Bi2O3-Assisted Sintering of Na3Zr2Si2PO12 Electrolyte for Solid-State Sodium Metal Batteries
Abstract
1. Introduction
2. Materials and Methods
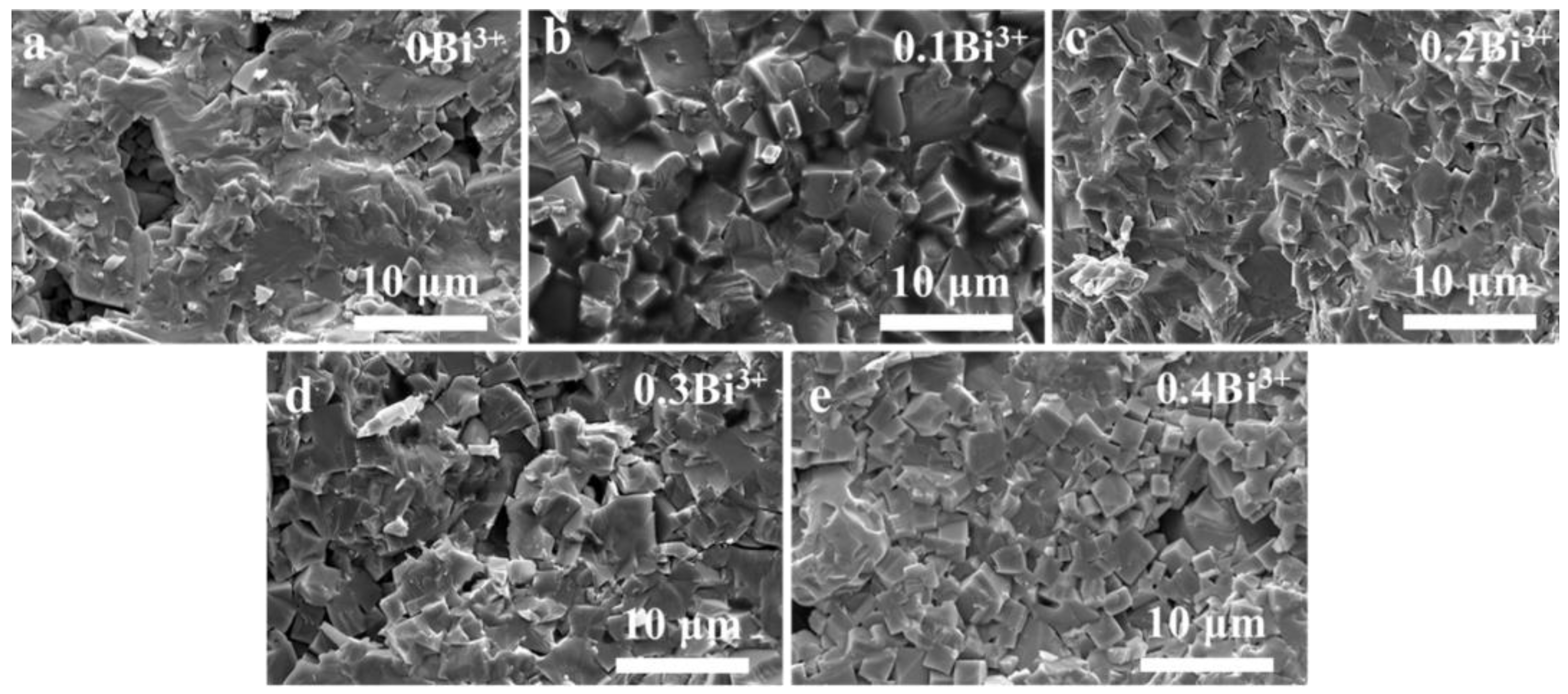
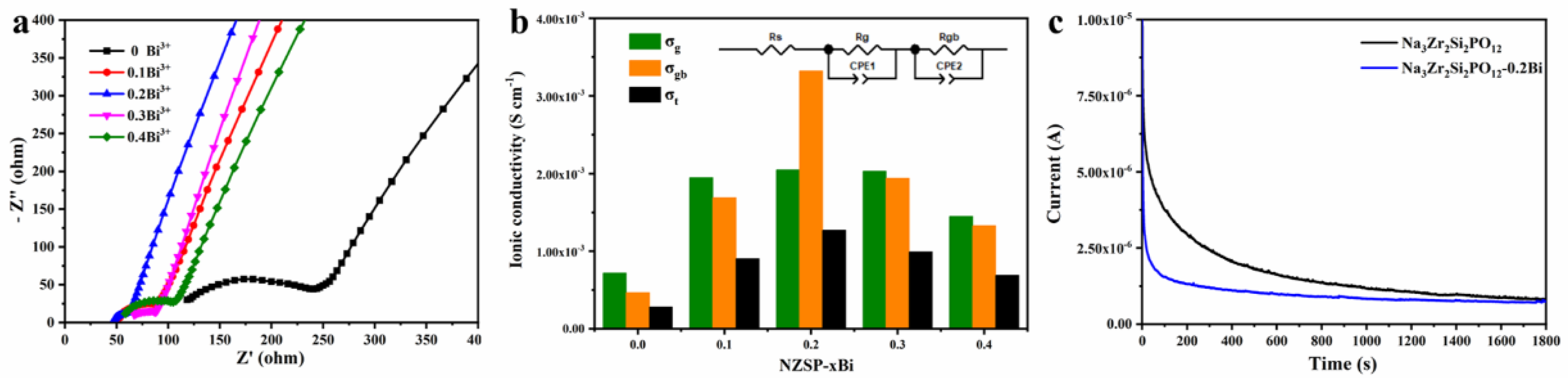
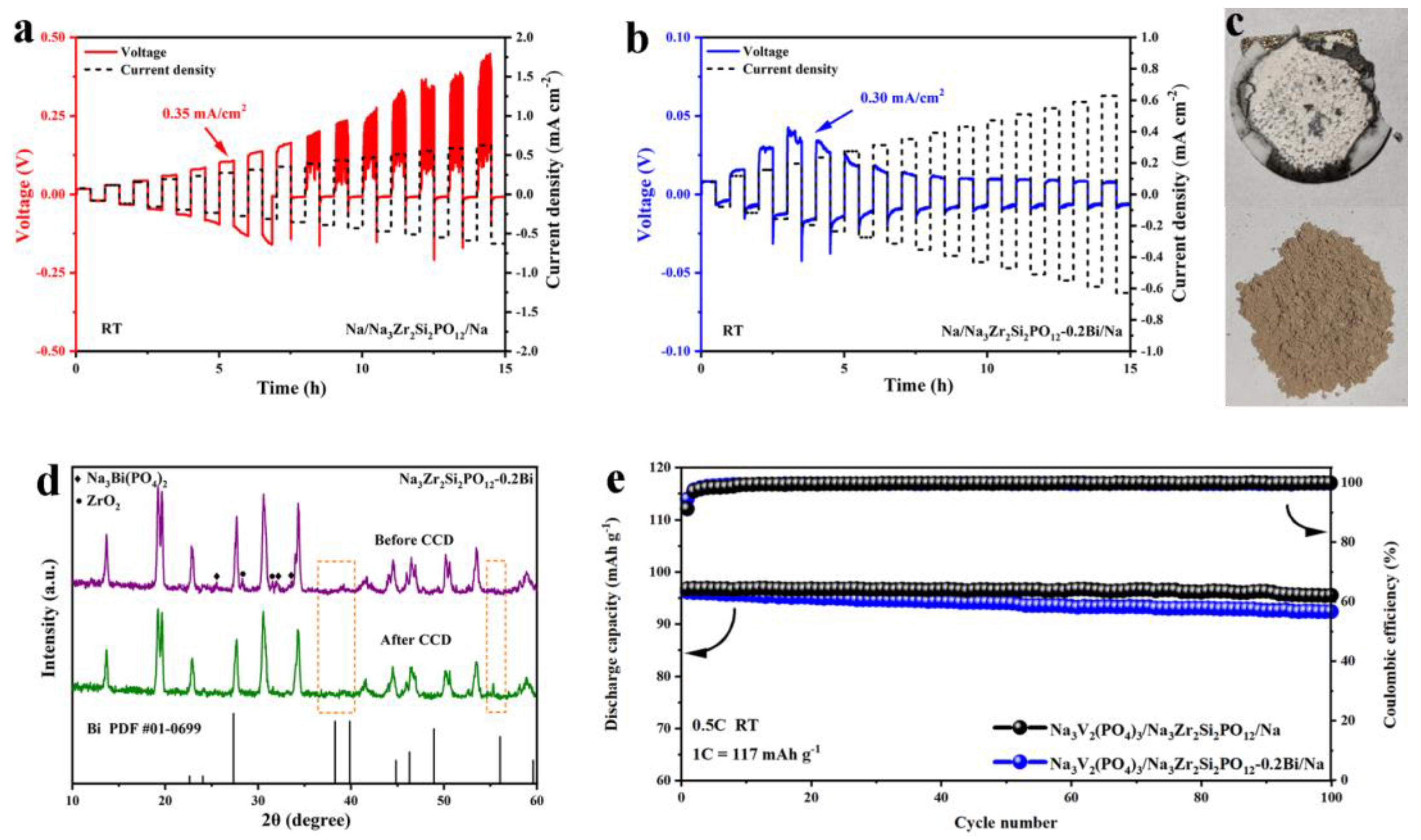
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, C.; Shen, L.; Zhu, C.; Huang, Y.; Xi, K.; Maier, J.; Yu, Y. Challenges and Perspectives for NASICON-Type Electrode Materials for Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700431. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, X.; Yu, X.; Weng, W.; Yang, J.; Zhou, D.; Xiao, R.; Chen, L.; Yao, X. Na10SnSb2S12: A nanosized air-stable solid electrolyte for all-solid-state sodium batteries. Chem. Eng. J. 2021, 420, 127692. [Google Scholar] [CrossRef]
- Wang, Y.; Richards, W.D.; Ong, S.P.; Miara, L.J.; Kim, J.C.; Mo, Y.; Ceder, G. Design principles for solid-state lithium superionic conductors. Nat. Mater. 2015, 14, 1026–1031. [Google Scholar] [CrossRef]
- Gandi, S.S.; Gandi, S.; Madduluri, V.R.; Katari, N.K.; Dutta, D.P.; Ravuri, B.R. Na3+x[CrxTi2−x(PO4)3] glass-ceramic electrolyte: Ionic conductivity and structural correlations for different heat treating temperatures and time schedules. Ionics 2019, 25, 4179–4188. [Google Scholar] [CrossRef]
- Leng, H.; Nie, J.; Luo, J. Combining cold sintering and Bi2O3-Activated liquid-phase sintering to fabricate high-conductivity Mg-doped NASICON at reduced temperatures. J. Mater. 2019, 5, 237–246. [Google Scholar] [CrossRef]
- Hong, H.Y.P. Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater. Res. Bull. 1976, 11, 173–182. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Sundar, G.S.; Suman, G.; Kumar, K.N.; Dutta, D.P.; Rao, R.B. Investigation on the applicability of high Na-ion conducting Na3+x[ZrxSc2-x(PO4)3] glass-ceramic electrolyte for use in safer Na-ion batteries. J. Phys. Chem. Solids 2019, 126, 209–218. [Google Scholar] [CrossRef]
- Lu, Y.; Alonso, J.A.; Yi, Q.; Lu, L.; Wang, Z.L.; Sun, C. A High-Performance Monolithic Solid-State Sodium Battery with Ca2+ Doped Na3Zr2Si2PO12 Electrolyte. Adv. Energy Mater. 2019, 9, 1901205. [Google Scholar] [CrossRef]
- Yang, J.; Wan, H.-L.; Zhang, Z.-H.; Liu, G.-Z.; Xu, X.-X.; Hu, Y.-S.; Yao, X.-Y. NASICON-structured Na3.1Zr1.95Mg0.05Si2PO12 solid electrolyte for solid-state sodium batteries. Rare Met. 2018, 37, 480–487. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Shi, J.; Chu, Y.S.; Yu, X.; Xu, K.; Ge, M.; Yan, H.; Li, W.; Gu, L.; et al. A Self-Forming Composite Electrolyte for Solid-State Sodium Battery with Ultralong Cycle Life. Adv. Energy Mater. 2017, 7, 1601196. [Google Scholar] [CrossRef]
- Miao, R.J.; Cao, X.G.; Wang, W.G.; Zhang, H.Y. Influence of Bi2O3 additive on the electrochemical performance of Na3.1Y0.1Zr1.9Si2PO12 inorganic solid electrolyte. Ceram. Int. 2021, 47, 17455–17462. [Google Scholar] [CrossRef]
- Chen, D.; Luo, F.; Zhou, W.; Zhu, D. Influence of Nb5+, Ti4+, Y3+ and Zn2+ doped Na3Zr2Si2PO12 solid electrolyte on its conductivity. J. Alloy. Compd. 2018, 757, 348–355. [Google Scholar] [CrossRef]
- Khakpour, Z. Influence of M: Ce4+, Gd3+ and Yb3+ substituted Na3+xZr2−xMxSi2PO12 solid NASICON electrolytes on sintering, microstructure and conductivity. Electrochim. Acta 2016, 196, 337–347. [Google Scholar] [CrossRef]
- Jolley, A.G.; Cohn, G.; Hitz, G.T.; Wachsman, E.D. Improving the ionic conductivity of NASICON through aliovalent cation substitution of Na3Zr2Si2PO12. Ionics 2015, 21, 3031–3038. [Google Scholar] [CrossRef]
- Huang, C.C.; Yang, G.M.; Yu, W.H.; Xue, C.; Zhai, Y.F.; Tang, W.P.; Hu, N.; Wen, Z.Y.; Lu, L.; Song, S.F. Gallium-substituted Nasicon Na3Zr2Si2PO12 solid electrolytes. J. Alloy. Compd. 2021, 855, 157501. [Google Scholar] [CrossRef]
- Ma, Q.L.; Guin, M.; Naqash, S.; Tsai, C.L.; Tietz, F.; Guillon, O. Scandium-Substituted Na3Zr2(SiO4)2(PO4) Prepared by a Solution Assisted Solid-State Reaction Method as Sodium-Ion Conductors. Chem. Mater. 2016, 28, 4821–4828. [Google Scholar] [CrossRef]
- Song, S.F.; Duong, H.M.; Korsunsky, A.M.; Hu, N.; Lu, L. A Na+ Superionic Conductor for Room-Temperature Sodium Batteries. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Mei, W.; Chen, J.; Wang, D.; Mao, Z. Rare Earth Oxide-Assisted Sintered NASICON Electrolyte Composed of a Phosphate Grain Boundary Phase with Low Electronic Conductivity. ACS Appl. Energy Mater. 2022, 5, 777–783. [Google Scholar] [CrossRef]
- Thirupathi, R.; Omar, S. A Strategic Co-doping Approach Using Sc3+ and Ce4+ toward Enhanced Conductivity in NASICON-Type Na3Zr2Si2PO12. J. Phys. Chem. C 2021, 125, 27723–27735. [Google Scholar] [CrossRef]
- Heo, E.; Wang, J.E.; Yun, J.H.; Kim, J.-H.; Kim, D.J.; Kim, D.K. Improving Room Temperature Ionic Conductivity of Na3–xKxZr2Si2PO12 Solid-Electrolytes: Effects of Potassium Substitution. Inorg. Chem. 2021, 60, 11147–11153. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Tsai, C.L.; Wei, X.K.; Heggen, M.; Tietz, F.; Irvine, J.T.S. Room temperature demonstration of a sodium superionic conductor with grain conductivity in excess of 0.01 S cm−1 and its primary applications in symmetric battery cells. J. Mater. Chem. A 2019, 7, 7766–7776. [Google Scholar] [CrossRef]
- Shen, L.; Yang, J.; Liu, G.; Avdeev, M.; Yao, X. High ionic conductivity and dendrite-resistant NASICON solid electrolyte for all-solid-state sodium batteries. Mater. Today Energy 2021, 20, 100691. [Google Scholar] [CrossRef]
- Oh, J.S.; He, L.; Plewa, A.; Morita, M.; Zhao, Y.; Sakamoto, T.; Song, X.; Zhai, W.; Zeng, K.; Lu, L. Composite NASICON (Na3Zr2Si2PO12) Solid-State Electrolyte with Enhanced Na+ Ionic Conductivity: Effect of Liquid Phase Sintering. ACS Appl. Mater. Interfaces 2019, 11, 40125–40133. [Google Scholar] [CrossRef]
- Shao, Y.J.; Zhong, G.M.; Lu, Y.X.; Liu, L.L.; Zhao, C.L.; Zhang, Q.Q.; Hu, Y.S.; Yang, Y.; Chen, L.Q. A novel NASICON-based glass-ceramic composite electrolyte with enhanced Na-ion conductivity. Energy Storage Mater. 2019, 23, 514–521. [Google Scholar] [CrossRef]
- Diouri, M.; Sadel, A.; Zahir, M.; Drache, M.; Conflant, P.; Wignacourt, J.P.; Boivin, J.C. Na3Bi(PO4)2 type solid solutions; investigation of structural and electrical properties. J. Alloy. Compd. 1992, 188, 206–210. [Google Scholar] [CrossRef]
- Cooper, K.R. Progress Toward Accurate Through-Plane Ion Transport Resistance Measurement of Thin Solid Electrolytes. J. Electrochem. Soc. 2010, 157, B1731. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Tang, Y.; Chen, J.; Wang, D.; Mao, Z. Low temperature and rapid microwave sintering of Na3Zr2Si2PO12 solid electrolytes for Na-Ion batteries. J. Power Sources 2021, 481, 228924. [Google Scholar] [CrossRef]
- Sun, F.; Xiang, Y.; Sun, Q.; Zhong, G.; Banis, M.N.; Li, W.; Liu, Y.; Luo, J.; Li, R.; Fu, R.; et al. Insight into Ion Diffusion Dynamics/Mechanisms and Electronic Structure of Highly Conductive Sodium-Rich Na3+xLaxZr2−xSi2PO12 (0 ≤ x ≤ 0.5) Solid-State Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 13132–13138. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.O.; Figueiredo, F.; Marques, F.M.B.; Franco, J.I. Reaction of NASICON with water. Solid State Ion. 2001, 139, 309–314. [Google Scholar] [CrossRef]
- He, S.; Xu, Y.; Chen, Y.; Ma, X. Enhanced ionic conductivity of an F−-assisted Na3Zr2Si2PO12 solid electrolyte for solid-state sodium batteries. J. Mater. Chem. A 2020, 8, 12594–12602. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.-Z.; Yuan, H.; Cheng, X.-B.; Huang, J.-Q.; Zhang, Q. Critical Current Density in Solid-State Lithium Metal Batteries: Mechanism, Influences, and Strategies. Adv. Funct. Mater. 2021, 31, 2009925. [Google Scholar] [CrossRef]
- Fang, W.; Fan, L.; Zhang, Y.; Zhang, Q.; Yin, Y.; Zhang, N.; Sun, K. Synthesis of carbon coated Bi2O3 nanocomposite anode for sodium-ion batteries. Ceram. Int. 2017, 43, 8819–8823. [Google Scholar] [CrossRef]
- Xu, R.; Kinderman, R.; Van der Lugt, W. Electrical resistivities of liquid Na-Bi and Rb-Bi alloys. J. Phys. Condens. Matter 1991, 3, 127–133. [Google Scholar] [CrossRef]


| Components | Ionic Conductivity (S cm−1) | Reference |
|---|---|---|
| Na3Zr1.9Ca0.1Si2PO12 | 1.67 × 10−3 | [11] |
| Na3Zr1.7La0.3Si2PO12 | 3.4 × 10−3 | [13] |
| Na3.1Y0.1Zr1.9Si2PO12 + 1 wt.% Bi2O3 | 1.21 × 10−3 | [14] |
| Na3Zr1.9Nb0.1Si2PO12 | 2.11 × 10−4 | [15] |
| Na3Zr1.9Ti0.1Si2PO12 | 5.97 × 10−4 | [15] |
| Na3Zr1.8Zn0.2Si2PO12 | 2.50 × 10−3 | [15] |
| Na3Zr1.9Yb0.1Si2PO12 | 1.7 × 10−4 | [16] |
| Na3Zr1.9Gd0.1Si2PO12 | 6.0 × 10−4 | [16] |
| Na3Zr1.9Ce0.1Si2PO12 | 9.0 × 10−4 | [16] |
| Na3Zr1.9Al0.1Si2PO12 | 4.39 × 10−4 | [17] |
| Na3Zr1.9Fe0.1Si2PO12 | 7.53 × 10−4 | [17] |
| Na3Zr1.9Co0.1Si2PO12 | 1.55 × 10−4 | [17] |
| Na3Zr1.9Ni0.1Si2PO12 | 6.18 × 10−4 | [17] |
| Na3Zr1.9Ga0.1Si2PO12 | 1.06 × 10−3 | [18] |
| Na3.4Zr1.6Sc0.4Si2PO12 | 4.0 × 10−3 | [19] |
| Na3.1Zr1.95Mg0.05Si2PO12 | 3.5 × 10−3 | [20] |
| Na3.2Zr1.8Sm0.2Si2PO12 | 1.36 × 10−3 | [21] |
| Na3.3Zr1.7Ho0.3Si2PO12 | 1.07 × 10−3 | [21] |
| Na3.33Ce0.02Sc0.33Zr1.65Si2PO12 | 2.44 × 10−3 | [22] |
| Na2.9K0.1Zr2Si2PO12 | 7.734 × 10−4 | [23] |
| Na3.4Zr2Si2.4P0.6O12 | 5.5 × 10−3 | [24] |
| Na3.4Zr1.9Mg0.1Si2.2P0.8O12 | 3.6 × 10−3 | [25] |
| Na3Zr2Si2PO12 + 5 wt.% Na2SiO3 | 1.45 × 10−3 | [26] |
| Na3.2Zr2Si2.2P0.8O12 + 0.5 NaF | 3.6 × 10−3 | [27] |
| NZSP-xBi | ρ (g cm−3) | Rg (Ω) | Rgb (Ω) | σb (S cm−1) | σgb (S cm−1) | σtotal (S cm−1) |
|---|---|---|---|---|---|---|
| x = 0 | 2.89 | 95.0 | 146.0 | 7.20 × 10−4 | 4.69 × 10−4 | 2.84 × 10−4 |
| x = 0.1 | 3.07 | 40.0 | 46.0 | 1.95 × 10−3 | 1.69 × 10−3 | 9.04 × 10−4 |
| x = 0.2 | 3.10 | 38.4 | 23.7 | 2.05 × 10−3 | 3.32 × 10−3 | 1.27 × 10−3 |
| x = 0.3 | 3.08 | 42.5 | 44.5 | 2.03 × 10−3 | 1.94 × 10−3 | 9.92 × 10−4 |
| x = 0.4 | 3.02 | 50.3 | 54.7 | 1.45 × 10−3 | 1.33 × 10−3 | 6.92 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cen, S.; Mei, W.; Xing, X.; Zeng, Y.; Mao, Z.; Wang, D.; Chen, J.; Dong, C. Bi2O3-Assisted Sintering of Na3Zr2Si2PO12 Electrolyte for Solid-State Sodium Metal Batteries. Coatings 2022, 12, 1774. https://doi.org/10.3390/coatings12111774
Cen S, Mei W, Xing X, Zeng Y, Mao Z, Wang D, Chen J, Dong C. Bi2O3-Assisted Sintering of Na3Zr2Si2PO12 Electrolyte for Solid-State Sodium Metal Batteries. Coatings. 2022; 12(11):1774. https://doi.org/10.3390/coatings12111774
Chicago/Turabian StyleCen, Shangxu, Wentao Mei, Xiangyuan Xing, Yiwei Zeng, Zhiyong Mao, Dajian Wang, Jingjing Chen, and Chenlong Dong. 2022. "Bi2O3-Assisted Sintering of Na3Zr2Si2PO12 Electrolyte for Solid-State Sodium Metal Batteries" Coatings 12, no. 11: 1774. https://doi.org/10.3390/coatings12111774
APA StyleCen, S., Mei, W., Xing, X., Zeng, Y., Mao, Z., Wang, D., Chen, J., & Dong, C. (2022). Bi2O3-Assisted Sintering of Na3Zr2Si2PO12 Electrolyte for Solid-State Sodium Metal Batteries. Coatings, 12(11), 1774. https://doi.org/10.3390/coatings12111774






