Domesticating a Halotolerant Bacterium of Vibrio sp. LY1024 with Heterotrophic Nitrification–Aerobic Denitrification Property for Efficient Nitrogen Removal in Mariculture Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Mediums
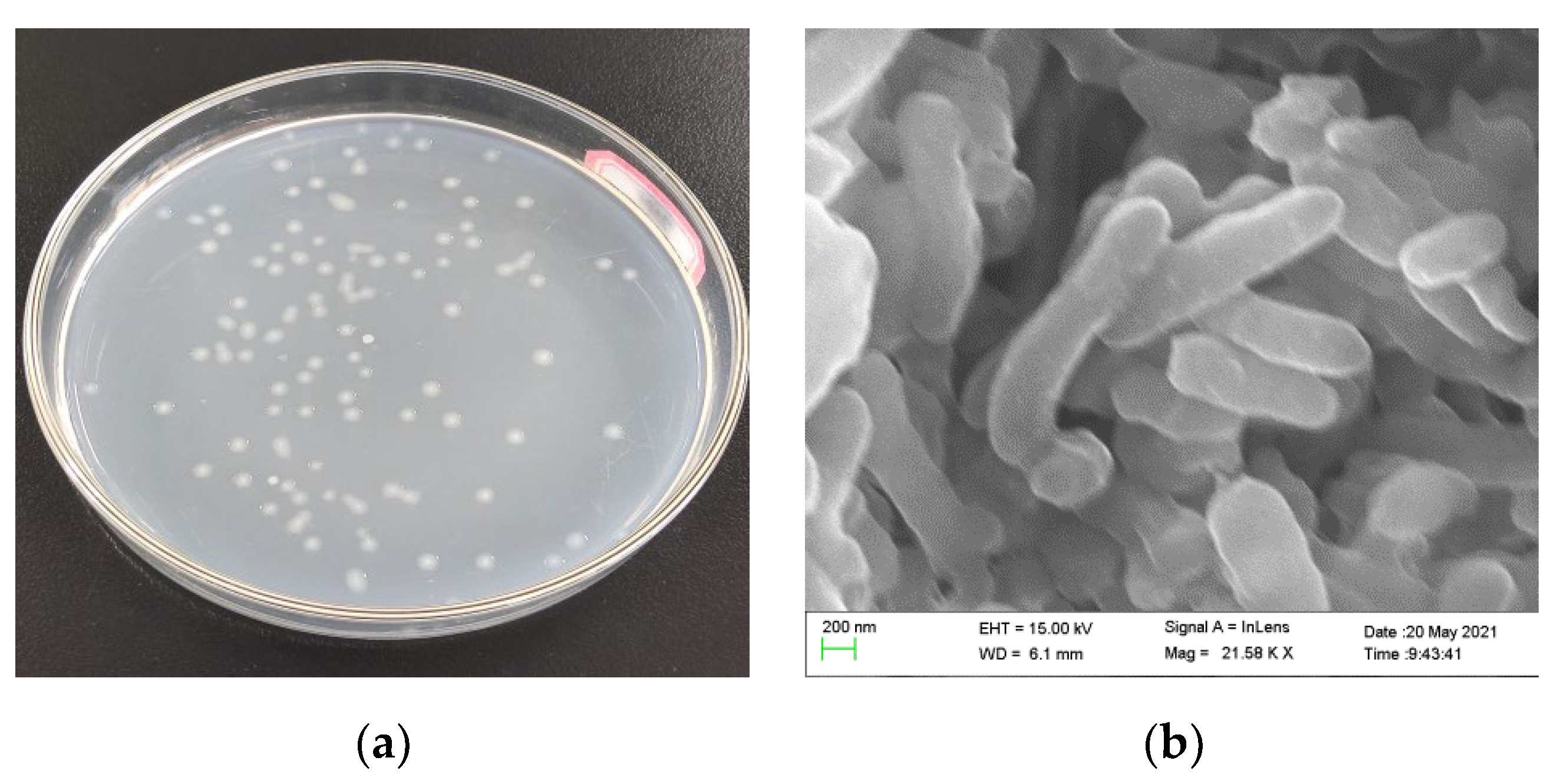
2.2. Domestication, Isolation, and Identification of Halotolerant HN-AD Bacteria
2.3. Identification and Phylogenetic Analysis of Isolates
2.4. Investigating the Influence of Different Operation Parameters on Nitrogen Removal over Strain LY1024
2.5. Treatment of Simulated Mariculture Wastewater by Strain LY1024
3. Results and Discussion
3.1. Identification of Isolates
3.2. Heterotrophic Nitrification and Aerobic Denitrification Ability of Strain LY1024 at High Salinity
3.3. Influence of C/N Ratio, Initial pH Value, Temperature, DO, and Salinity on Nitrogen Removal over Strain LY1024
3.4. Aerobic Nitrogen Removal Mechanism and Pathway by Strain LY1024
3.5. Treatment of Stimulated Mariculture Wastewater by Vibrio sp. LY1024
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piedrahita, R.H. Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation. Aquaculture 2003, 226, 35–44. [Google Scholar] [CrossRef]
- Kang, P.; Xu, S. The impact of mariculture on nutrient dynamics and identification of the nitrate sources in coastal waters. Environ. Sci. Pollut. Res. Int. 2016, 23, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.R.; Asghari, M.; Mahmoodi, N.M. Chitosan-wrapped multiwalled carbon nanotube as filler within PEBA thin film nanocomposite (TFN) membrane to improve dye removal. Carbohydr. Polym. 2020, 237, 116128. [Google Scholar] [CrossRef]
- Hosseini, F.; Sadighian, S.; Monfared, H.H.; Mahmoodi, N.M. Dye removal and kinetics of adsorption by magnetic chitosan nanoparticles. Desalin. Water Treat. 2016, 57, 24378–24386. [Google Scholar] [CrossRef]
- Healy, M.G.; Ibrahim, T.G.; Lanigan, G.J.; Serrenho, A.J.; Fenton, O. Nitrate removal rate, efficiency and pollution swapping potential of different organic media in laboratory denitrification bioreactors. Ecol. Eng. 2011, 40, 198–209. [Google Scholar] [CrossRef]
- Camargo, J.; Alonso, A. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Oveisi, M.; Bakhtiari, M.; Hayati, B.; Shekarchi, A.A.; Bagheri, A.; Rahimi, S. Environmentally friendly ultrasound-assisted synthesis of magnetic zeolitic imidazolate framework—Graphene oxide nanocomposites and pollutant removal from water. J. Mol. Liq. 2019, 282, 115–130. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Mokhtari-Shourijeh, Z. Preparation of PVA-chitosan blend nanofiber and its dye removal ability from colored wastewater. Fibers Polym. 2015, 16, 1861–1869. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Liu, Y.; Gao, X.Y.; Ai, G.M.; Miao, L.L.; Liu, Z.P. Characterization of a marine origin aerobic nitrifying-denitrifying bacterium. J. Biosci. Bioeng. 2012, 114, 33–37. [Google Scholar] [CrossRef]
- Uygur, A. Specific nutrient removal rates in saline wastewater treatment using sequencing batch reactor. Process Biochem. 2006, 41, 61–66. [Google Scholar] [CrossRef]
- Kong, Q.X.; Wang, X.W.; Jin, M.; Shen, Z.Q.; Li, J.W. Development and application of a novel and effective screening method for aerobic denitrifying bacteria. FEMS Microbiol. Lett. 2010, 260, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Luo, P.; Zuo, H.; Chen, J.; Chem, M.; Wang, W. Vibrio zhanjiangensis sp. nov., isolated from sea water of shrimp farming pond. Antonie Van Leeuwenhoek 2010, 101, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Li, Y. Nitrogen removal characteristics of heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis C16. Chin. J. Chem. Eng. 2015, 23, 827–834. [Google Scholar] [CrossRef]
- Chen, P.; Ji, L.; Li, Q.X. Simultaneous heterotrophic nitrification and aerobic denitrification by bacterium Rhodococcus sp. CPZ24. Bioresour. Technol. 2012, 116, 266–270. [Google Scholar] [CrossRef]
- Zhao, B.; Tian, M.; An, Q. Characteristics of a heterotrophic nitrogen removal bacterium and its potential application on treatment of ammonium-rich wastewater. Bioresour. Technol. 2016, 226, 46–54. [Google Scholar] [CrossRef]
- Richardson, D.J.; Wehrfritz, J.M.; Keech, A.; Crossman, L.C.; Spiro, S. The diversity of redox proteins involved in bacterial heterotrophic nitrification and aerobic denitrification. Biochem. Soc. Trans. 1998, 26, 401–408. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, H.; Wu, D.; Li, C.; Wang, R.; Tanaka, S. Physiological and molecular biological characteristics of heterotrophic ammonia oxidation by Bacillus sp. LY. World J. Microbiol. Biotechnol. 2010, 26, 1605–1612. [Google Scholar] [CrossRef]
- Yang, X.P.; Wang, S.M.; Zhang, D.W.; Zhou, L.X. Isolation and nitrogen removal characteristics of an aerobic heterotrophic nitrifying-denitrifying bacterium, Bacillus subtilis A1. Bioresour. Technol. 2011, 102, 854–862. [Google Scholar] [CrossRef]
- Wang, T.; Wei, H.; Hu, Z.; Chai, H.; Zhao, H. Isolation and identification of a heterotrophic nitrifying and aerobic denitrifying strain and its denitrification characteristics. Acta Scien. Circum. 2017, 37, 945–953. [Google Scholar]
- Chen, M.; Wang, W.; Feng, Y.; Zhu, X.; Zhou, H.; Tan, Z. Impact resistance of different factors on ammonia removal by heterotrophic nitrification-aerobic denitrification bacterium Aeromonas sp. HN-02. Bioresour. Technol. 2014, 167, 456–461. [Google Scholar] [CrossRef]
- Fei, H.; Pan, L.; Na, L.; Tang, X. Characterization of novel Bacillus strain N31 from mariculture water capable of halophilic heterotrophic nitrification-aerobic denitrification. J. Biosci. Bioeng. 2017, 124, 564–571. [Google Scholar]
- Lei, Y.; Wang, Y.; Liu, H.; Xi, C.; Song, L. A novel heterotrophic nitrifying and aerobic denitrifying bacterium, Zobellella taiwanensis DN-7, can remove high-strength ammonium. Appl. Microbiol. Biotechnol. 2016, 100, 4219–4229. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.R.; Wang, Y.; Chen, H.; Lyu, Y.K. Ammonium removal characteristics of an acid-resistant bacterium Acinetobacter sp. JR1 from pharmaceutical wastewater capable of heterotrophic nitrification-aerobic denitrification. Bioresour. Technol. 2019, 274, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ou, L.; Pan, F.; Wang, Y.; Fan, G.; Liu, G.; Wang, W. Isolation of a Novel Heterotrophic Nitrification–Aerobic Denitrification Bacterium Serratia marcescens CL1502 from Deep-Sea Sediment. Environ. Eng. Sci. 2017, 34, 453–459. [Google Scholar] [CrossRef]
- Uygur, A.; Karg, F. Salt inhibition on biological nutrient removal from saline wastewater in a sequencing batch reactor. Enzym. Microb. Technol. 2004, 34, 313–318. [Google Scholar] [CrossRef]
- Ren, Y.X.; Lei, Y.; Liang, X. The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Bioresour. Technol. 2014, 171, 1–9. [Google Scholar] [CrossRef]
- Teng, W.; Dang, Q.; Liu, C.; Yan, J.; Zhang, Y. Heterotrophic nitrogen removal by a newly-isolated alkalitolerant microorganism, Serratia marcescens W5. Bioresour. Technol. 2016, 211, 618–627. [Google Scholar]
- Xiao, J.B.; Jiang, H.X.; Chu, S.Y. Denitrification characteristics of an aerobic denitrifying bacterium Defluoibacter lusatiensis str. DN7 using different sources of nitrogen. Acta Ecol. Sin. 2012, 32, 6463–6470. [Google Scholar] [CrossRef][Green Version]
- Qian, C.; Ni, J. Ammonium removal by Agrobacterium sp. LAD9 capable of heterotrophic nitrification-aerobic denitrification. J. Biosci. Bioeng. 2012, 113, 619–623. [Google Scholar]
- Qian, C.; Ni, J. Heterotrophic nitrification-aerobic denitrification by novel isolated bacteria. J. Ind. Microbiol. Biotechnol. 2011, 38, 1305–1310. [Google Scholar]
- Sun, Q.H.; Yu, D.S.; Zhang, P.Y. Identification and nitrogen removal characteristics of a heterotrophic nitrification-aerobic denitrification strain isolated from marine environment. Environ. Sci. 2016, 37, 1089–1097. [Google Scholar]
- Zhao, B.; Yi, L.H.; Hughes, J.; Xiao, F.Z. Heterotrophic nitrogen removal by a newly isolated Acinetobacter calcoaceticus HNR. Bioresour. Technol. 2010, 101, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Liu, Y.; Shi, F.Y.; Cheng, B.; Song, B.B. Study on total ammonia nitrogen removal performance of marine biofilters and nitrification kinetics. Chin. J. Environ. Eng. 2010, 4, 1697–1703. [Google Scholar]
- Hu, B.B.; Zhang, Y.C.; Wan, J.F. Effect of nitrate nitrogen concentration on denitrification efficiency of short cut denitrification. Appl. Chem. Ind. 2021, 50, 1468–1471+1477. [Google Scholar]











| Description (16S Ribosomal RNA Gene Partial Sequence) | Per. Ident (%) |
|---|---|
| Vibrio sp. A5-15 | 99.66 |
| Vibrio sp. B2-5-1 | 99.59 |
| Vibrio sp. A5-5 | 99.52 |
| Vibrio sp. B2-23 | 99.52 |
| Vibrio sp. C4-5 | 99.52 |
| Initial pH Value | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|
| pH value after 24 h | 5.12 | 6.80 | 7.75 | 8.67 | 9.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Fu, Y.; Wang, S.; Ye, F.; Cui, E.; Sun, Q. Domesticating a Halotolerant Bacterium of Vibrio sp. LY1024 with Heterotrophic Nitrification–Aerobic Denitrification Property for Efficient Nitrogen Removal in Mariculture Wastewater Treatment. Coatings 2022, 12, 1786. https://doi.org/10.3390/coatings12111786
Wang L, Fu Y, Wang S, Ye F, Cui E, Sun Q. Domesticating a Halotolerant Bacterium of Vibrio sp. LY1024 with Heterotrophic Nitrification–Aerobic Denitrification Property for Efficient Nitrogen Removal in Mariculture Wastewater Treatment. Coatings. 2022; 12(11):1786. https://doi.org/10.3390/coatings12111786
Chicago/Turabian StyleWang, Lu, Yutong Fu, Shuaijie Wang, Fei Ye, Enming Cui, and Qina Sun. 2022. "Domesticating a Halotolerant Bacterium of Vibrio sp. LY1024 with Heterotrophic Nitrification–Aerobic Denitrification Property for Efficient Nitrogen Removal in Mariculture Wastewater Treatment" Coatings 12, no. 11: 1786. https://doi.org/10.3390/coatings12111786
APA StyleWang, L., Fu, Y., Wang, S., Ye, F., Cui, E., & Sun, Q. (2022). Domesticating a Halotolerant Bacterium of Vibrio sp. LY1024 with Heterotrophic Nitrification–Aerobic Denitrification Property for Efficient Nitrogen Removal in Mariculture Wastewater Treatment. Coatings, 12(11), 1786. https://doi.org/10.3390/coatings12111786







