Synthesis of a Hydroxyl-Containing Corrosion Inhibitor and Its Inhibitory Performance on N80 Steel in Hydrochloric Acid Solution
Abstract
:1. Introduction
2. Experimental Part
2.1. Synthesis of Target Corrosion Inhibitors

2.2. Static Weight Loss Test
2.3. Electrochemical Measurements
2.4. Surface Analysis
3. Results and Discussion
3.1. Characterization of Target Corrosion Inhibitors
3.2. Influence of Inhibitor Concentration on Corrosion Inhibition Performance
3.3. Electrochemical Measurements
3.3.1. Potentiodynamic Polarization Measurements
3.3.2. Electrochemical Impedance Spectroscope (EIS)
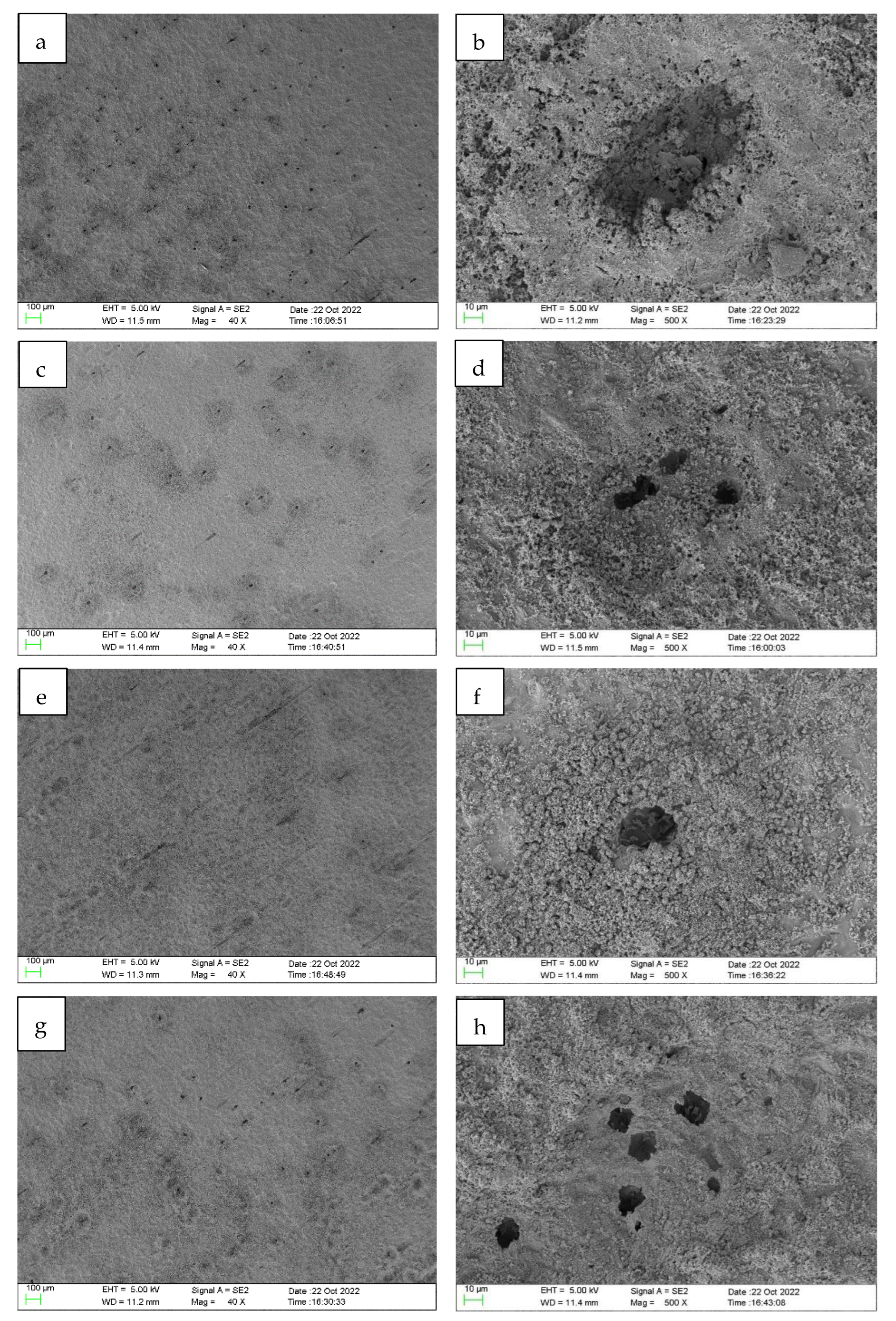
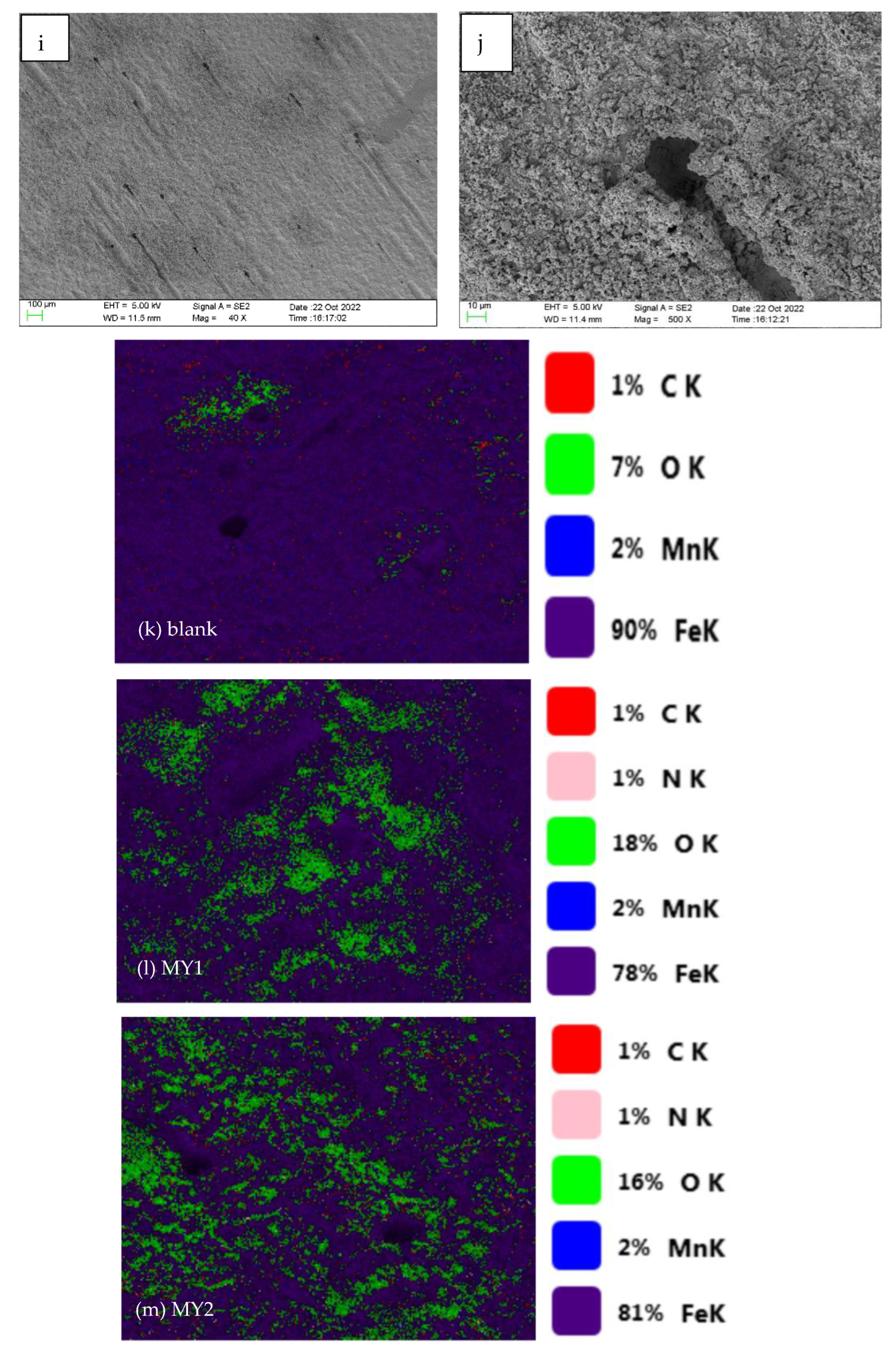
3.4. Corrosion Morphology Analysis of N80 Steel Specimens
3.5. Adsorption and Thermodynamic Studies
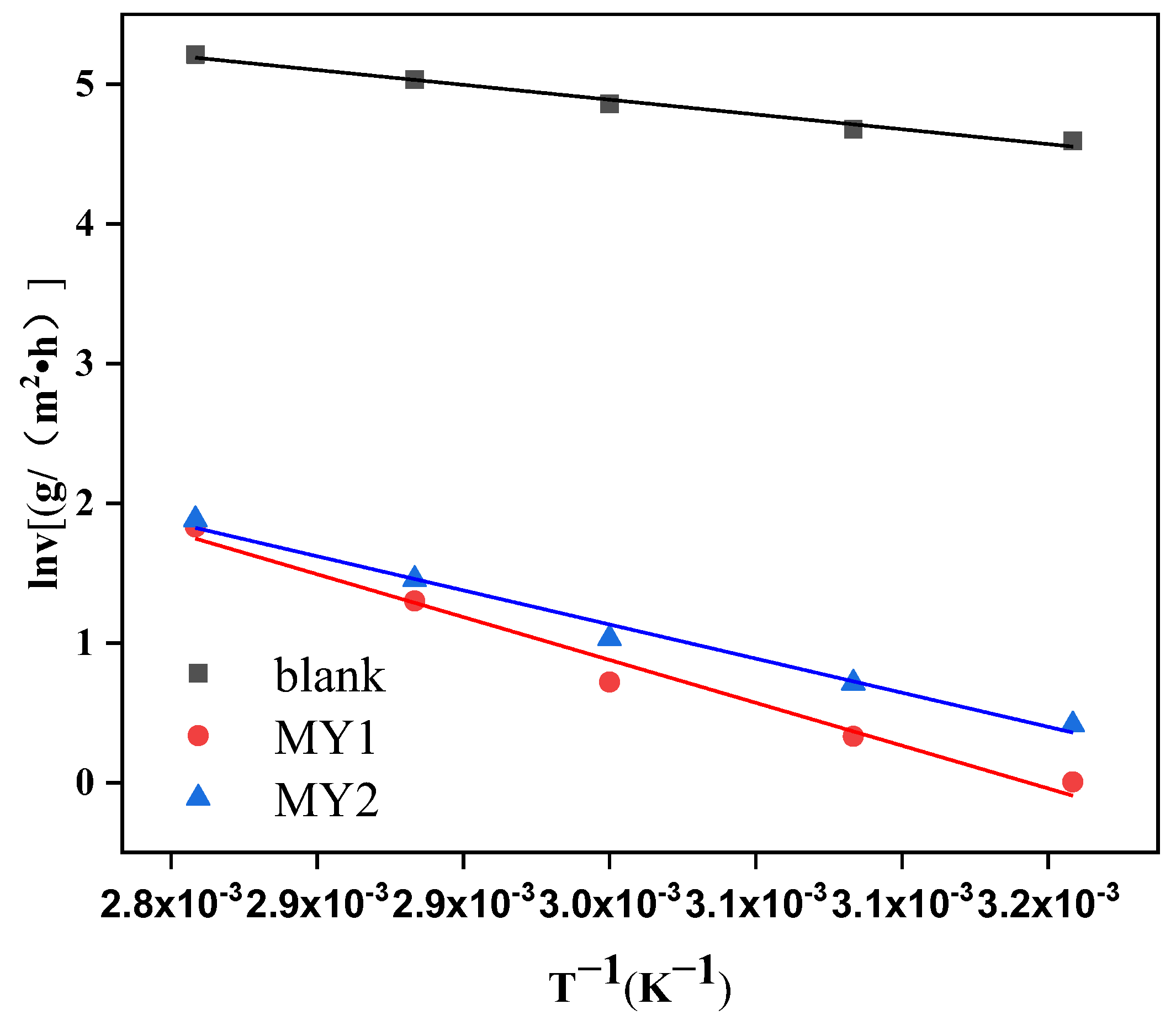
3.5.1. Arrhenius Curve Analysis
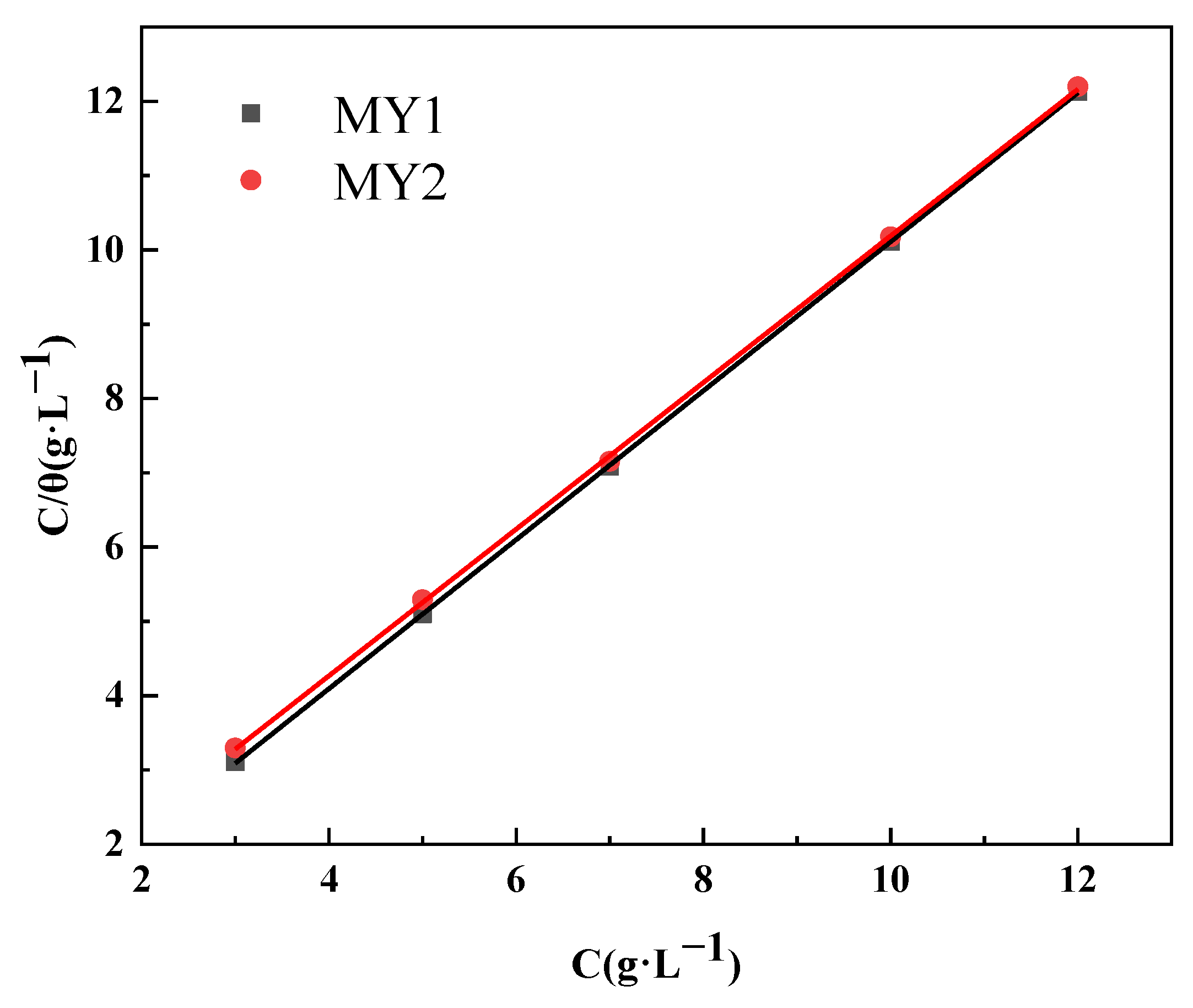
3.5.2. Adsorption Isotherm Analysis
3.6. Contact Angle Test
3.7. Dissolution and Dispersion Test
3.8. Corrosion Inhibition Mechanism Analysis
4. Conclusions
- The corrosion inhibition rates of synthesized MY1 and MY2 in 15% hydrochloric acid solution with a concentration of 0.7% were 98.8% and 97.8%, respectively, indicating that these two corrosion inhibitors have obvious effects on corrosion inhibition. Moreover, the corrosion inhibition effect of MY1 is better than that of MY2.
- From the electrochemical test results, it can be seen that the concentration of the corrosion inhibitor is increased, and the corrosion current density is decreased, indicating that the two corrosion inhibitors can effectively inhibit the electrochemistry reaction. When the corrosion inhibitor is added, the ability of the anode metal surface to lose electrons and the ability of the cathode surface to gather electrons are obviously inhibited, but the Ecorr value is obviously shifted to the positive direction compared with the blank solution, indicating that the inhibitor molecules have a great hindering effect on the anode process, so MY1 and MY2 are high-efficiency corrosion inhibitors of a mixed control type and mainly inhibiting anodes.
- The adsorption behavior of MY1 and MY2 on the metal surface conforms to the Langmuir adsorption isotherm, indicating that the adsorption of inhibitor molecules on the metal surface belongs to monolayer adsorption. According to the Gibbs free energy (∆G) of MY1 and MY2, the adsorption process of the two corrosion inhibitors is spontaneous adsorption and belongs to the mixed adsorption type.
- Through SEM, EDS elemental mapping and contact angle measurement. A layer of hydrophobic protective film was indeed formed on the metal surface after the addition of MY1 and MY2, which weakens the corrosion degree of the metal surface and enhances the hydrophobicity of the metal surface.
- According to the dispersion evaluation experiment of corrosion inhibitor, the hydroxyl group contained in MY1 can promote the dissolution of corrosion inhibitor in an aqueous solution so that the molecules of corrosion inhibitor can form a more dense adsorption film on the metal surface, thus achieving a better effect of corrosion inhibitor.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turnbull, A.; Griffiths, A. Corrosion and cracking of weldable 13 wt% Cr martensitic stainless steels for application in the oil and gas industry. Corros. Eng. Sci. Technol. 2003, 38, 21–50. [Google Scholar] [CrossRef]
- Sainia, N.; Kumaa, R.; Pahujaa, P.; Malika, R.; Malika, R.; Singhalb, S.; Lataa, S. Exploring the Capability of Synthesized PVP-Oxime for Corrosion Inhibition of a Mild Steel Surface in a 1 M H2SO4 Solution. Port. Electrochim. Acta 2020, 38, 43–58. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Chauhan, D.S.; Quraishi, M.; Obot, I. Influence of hydrodynamic condition on 1,3,5-tris (4-methoxyphenyl)-1, 3,5-triazinane as a novel corrosion inhibitor formulation for oil and gas industry. Corros. Eng. Sci. Technol. 2021, 56, 154–161. [Google Scholar] [CrossRef]
- Queiroz Baddini, A.L.; Cardoso, S.P.; Hollauer, E.; Gomes, J.A.d.C.P. Statistical analysis of a corrosion inhibitor family on three steel surfaces (duplex, super-13 and carbon) in hydrochloric acid solutions. Electrochim. Acta 2007, 53, 434–446. [Google Scholar] [CrossRef]
- Barmatov, E.; Geddes, J.; Hughes, T.; Nagl, M. Research on corrosion inhibitors for acid stimulation. In Proceedings of the NACE —International Corrosion Conference Series, Salt Lake City, UT, USA, March 2012. [Google Scholar]
- Ayeni, A.O.; Akinyele, O.F.; Hosten, E.C.; Fakola, E.G.; Olalere, J.T.; Egharevba, G.O.; Watkins, G.M. Synthesis, crystal structure, experimental and theoretical studies of corrosion inhibition of 2-((4-(2-hydroxy-4-methylbenzyl) piperazin-1-yl) methyl)-5-methylphenol–A Mannich base. J. Mol. Struct. 2020, 1219, 128539. [Google Scholar] [CrossRef]
- Ita, B.; Offiong, O. The study of the inhibitory properties of benzoin, benzil, benzoin-(4-phenylthiosemicarbazone) and benzil-(4-phenylthiosemicarbazone) on the corrosion of mild steel in hydrochloric acid. Mater. Chem. Phys. 2001, 70, 330–335. [Google Scholar] [CrossRef]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Rahoma, A.A.B.; Mesmari, H. Electrochemical and quantum chemical calculations on 4,4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. J. Mol. Struct. 2010, 969, 233–237. [Google Scholar] [CrossRef]
- Emregül, K.C.; Hayvalí, M. Studies on the effect of a newly synthesized Schiff base compound from phenazone and vanillin on the corrosion of steel in 2 M HCl. Corros. Sci. 2006, 48, 797–812. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.; Lin, Y.; Quraishi, M.; Lgaz, H.; Chung, I.-M. Corrosion inhibition performance of imidazolidine derivatives for J55 pipeline steel in acidic oilfield formation water: Electrochemical, surface and theoretical studies. J. Taiwan Inst. Chem. Eng. 2019, 95, 341–356. [Google Scholar] [CrossRef]
- Obot, I.; Onyeachu, I.B.; Kumar, A.M. Sodium alginate: A promising biopolymer for corrosion protection of API X60 high strength carbon steel in saline medium. Carbohydr. Polym. 2017, 178, 200–208. [Google Scholar] [CrossRef]
- Xu, C.; Zhan, W.; Tang, X.; Mo, F.; Fu, L.; Lin, B. Self-healing chitosan/vanillin hydrogels based on Schiff-base bond/hydrogen bond hybrid linkages. Polym. Test. 2018, 66, 155–163. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Deng, C.; Yang, L.; Lv, J.; Fu, L. Novel carbon dots as effective corrosion inhibitor for N80 steel in 1 M HCl and CO2-saturated 3.5 wt% NaCl solutions. J. Mol. Struct. 2022, 1250, 131897. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Zhan, F.; Chen, W.; Ding, M.; Qian, C.; Wang, R.; Hou, B. Indolizine quaternary ammonium salt inhibitors, part III: Insights into the highly effective low-toxicity acid corrosion inhibitor–synthesis and protection performance. New J. Chem. 2019, 43, 18461–18475. [Google Scholar] [CrossRef]
- Nwokolo, I.K.; Shi, H.; Ikeuba, A.I.; Gao, N.; Li, J.; Ahmed, S.; Liu, F. Synthesis, Characterization and Investigation of Anticorrosion Properties of an Innovative Metal–Organic Framework, ZnMOF-BTA, on Carbon Steel in HCl Solution. Coatings 2022, 12, 1288. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Gao, K.; Yan, L.; Pang, X. Inhibition of the corrosion of X70 and Q235 steel in CO2-saturated brine by imidazoline-based inhibitor. J. Electroanal. Chem. 2017, 791, 83–94. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.; Kumar, A.; Liu, W.; Songsong, C.; Lin, Y. Electrochemical, surface and quantum chemical studies of novel imidazole derivatives as corrosion inhibitors for J55 steel in sweet corrosive environment. J. Alloys Compd. 2017, 712, 121–133. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, H.; Jiang, R. Synergistic corrosion inhibition effect of quinoline quaternary ammonium salt and Gemini surfactant in H2S and CO2 saturated brine solution. Corros. Sci. 2015, 91, 108–119. [Google Scholar] [CrossRef]
- Yang, Z.; Zhan, F.; Pan, Y.; LYu, Z.; Han, C.; Hu, Y.; Ding, P.; Gao, T.; Zhou, X.; Jiang, Y. Structure of a novel Benzyl Quinolinium Chloride derivative and its effective corrosion inhibition in 15 wt.% hydrochloric acid. Corros. Sci. 2015, 99, 281–294. [Google Scholar] [CrossRef]
- Singh, A.; Bedi, P.; Ansari, K.; Pramanik, T.; Chaudhary, D.; Santra, S.; Alanazi, A.K.; Das, S.; Quraishi, M.; Lin, Y. Inhibition effect of newly synthesized benzoxanthonesderivative on hydrogen evolution and Q235 steel corrosion in 15% HCl under hydrodynamic condition: Combination of experimental, surface and computational study. Int. J. Hydrogen Energy 2021, 46, 37995–38007. [Google Scholar] [CrossRef]
- Elewady, G.; Mostafa, H. Ketonic secondary Mannich bases as corrosion inhibitors for aluminium. Desalination 2009, 247, 573–582. [Google Scholar] [CrossRef]
- Flores, E.A.; Olivares, O.; Likhanova, N.V.; Domínguez-Aguilar, M.A.; Nava, N.; Guzman-Lucero, D.; Corrales, M. Sodium phthalamates as corrosion inhibitors for carbon steel in aqueous hydrochloric acid solution. Corros. Sci. 2011, 53, 3899–3913. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Zhang, Z.; Li, Q.; Lv, R.; Wu, W. Bis-Mannich bases as effective corrosion inhibitors for N80 steel in 15% HCl medium. J. Mol. Liq. 2022, 347, 117957. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Tharini, K.; Sethuraman, M. Studies on a few substituted piperidin-4-one oximes as corrosion inhibitor for mild steel in HCl. J. Mater. Eng. Perform. 2011, 20, 969–977. [Google Scholar] [CrossRef]
- Quraishi, M.A. 2-Amino-3, 5-dicarbonitrile-6-thio-pyridines: New and effective corrosion inhibitors for mild steel in 1 M HCl. Ind. Eng. Chem. Res. 2014, 53, 2851–2859. [Google Scholar]



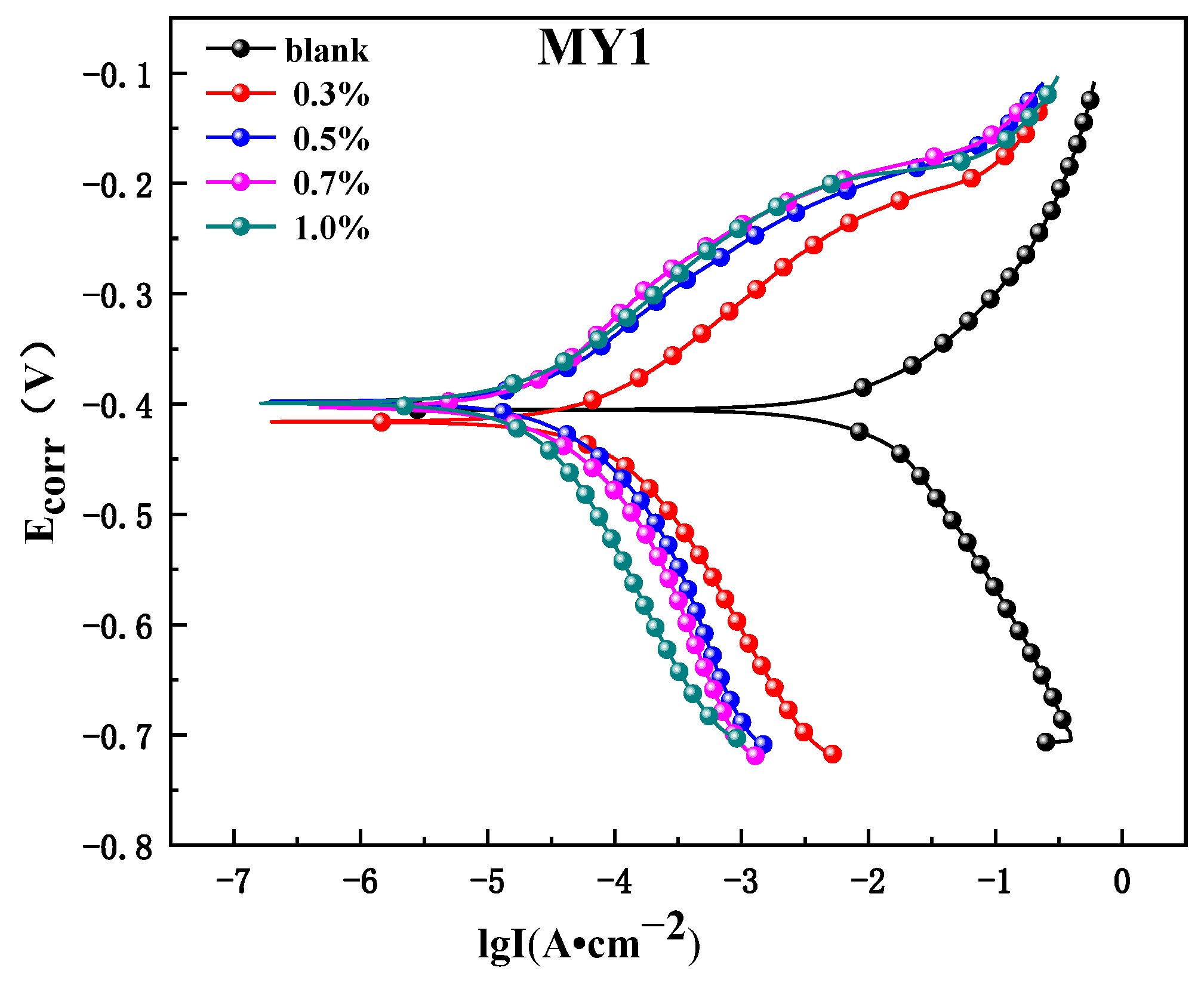
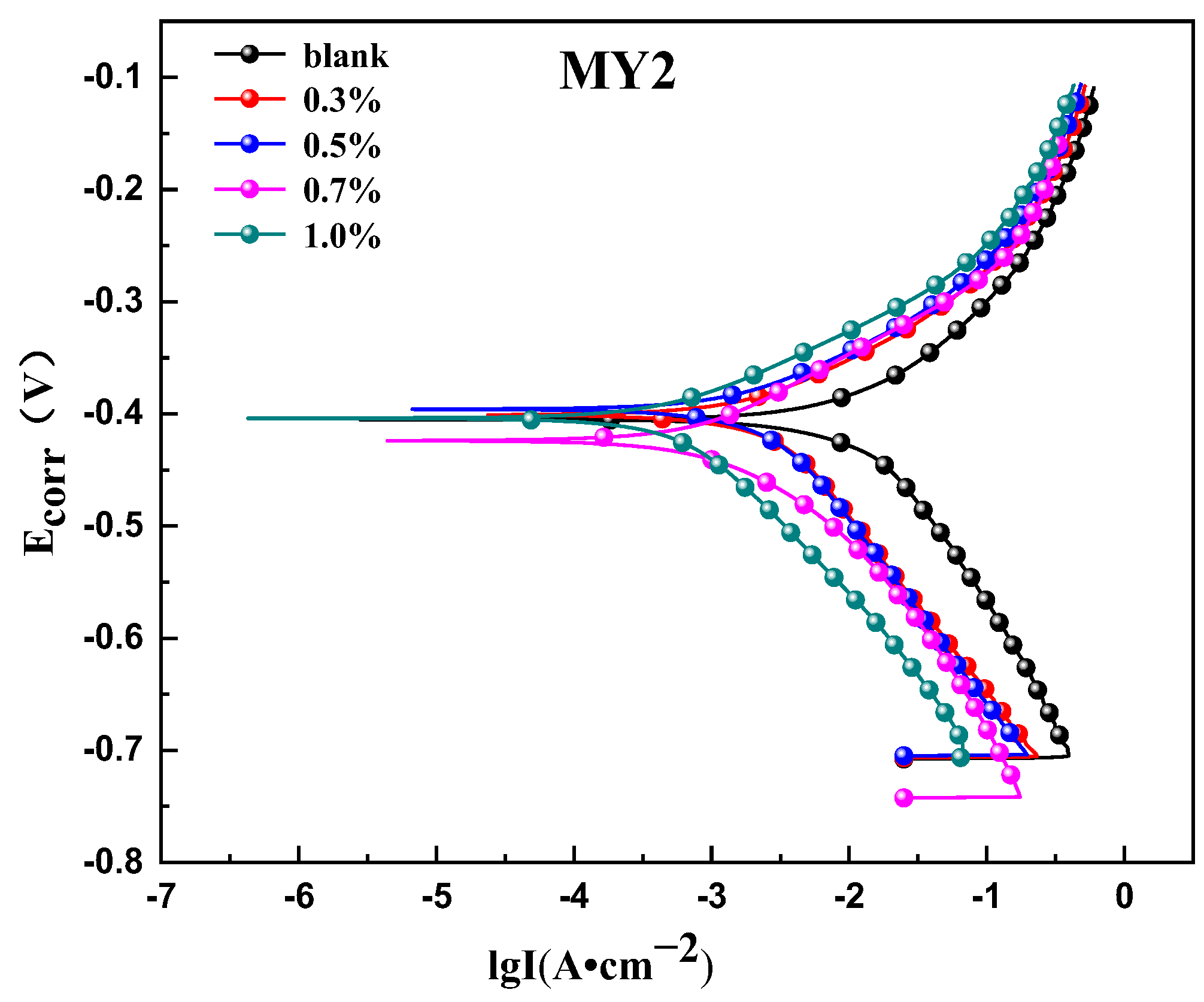
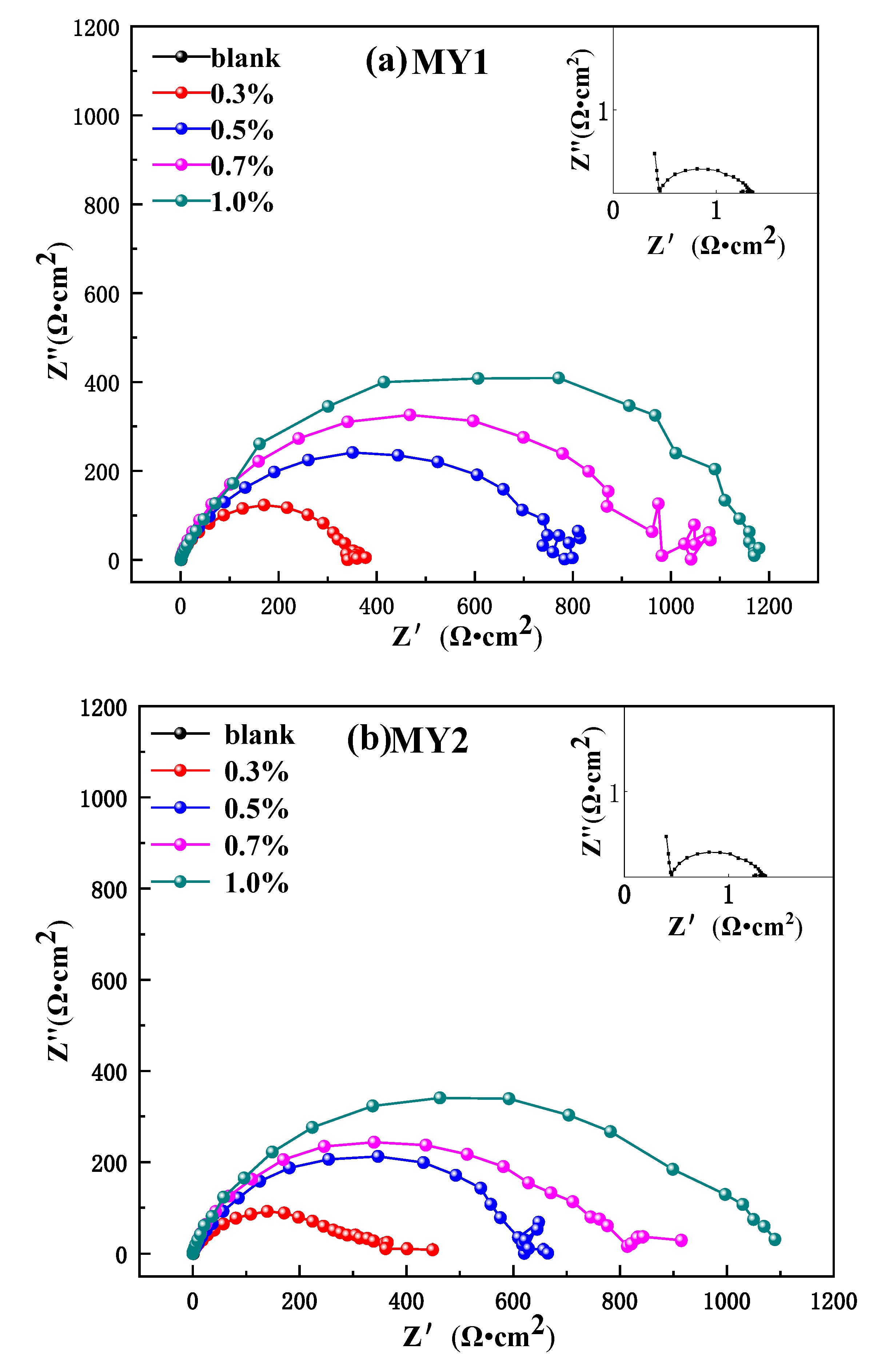

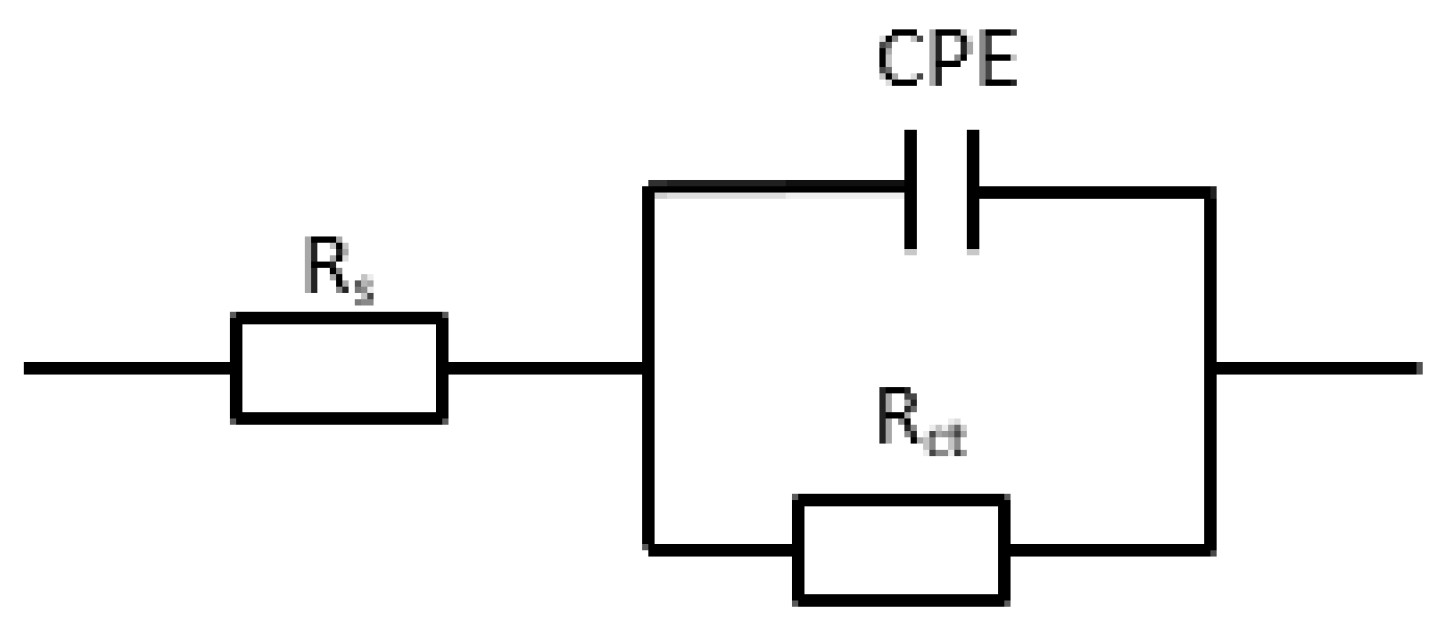


| Inhibitor | C (%) | Ecorr (V) | Icorr (A·cm−2) | Ba (mV·dec−1) | Bc (mV·dec−1) |
|---|---|---|---|---|---|
| blank | 0 | −0.405 | 6.4611 × 10−3 | 69.093 | 78.43 |
| MY1 | 0.3 | −0.416 | 8.7752 × 10−5 | 108.11 | 159.7 |
| 0.5 | −0.398 | 3.4208 × 10−5 | 119.25 | 118.07 | |
| 0.7 | −0.403 | 2.8485 × 10−5 | 143.73 | 118.45 | |
| 1.0 | −0.399 | 2.5375 × 10−5 | 107.17 | 208.28 | |
| MY2 | 0.3 | −0.401 | 3.904 × 10−3 | 122.88 | 174.97 |
| 0.5 | −0.396 | 2.4332 × 10−3 | 89.741 | 129.16 | |
| 0.7 | −0.424 | 9.8526 × 10−4 | 83.096 | 79.171 | |
| 1.0 | −0.404 | 7.165 × 10−4 | 76.624 | 153.25 |
| Inhibitor | C (%) | Rs (Ω·cm2) | Rct (Ω·cm2) | Cdl (μF·cm−2) | n |
|---|---|---|---|---|---|
| blank | 0 | 0.5695 | 0.731 | 256.40 | 0.9765 |
| MY1 | 0.3 | 0.5936 | 342.1 | 18.91 | 0.8682 |
| 0.5 | 0.5529 | 744.9 | 14.00 | 0.8434 | |
| 0.7 | 0.547 | 973 | 11.42 | 0.8567 | |
| 1.0 | 0.5542 | 1151 | 10.79 | 0.8314 | |
| MY2 | 0.3 | 0.1732 | 332.4 | 25.58 | 0.7806 |
| 0.5 | 0.086 | 632.1 | 25.64 | 0.7792 | |
| 0.7 | 0.2265 | 777.9 | 18.09 | 0.8332 | |
| 1.0 | 0.2372 | 995.6 | 19.74 | 0.8455 |
| Inhibitor | Ea (kJ/mol) | R2 |
|---|---|---|
| blank | 14.70 | 0.983 |
| MY1 | 42.51 | 0.980 |
| MY2 | 33.85 | 0.987 |
| Inhibitor | ∆G (kJ/mol) | R2 |
|---|---|---|
| MY1 | −26.117 | 0.999 |
| MY2 | −22.457 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Li, Y.; Gao, K.; Cheng, L.; Yang, X.; Liao, R. Synthesis of a Hydroxyl-Containing Corrosion Inhibitor and Its Inhibitory Performance on N80 Steel in Hydrochloric Acid Solution. Coatings 2022, 12, 1975. https://doi.org/10.3390/coatings12121975
Sun S, Li Y, Gao K, Cheng L, Yang X, Liao R. Synthesis of a Hydroxyl-Containing Corrosion Inhibitor and Its Inhibitory Performance on N80 Steel in Hydrochloric Acid Solution. Coatings. 2022; 12(12):1975. https://doi.org/10.3390/coatings12121975
Chicago/Turabian StyleSun, Shoumin, Yang Li, Kejing Gao, Li Cheng, Xue Yang, and Ruiquan Liao. 2022. "Synthesis of a Hydroxyl-Containing Corrosion Inhibitor and Its Inhibitory Performance on N80 Steel in Hydrochloric Acid Solution" Coatings 12, no. 12: 1975. https://doi.org/10.3390/coatings12121975





