Chemical Agglomeration to Enhance Blast Furnace Dust Capture Efficiency in Wet Electrostatic Precipitators
Abstract
1. Introduction
2. Materials and Methods
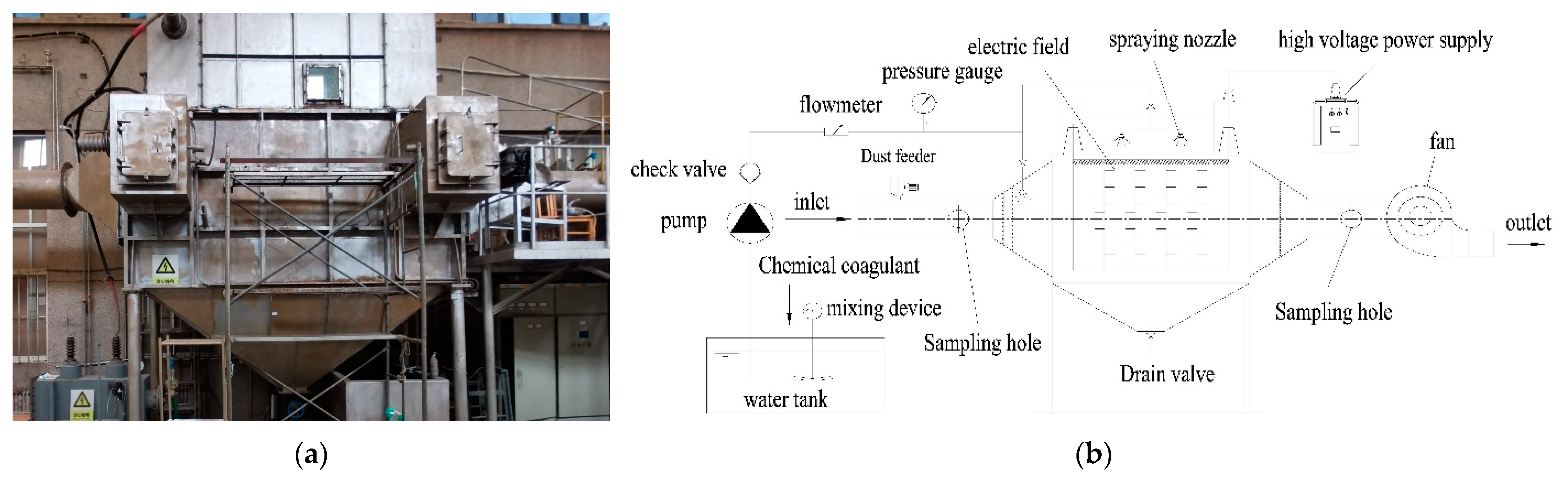
2.1. Experimental Setup
2.1.1. Wet Electrostatic Precipitators System
2.1.2. Sampling System
2.1.3. Particle Size Distribution Measurement Device
2.1.4. Representation
2.2. Experimental Materials
3. Analysis and Discussion
3.1. Blast Furnace Dust Particle Size Distribution and Morphology
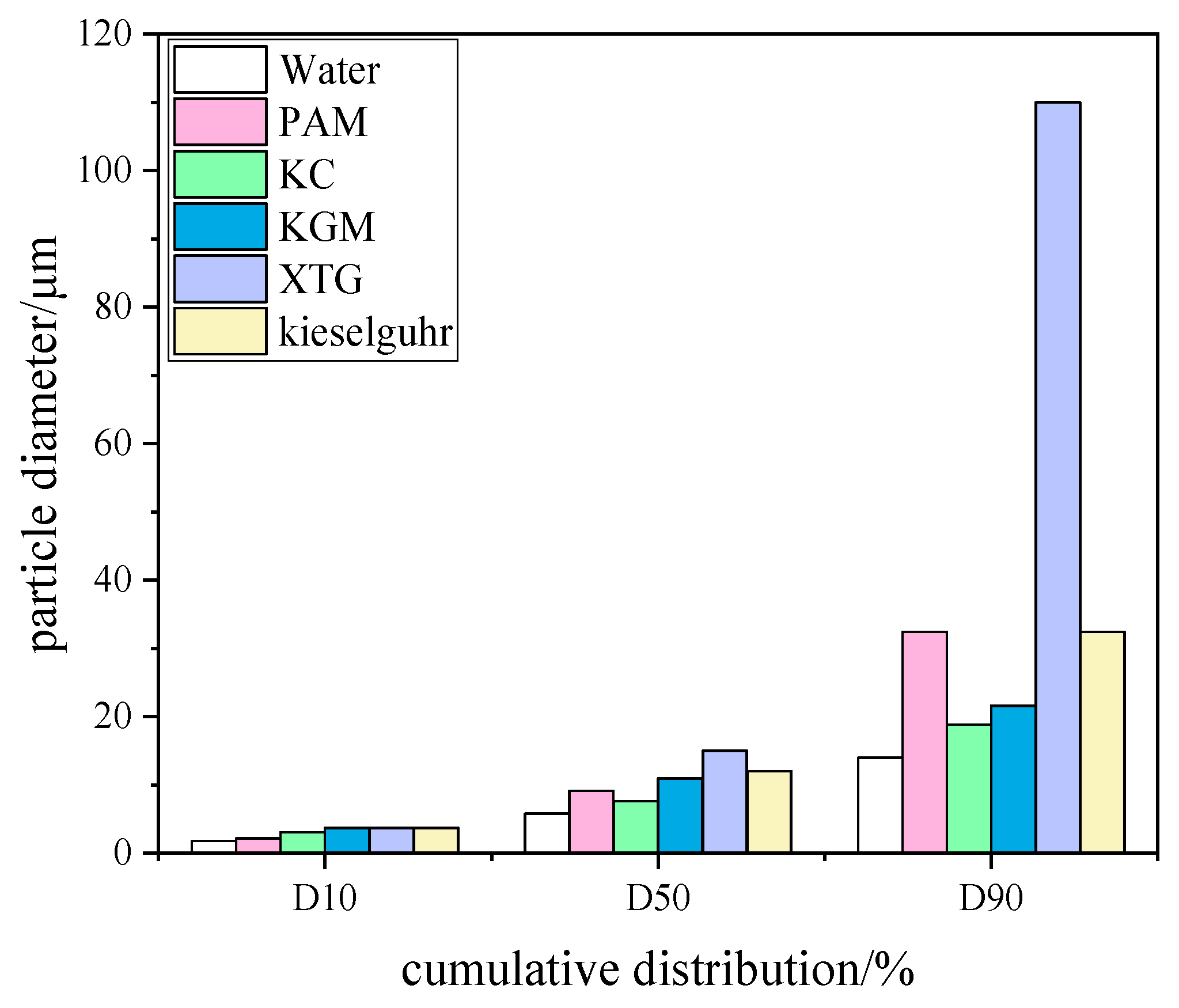
3.1.1. Particle Size Distribution Analysis
3.1.2. Morphological Analysis
3.2. Influence of Chemical Coagulant on the Effect of Blast Furnace Dust Capture
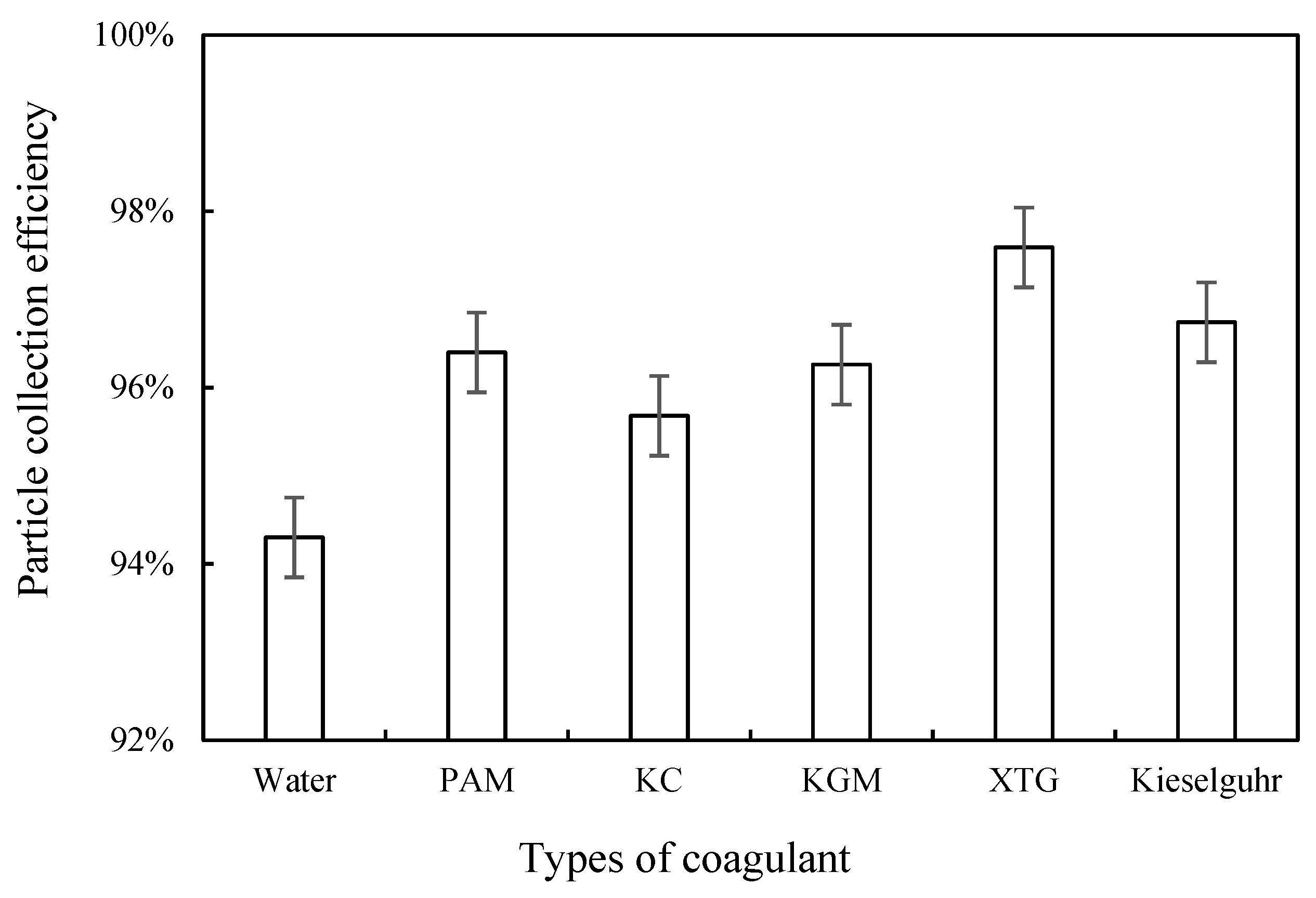
3.2.1. Effect of Coagulant Type
Coagulation Effect
Dust Removal Efficiency
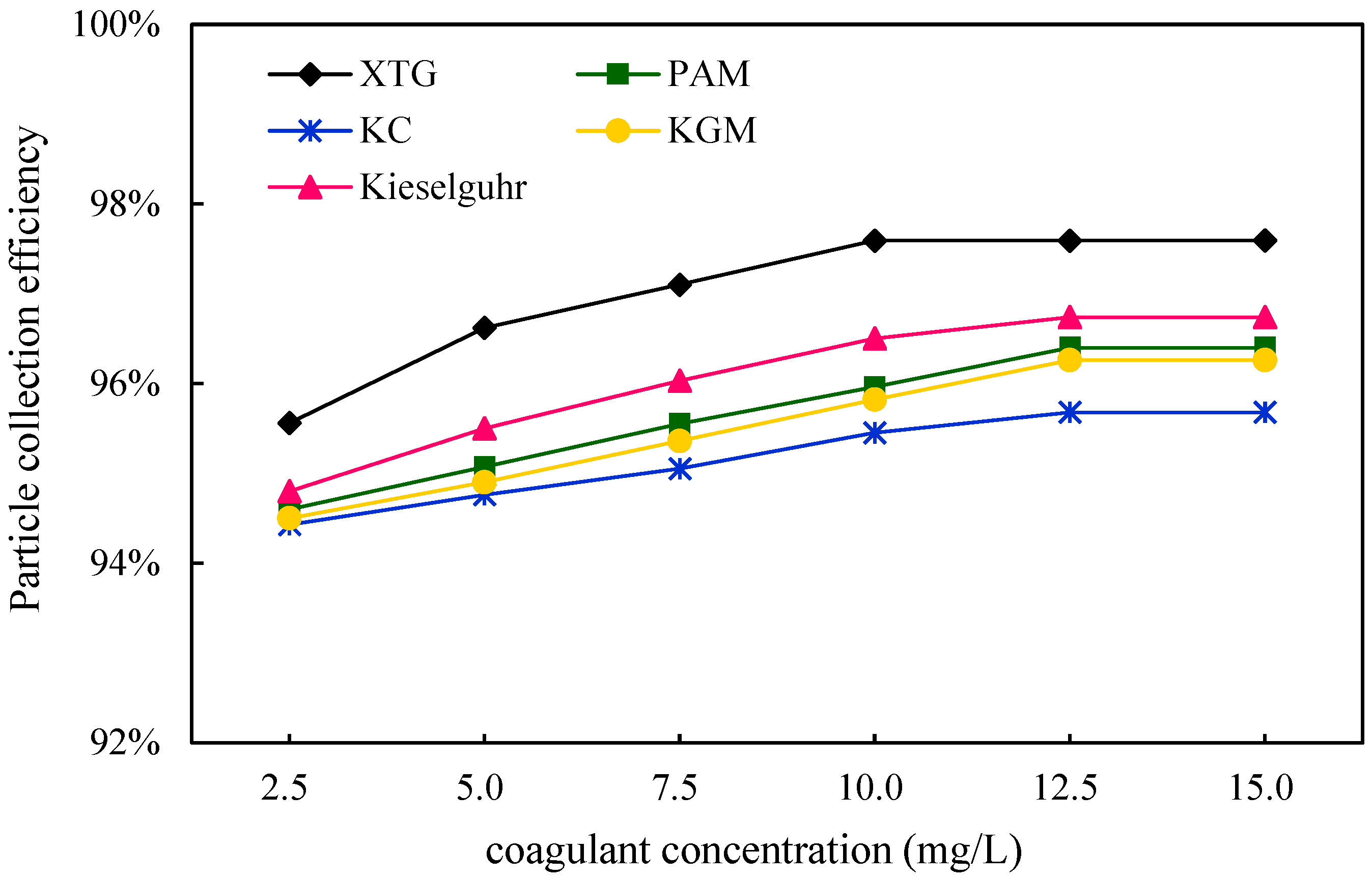
3.2.2. Effect of Coagulant Concentration
Coagulation Effect
Dust Removal Efficiency
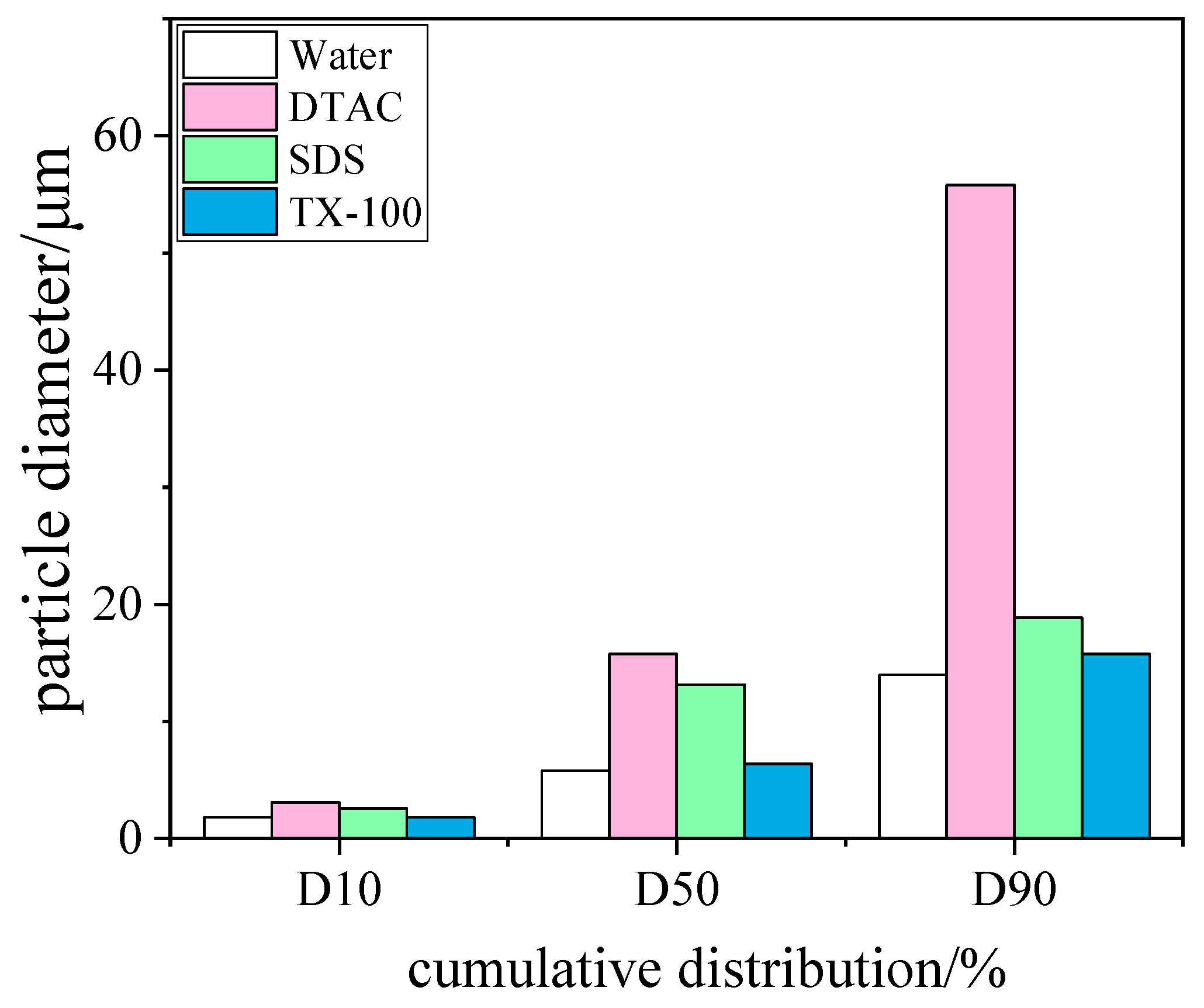
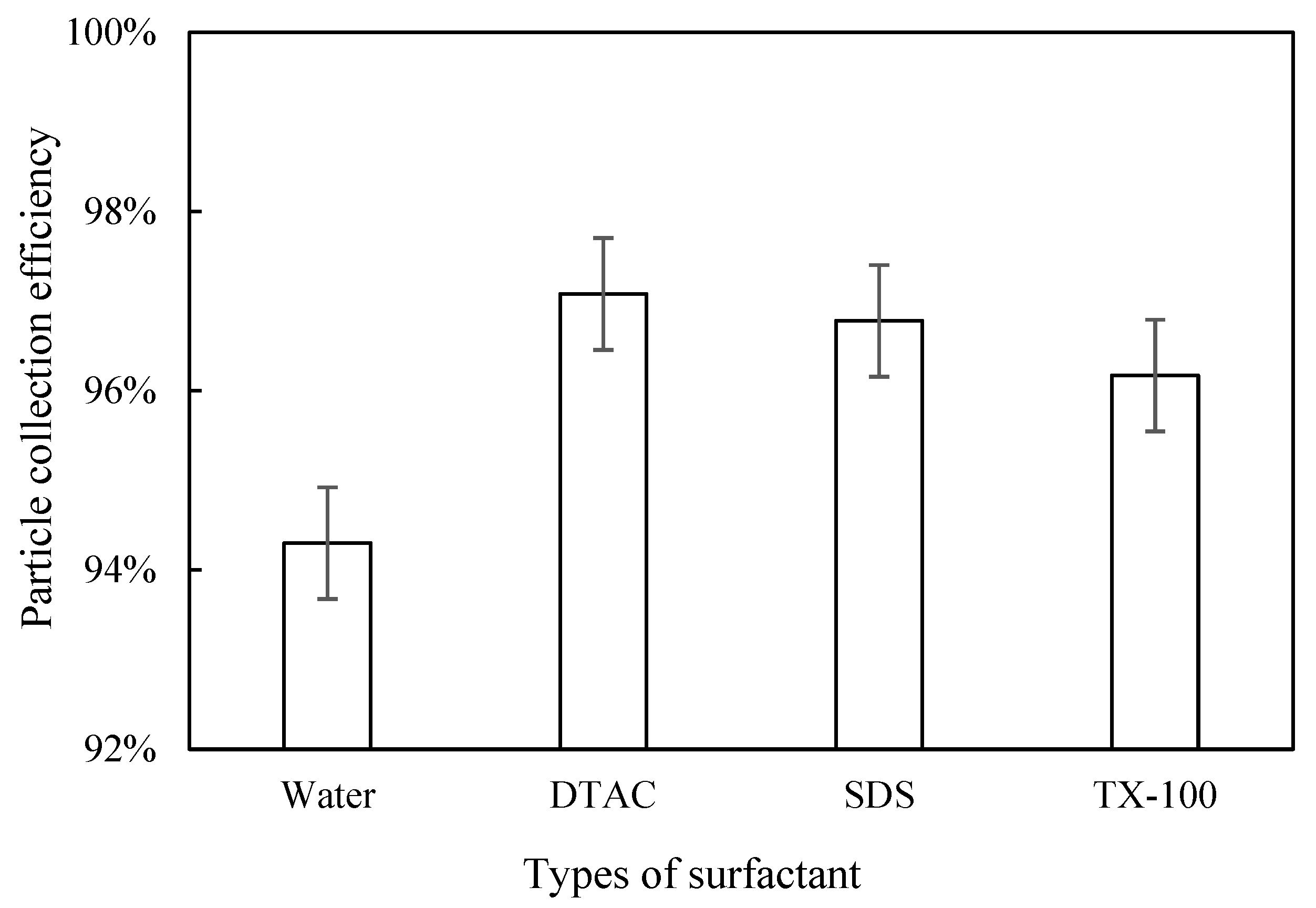
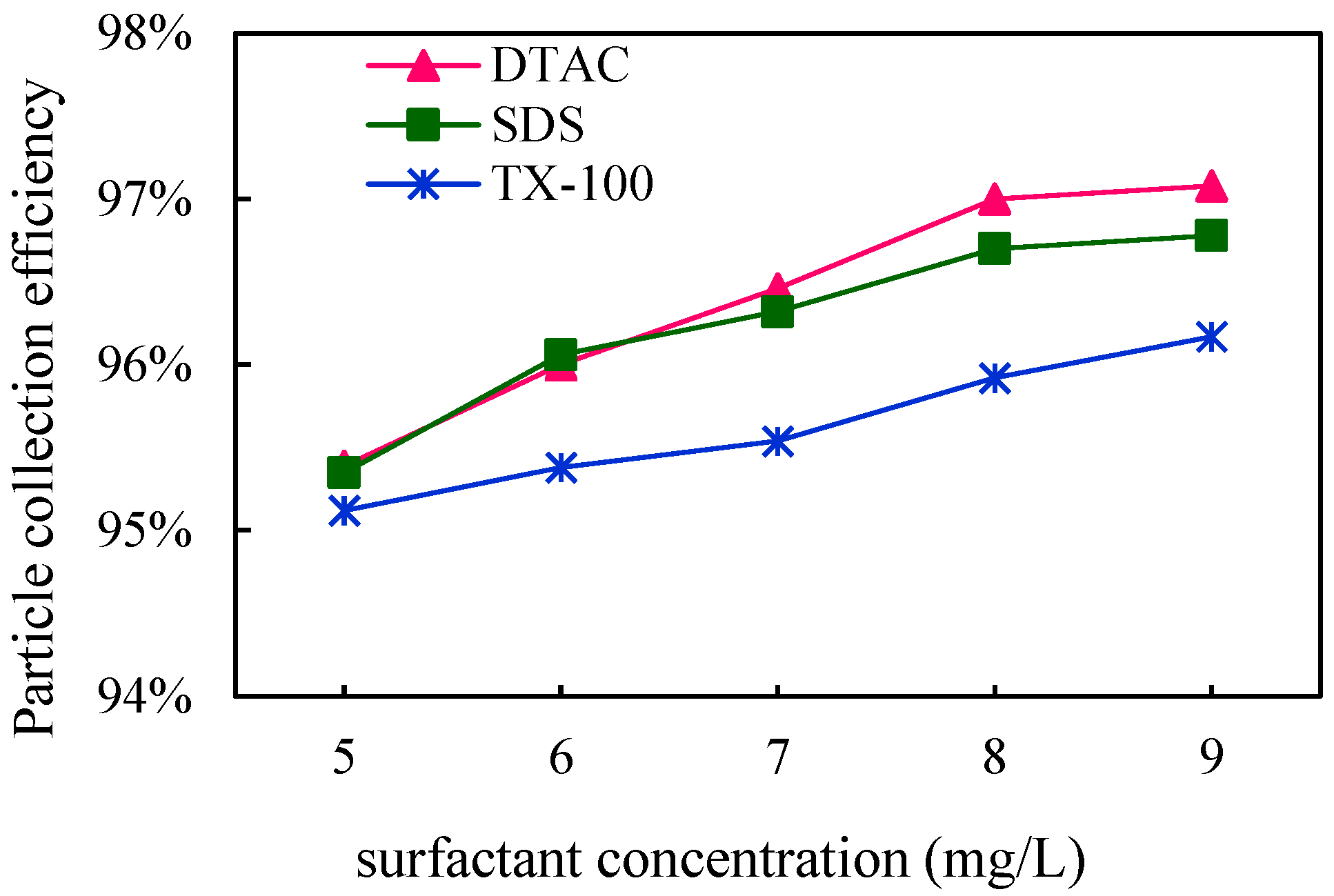
3.3. Effect of Surfactants on Blast Furnace Dust Capture
3.3.1. Influence of Surfactant Type
Coagulation Effect
Dust Removal Efficiency
3.3.2. Effect of Surfactant Concentration
Coagulation Effect
Dust Removal Efficiency
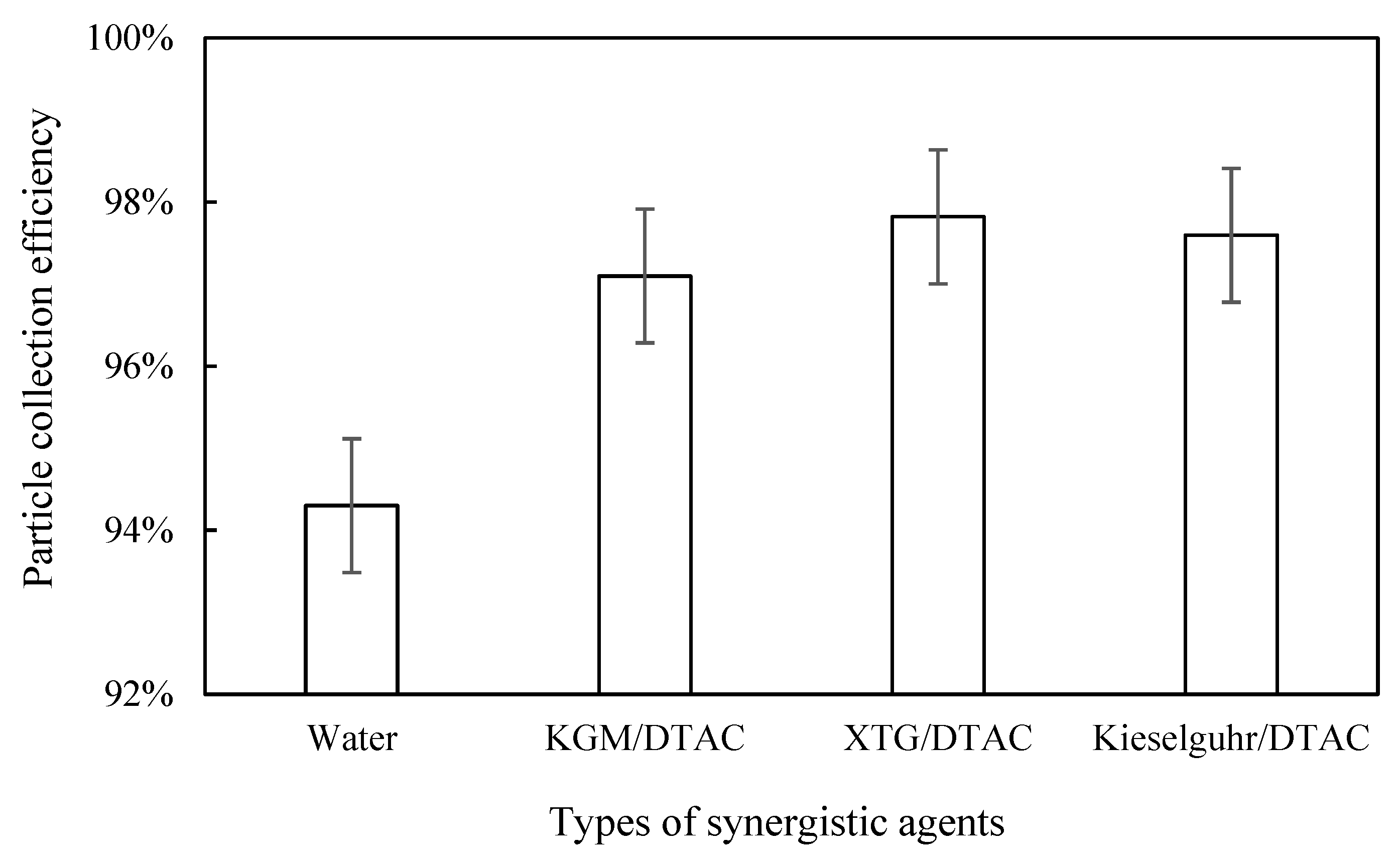
3.4. Effect of Synergy on Blast Furnace Dust Capture
3.4.1. Impact of Synergistic Species
3.4.2. Dust Removal Efficiency
4. Conclusions
- (1)
- All five chemical coagulants selected in this study can effectively promote the capture of blast furnace dust. The D50 of blast furnace dust and the proportion of large particles increased after the addition of coagulants. XTG had the best agglomeration effect on blast furnace dust, KC had the worst agglomeration effect, and their corresponding WESP removal efficiencies were 97.59% and 95.68%, respectively. With an increase in the XTG concentration, the agglomeration effect was better, and the dust removal efficiency reached its highest level when the XTG concentration was 10 mg/L.
- (2)
- All three surfactants effectively improved the trapping efficiency of blast furnace dust in a WESP. As far as the agglomeration effect of the blast furnace dust was concerned, DTAC had the best agglomeration effect, and TX-100 had the worst agglomeration effect, corresponding to 97.08% and 96.17% removal efficiency of WESP, respectively; with an increase in the surfactant concentration, the blast furnace dust particles grew better.
- (3)
- The synergistic effect of a chemical coagulant and a surfactant can improve the agglomeration effect and capture efficiency of furnace dust, but this effect was more obvious when the dust removal efficiency of the chemical coagulant was low. When XTG at 10 mg/L and DTAC at 9 mg/L acted synergistically, the number of respirable particles was significantly reduced, and the dust removal efficiency reached its maximum of 97.82%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| d | Particle diameter |
| D10 | Diameter when the cumulative distribution of dust is 10% |
| D50 | Diameter when the cumulative distribution of dust is 50% |
| D90 | Diameter when the cumulative distribution of dust is 90% |
References
- BP. BP Statistical Review of China Energy 2015. Available online: http://www.bp.com/zh_cn/china/reports-and-publications/bp_20351.html (accessed on 30 October 2022).
- Meij, R.; Te Winkel, B. The emissions and environmental impact of PM10 and trace elements from a modern coal-fired power plant equipped with ESP and wet FGD. Fuel Process. Technol. 2004, 85, 641–656. [Google Scholar] [CrossRef]
- Senior, C.L.; Helble, J.J.; Sarofim, A.F. Emissions of mercury, trace elements, and fine particles from stationary combustion sources. Fuel Process. Technol. 2000, 65, 263–288. [Google Scholar] [CrossRef]
- Pope Iii, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Neas, L.M. Fine particulate matter and cardiovascular disease. Fuel Process. Technol. 2000, 65, 55–67. [Google Scholar] [CrossRef]
- Tai, A.P.K.; Mickley, L.J.; Jacob, D.J. Correlations between fine particulate matter (PM 2.5) and meteorological variables in the United States: Implications for the sensitivity of PM 2.5 to climate change. Atmos. Environ. 2010, 4, 3976–3984. [Google Scholar] [CrossRef]
- Avise, J.; Chen, J.; Lamb, B.; Wiedinmyer, C.; Guenther, A.; Salathe, E.; Mass, C. Attribution of projected changes in summertime US ozone and PM 2.5 concentrations to global changes. Atmos. Chem. Phys. 2009, 9, 1111–1124. [Google Scholar] [CrossRef]
- Ylätalo, S.I.; Hautanen, J. Electrostatic precipitator penetration function for pulverized coal combustion. Aerosol. Sci. Technol. 1998, 29, 17–30. [Google Scholar] [CrossRef]
- China Iron and Steel Industry Association: National Steel Production in June 2021. Available online: www.chinaisa.org.cn (accessed on 15 July 2021). (In Chinese).
- China Iron and Steel Industry Association: Environmental Protection of Member Enterprises in June 2021. Available online: www.chinaisa.org.cn (accessed on 21 July 2021). (In Chinese).
- Matino, I.; Dettori, S.; Colla, V.; Weber, V.; Salame, S. Forecasting blast furnace gas production and demand through echo state neural network-based models: Pave the way to off-gas optimized management. Appl. Energy 2019, 253, 113578. [Google Scholar] [CrossRef]
- Zhao, Q.E. Trend analysis of sulfur in titanium slag from off-sheet titanium concentrate smelting. Steel Vanadium Titan. 2018, 39, 97–101. (In Chinese) [Google Scholar]
- Guo, Y.H. Current status and future development trend of blast furnace gas purification and quality utilization technology. J. Iron Steel Res. 2020, 32, 525–531. (In Chinese) [Google Scholar]
- Durham, M.D.; Schlager, R.J.; Ebner, T.G.; Stewart, R.M.; Bustard, C.J. Method and Apparatus for Decreased Undesired Particle Emissions in Gas Streams. U.S. Patent 5,893,943, 13 April 1999. [Google Scholar]
- Johansen, A.; Schæfer, T. Effects of physical properties of powder particles on binder liquid requirement and agglomerate growth mechanisms in a high shear mixer. Eur. J. Pharm. Sci. 2001, 14, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhang, J.Y.; Wang, C.M.; Zheng, C.G. Research progress of coal combustion ultrafine particle agglomeration promotion technology. Coal Convers. 2003, 26, 27–31. (In Chinese) [Google Scholar]
- Zhao, Y.C.; Zhang, J.Y.; Wei, F.; Chen, J.; Zheng, C.G. Experimental study on the agglomeration promotion mechanism of ultrafine particulate matter from coal combustion. J. Chem. Eng. 2007, 58, 2876–2881. (In Chinese) [Google Scholar]
- Rajniak, P.; Mancinelli, C.; Chern, R.T.; Stepanek, F.; Farber, L.; Hill, B.T. Experimental study of wet granulation in fluidized bed: Impact of the binder properties on the granule morphology-ScienceDirect. Int. J. Pharm. 2007, 334, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Zhang, J.Y.; Zhao, Y.C.; Ding, F.; Zhao, C.G. Experimental study on solid-liquid agglomeration of fine coal combustion particles. Chin. J. Electr. Eng. 2009, 29, 62–66. (In Chinese) [Google Scholar]
- Campo, V.L.; Kawano, D.F.; Campo, V.L.; Kawano, D.F.; Da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications, and structural analysis–A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Forbes, E. Shear, selective and temperature responsive flocculation: A comparison of fine particle flotation techniques. Int. J. Miner. Process. 2011, 99, 1–10. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Bao, J.J.; Geng, J.F.; Yang, L.J. Experimental study of chemical agglomeration to promote the removal of fine particulate matter from coal combustion. Chin. J. Electr. Eng. 2013, 33, 52–58. (In Chinese) [Google Scholar]
- Thonglek, V.; Kiatsiriroat, T. Agglomeration of sub-micron particles by a non-thermal plasma electrostatic precipitator. J. Electrost. 2013, 72, 33–38. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.J.; Pan, D.P.; Huang, R.T. Experiments on the Removal of Fine Particles in Existing Air Pollution Control Devices by Chemical Agglomeration. Adv. Mater. Res. 2014, 3384, 756–760. [Google Scholar]
- Balakin, B.V.; Kutsenko, K.V.; Lavrukhin, A.A.; Kosinski, P. The collision efficiency of liquid bridge agglomeration. Chem. Eng. Sci. 2015, 137, 590–600. [Google Scholar]
- Liu, Y.; Hu, B.; Zhou, L.; Jiang, Y.; Yang, L. Improving the Removal of Fine Particles with an Electrostatic Precipitator by Chemical Agglomeration. Energy Fuels 2016, 30, 8441–8447. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Zhao, Y.; Wang, S.; Jiang, C.; Zheng, C. Chemical agglomeration of fine particles in coal combustion flue gas: Experimental evaluation. Fuel 2017, 203, 557–569. [Google Scholar] [CrossRef]
- Hu, B.; Yang, Y.; Zhou, L.; Ao, S.; Cai, L.; Linjun, Y.; Roszak, S. Experimental and DFT studies of PM2.5 removal by chemical agglomeration. Fuel 2018, 212, 27–33. [Google Scholar]
- Sun, Z.; Yang, L.; Shen, A.; Zhou, L.; Wu, H. Combined effect of chemical and turbulent agglomeration on improving the removal of fine particles by different coupling mode. Powder Technol. 2019, 344, 242–250. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, W.; Wu, H.; Shen, A.; Yuan, Z.; Yang, L. Investigation on the relationship of droplet atomization performance and fine particle abatement during the chemical agglomeration process. Fuel 2019, 245, 65–77. [Google Scholar] [CrossRef]
- Li, R.; Li, C.; Zhuang, J.; Zhu, H.; Fang, L.; Sun, D. Mechanistic Influence of Chemical Agglomeration Agents on Removal of Inhalable Particles from Coal Combustion. ACS Omega 2020, 5, 25906–25912. [Google Scholar] [CrossRef]
- Gao, S.; Xiao, L.C. Study on Dust Turbulence-Chemical Agglomeration for Electrostatic Precipitation Technology. E3S Web Conf. 2021, 245, 03011. [Google Scholar]
- Yang, G.Z.; Zhao, Y.C.; Xiong, Z.; Gong, B.G.; Gao, T.; Zhang, J.Y. Study on chemical agglomeration enhanced dedusting and co-desulfurization of 300MW coal-fired power plant with zero wastewater discharge. Chin. J. Electr. Eng. 2021, 41, 5274–5282. (In Chinese) [Google Scholar]
- Lyklema, J. Modern Trends of Colloid Science in Chemistry and Biology. In Adsorption of Polyelectrolytes and Their Effect on the Interaction of Colloid Particles; Eicke, H.F., Ed.; Birkhäuser: Basel, Switzerland, 1985; pp. 55–73. [Google Scholar]
- Zhang, J.G.; Liu, Y.T.; Wang, M.; Wang, Y.; Li, H.; Xie, J.; Zhao, W.; Zhou, W.; Ye, S.; Li, L.; et al. Mechanism of non-ionic surfactant effect on coal wettability based on molecular dynamics simulation. Eng. Sci. Technol. 2022, 54, 191–202. (In Chinese) [Google Scholar]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J. (Ed.) Principles of Chemical Flocculant Action; Science Press: Beijing, China, 2005; pp. 86–92. (In Chinese) [Google Scholar]






| Species | Name | Manufacturer |
|---|---|---|
| Chemical coagulant | PAM (polyacrylamide) | Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China |
| KC (k-carrageenan) | Henan Wanbang Chemical Technology Co., Ltd., Zhengzhou, China | |
| KGM (konjac glucomannan) | Henan Wanbang Chemical Technology Co., Ltd., Zhengzhou, China | |
| XTG (xanthan gum) | Tianjin Guangfu Fine Chemical Research Institute, Tianjin, China | |
| Kieselguhr | Tianjin Dengfeng Chemical Reagent Factory, Tianjin, China | |
| Surfactant | DTAC (Dodecyl trimethyl ammonium chloride) | Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China |
| SDS (sodium dodecyl sulfate) | Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China | |
| TX-100 (Octylphenyl polyoxyethylene ether) | Tianjin Guangfu Fine Chemical Research Institute, Tianjin, China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Xiao, L.; Chen, H. Chemical Agglomeration to Enhance Blast Furnace Dust Capture Efficiency in Wet Electrostatic Precipitators. Coatings 2022, 12, 1937. https://doi.org/10.3390/coatings12121937
Han Y, Xiao L, Chen H. Chemical Agglomeration to Enhance Blast Furnace Dust Capture Efficiency in Wet Electrostatic Precipitators. Coatings. 2022; 12(12):1937. https://doi.org/10.3390/coatings12121937
Chicago/Turabian StyleHan, Yingying, Lichun Xiao, and Hongrui Chen. 2022. "Chemical Agglomeration to Enhance Blast Furnace Dust Capture Efficiency in Wet Electrostatic Precipitators" Coatings 12, no. 12: 1937. https://doi.org/10.3390/coatings12121937
APA StyleHan, Y., Xiao, L., & Chen, H. (2022). Chemical Agglomeration to Enhance Blast Furnace Dust Capture Efficiency in Wet Electrostatic Precipitators. Coatings, 12(12), 1937. https://doi.org/10.3390/coatings12121937






