Effect of a Thermal Catalyst on Organosilanes Treatment to Improve Durability and Stability of Canadian Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solutions Preparation and Wood Treatment
2.3. Dimensional Stability
2.4. Hydroxyl Quantification by Water Vapor Sorption
2.5. Leaching Test
2.6. Brown Rot Resistance
3. Results
3.1. Retention and Weight Percant Gain (WPG)
3.2. Dimensional Stability
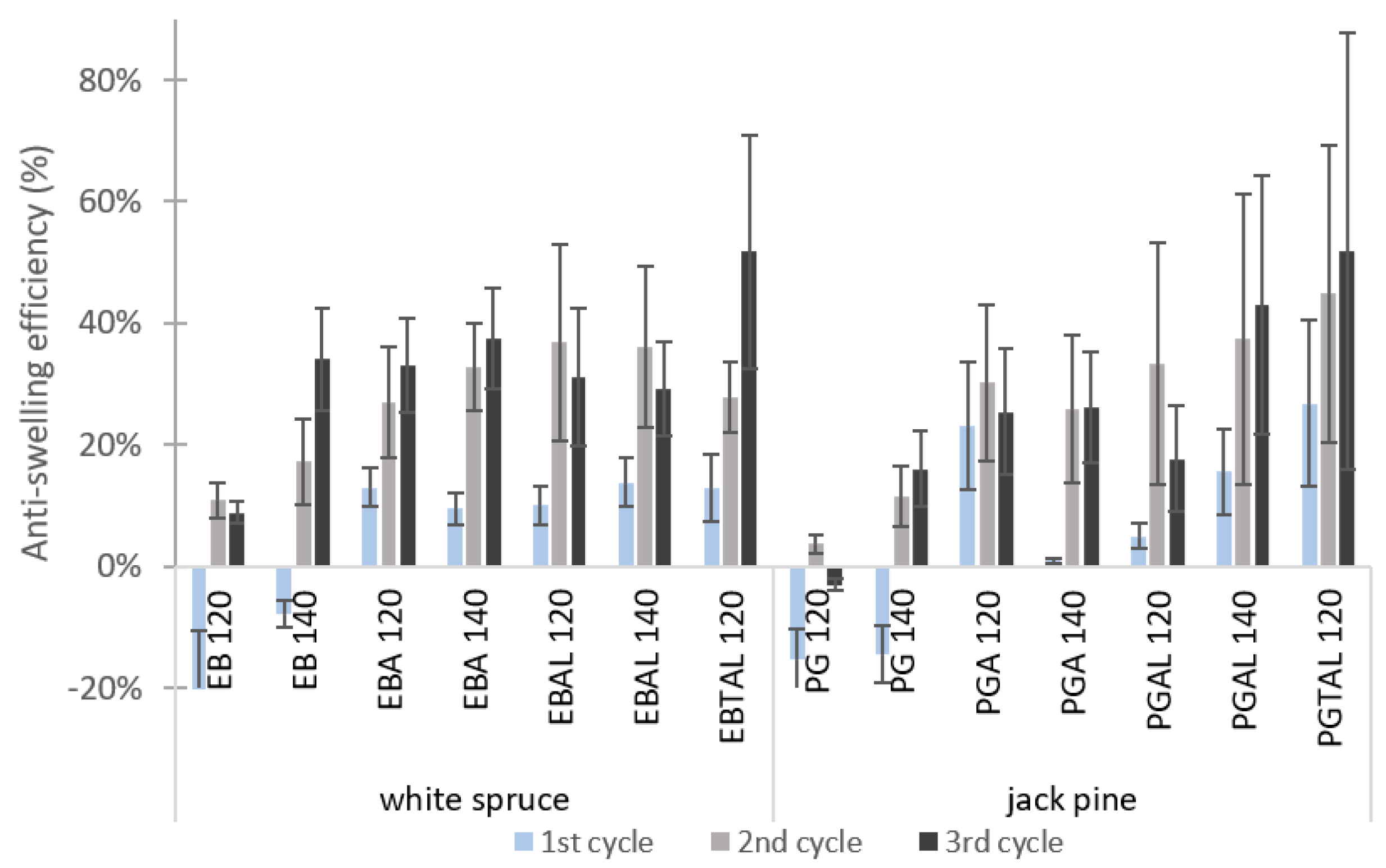
3.2.1. Dimensional Stability under Humidity Cycling
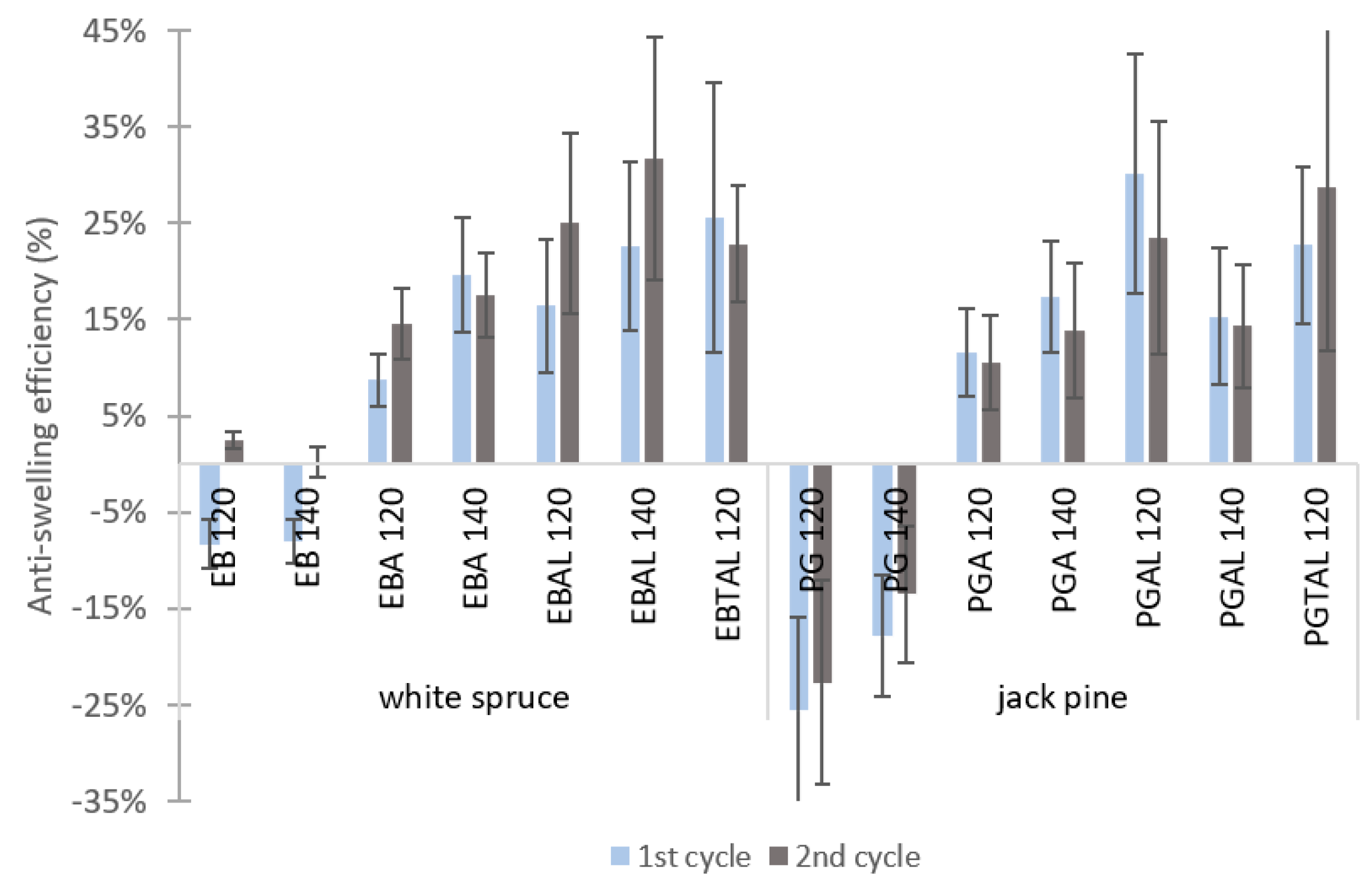
3.2.2. Dimensional Stability under Water Immersion
3.3. Hydroxyl Quantification by Water Vapor Sorption
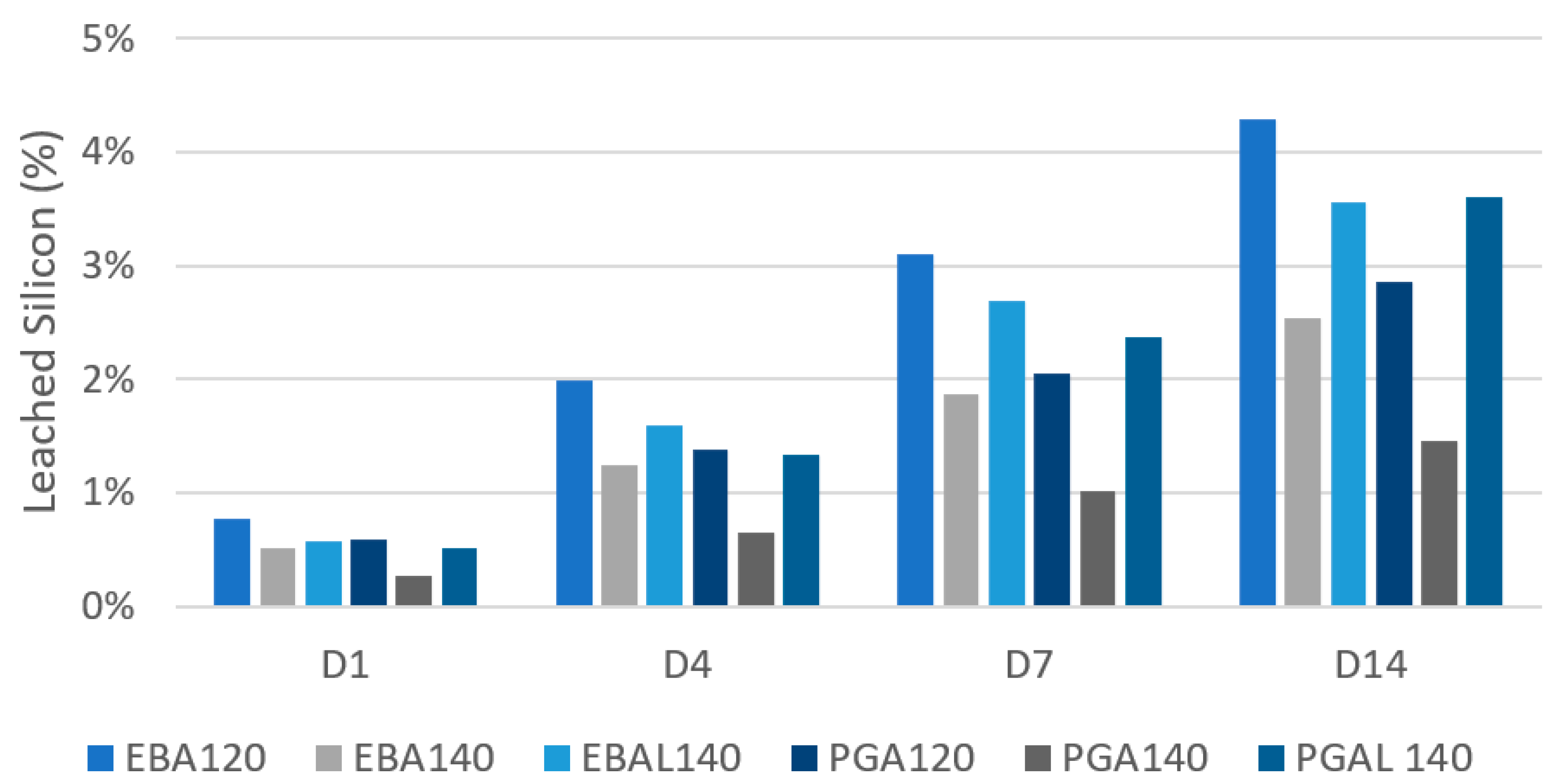
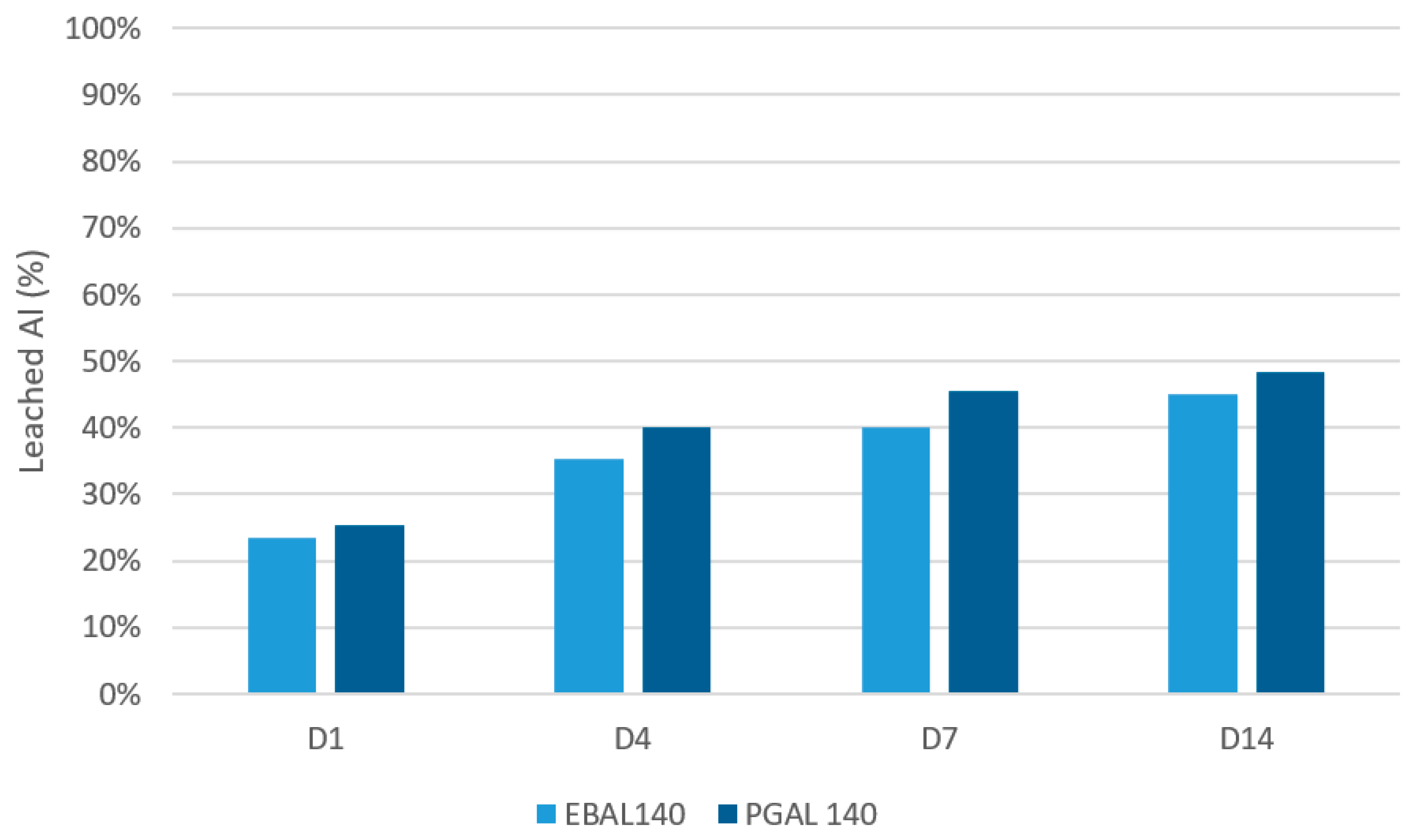
3.4. Leaching Test
3.5. Brown Rot Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandak, A.; Sandak, J.; Dimitriou, A.; Burud, I.; Thiis, T.K.; Gobakken, L.R.; Ormondroyd, G.A.; Kraniotis, D. Assessment and monitoring of aesthetic appearance of building biomaterials during the service life. WIT Trans. Ecol. Environ. 2017, 226, 527–536. [Google Scholar]
- Ratu, R.; Weizenegger, J.; Evans, P.D. Preliminary observations of the effect of kerfing on the surface checking and warping of flat sawn southern pine decking. In Proceedings of the thirty eighth annual meeting of international research group on wood protection, Jackson Lake Lodge, WY, USA, 20–24 May 2007; International Research Group on Wood Protection: Stockholm, Sweden, 2007; pp. 1–7. [Google Scholar]
- Cheng, K.J.; Evans, P.D. Manufacture of profiled amabilis fir deckboards with reduced susceptibility to surface checking. J. Manuf. Mater. Process 2018, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Nejad, M.; Cooper, C. Exterior wood coatings. Part-1: Performance of semitransparent stains on preservative-treated wood. J. Coat. Technol. Res. 2011, 8, 449–458. [Google Scholar] [CrossRef]
- Schauwecker, C.; Preston, A.; Morrell, J.J. A new look at the weathering performance of solid-wood decking materials. J. Coat. Technol. 2009, 6, 32–38. [Google Scholar]
- Esteves, B.M.; Pereira, H.M. Wood modification by heat treatment: A review. Bioresources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Titan Wood Limited, Process for Wood Acetylation and Product Thereof. U.S. Patent 8512815 B2, 20 August 2013.
- Brelid, L.P.; Simonson, R.; Risman, P.O. Acetylation of solid wood using microwave heating Part 1: Studies of dielectric properties. Holz Als Roh Werkst. 1999, 57, 259–263. [Google Scholar] [CrossRef]
- Teacă, C.-A.; Bodîrlău, R.; Spiridon, I. Maleic anhydride treatment of softwood-Effect on wood structure and properties. Cell. Chem. Technol. 2014, 48, 863–868. [Google Scholar]
- Berube, M.A.; Schorr, D.; Ball, R.J.; Landry, V.; Blanchet, P. Determination of in situ esterification parameters of citric acid-glycerol based polymers for wood impregnation. J. Polym. Environ. 2018, 26, 970–979. [Google Scholar] [CrossRef]
- Larnøy, E.; Karaca, A.; Gobakken, L.R.; Hill, C.A.S. Polyesterification of wood using sorbitol and citric acid under aqueous conditions. Int. Wood Prod. 2018, 9, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Mubarok, M.; Militz, H.; Dumarçay, S.; Gérardin, P. Beech wood modification based on in situ esterification with sorbitol and citric acid. Wood Sci. Technol. 2020, 54, 479–502. [Google Scholar] [CrossRef] [Green Version]
- Pries, M. Treatment of Solid Wood with Silanes, Polydimethylsiloxanes and Silica Sols. Ph.D. Thesis, Georg-August University School of Science, Göttingen, Germany, 2013. Available online: https://d-nb.info/106277065X/34 (accessed on 13 July 2022).
- Donath, S.; Militz, H.; Mai, C. Wood modification with alkoxysilanes. Wood Sci. Technol. 2004, 38, 555–566. [Google Scholar] [CrossRef]
- Pépin, S.; Blanchet, P.; Landry, V. Performances of white pine and white spruce treated with organic fungicides using an aqueous buffered amine oxide preservation system. Bioresources 2019, 14, 264–288. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Kamperidou, V.; Ratajczak, I.; Perdoch, W.; Mazela, B. Impact of thermal modification combined with silicon compounds treatment on wood structure. Wood Res. 2022, 67, 773–784. [Google Scholar] [CrossRef]
- Broda, M.; Majika, J.; Olek, W.; Mazela, B. Dimensional stability and hygroscopic properties of waterlogged archaeological wood treated with alkoxysilanes. Int. Biodeterior. Biodegrad. 2018, 133, 34–41. [Google Scholar] [CrossRef]
- Schorr, D.; Blanchet, P. Improvement of white spruce wood dimensional stability by organosilanes sol-gel impregnation and heat treatment. Materials 2020, 13, 973. [Google Scholar] [CrossRef] [Green Version]
- Schorr, D.; Boivin, G. Modification of white spruce to improve dimensional stability of exterior wood cladding. In Proceedings of the 10th ECWM, Nancy, France, 25–26 April 2022. [Google Scholar]
- Schorr, D.; Boivin, G. Influence of basic catalyst and organosilane types on dimensional stability and durability of exterior wood cladding. Wood Mater. Sci. Eng. 2022, 1–6. [Google Scholar] [CrossRef]
- Qu, L.; Wang, Z.; Qian, J.; He, Z.; Yi, S. Effects of aluminum sulfate soaking pretreatment on dimensional stability and thermosta-bility of heat-treated wood. Eur. J. Wood Wood Prod. 2021, 79, 189–198. [Google Scholar] [CrossRef]
- DIN 52 184; Testing of Wood; Determination of swelling and shrinkage. Deutsches institut für normung E.V.: Berlin, Germany, 1979.
- Thybring, E.E.; Thygesen, L.G.; Burgert, I. Hydroxyl accessibility in wood cell walls as affected by drying and re-wetting procedures. Cellulose 2017, 24, 2375–2384. [Google Scholar] [CrossRef] [Green Version]
- AWPA E11-16 (R2022); Standard method for accelerated evaluation of preservative leaching. Book of standards. American Wood Protection Association: Birmingham, AL, USA, 2016; 6p.
- AWPA E10-22; Laboratory method for evaluating the decay resistance of wood-based materials against pure basidiomycete cultures: Soil/block test. Book of Standards. American Wood Protection Association: Birmingham, AL, USA, 2022; 9p.
- Eaton, R.A.; Hale, M.D.C. Wood: Decay, Pests and Protection; Chapman and Hall: London, UK, 1993; pp. 119, 457–461. [Google Scholar]
- Siau, J.F.; Shaw, J.S. The treatability of refractory softwoods. Wood Fiber Sci. 1979, 3, 1–12. [Google Scholar]
- Furuno, T.; Imamura, Y. Combinations of wood and silicate Part 6. Biological resistances of wood-mineral composites using water glass-boron compound system. Wood Sci.Technol. 1998, 32, 161–170. [Google Scholar] [CrossRef]
- Ximenes, F.A.; Evans, P.D. Protection of wood using oxy-aluminum compounds. For. Prod. J. 2006, 56, 116–122. [Google Scholar]
- Vidholdová, Z.; Kačík, F.; Reinprecht, L.; Kučerová, V.; Luptáková, J. Changes in chemical structure of thermally modified spruce wood due to decaying fungi. J. Fungi 2022, 8, 739. [Google Scholar] [CrossRef] [PubMed]

| Treatment Parameters | 19 mm × 19 mm × 19 mm Specimens | 50 mm × 50 mm × 19 mm Specimens |
|---|---|---|
| Vacuum (85 kPa) | 15 min | 30 min |
| Pressure (620 kPa) | 45 min | 2 h |
| Ethanol evaporation | Min. 12 h | Min. 12 h |
| Oven temperature (°C) | 120 or 140 | 120 or 140 |
| Time in oven (h) | 17 | 24 |
| Species | ID | Treatment |
|---|---|---|
| White spruce (EB) | EB 120 | TT at 120 °C |
| EB 140 | TT at 140 °C | |
| EBA 120 | MTMS + HDTMS treatment (A) + TT at 120 °C | |
| EBA 140 | MTMS + HDTMS treatment (A) + TT at 140 °C | |
| EBAL 120 | MTMS + HDTMS treatment (A)+ Al treatment (L)+ TT at 120 °C | |
| EBAL 140 | MTMS + HDTMS treatment (A)+ Al treatment (L)+ TT at 140 °C | |
| EBTAL 120 | Al treatment (TAL) + TT at 120 °C | |
| Jack pine (PG) | PG 120 | TT at 120 °C |
| PG 140 | TT at 140 °C | |
| PGA 120 | MTMS + HDTMS treatment (A) + TT at 120 °C | |
| PGA 140 | MTMS + HDTMS treatment (A) + TT at 140 °C | |
| PGAL 120 | MTMS + HDTMS treatment (A) + Al treatment (L) + TT at 120 °C | |
| PGAL 140 | MTMS + HDTMS treatment (A) + Al treatment (L) + TT at 140 °C | |
| PGTAL 120 | Al treatment (TAL) + TT at 120 °C |
| Treatment ID | Description |
|---|---|
| EBNT | Untreated white spruce |
| EBA | White spruce + organosilanes |
| EBAL | White spruce + organosilanes + aluminum sulfate |
| EBACQ | White spruce treated with ACQ-C |
| PGNT | Untreated jack pine |
| PGA | Jack pine + organosilanes |
| PGAL | Jack pine + organosilanes + aluminum sulfate |
| PGACQ | Jack pine treated with ACQ-C |
| PP sapwood | Untreated Ponderosa pine sapwood |
| Rhodonia placenta (Fr.) M. Lars. et Lomb. | Ftk 120F |
|---|---|
| Gloeophyllum trabeum (Pers. Ex FR.) Murr. | Ftk 47D |
| Fibroporia radiculosa (Peck) Gilb. & Ryvarden | TFFH 294 |
| - | White Spruce | Jack Pine | ||||||
|---|---|---|---|---|---|---|---|---|
| - | 120 °C (18 h) | 140 °C (18 h) | 120 °C (18 h) | 140 °C (18 h) | ||||
| - | EBA | EBAl | EBA | EBAl | PGA | PGAl | PGA | PGAl |
| OSi retention before thermal treatment (%) | 79 | 84 | 79 | 84 | 54 | 58 | 52 | 50 |
| Al retention before thermal treatment (%) | - | 36 | - | 36 | - | 36 | - | 34 |
| WPG after thermal treatment and stabilization at 20 °C/50% RH | 11 | 12 | 11 | 12 | 8 | 8 | 7 | 4 |
| - | EBA 120 | EBA 140 | EBAl 120 | PGA 120 | PGA 140 | PGAl 120 |
|---|---|---|---|---|---|---|
| Decrease in OH accessibility (WVS) | 33% | 21% | 37% | 15% | 17% | 17% |
| Product ID | Treatment ID | Moisture Content (%) | Corrected Weight Loss (%) | ||
|---|---|---|---|---|---|
| AVG | STD | AVG | STD | ||
| White Spruce | Untreated | 236.7 | 33.0 | 59.3 | 7.0 |
| White Spruce | Si | 97.5 | 20.4 | 48.8 | 6.7 |
| White Spruce | Si + Al | 100.4 | 16.5 | 5.8 | 2.9 |
| White Spruce | ACQ-C | 39.4 | 4.8 | 0.3 | 0.1 |
| Jack pine | Untreated | 220.0 | 15.9 | 51.5 | 5.6 |
| Jack pine | Si | 147.2 | 26.3 | 51.6 | 2.2 |
| Jack pine | Si + Al | 96.7 | 11.3 | 1.8 | 0.9 |
| Jack pine | ACQ-C | 38.3 | 2.3 | 0.0 | 0.0 |
| Ponderosa Pine sapwood | Untreated | 188.0 | 42.0 | 57.7 | 5.5 |
| Product ID | Treatment ID | Moisture Content (%) | Corrected Weight Loss (%) | ||
|---|---|---|---|---|---|
| AVG | STD | AVG | STD | ||
| White Spruce | Untreated | 224.1 | 54.9 | 46.1 | 4.1 |
| White Spruce | Si | 86.4 | 41.5 | 23.9 | 4.9 |
| White Spruce | Si + Al | 91.6 | 31.1 | 1.8 | 0.8 |
| White Spruce | ACQ-C | 31.9 | 3.1 | 0.1 | 0.3 |
| Jack pine | Untreated | 192.3 | 34.8 | 49.3 | 6.4 |
| Jack pine | Si | 117.4 | 36.2 | 34.2 | 3.5 |
| Jack pine | Si + Al | 65.8 | 8.0 | 1.0 | 1.1 |
| Jack pine | ACQ-C | 31.3 | 3.9 | 0.0 | 0.1 |
| Ponderosa Pine sapwood | Untreated | 167.1 | 20.6 | 59.6 | 4.6 |
| Product ID | Treatment ID | Moisture Content (%) | Corrected Weight Loss (%) | ||
|---|---|---|---|---|---|
| AVG | STD | AVG | STD | ||
| White Spruce | Untreated | 233.5 | 15.1 | 52.2 | 4.2 |
| White Spruce | Si | 109.9 | 48.7 | 28.3 | 9.4 |
| White Spruce | Si + Al | 95.7 | 14.8 | 1.6 | 0.9 |
| White Spruce | ACQ-C | 37.3 | 4.3 | 0.2 | 0.1 |
| Jack pine | Untreated | 194.6 | 22.8 | 35.4 | 8.5 |
| Jack pine | Si | 152.4 | 30.1 | 33.8 | 9.7 |
| Jack pine | Si + Al | 100.1 | 10.6 | 1.2 | 0.4 |
| Jack pine | ACQ-C | 35.7 | 1.9 | 0.1 | 0.1 |
| Ponderosa Pine sapwood | Untreated | 195.2 | 53.6 | 50.7 | 9.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schorr, D.; Boivin, G.; Stirling, R. Effect of a Thermal Catalyst on Organosilanes Treatment to Improve Durability and Stability of Canadian Wood. Coatings 2022, 12, 1867. https://doi.org/10.3390/coatings12121867
Schorr D, Boivin G, Stirling R. Effect of a Thermal Catalyst on Organosilanes Treatment to Improve Durability and Stability of Canadian Wood. Coatings. 2022; 12(12):1867. https://doi.org/10.3390/coatings12121867
Chicago/Turabian StyleSchorr, Diane, Gabrielle Boivin, and Rod Stirling. 2022. "Effect of a Thermal Catalyst on Organosilanes Treatment to Improve Durability and Stability of Canadian Wood" Coatings 12, no. 12: 1867. https://doi.org/10.3390/coatings12121867






