Pitting Performance of Cold- and Hot-Rolled Nickel-Saving High-Strength Metastable Austenitic Stainless Steel
Abstract
:1. Introduction
2. Experimental
2.1. Material Preparation
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
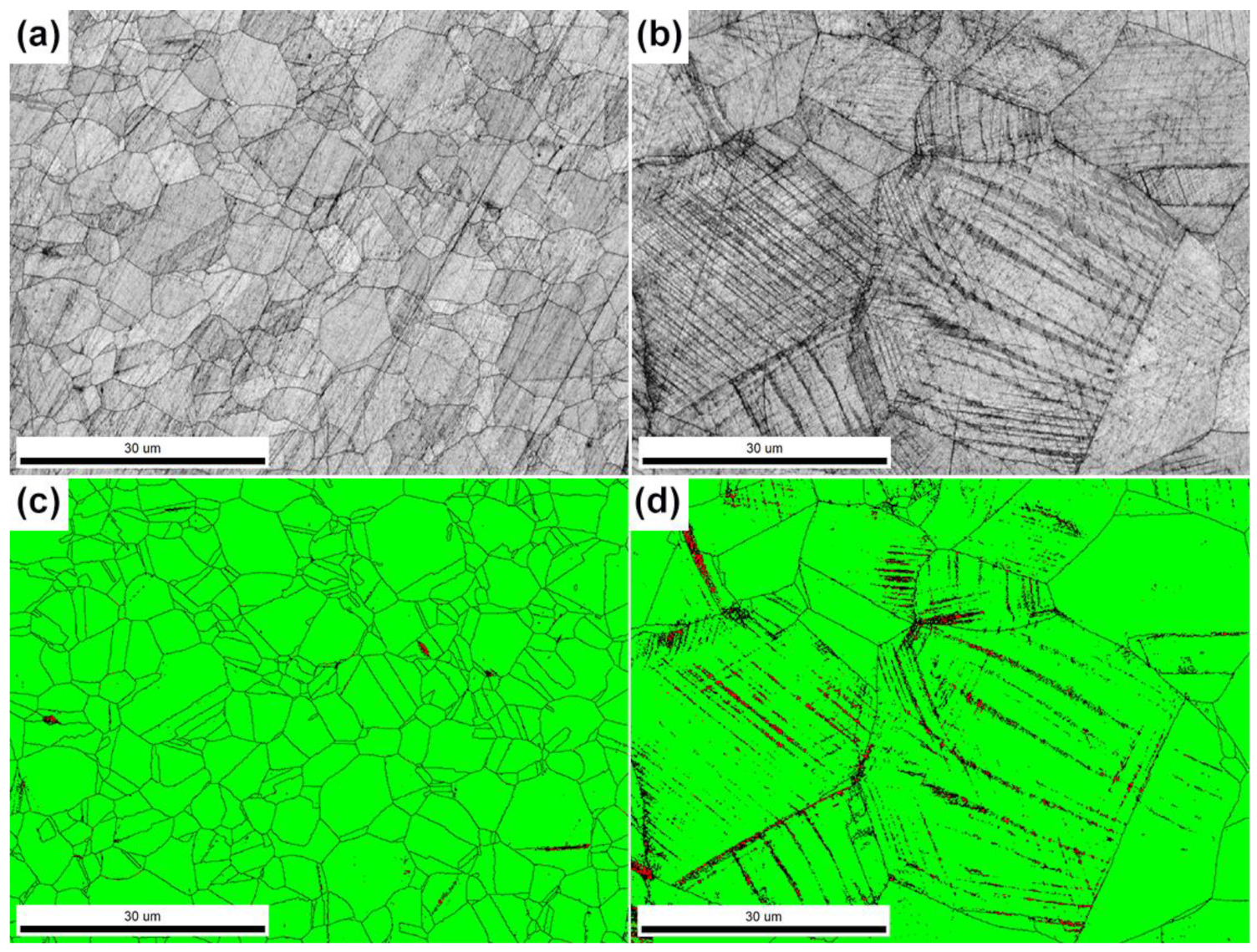
3.1. Microstructures Characterization
3.1.1. Overview of the Steel Microstructure
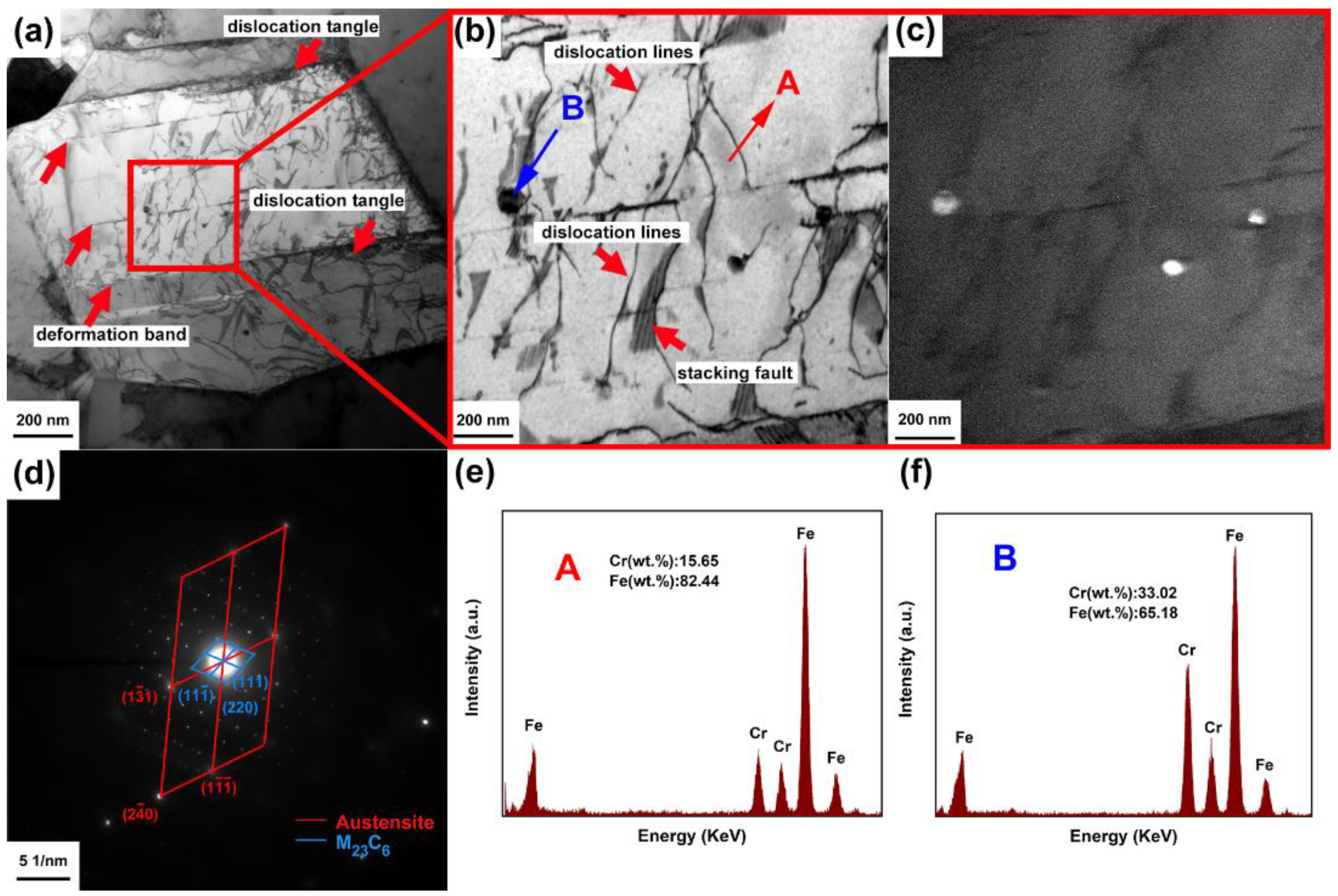
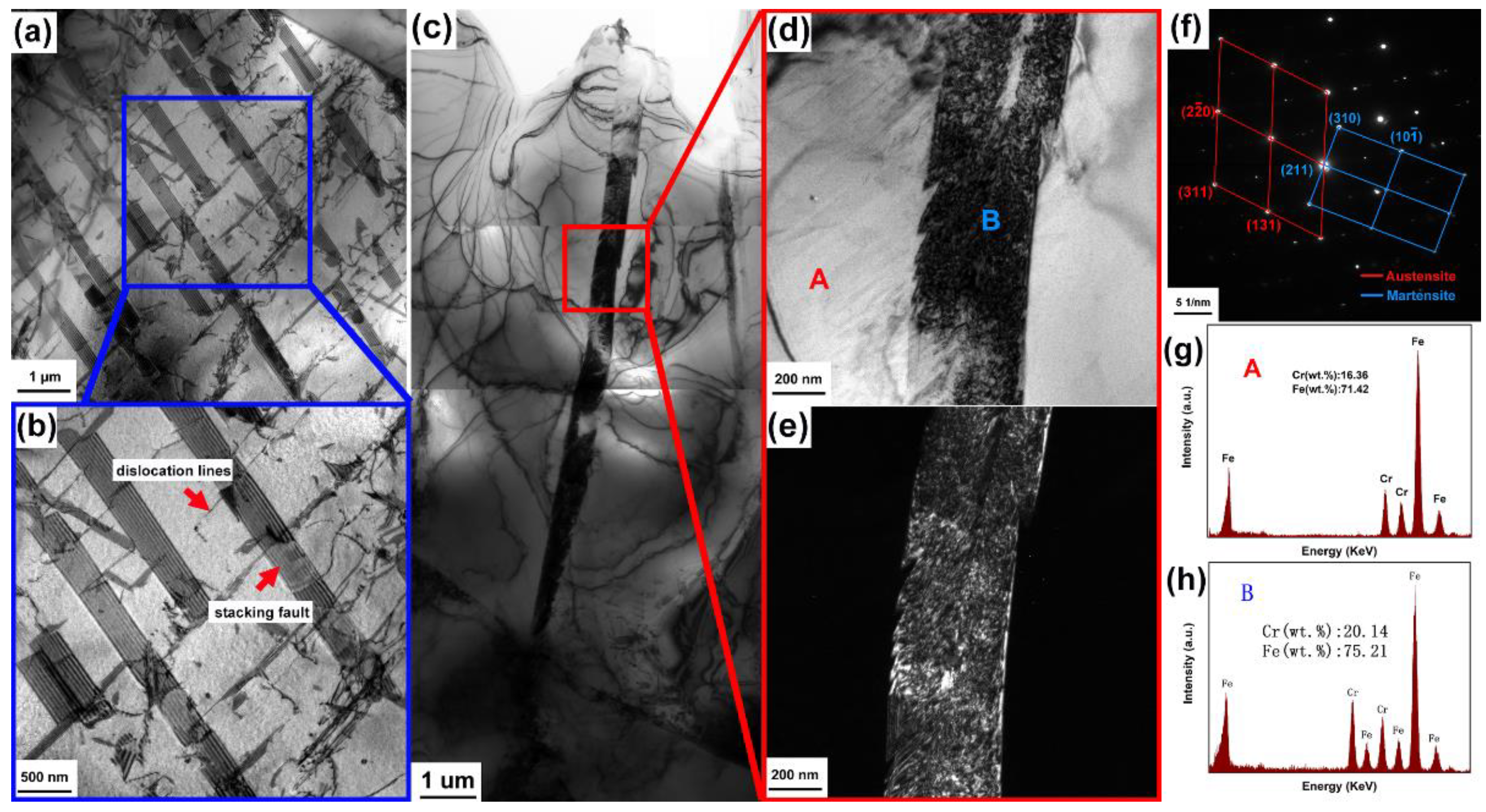
3.1.2. TEM Characterization
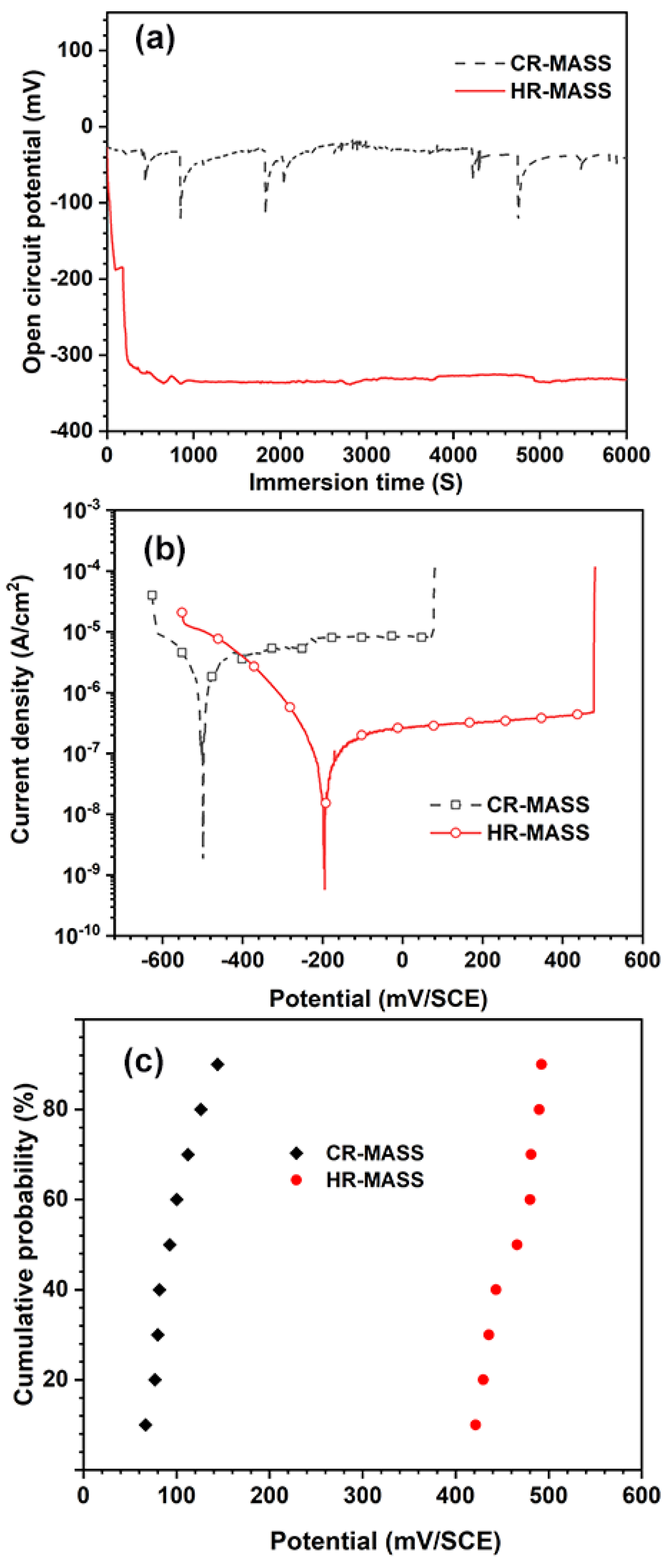
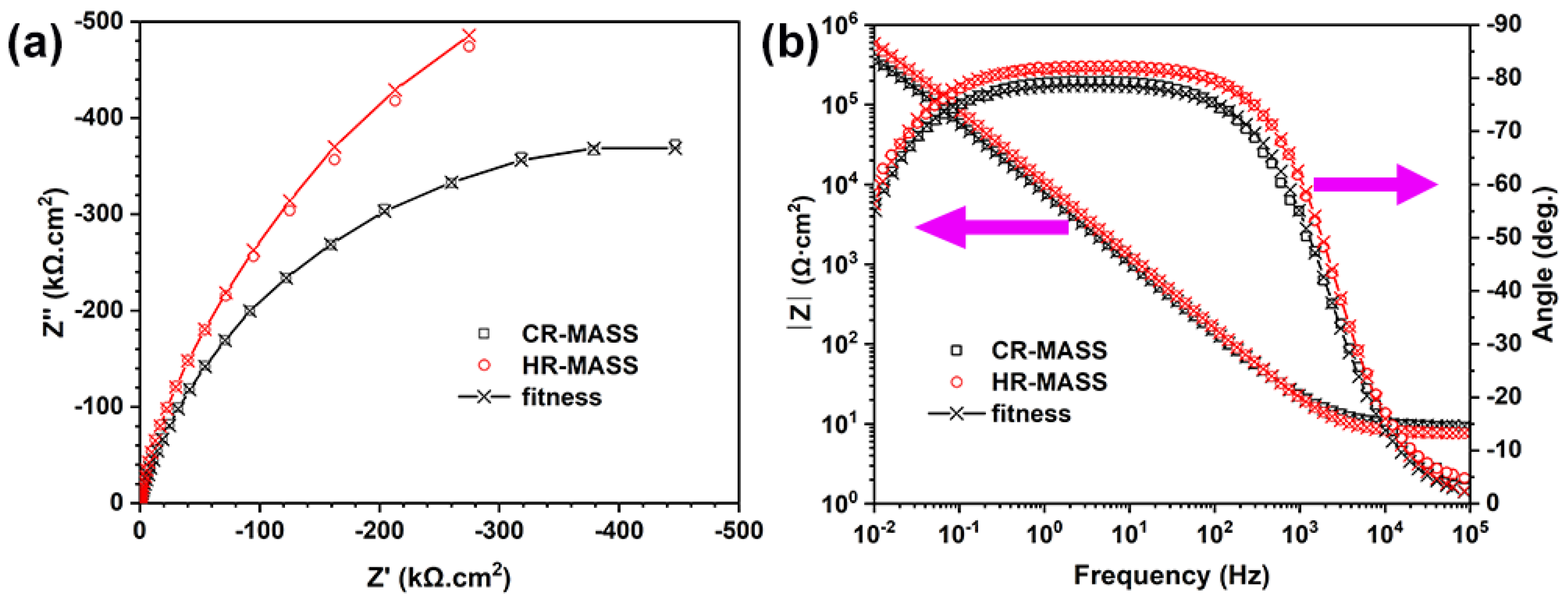
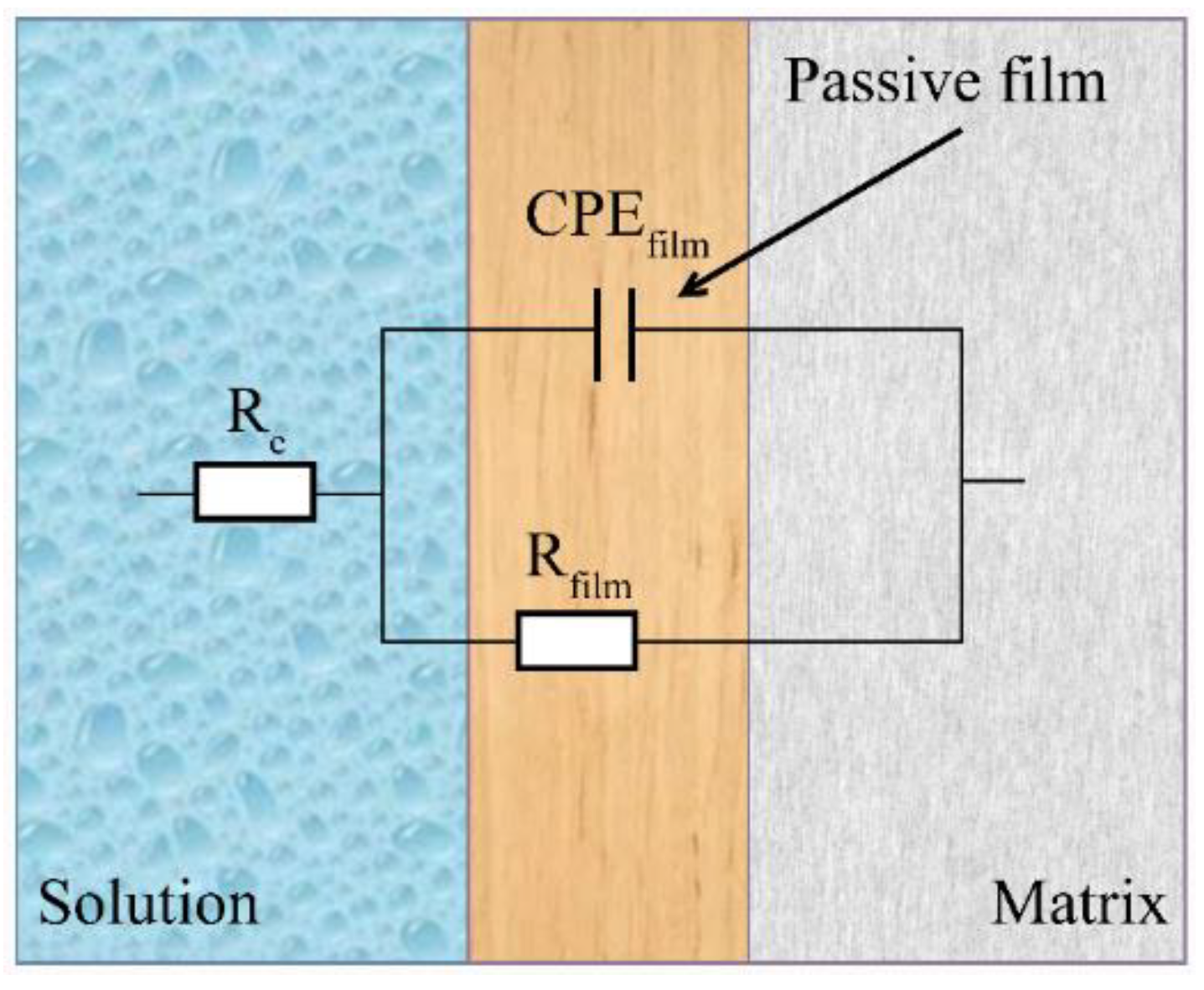
3.2. Electrochemical Performance of the Experimental MASS
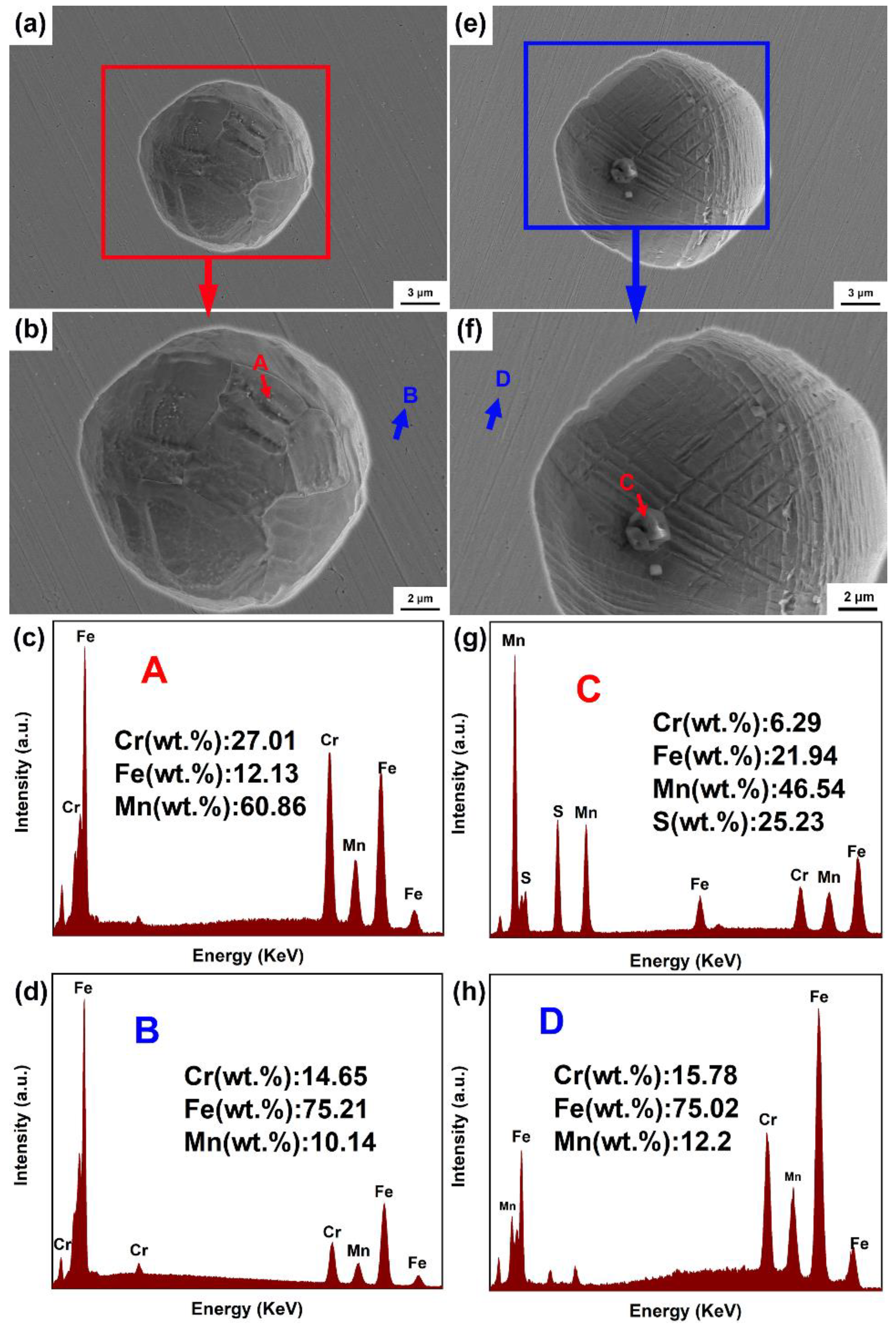
3.3. Pitting Morphology Analysis after Immersion Tests
4. Discussion
4.1. Formation of the Cr-Rich M23C6 Carbides in CR-MASS
4.2. Pitting Mechanism of the CR- and HR-MASS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Backhouse, A.; Baddoo, N. Recent developments of stainless steels in structural applications. CE/Papers 2021, 4, 2349–2355. [Google Scholar] [CrossRef]
- Hariharan, K.; Balachandran, G.; Prasad, M.S. Application of Cost-Effective Stainless Steel for Automotive Components. Mater. Manuf. Process. 2009, 24, 1442–1452. [Google Scholar] [CrossRef]
- Hofmann, H.; Mattissen, D.; Schaumann, T.W. Advanced Cold Rolled Steels for Automotive Applications. Steel Res. Int. 2009, 80, 22–28. [Google Scholar] [CrossRef]
- De Vecchis, R.R.; Wang, X.; Sridar, S.; Wang, Z.; Pataky, G.J.; Xiong, W. Introducing Heusler intermetallics for synergic effect of grain refinement and precipitation strengthening in high-strength low-alloy steels. J. Alloy. Compd. 2022, 904, 163885. [Google Scholar] [CrossRef]
- Taylor, T.; Clough, A. Critical review of automotive hot-stamped sheet steel from an industrial perspective. Mater. Sci. Technol. 2017, 34, 809–861. [Google Scholar] [CrossRef]
- Boes, J.; Röttger, A.; Theisen, W. Microstructure and properties of high-strength C+N austenitic stainless steel processed by laser powder bed fusion. Addit. Manuf. 2020, 32, 101081. [Google Scholar] [CrossRef]
- Hu, L.; Peng, H.; Baker, I.; Li, L.; Zhang, W.; Ngai, T. Characterization of high-strength high-nitrogen austenitic stainless steel synthesized from nitrided powders by spark plasma sintering. Mater. Charact. 2019, 152, 76–84. [Google Scholar] [CrossRef]
- Kim, D.G.; Jo, Y.H.; Song, T.; Kim, H.S.; Lee, B.-J.; Sohn, S.S.; Lee, S. Excellent strength-ductility combination of multi-layered sheets composed of high-strength V10Cr10Fe50Co30 high entropy alloy and 304 austenitic stainless steel. Mater. Sci. Eng. A 2021, 823, 141727. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, K.-A. Direct energy deposition of high strength austenitic stainless steel matrix nanocomposite with superior ductility: Microstructure, tensile properties, and deformation behavior. Mater. Charact. 2021, 179, 111358. [Google Scholar] [CrossRef]
- Schmitt, J.-H.; Iung, T. New developments of advanced high-strength steels for automotive applications. C. R. Phys. 2018, 19, 641–656. [Google Scholar] [CrossRef]
- Vukkum, V.; Gupta, R. Review on corrosion performance of laser powder-bed fusion printed 316L stainless steel: Effect of processing parameters, manufacturing defects, post-processing, feedstock, and microstructure. Mater. Des. 2022, 221, 110874. [Google Scholar] [CrossRef]
- Cheng, M.; He, P.; Lei, L.; Tan, X.; Wang, X.; Sun, Y.; Li, J.; Jiang, Y. Comparative studies on microstructure evolution and corrosion resistance of 304 and a newly developed high Mn and N austenitic stainless steel welded joints. Corros. Sci. 2021, 183, 109338. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Liang, Y.; Liu, P.; Yang, L.; Wang, Y. Effects of heat input and cooling rate during welding on intergranular corrosion behavior of high nitrogen austenitic stainless steel welded joints. Corros. Sci. 2020, 166, 108445. [Google Scholar] [CrossRef]
- Hu, L.; Ngai, T.; Peng, H.; Li, L.; Zhou, F.; Peng, Z. Microstructure and Properties of Porous High-N Ni-Free Austenitic Stainless Steel Fabricated by Powder Metallurgical Route. Materials 2018, 11, 1058. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, C.; Lu, J.; Chen, R.; Chen, D.; Cui, G. A review of progress on high nitrogen austenitic stainless-steel research. Mater. Express 2021, 11, 1901–1925. [Google Scholar] [CrossRef]
- Di Schino, A.; Kenny, J.M.; Mecozzi, M.G.; Barteri, M. Development of high nitrogen, low nickel, 18%Cr austenitic stainless steels. J. Mater. Sci. 2000, 35, 4803–4808. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, K.Y.; Lee, Y.D. Nitrogen-Alloyed, Metastable Austenitic Stainless Steel for Automotive Structural Applications. Mater. Manuf. Process. 2004, 19, 51–59. [Google Scholar] [CrossRef]
- Wállen, B.; Liljas, M.; Stenvall, P. A new high-molybdenum, high-nitrogen stainless steel. Mater. Des. 1992, 13, 329–333. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Bi, H.; Chen, D.; Gu, J.; Chang, E. Mechanical properties of cold-rolled metastable Cr–Mn–Ni–N austenitic stainless steel at low ambient temperature. Mater. Sci. Eng. A 2019, 759, 224–233. [Google Scholar] [CrossRef]
- Fukumoto, S.; Oikawa, Y.; Tsuge, S.; Nomoto, S. Prediction of σ Phase Formation in Fe–Cr–Ni–Mo–N Alloys. ISIJ Int. 2010, 50, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.-s.; Li, J.; Liu, W.-c.; Zhang, W.-n.; Liu, Z.-y. On microstructure characterization of Fe–Cr–Ni–Mo–N super-austenitic stainless steel during hot deformation. J. Iron Steel Res. Int. 2019, 26, 1080–1087. [Google Scholar] [CrossRef]
- Pu, E.; Zheng, W.; Song, Z.; Feng, H.; Zhu, Y. Characterization of Hot Deformation Behavior of a Fe-Cr-Ni-Mo-N Superaustenitic Stainless Steel Using Dynamic Materials Modeling. J. Mater. Eng. Perform. 2017, 26, 1424–1432. [Google Scholar] [CrossRef]
- Tao, X.; Li, X.; Dong, H.; Matthews, A.; Leyland, A. Evaluation of the sliding wear and corrosion performance of triode-plasma nitrided Fe-17Cr-20Mn-0.5N high-manganese and Fe-19Cr-35Ni-1.2Si high-nickel austenitic stainless steels. Surf. Coat. Technol. 2021, 409, 126890. [Google Scholar] [CrossRef]
- Vats, V.; Baskaran, T.; Arya, S.B. Tribo-corrosion study of nickel-free, high nitrogen and high manganese austenitic stainless steel. Tribol. Int. 2018, 119, 659–666. [Google Scholar] [CrossRef]
- Niederhofer, P.; Richrath, L.; Huth, S.; Theisen, W. Influence of conventional and powder-metallurgical manufacturing on the cavitation erosion and corrosion of high interstitial CrMnCN austenitic stainless steels. Wear 2016, 360–361, 67–76. [Google Scholar] [CrossRef]
- Shi, F.; Tian, P.; Jia, N.; Ye, Z.; Qi, Y.; Liu, C.; Li, X. Improving intergranular corrosion resistance in a nickel-free and manganese-bearing high-nitrogen austenitic stainless steel through grain boundary character distribution optimization. Corros. Sci. 2016, 107, 49–59. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting Corrosion of Metals: A Review of the Critical Factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Krawczyk, B.; Cook, P.; Hobbs, J.; Engelberg, D.L. Corrosion Behavior of Cold Rolled Type 316L Stainless Steel in HCl-Containing Environments. Corrosion 2017, 73, 1346–1358. [Google Scholar] [CrossRef]
- Krüger, L.; Wolf, S.; Martin, U.; Martin, S.; Scheller, P.R.; Jahn, A.; Weiß, A. The influence of martensitic transformation on mechanical properties of cast high alloyed CrMnNi-steel under various strain rates and temperatures. J. Phys. Conf. Ser. 2010, 240, 012098. [Google Scholar] [CrossRef]
- Han, D.-X.; Du, L.-X.; Dong, Y.; Misra, R.D.K. The Impact of Deformation Conditions on Divorced Eutectoid Transformation in Bearing Steels. Steel Res. Int. 2018, 90, 1800384. [Google Scholar] [CrossRef]
- Kumar, B.R.; Singh, R.; Mahato, B.; De, P.; Bandyopadhyay, N.; Bhattacharya, D. Effect of texture on corrosion behavior of AISI 304L stainless steel. Mater. Charact. 2005, 54, 141–147. [Google Scholar] [CrossRef]
- Mudali, U.K.; Shankar, P.; Ningshen, S.; Dayal, R.; Khatak, H.; Raj, B. On the pitting corrosion resistance of nitrogen alloyed cold worked austenitic stainless steels. Corros. Sci. 2002, 44, 2183–2198. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Bi, H. The mechanism of pitting initiation and propagation at deformation bands intersection of cold-rolled metastable stainless steel in acidic ferric chloride solution. J. Mater. Sci. 2019, 54, 14914–14925. [Google Scholar] [CrossRef]
- Franck, F.J.; Tambuyser, P.; Zubani, I. X-ray powder diffraction evidence for the incorporation of W and Mo into M23C6 extracted from high-temperature alloys. J. Mater. Sci. 1982, 17, 3057–3065. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Yao, K.-F.; Chen, Y.-B.; Wang, M.-H.; Shao, Y.; Ge, X.-Y. Effects of austenitizing temperature on the microstructure and electrochemical behavior of a martensitic stainless steel. J. Appl. Electrochem. 2015, 45, 375–383. [Google Scholar] [CrossRef]
- Cao, C.; Fu, J.; Tong, T.; Hao, Y.; Gu, P.; Hao, H.; Peng, L. Intermediate-Temperature Creep Deformation and Microstructural Evolution of an Equiatomic FCC-Structured CoCrFeNiMn High-Entropy Alloy. Entropy 2018, 20, 960. [Google Scholar] [CrossRef] [Green Version]
- Fukunaga, T.; Kaneko, K.; Kawano, R.; Ueda, K.; Yamada, K.; Nakada, N.; Kikuchi, M.; Barnard, J.S.; Midgley, P.A. Formation of Intergranular M23C6 in Sensitized Type-347 Stainless Steel. ISIJ Int. 2014, 54, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.; Moon, J.; Bae, J.W.; Park, J.M.; Son, S.; Do, H.-S.; Lee, B.-J.; Kim, H.S. Precipitation-driven metastability engineering of carbon-doped CoCrFeNiMo medium-entropy alloys at cryogenic temperature. Scr. Mater. 2020, 188, 140–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Bi, H.; Gu, J.; Chen, D.; Chang, E.; Zhang, W. Martensite transformation behavior and mechanical properties of cold-rolled metastable Cr-Mn-Ni-N austenitic stainless steels. Mater. Sci. Eng. A 2018, 724, 411–420. [Google Scholar] [CrossRef]
- Han, D.-X.; Du, L.-X.; Yao, C.-X.; Misra, R.D.K. The Evolution of Deformation-Induced Carbides during Divorced Eutectoid Transformation in GCr15 Steels. J. Mater. Eng. Perform. 2019, 28, 5277–5288. [Google Scholar] [CrossRef]
- Han, D.-X.; Du, L.-X.; Zhang, B.; Misra, R.D.K. Effect of deformation on deformation-induced carbides and spheroidization in bearing steel. J. Mater. Sci. 2018, 54, 2612–2627. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, Z.; Zhang, W.; Fang, F.; Tu, Y.; Jiang, J. Deformation-Induced Carbide Transformation in M2 High-Speed Steel. Met. Mater. Trans. A 2019, 51, 568–573. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, L.; Ni, H.; Hao, W.; Man, C.; Chen, S.; Wang, X.; Liu, Z.; Li, X. Influence of temperature on the electrochemical and passivation behavior of 2507 super duplex stainless steel in simulated desulfurized flue gas condensates. Corros. Sci. 2017, 118, 31–48. [Google Scholar] [CrossRef]
- Khalaj, G.; Pouraliakbar, H.; Arab, N.; Nazerfakhari, M. Correlation of passivation current density and potential by using chemical composition and corrosion cell characteristics in HSLA steels. Measurement 2015, 75, 5–11. [Google Scholar] [CrossRef]
- King, A.; Birbilis, N.; Scully, J. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Duan, Z.; Man, C.; Dong, C.; Cui, Z.; Kong, D.; Wang, L.; Wang, X. Pitting behavior of SLM 316L stainless steel exposed to chloride environments with different aggressiveness: Pitting mechanism induced by gas pores. Corros. Sci. 2020, 167, 108520. [Google Scholar] [CrossRef]
- Chen, W.-C.; Wen, T.-C.; Teng, H. Polyaniline-deposited porous carbon electrode for supercapacitor. Electrochim. Acta 2003, 48, 641–649. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Ruan, H.M.; Zhang, G.A. Heterogeneous corrosion of mild steel under SRB-biofilm characterised by electrochemical mapping technique. Corros. Sci. 2011, 53, 2978–2987. [Google Scholar] [CrossRef]
- Macdonald, D.D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Golozar, M.; Saatchi, A.; Raeissi, K. Effect of solution concentration on semiconducting properties of passive films formed on austenitic stainless steels. Corros. Sci. 2010, 52, 205–209. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Cui, Z.; Xiao, K.; Yu, Q.; Li, X. A comparative study of primary and secondary passive films formed on AM355 stainless steel in 0.1 M NaOH. Appl. Surf. Sci. 2018, 427, 763–773. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fan, L.; Zhong, M.; Cheng, L.; Cui, Z. Understanding the effect of fluoride on corrosion behavior of pure titanium in different acids. Corros. Sci. 2021, 192, 109812. [Google Scholar] [CrossRef]
- Bonagani, S.K.; Bathula, V.; Kain, V. Influence of tempering treatment on microstructure and pitting corrosion of 13 wt.% Cr martensitic stainless steel. Corros. Sci. 2018, 131, 340–354. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Liu, Z.; Zhang, D.; Li, X.; Terryn, H. Effect of inclusions modified by rare earth elements (Ce, La) on localized marine corrosion in Q460NH weathering steel. Corros. Sci. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Korshunov, L.G.; Zamatovskii, A.E.; Litvinov, A.V.; Sagaradze, V.; Kositsyna, I.I. Deformation-induced dissolution of carbides of the Me(V, Mo)-C Type in high-manganese steels upon the friction effect. Phys. Met. Met. 2012, 113, 914–921. [Google Scholar] [CrossRef]
- Ryan, M.P.; Williams, D.E.; Chater, R.J.; Hutton, B.M.; McPhail, D.S. Why stainless steel corrodes. Nature 2002, 415, 770–774. [Google Scholar] [CrossRef]
- Tokuda, S.; Muto, I.; Sugawara, Y.; Hara, N. Pit initiation on sensitized Type 304 stainless steel under applied stress: Correlation of stress, Cr-depletion, and inclusion dissolution. Corros. Sci. 2020, 167, 108506. [Google Scholar] [CrossRef]
- Bruemmer, S.M.; Chariot, L.A.; Arey, B.W. Sensitization Development in Austenitic Stainless Steel: Correlation between STEM-EDS and EPR Measurements. Corrosion 1988, 44, 328–333. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, W.; Zhang, T.; Sun, Z.; Wang, Y.; Fan, Y.; Dong, B. Assessment of the correlation between M23C6 precipitates and pitting corrosion resistance of 0Cr13 martensitic stainless steel. Corros. Sci. 2021, 189, 109580. [Google Scholar] [CrossRef]


| Steel | Chemical Composition, wt.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Ni | Cr | Mn | Cu | Si | P | S | N | |
| MASS | 0.015 | 1.025 | 13.2 | 9.15 | 0.25 | 0.5 | 0.023 | 0.003 | 0.145 |
| Sample | OCP (mV/SCE) | ipass (μA/cm2) | Ecorr (mV/SCE) | Epit (mV/SCE) |
|---|---|---|---|---|
| CR-MASS | −57.64 ± 8.24 | 8.37 ± 0.15 | −173.36 ± 18.96 | 105.42 ± 16.34 |
| HR-MASS | −331.82 ± 6.08 | 3.74 ± 0.08 | −451.68 ± 9.74 | 460.40 ± 8.59 |
| Specimen | Re (Ω·cm2) ± 0.5% | CPEf | Rf (kΩ·cm2) ± 2.6% | |
|---|---|---|---|---|
| Qf (MΩ−1·cm−2·sα) ± 1.5% | αf ± 0.3% | |||
| CR-MASS | 4.13 | 23.34 | 0.92 | 607.87 |
| HR-MASS | 9.34 | 16.10 | 0.91 | 1640.11 |
| Sample | Cf (μF·cm−2) | df (nm) |
|---|---|---|
| CR-MASS | 53.60 | 2.58 |
| HR-MASS | 44.07 | 3.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Zou, C.; Huang, R.; Qiu, Y.; Qiu, S.; Zhang, C. Pitting Performance of Cold- and Hot-Rolled Nickel-Saving High-Strength Metastable Austenitic Stainless Steel. Coatings 2022, 12, 1869. https://doi.org/10.3390/coatings12121869
Lu S, Zou C, Huang R, Qiu Y, Qiu S, Zhang C. Pitting Performance of Cold- and Hot-Rolled Nickel-Saving High-Strength Metastable Austenitic Stainless Steel. Coatings. 2022; 12(12):1869. https://doi.org/10.3390/coatings12121869
Chicago/Turabian StyleLu, Siyuan, Chaoyang Zou, Riqing Huang, Yiming Qiu, Shuheng Qiu, and Chi Zhang. 2022. "Pitting Performance of Cold- and Hot-Rolled Nickel-Saving High-Strength Metastable Austenitic Stainless Steel" Coatings 12, no. 12: 1869. https://doi.org/10.3390/coatings12121869




