Corrosion Properties of DLC Film in Weak Acid and Alkali Solutions
Abstract
:1. Introduction
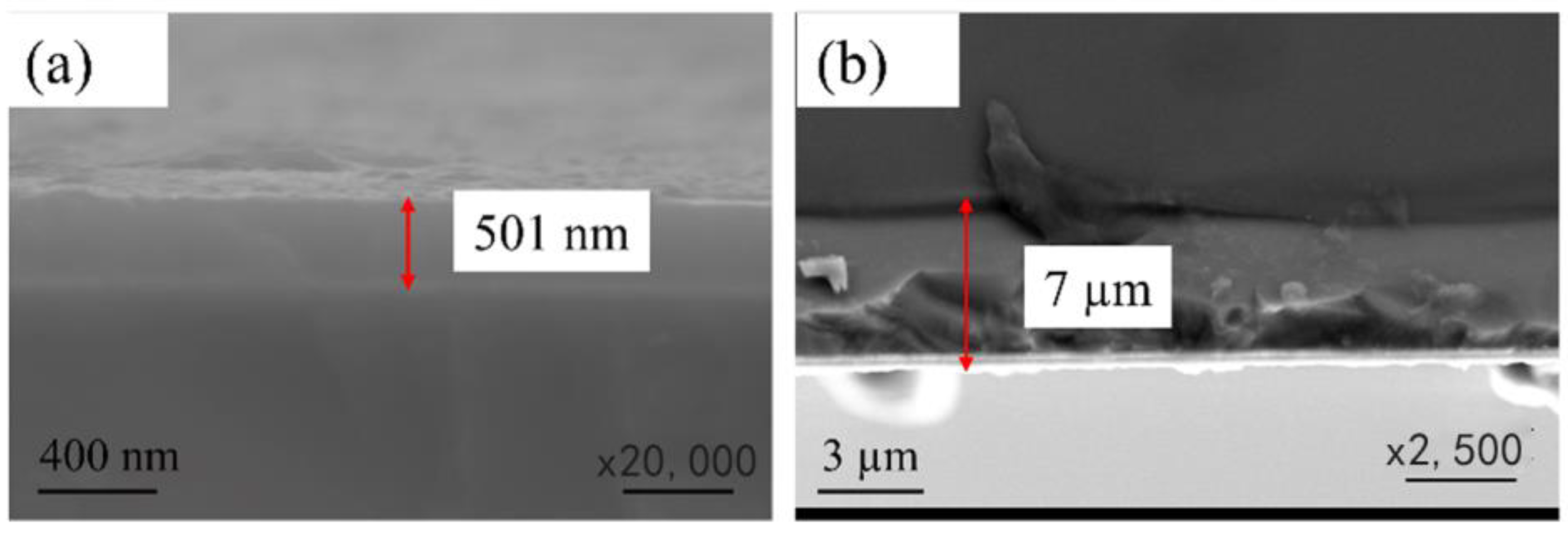
2. Preparation and Characterization
3. Results and Discussion
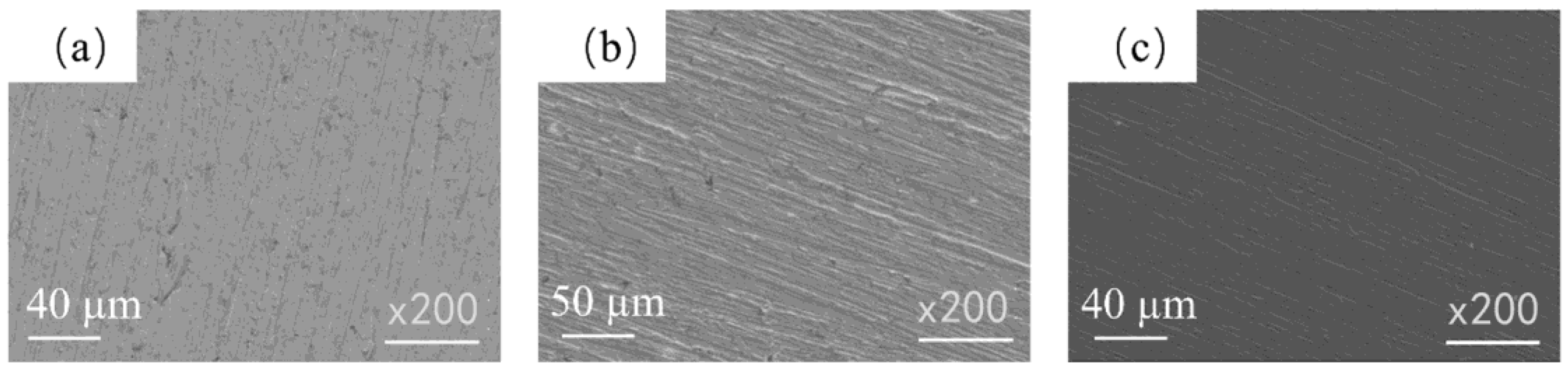
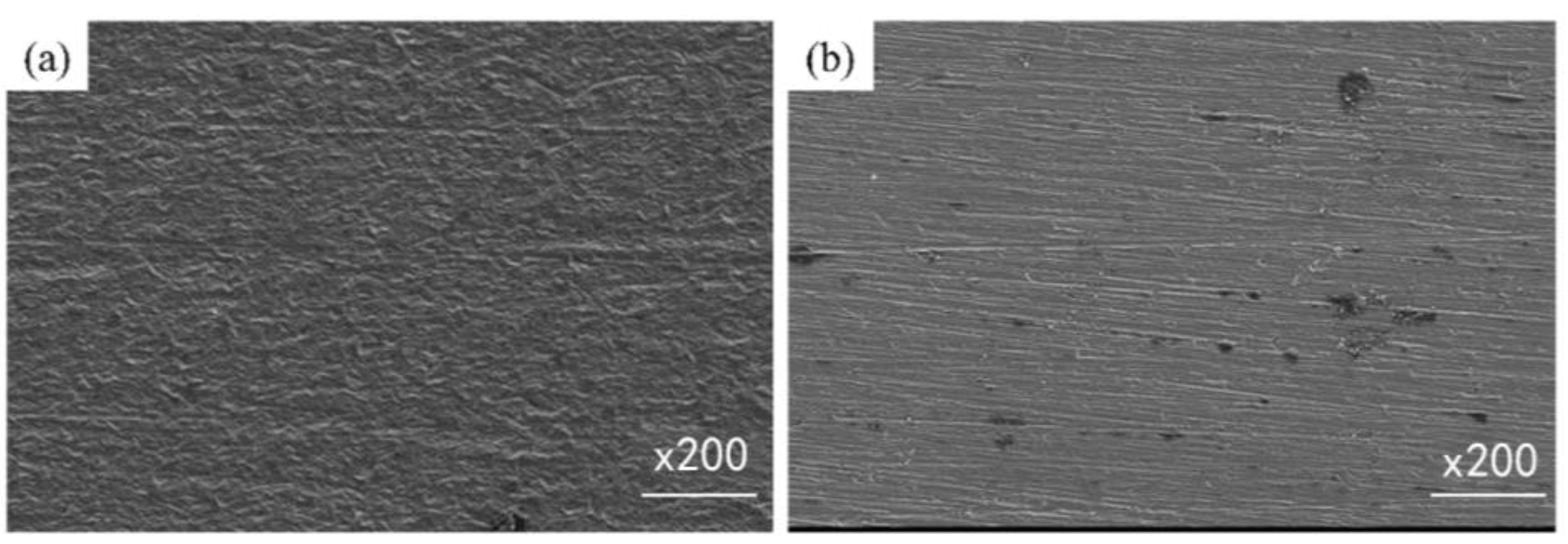
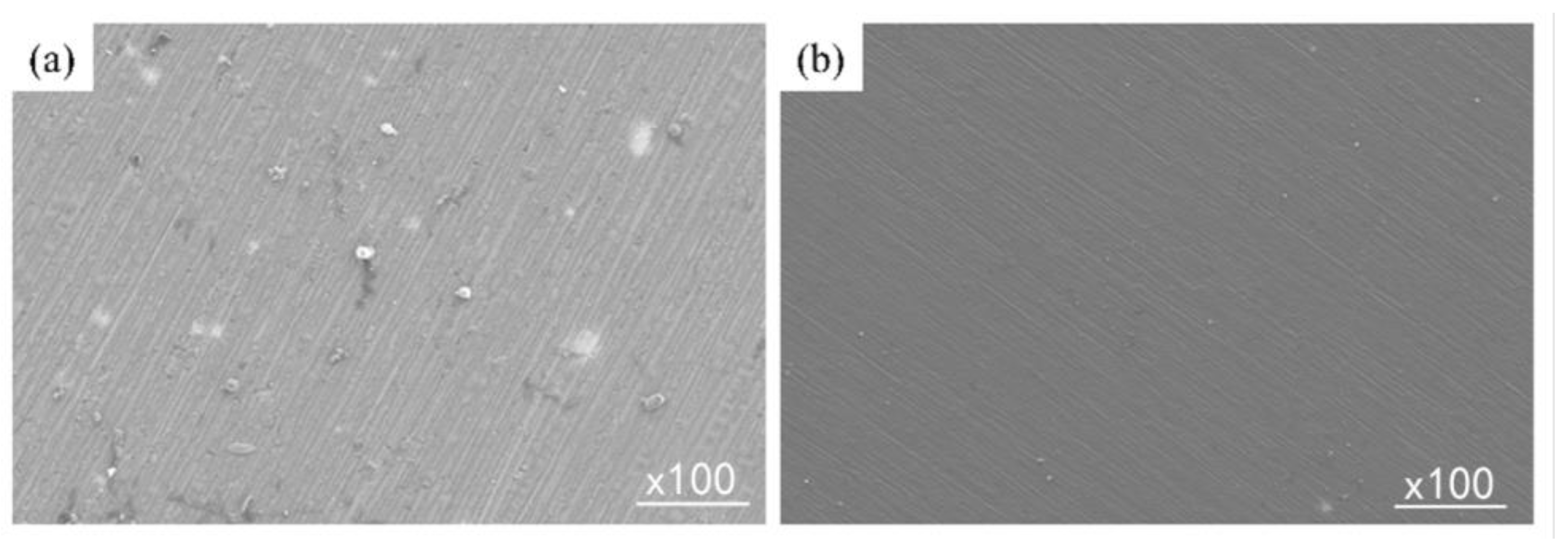
3.1. Surface Morphology
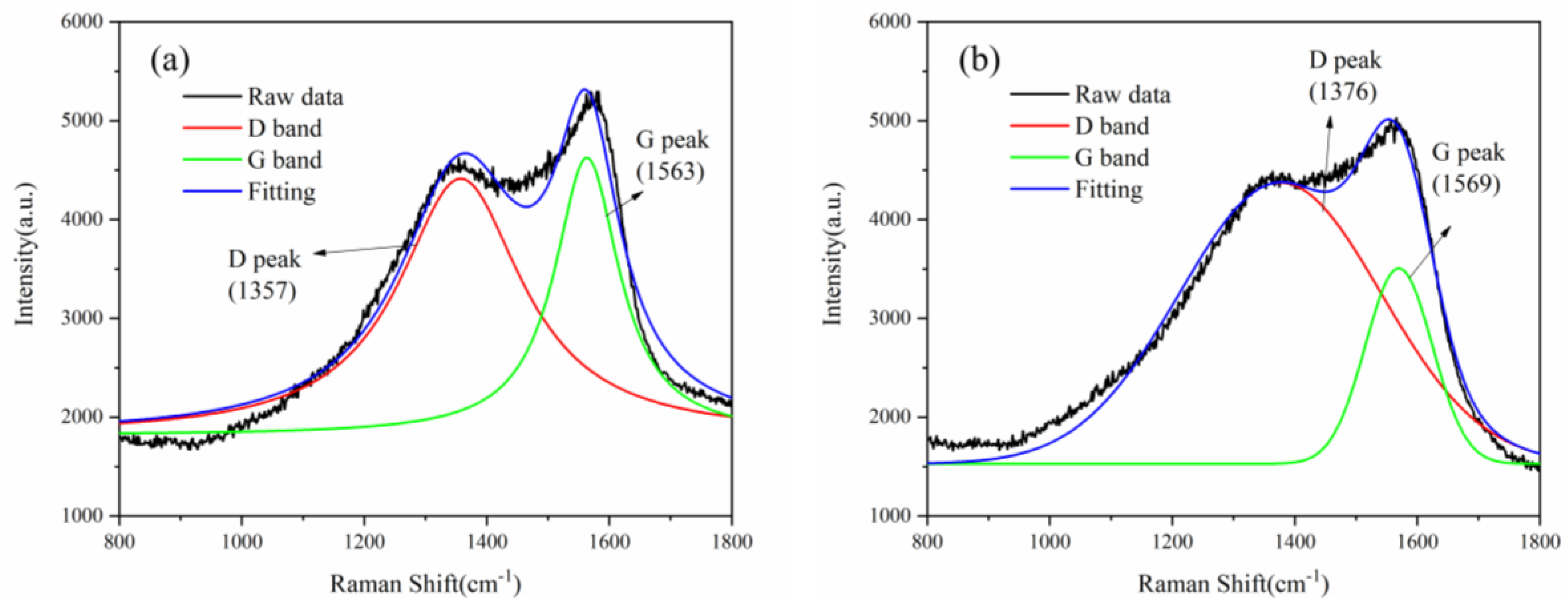
3.2. Raman Spectroscopy
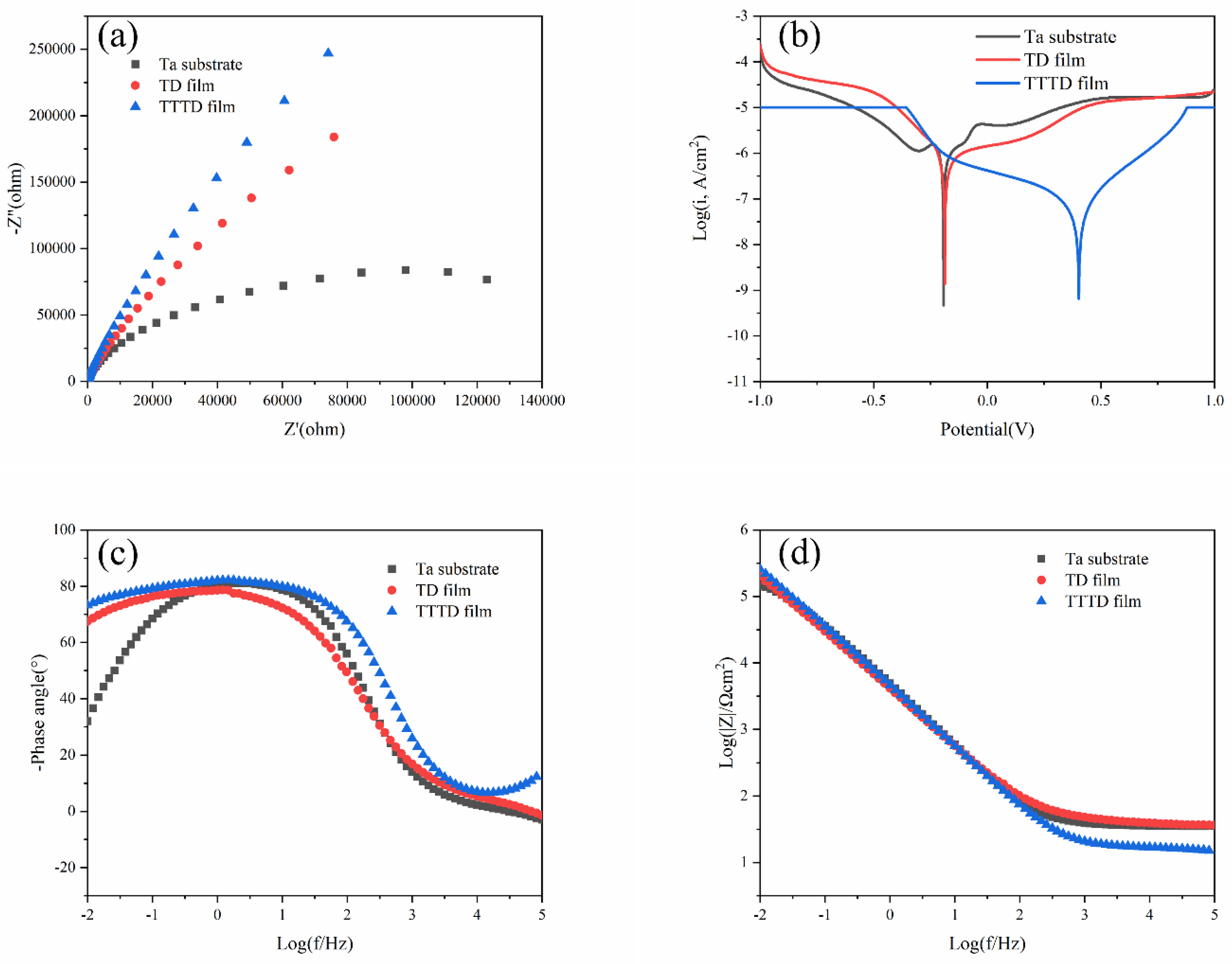
3.3. Corrosion Pattern
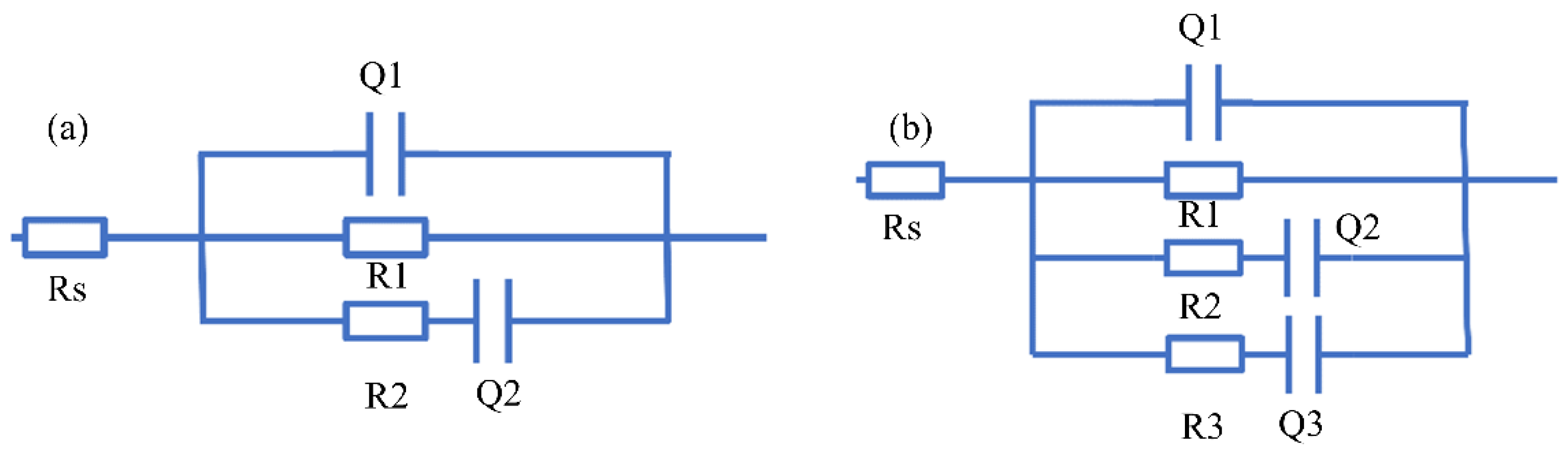
3.4. Corrosion Analysis
3.4.1. Weak Alkaline Environment
3.4.2. Weak Acid Environment
3.5. Contact Angle and Roughness
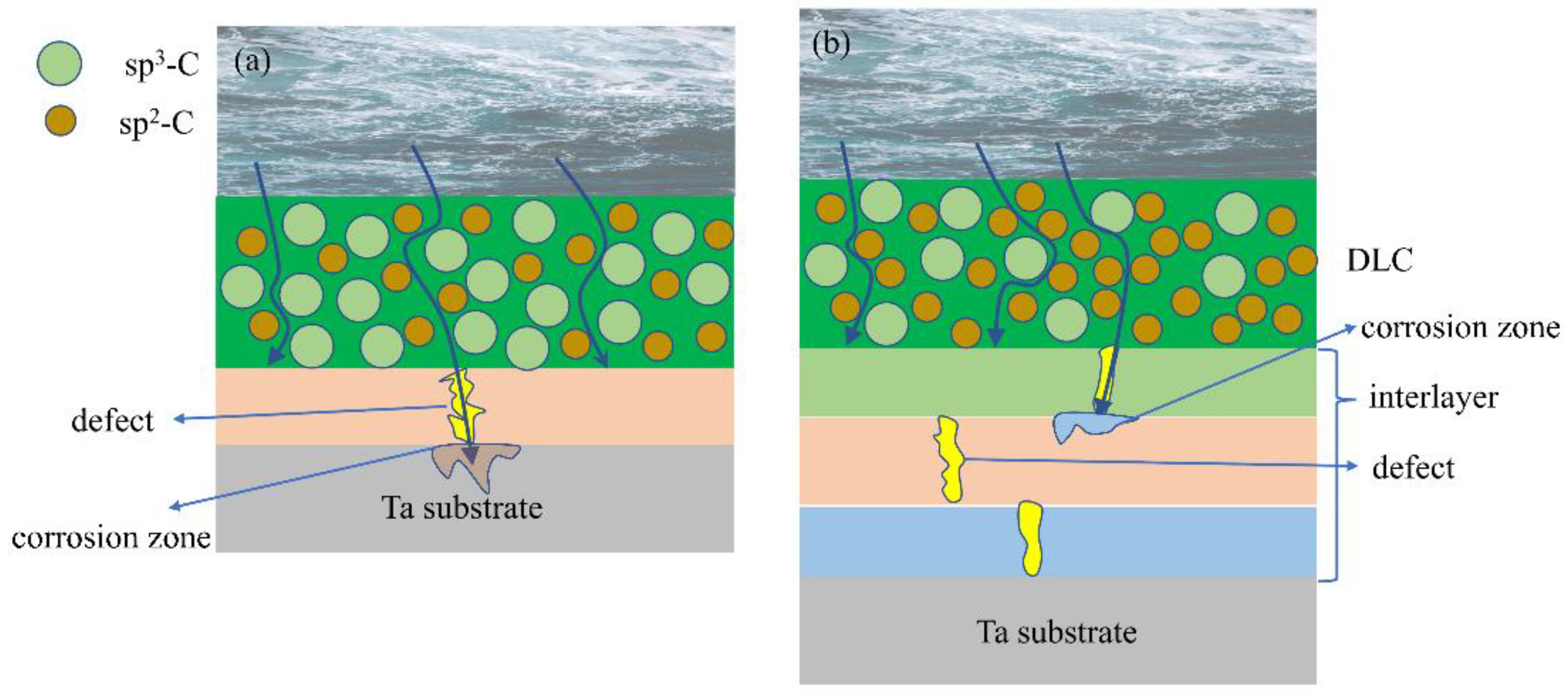
3.6. Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, D.; Wei, J.; Duan, X.; Zhang, R. Analysis of the disease burden of osteoarthritisi in China from global perspective. Mod. Prev. Med. 2022, 49, 2312–2316. [Google Scholar]
- Wu, B.J.; Deng, Q.Y.; Leng, Y.X.; Wang, C.M.; Huang, N. Characterization of adsorption and lubrication of synovial fluid proteins and HA on DLC joint bearings surface. Surf. Coat. Technol. 2017, 320, 320–332. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, G.; Song, G.; Zhang, C.; Liu, Z.; Chen, D. Research progress of bioprosthesis materials for artificial joints. J. Med. Res. 2020, 49, 171–173+178. [Google Scholar]
- Gong, Y.L.; Jing, P.P.; Zhou, Y.J.; Ma, D.L.; Deng, Q.Y.; Shen, R.; Huang, N.; Leng, Y.X. Formation of Rod-shaped Wear Debris and the Graphitization Tendency of Cu-doped Hydrogenated Diamond-like Carbon Films. Diam. Relat. Mater. 2020, 102, 107654. [Google Scholar] [CrossRef]
- Wu, X. Study on Surface Treatment of Metal Materials for Artificial Joints. China Plant Eng. 2021, 17, 102–103. [Google Scholar]
- Yi, X. Effects of Artificial Joint Replacement on the Quality of Life and Pain Degree of Elderly Patients with Femoral Intertrochanteric Fractures. WJNM 2022, 8, 23–26+31. [Google Scholar]
- Shi, X.; Wu, X.; Ma, X.; Liu, Y.; He, P.; Luo, Y. Biomedical applications of tantalum-based materials and the underlying mechanisms. J. Func. Mater. 2019, 50, 12001–12006. [Google Scholar]
- Chen, H.; Song, Z.; Luo, Y. Study on Precision Cutting Process of the Ta-12W Alloy. Mach. Des. Manuf. 2012, 9, 164–166. [Google Scholar]
- Deng, Z.; Yan, X.; Dong, D.; Wang, Y.; Ma, W.; Liu, M. Study on Process for Medical Pure Tantalum Parts Prepared by Selective Laser Melting (SLM) and Mechanical Properties. Hot Work. Tech 2021, 50, 76–81. [Google Scholar]
- Zhang, X.; Xu, R.; Wu, G.; Bai, X. Different biomaterial prostheses in joint replacement. CJTER 2012, 16, 8869–8874. [Google Scholar]
- Shi, W.; Wang, Y. Handering Treatment on Tantalum Surface. Heat Treat. Met. 2000, 5, 27–29. [Google Scholar]
- Yao, J.; Wang, Y. The Research of Crystalline Diamond Films Depositing on Ta Spinneret. New Tec. New Pro 2007, 2, 74–76+74. [Google Scholar]
- Pornwasa, W.; Nattapo, L.P.; Kanjana, T.; Chanan, E. Improvement of corrosion resistance and biocompatibility of 316L stainless steel for joint replacement application by Ti-doped and Ti-interlayered DLC films. Surf. Coat. Technol. 2021, 425, 127734. [Google Scholar]
- Nakatani, T.; Takeuchi, H.; Wada, A.; Yamashita, S. Investigation of Anti-Corrosive Performance of a Si-Doped DLC-Coated Magnesium Alloy Stent Deposited by RF-Plasma CVD. J. Photopolym. Sci. Tec. 2019, 32, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Sara, K.; Eiman, A.; Mohammad, M.; Saeb, M.; Vahabi, H.; Kokanyan, N.; Laheurte, P. Magnetron-sputtered copper/diamond-like carbon composite thin films with super anti-corrosion properties. Surf. Coat. Technol. 2018, 333, 148–157. [Google Scholar]
- Shang, L.; Gou, C.; Li, W.; He, D.; Wang, S. Effect of microstructure and mechanical properties on the tribological and electrochemical performances of Si/DLC films under HCl corrosive environment. Diam. Relat. Mater. 2021, 116, 108385. [Google Scholar] [CrossRef]
- Maxime, C.; Stéphane, T.; Yan, B.; Tatoulian, M.; Pireaux, J.; Mantovani, D. Controlled distribution and clustering of silver in Ag-DLC nanocomposite coatings using a hybrid plasma approach. ACS Appl. Mater. Inter. 2016, 8, 21020–21027. [Google Scholar]
- Dorota, L.; Błażej, B.; Witold, K.; Grabarczyk, J.; Svobodova, L.; Szatkiewicz, T.; Mitura, K. The DLC Coating on 316L Stainless Steel Stochastic Voronoi Tessellation Structures Obtained by Binder Jetting Additive Manufacturing for Potential Biomedical Applications. Coatings 2022, 12, 1373. [Google Scholar]
- Guo, Z.; Wang, R.; Yang, H.; Chen, J.; Lin, R.; Wei, S.; Li, B. Preparation and Performance Study of Si-DLC Based on Ion Deposition of Different Multiple Gradient Transition Layers. Coatings 2022, 12, 882. [Google Scholar] [CrossRef]
- Cui, M.; Pu, J.; Zhang, G.; Wang, L.; Xue, Q. The corrosion behaviors of multilayer diamond-like carbon coatings: Influence of deposition periods and corrosive medium. RSC Adv. 2016, 6, 28570. [Google Scholar] [CrossRef]
- Cao, X.; Shang, L.; Zhang, G.; Ding, Q. Simultaneously Improving the Corrosion Resistance and Wear Resistance of Internal Surface of Aluminum Pipe by Using Multilayer Diamond-Like Carbon-Si Coatings. J. Mater. Eng. Perform. 2022, 22, 6678–6685. [Google Scholar] [CrossRef]
- Iijima, Y.; Harigai, T.; Isono, R.; Degai, S.; Tanimoto, T.; Suda, Y.; Takikawa, H.; Yasui, H.; Kaneko, S.; Kunitsugu, S.; et al. Preparation of multi-layer film consisting of hydrogen-free DLC and nitrogen-containing DLC for conductive hard coating. AIP Conf. Proc. 2018, 1929, 20024. [Google Scholar]
- Dai, W.; Gao, X.; Liu, J.; Kwon, S.-H.; Wang, Q. Compositionally modulated multilayer diamond-like carbon coatings with AlTiSi multi-doping by reactive high power impulse magnetron sputtering. Appl. Surf. Sci. 2017, 425, 855–861. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Y.; Wang, J.; Xue, Q. Adaptive capacities of chromium doped graphite-like carbon films in aggressive solutions with variable pH. Tribol. Int. 2016, 96, 307–316. [Google Scholar] [CrossRef]
- Ding, J.C.; Mei, H.; Jeong, S.; Zheng, J.; Wang, Q.M.; Kim, K.H. Effect of bias voltage on the microstructure and properties of Nb-DLC films prepared by a hybrid sputtering system. J. Alloy. Compd. 2021, 861, 158505. [Google Scholar] [CrossRef]
- Catena, A.; Guo, Q.; Kunze, M.R.; Agnello, S.; Gelardi, F.M.; Wehner, S.; Fischer, C.B. Morphological and Chemical Evolution of Gradually Deposited Diamond-Like Carbon Films on Polyethylene Terephthalate: From Subplantation Processes to Structural Reorganization by Intrinsic Stress Release Phenomena. ACS Appl. Mater. Inter. 2016, 8, 10636–10646. [Google Scholar] [CrossRef] [Green Version]
- Seyed, E.M.; Nastaran, N.; Mohabbat, A.; Shammakhi, H. Corrosion performance and tribological behavior of diamond-like carbon based coating applied on Ni−Al−bronze alloy. T Nonferr. Metal. Soc. 2021, 31, 499–511. [Google Scholar]
- Zhang, L.; Pu, J.; Wang, L.; Xue, Q. Frictional dependence of graphene and carbon nanotube in diamond-like carbon/ionic liquids hybrid films in vacuum. Carbon 2014, 80, 734–745. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, S.; Zhuang, X.; Wang, Y.; Ma, L. Wettability and Corrosion Resistance of Iron-containing Diamond-like Film. Surf. Tech. 2017, 46, 169–174. [Google Scholar]
- Li, X.; Jiang, L.; Li, L.; Yan, Y. Influence of Acid Etching on Wettability of Ion-exchanged Aluminosilicate Float Glass. J. Aeronaut. Mater. 2016, 36, 54–60. [Google Scholar]
- Simič, R.; Kalin, M.; Kovač, J.; Jakša, G. Adsorption of alcohols and fatty acids onto hydrogenated (a-C:H) DLC coatings. Appl. Surf. Sci. 2016, 363, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Hu, C.; Chen, L. Structure, mechanical properties and thermal stability of CrAlNbN/TiN multilayers. Vacuum 2021, 188, 110182. [Google Scholar] [CrossRef]
- Sui, X.; Xu, R.; Liu, J.; Zhang, S.; Wu, Y.; Yang, J.; Hao, J. Tailoring the Tribocorrosion and Antifouling Performance of (Cr, Cu)-GLC Coatings for Marine Application. ACS Appl. Mater. Inter. 2018, 10, 36531–36539. [Google Scholar] [CrossRef] [PubMed]

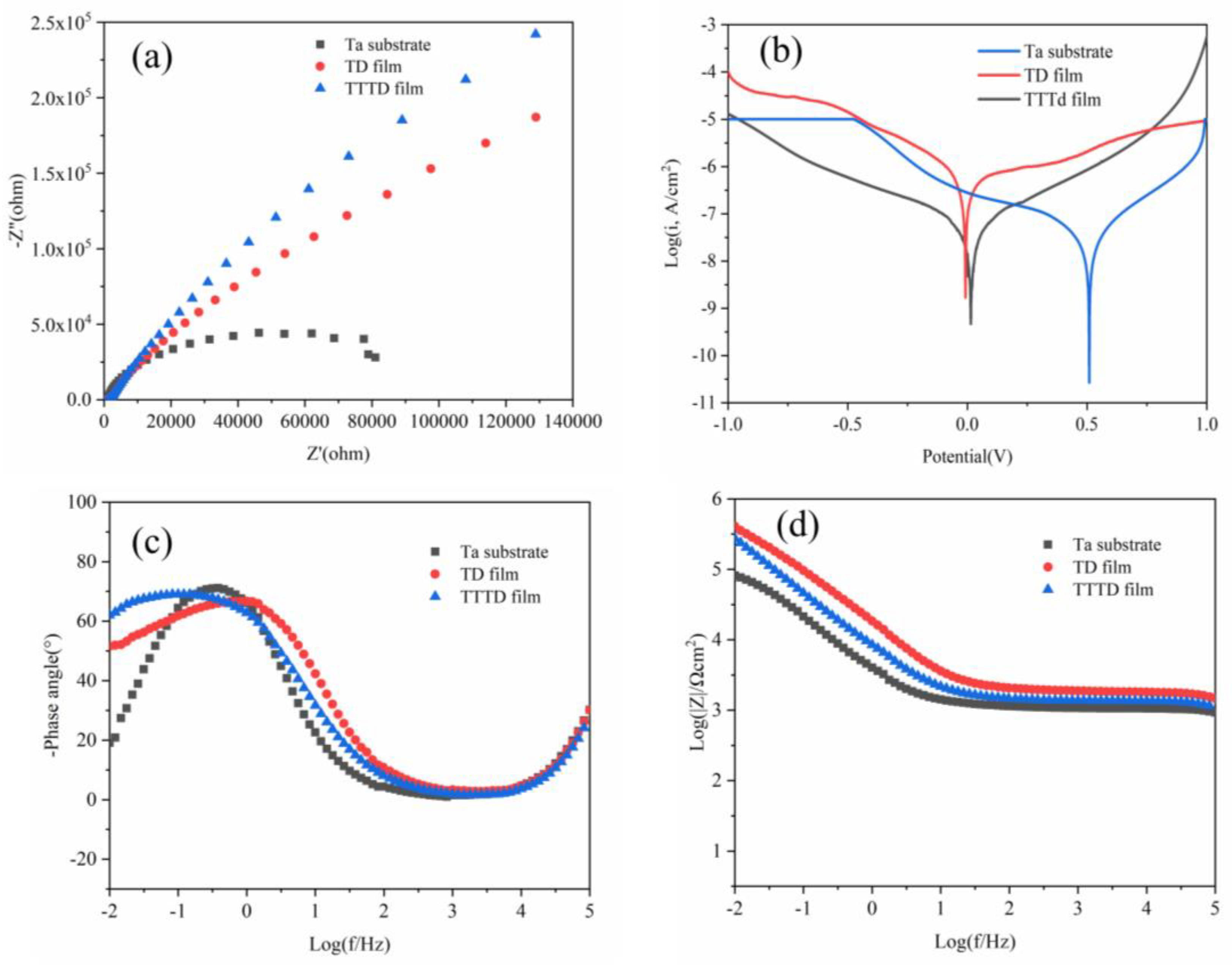



| Sample | Icorr (A/cm2) | Ecorr (V) | Rp (Ω cm2) |
|---|---|---|---|
| Ta | 2.74 × 10−6 | −0.193 | 2.2 × 104 |
| TD film | 1.552 × 10−6 | −0.187 | 4.0 × 104 |
| TTTD film | 5.586 × 10−6 | 0.403 | 7.38 × 105 |
| Sample | Rs (Ω cm2) | R1 (Ω cm2) | Q1 (F cm−2) | R2 (Ω cm2) | Q2 (F cm−2) | R3 (Ω cm2) | Q3 (F cm−2) |
|---|---|---|---|---|---|---|---|
| TD film | 21.65 | 6.065 × 105 | 2.718 × 10−5 | 154 | 5.478 × 10−5 | - | - |
| TTTD film | 3.163 × 10−2 | 2.408 × 106 | 2.328 × 10−8 | 89.53 | 5.413 × 10−7 | 18.82 | 3.725 × 10−5 |
| Sample | Icorr (A/cm2) | Ecorr (V) | Rp (Ω cm2) |
|---|---|---|---|
| Ta | 3.049 × 10−8 | 0.014 | 1.35 × 106 |
| TD film | 6.025 × 10−7 | −0.008 | 9.6 × 104 |
| TTTD film | 2.389 × 10−8 | 0.509 | 1.7 × 106 |
| Sample | Rs (Ω cm2) | R1 (Ω cm2) | Q1 (F cm−2) | R2 (Ω cm2) | Q2 (F cm−2) | R3 (Ω cm2) | Q3 (F cm−2) |
|---|---|---|---|---|---|---|---|
| TD film | 241.5 | 829.4 | 1.199 × 10−9 | 104.5 | 6.372 × 10−5 | 141.9 | 1.712 × 10−5 |
| TTTD film | 346.5 | 1013 | 1.259 × 10−9 | 2.044 × 106 | 3.083 × 10−5 | 54.93 | 2.914 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Zhang, S.; Huang, T.; Wu, S.; Ma, X.; Guo, F. Corrosion Properties of DLC Film in Weak Acid and Alkali Solutions. Coatings 2022, 12, 1776. https://doi.org/10.3390/coatings12111776
Guo D, Zhang S, Huang T, Wu S, Ma X, Guo F. Corrosion Properties of DLC Film in Weak Acid and Alkali Solutions. Coatings. 2022; 12(11):1776. https://doi.org/10.3390/coatings12111776
Chicago/Turabian StyleGuo, Dong, Shuling Zhang, Tenglong Huang, Shuaizheng Wu, Xinghua Ma, and Feng Guo. 2022. "Corrosion Properties of DLC Film in Weak Acid and Alkali Solutions" Coatings 12, no. 11: 1776. https://doi.org/10.3390/coatings12111776





