef]
- Cody, D.; Gribbin, S.; Mihaylova, E.; Naydenova, I. Low-Toxicity Photopolymer for Reflection Holography. ACS Appl. Mater. Interfaces 2016, 8, 18481–18487. [Google Scholar] [CrossRef] [PubMed]




| Photopolymer | Main Monomer | Dye | Binder | DEmax, % | Key Characteristics of the Photopolymer |
|---|---|---|---|---|---|
| Biophotopol [23,24] | NaAO | PRF | PVA, Mw = 130,000, 87.7% hydrolysed | 77 in TR | Low-toxicity; Multiplexing; |
| NIPA-based [27,28] | NIPA | Erythrosin B | PVA, Mw = 9000–10,000, 80% hydrolysed | 80 in TR; 20 in RE | Low-toxicity; Temperature sensitive; |
| DA-based [12,29,37] | DA | Erythrosin B; or MB | PVA, Mw = 9000–10,000, 80% hydrolysed | 90 in TR; 50 in RE | Low-toxicity; Pressure sensitive; High DE in TR; High DE in RE; |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaylova, E.M. Water-Soluble Holographic Photopolymers for a Sustainable Future—A Review. Coatings 2022, 12, 1765. https://doi.org/10.3390/coatings12111765
Mihaylova EM. Water-Soluble Holographic Photopolymers for a Sustainable Future—A Review. Coatings. 2022; 12(11):1765. https://doi.org/10.3390/coatings12111765
Chicago/Turabian StyleMihaylova, Emilia Mitkova. 2022. "Water-Soluble Holographic Photopolymers for a Sustainable Future—A Review" Coatings 12, no. 11: 1765. https://doi.org/10.3390/coatings12111765





