An In Vitro Study of the Antifungal Efficacy of Zinc Oxide Nanoparticles against Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of ZnO NPs
2.2. Preparation of ZnO NPs Suspension
2.3. Exposure of Yeast to ZnO NPs
2.4. Growth Inhibition Test
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. SEM-EDX Analysis
2.7. Statistical Analysis
3. Results and Discussion
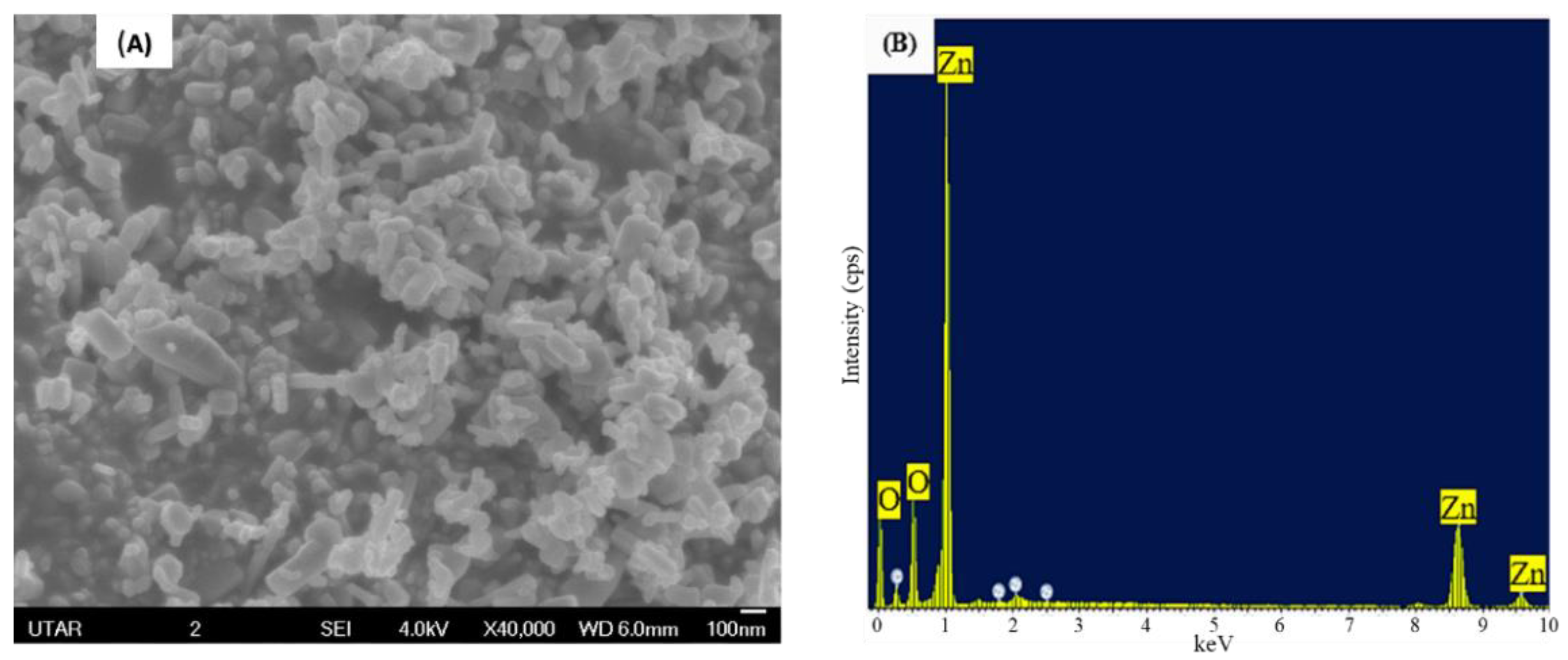
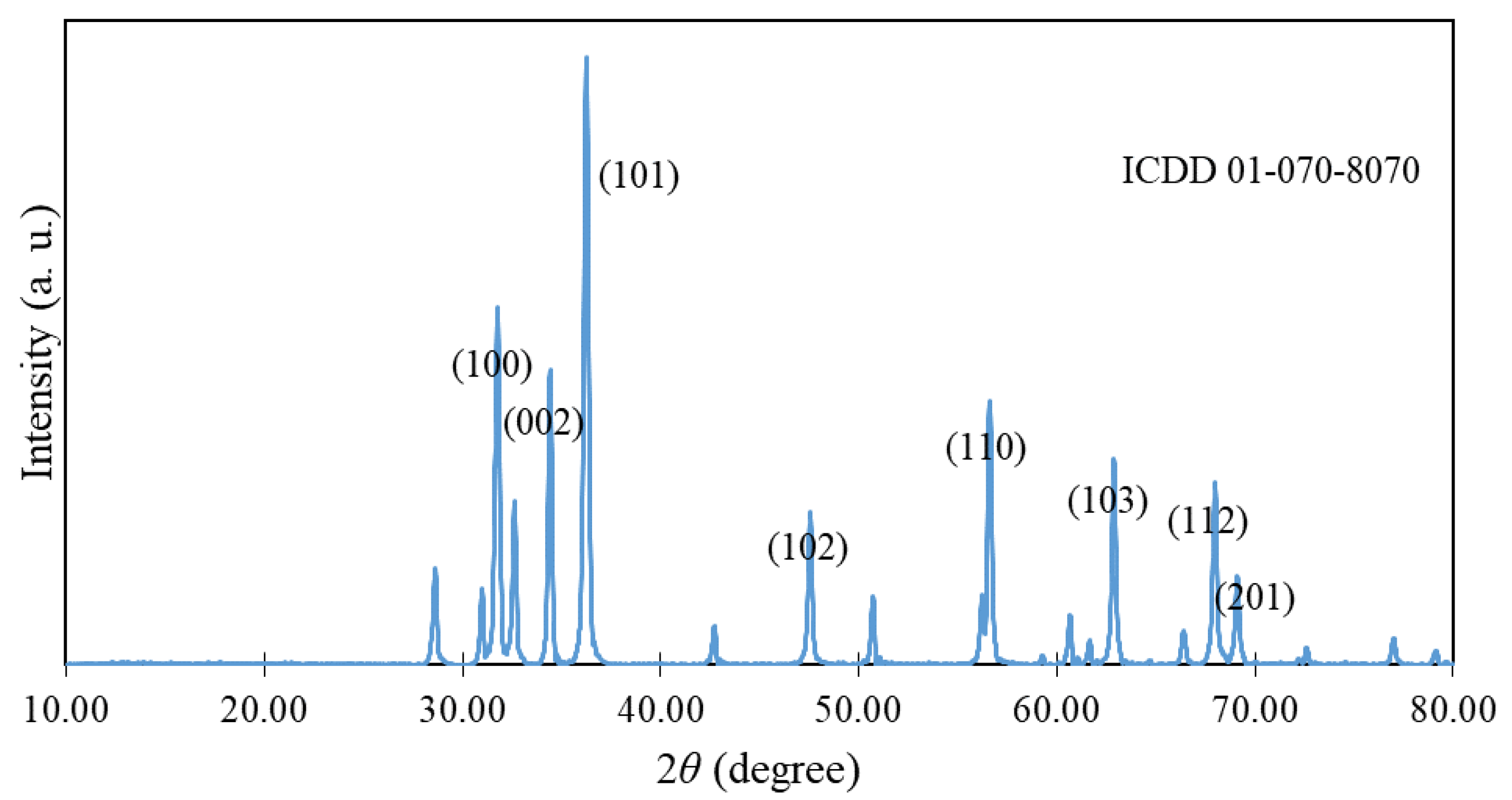
3.1. Characterization of ZnO NPs
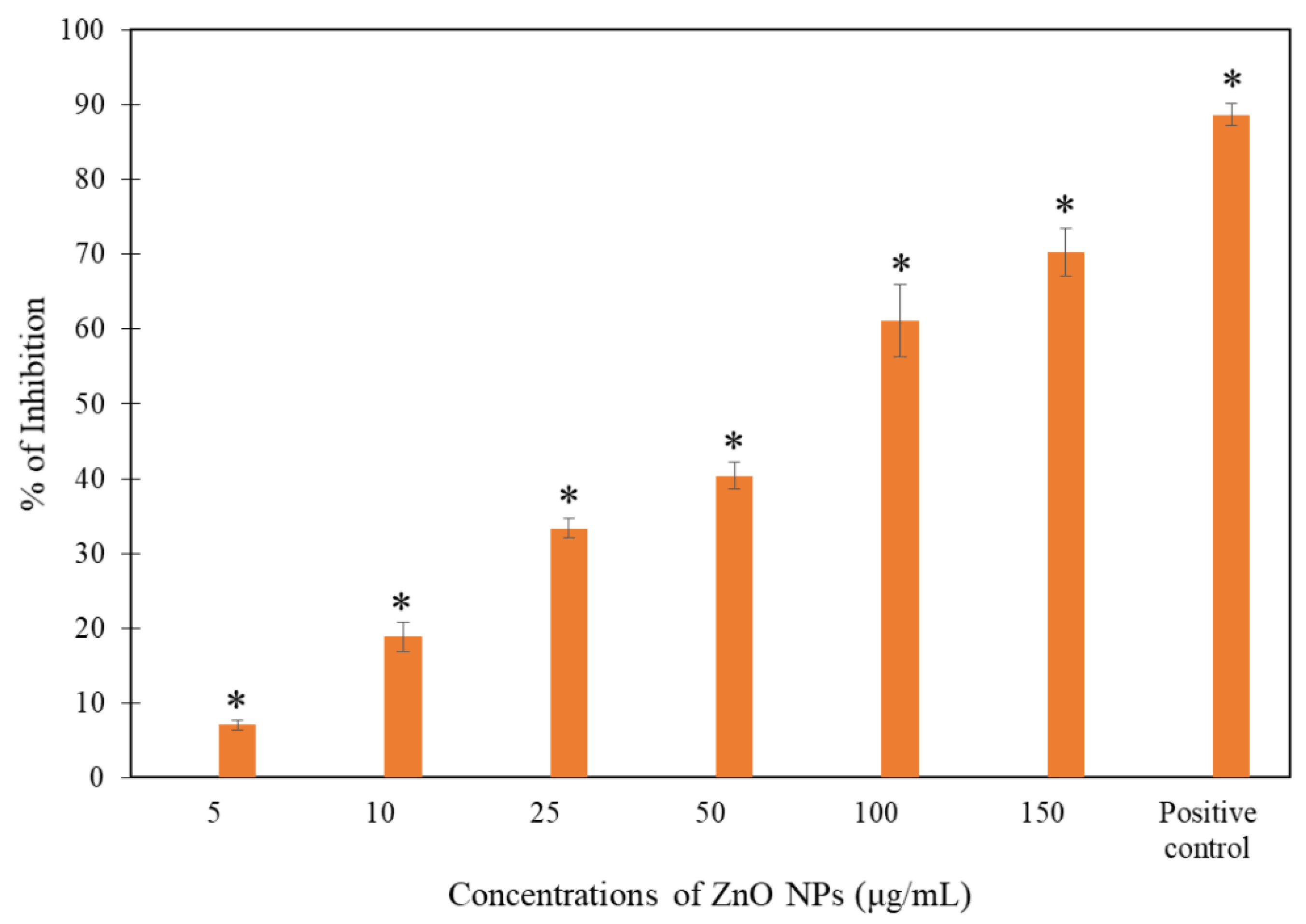
3.2. Growth Inhibition Test
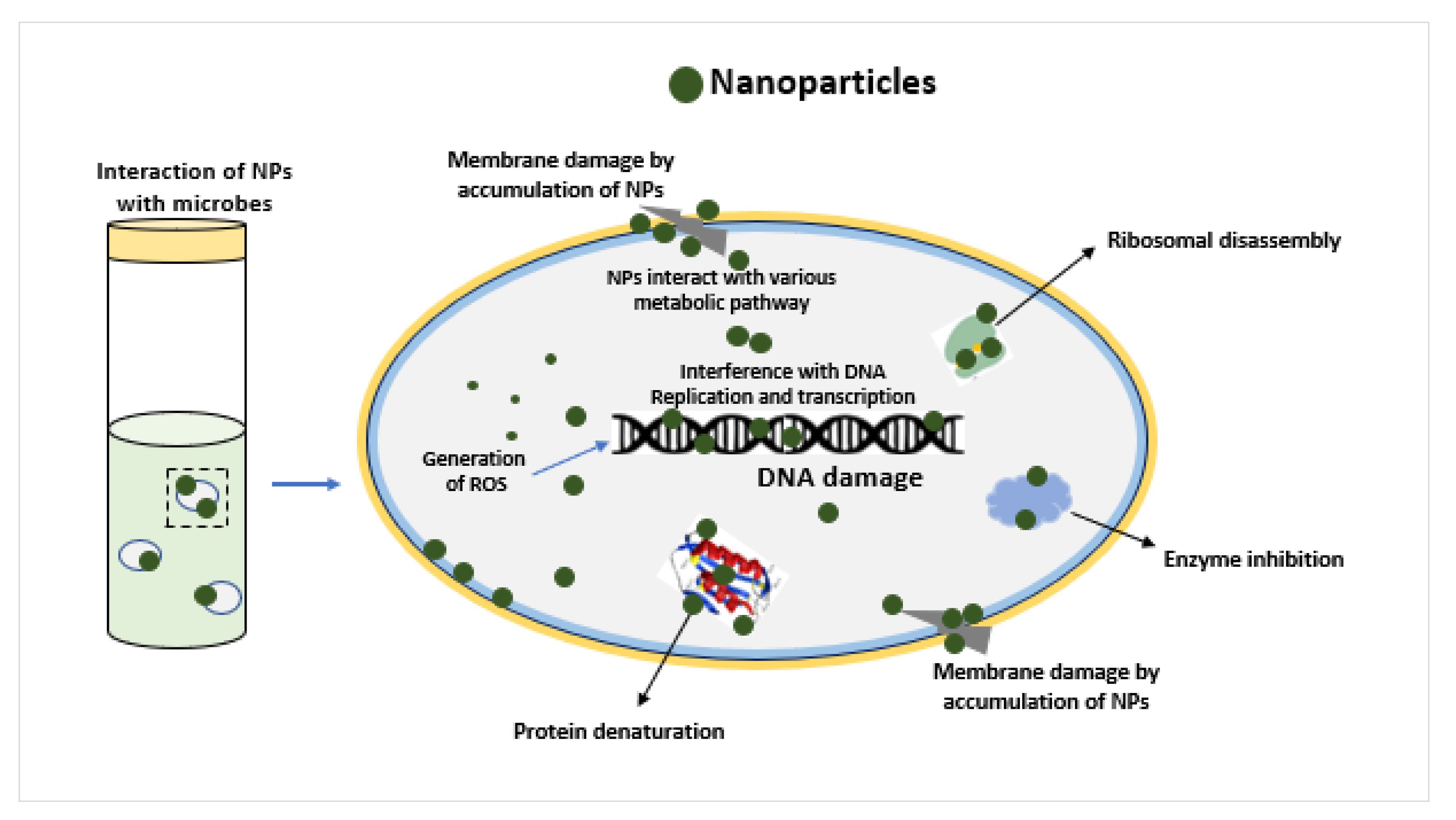
3.3. Surface Interaction of ZnO NPs on the Yeast Cell Wall
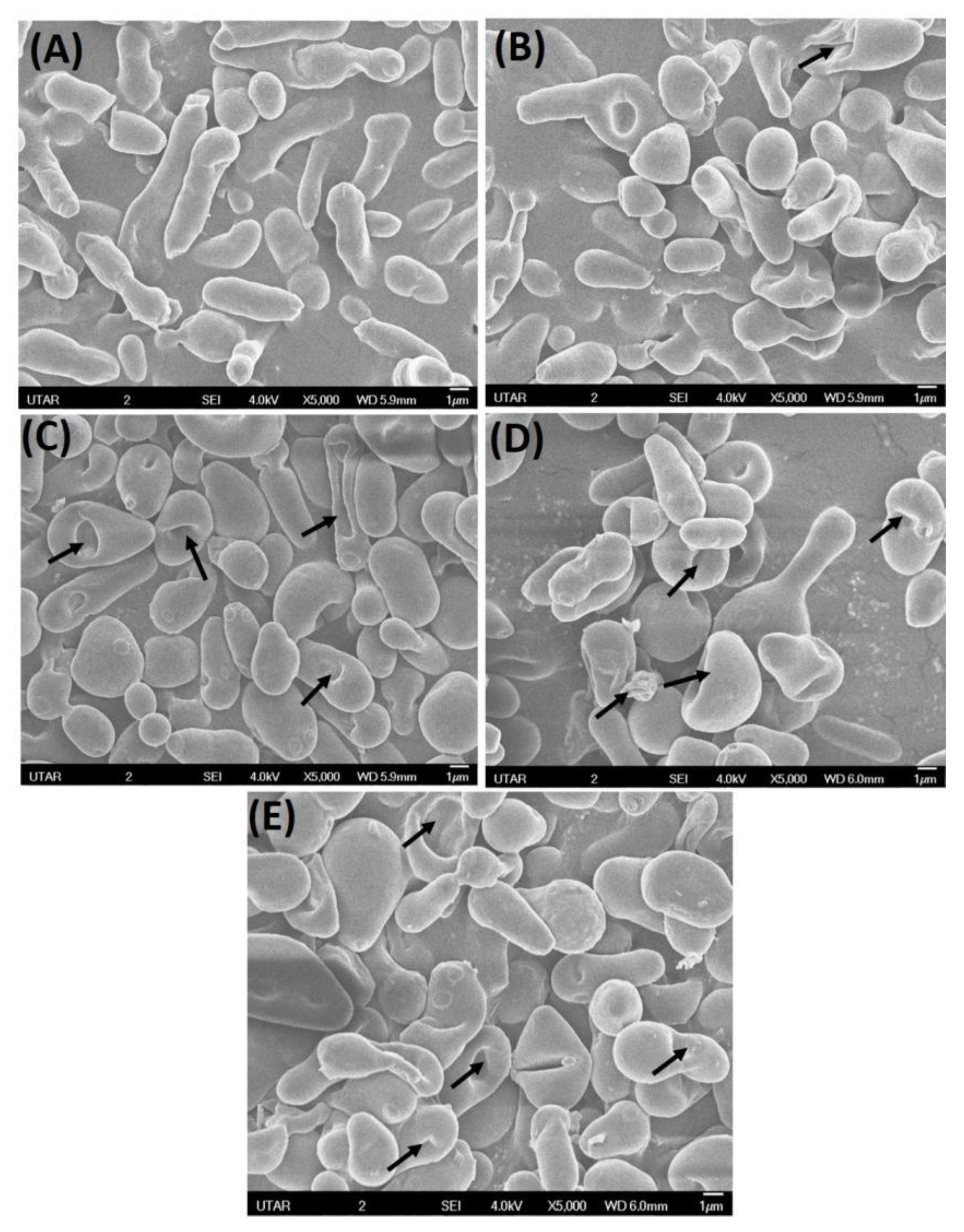
3.4. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Zhu, A. Emerging invasive fungal infections: Clinical features and controversies in diagnosis and treatment processes. Infect. Drug Resist. 2020, 13, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Yu, S.-J.; Heitman, J.; Wellington, M.; Chen, Y.-L. New facets of antifungal therapy. Virulence 2016, 8, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.; Amaral, A.C. Antifungal Therapy for Systemic Mycosis and the Nanobiotechnology Era: Improving Efficacy, Biodistribution and Toxicity. Front. Microbiol. 2017, 8, 336. [Google Scholar] [CrossRef]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current insights on antifungal therapy: Novel nanotechnology approaches for drug delivery systems and new drugs from natural sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Djearamane, S.; Wong, L.S.; Lim, Y.M.; Lee, P.F. Oxidative stress effects of zinc oxide nanoparticles on fresh water microalga Haematococcus pluvialis. Ecol. Environ. Conserv. 2020, 26, 2020–2663. [Google Scholar]
- Zhang, Z.; Wang, H.; Chen, Z.; Wang, X.; Choo, J.; Chen, L. Plasmonic colorimetric sensors based on etching and growth of noble metal nanoparticles: Strategies and applications. Biosens Bioelectron. 2018, 114, 52–65. [Google Scholar] [CrossRef]
- Soto-Robles, C.A.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Nava, O. Vilchis-Nestor, A.R.; Lugo-Medina, E. Ranjithkumar. R. Castro-Beltrán, A. Study on the effect of the concentration of Hibiscus sabdariffa extract on the green synthesis of ZnO nanoparticles. Results Phys. 2019, 15, 1–8. [Google Scholar] [CrossRef]
- Djearamane, S.; Wong, L.S.; Lim, Y.M.; Lee, P.F. Cytotoxic Effects of Zinc Oxide Nanoparticles on Chlorella vulgaris. Pollut. Res. 2019, 38, 479–484. [Google Scholar]
- Ranjithkumar, R.; Selvam, K.; Shanmugavadivu, M. Antimicrobial coated textile via biogenic synthesis silver nanoparticles. J. Green Sci. Technol. 2013, 1, 111–113. [Google Scholar]
- Rauf, M.A.; Oves, M.; Rehman, F.U.; Khan, A.R.; Husain, N. Bougainvillea flower extract mediated zinc oxide’s nanomaterials for antimicrobial and anticancer activity. Biomed. Pharmacother. 2019, 116, 108983. [Google Scholar] [CrossRef] [PubMed]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Oves, M.; Hussain, S.Z.; Khan, I.U.; Abdel-wahab, M.S. Structural and optical characteristics, and bacterial decolonization studies on non-reactive RF sputtered Cu–ZnO@ graphene-based nanoparticles thin films. J. Mater. Sci. 2019, 54, 6515–6529. [Google Scholar] [CrossRef]
- Djearamane, S.; Ravintharan, T.; Liang, S.X.T.; Wong, L.S. Sensitivity of Proteus vulgaris to Zinc Oxide Nanoparticles. Sains Malays. 2022, 51, 1353–1362. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Chandar Shekar, B.; Bhuvaneshwari, V. Preparation of chitosan coated zinc oxide nanocomposites for enhanced antibacterial and photocatalytic activity: As a bionanocomposite. Int. J. Biol. Macromol. 2019, 129, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-E.; Jin, H.-E. Antimicrobial Activity of Zinc Oxide Nano/Microparticles and Their Combinations against Pathogenic Microorganisms for Biomedical Applications: From Physicochemical Characteristics to Pharmacological Aspects. Nanomaterials 2021, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.U.; Ahmed, A.; Usman, M.; Salman, S. Recent advances in nanotechnology-aided materials in combating microbial resistance and functioning as antibiotics substitutes. Int. J. Nanomed. 2020, 15, 7329. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlén, A. The global preclinical antibacterial pipeline. Nat. Rev. Genet. 2019, 18, 275–285. [Google Scholar] [CrossRef]
- Li, W.; Separovic, F.; O’Brien-Simpson, N.M.; Wade, J.D. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 2021, 50, 4932–4973. [Google Scholar] [CrossRef]
- Djearamane, S.; Lim, Y.M.; Wong, L.S.; Lee, P.F. Cellular accumulation and cytotoxic effects of zinc oxide nanoparticles in microalga Haematococcus pluvialis. PeerJ 2019, 7, e7582. [Google Scholar] [CrossRef]
- Maheswaran, H.; Wong, L.S.; Dhanapal, A.C.T.A.; Narendhirakannan, R.T.; Janakiraman, A.K.; Djearamane, S. Toxicity of zinc oxide nanoparticles on human skin dermal cells. J. Exp. Biol. Agric. Sci. 2021, 9, S95–S100. [Google Scholar] [CrossRef]
- Djearamane, S.; Loh, Z.C.; Lee, J.J.; Wong, L.S.; Rajamani, R.; Luque, P.A.; Gupta, P.K.; Liang, S.X.T. Remedial Aspect of Zinc Oxide Nanoparticles Against Serratia marcescens and Enterococcus faecalis. Front. Pharmacol. 2022, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Munoz, P.; Bouza, E.; Cuenca-Estrella, M.; Eiros, J.M.; Pérez, M.J.; Sánchez-Somolinos, M.; Peláez, T. Saccharomyces cerevisiae Fungemia: An Emerging Infectious Disease Source: Clinical Infectious Diseases. Oxf. J. 2005, 40, 1625–1634. [Google Scholar]
- Bojsen, R.; Regenberg, B.; Folkesson, A. Saccharomyces cerevisiae biofilm tolerance towards systemic antifungals depends on growth phase. BMC Microbiol. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Michlik, T.; Schmid, M.; Rosin, A.; Gerdes, T.; Moos, R. Mechanical coating of zinc particles with Bi2O3-Li2O-ZnO glasses as anode material for rechargeable zinc-based batteries. Batteries 2018, 4, 12. [Google Scholar] [CrossRef]
- Ahmad, W.; Kalra, D. Green synthesis, characterization and anti-microbial activities of ZnO nanoparticles using Euphorbia hirta leaf extract. J. King Saud Univ.-Sci. 2020, 32, 2358–2364. [Google Scholar] [CrossRef]
- Saini, M.; Yadav, S.; Rani, N.; Mushtaq, A.; Rawat, S.; Saini, K. Antibacterial study of nanosized zinc oxide (F1) against various gram-positive and gram-negative bacteria. Mater. Today Proc. 2022, 67, 852–857. [Google Scholar] [CrossRef]
- Nasrollahi, A.; Pourshamsian, K.H.; Mansourkiaee, P. Antifungal activity of silver nanoparticles on some of fungi. Int. J. Nano Dim. 2011, 1, 233–239. [Google Scholar]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Aliev, G. Flower-shaped ZnO nanoparticles synthesized by a novel approach at near-room temperatures with antibacterial and antifungal properties. Int. J. Nanomed. 2014, 9, 853–864. [Google Scholar] [CrossRef]
- Miri, A.; Mahdinejad, N.; Ebrahimy, O.; Khatami, M.; Sarani, M. Zinc oxide nanoparticles: Biosynthesis, characterization, antifungal and cytotoxic activity. Mater. Sci. Eng. C 2019, 104, 109981. [Google Scholar] [CrossRef] [PubMed]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ.–Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Naumann, D. FT-Infrared and FT-Raman spectroscopy in biomedical research. Appl. Spectrosc. Rev. 2001, 36, 239–298. [Google Scholar] [CrossRef]
- Burattini, E.; Cavagna, M.; Dell’Anna, R.; Campeggi, F.M.; Monti, F.; Rossi, F.; Torriani, S. A FTIR microspectroscopy study of autolysis in cells of the wine yeast Saccharomyces cerevisiae. Vib. Spectrosc. 2008, 47, 139–147. [Google Scholar] [CrossRef]
- Kiwi, J.; Nadtochenko, V. Evidence for the mechanism of photocatalytic degradation of the bacterial wall membrane at the TiO2 interface by ATR–FTIR and laser kinetic spectroscopy. Langmuir 2005, 21, 4631–4641. [Google Scholar] [CrossRef]
- Fang, J.; Lyon, D.Y.; Wiesner, M.R.; Dong, J.; Alvarez, P.J. Effect of a fullerene water suspension on bacterial phospholipids and membrane phase behaviour. Environ. Sci. Technol. 2007, 41, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Sivakesava, S.; Irudayaraj, J.; Debroy, C. Differentiation of microorganisms by FTIR-AIR and NIR spectroscopy. Trans. ASAE 2004, 47, 951–957. [Google Scholar] [CrossRef]
- Mura, S.; Greppi, G.; Marongiu, M.L.; Roggero, P.P.; Ravindranath, S.P.; Mauer, L.J.; Irudayaraj, J. FTIR nanobiosensors for Escherichia coli detection. Beilstein J. Nanotechnol. 2012, 3, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.; Nethradevi, C.; Renganathan, S. Synthesis of silver nanoparticles using Lantana Camara fruit extract and its effect on pathogens. Asian J. Pharm. Clin. Res. 2012, 5, 97–101. [Google Scholar]
- Burgula, Y.; Khali, D.; Kim, S.; Krishnan, S.S.; Cousin, M.A.; Gore, J.P.; Mauer, L.J. Detection of Escherichia coli O157:H7 and Salmonella typhimurium using filtration followed by Fourier-transform infrared spectroscopy. J. Food Prot. 2006, 69, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio Silver: Its Interactions with Peptides and Bacteria, and Its Uses in Medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef] [PubMed]
- Correa-Garcia, S.; Bermudez-Moretti, M.; Travo, A.; Dekeris, G.; Fortar, I. FTIR spectroscopic metabolome analysis of lyophilized and fresh Saccharomyces cerevisiae cells. Anal. Methods 2014, 6, 1855–1861. [Google Scholar] [CrossRef]
- Gorgulu, S.T.; Dogan, M.; Severcan, F. The characterization and differentiation of higher plants by Fourier transform infrared spectroscopy. Appl. Spectrosc. 2007, 61, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Lasch, P.; Boese, M.; Pacifico, A.; Diem, M. FT-IR spectroscopic investigations of single cells on the subcellular level. Vib. Spectrosc. 2002, 28, 147–157. [Google Scholar] [CrossRef]
- Cieślik-Boczula, K.; Czarnik-Matusewicz, B.; Perevozkina, M.; Rospenk, M. MCR-ALS as an effective tool for monitoring subsequent phase transitions in pure and doped DPPC liposomes. R. Soc. Chem. Adv. 2015, 5, 40455–40464. [Google Scholar] [CrossRef]
- Casal, H.L.; Mantsch, H.H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim. Biophys. Acta 1984, 779, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Plata, M.R.; Koch, C.; Wechselberger, P.; Herwig, C.; Lendl, B. Determination of carbohydrates present in Saccharomyces cerevisiae using mid-infrared spectroscopy and partial least squares regression. Anal. Bioanal. Chem. 2013, 405, 8241–8250. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yang, K.; Vachet, R.W.; Xing, B. Interaction between oxide nanoparticles and biomolecules of the bacterial cell envelope as examined by infrared spectroscopy. Langmuir 2010, 26, 18071–18077. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kurcz, A. Effects of Selenium on Morphological Changes in Candida utilis ATCC 9950 Yeast Cells. Biol. Trace Elem. Res. 2015, 169, 387–393. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Q.; Jia, S.; Qiao, C. Effects of high pressure on the accumulation of trehalose and glutathione in the Saccharomyces cerevisiae cells. Biochem. Eng. J. 2007, 37, 226–230. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S. Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int. J. Nanomed. 2018, 13, 2647–2663. [Google Scholar] [CrossRef]
- Liang, S.X.T.; Wong, L.S.; Lim, Y.M.; Lee, P.F.; Djearamane, S. Effects of zinc oxide nanoparticles on Streptococcus pyogenes. South Afr. J. Chem. Eng. 2020, 34, 63–71. [Google Scholar] [CrossRef]
- Dhanasegaran, K.; Djearamane, S.; Liang, S.X.T.; Wong, L.S.; Kasivelu, G.; Lee, P.F.; Lim, Y.M. Antibacterial properties of zinc oxide nanoparticles on Pseudomonas aeruginosa (ATCC 27853). Sci. Iran. 2022, 28, 3806–3815. [Google Scholar]
- Eskandari, M.; Haghighi, N.; Ahmadi, V.; Haghighi, F.; Mohammadi, S.R. Growth and investigation of antifungal properties of ZnO nanorod arrays on the glass. Phys. B Condens. Matter 2011, 406, 112–114. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ghose, R. Synthesis of zinc oxide nanoparticles by homogeneous precipitation method and its application in antifungal activity against Candida albicans. Ceram. Int. 2014, 41, 967–975. [Google Scholar] [CrossRef]
- Kasemets, K.; Ivask, A.; Dubourguier, H.C.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. In Vitro 2009, 23, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Galván Márquez, I.; Ghiyasvand, M.; Massarsky, A.; Babu, M.; Samanfar, B.; Omidi, K.; Golshani, A. Zinc oxide and silver nanoparticles toxicity in the baker’s yeast, Saccharomyces cerevisiae. PLoS ONE 2018, 13, e0193111. [Google Scholar] [CrossRef]
- Abdul, A.T.; Ali, A.F. Effect of zinc oxide nanoparticles on candida albicans of human saliva (in vitro study). Int. J. Res. Dev. Pharm. Life Sci. 2015, 4, 1892–1900. [Google Scholar]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G. Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by aone-potsol–gel method. Environ. Sci. Technol. 2009, 43, 2905–2910. [Google Scholar] [CrossRef]
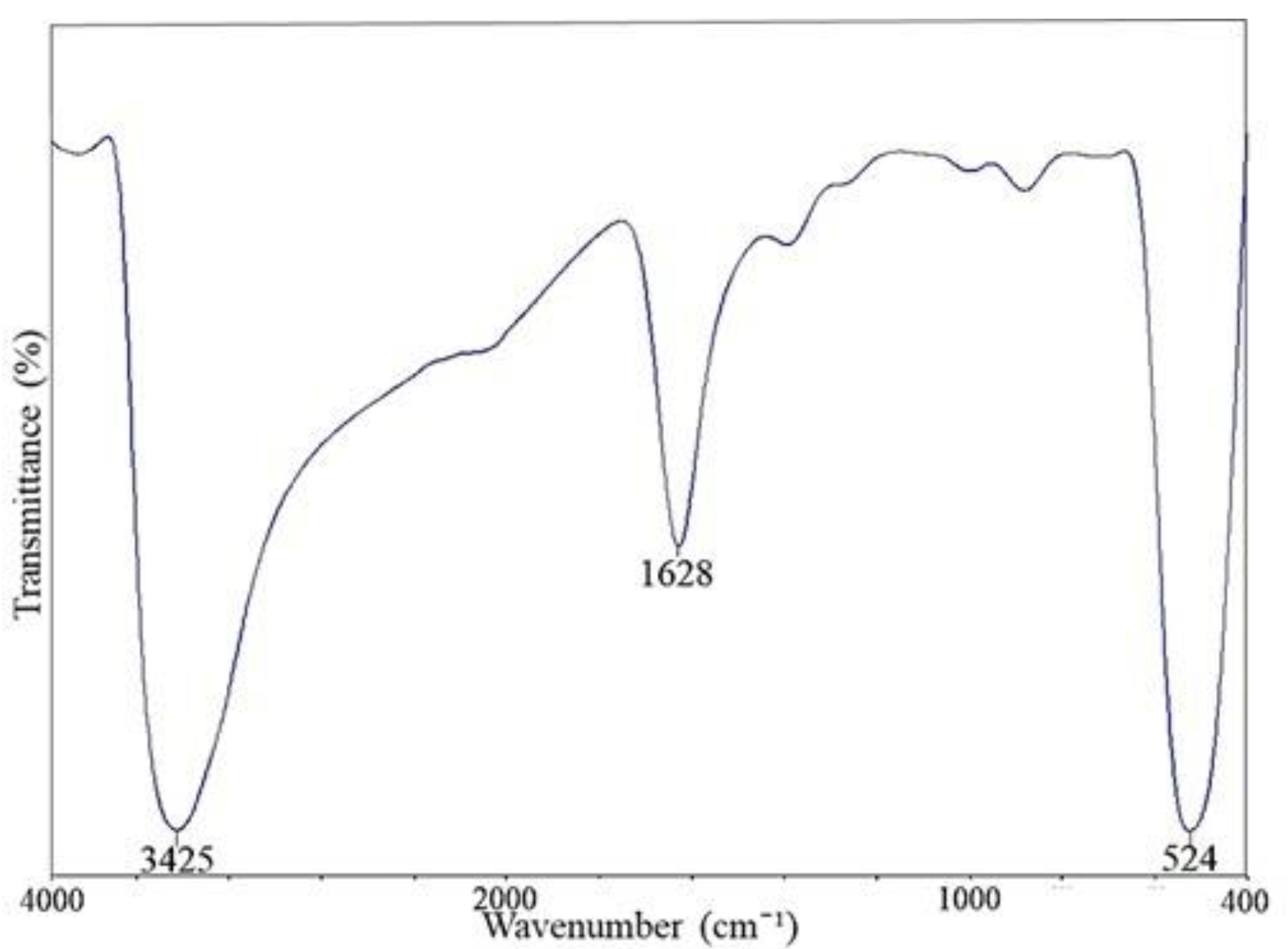
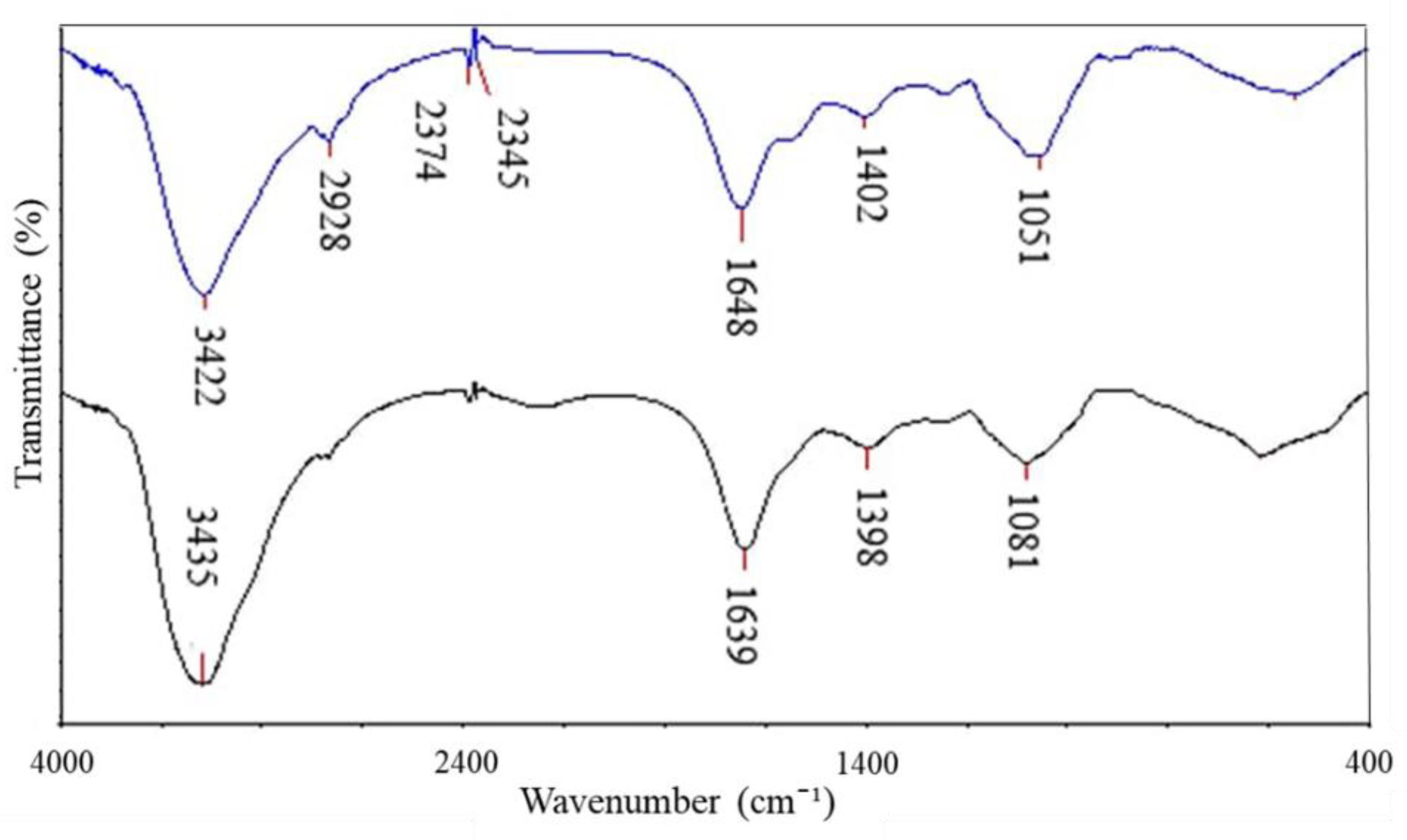
| Absorption (cm−1) | Molecular Motion | Functional Group | Biomolecules |
|---|---|---|---|
| 3435 → 3422 | O-H with N-H stretching modes | Alcohol, secondary amide | Proteins, Polysaccharides, Chitin |
| 2928 | C-H groups | Ergosterol | Lipids |
| 2374, 2345 | CH2 vibration | Alkene group | Hydrocarbon |
| 1639 → 1648 | C=O stretching, N-H bending | Amide I | Polypeptides |
| 1398 → 1402 | C=O of COO− symmetric stretching vibrations in proteins, CH2 wagging vibrations in lipids, and β (1–3) glucans | Carbonyl group, Alkene group | Lipids, Proteins, Polysaccharides |
| 1081 → 1051 | C-O mainly by vibrations and absorptions of polysaccharides and phosphate groups | Polysaccharides, phosphate group | Polysaccharides, mainly glucans and mannans & Phospholipids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, E.P.; Djearamane, S.; Wong, L.S.; Rajamani, R.; Tanislaus Antony, A.C.; Subbaih, S.K.; Janakiraman, A.K.; Aminuzzaman, M.; Subramaniyan, V.; Sekar, M.; et al. An In Vitro Study of the Antifungal Efficacy of Zinc Oxide Nanoparticles against Saccharomyces cerevisiae. Coatings 2022, 12, 1988. https://doi.org/10.3390/coatings12121988
Tan EP, Djearamane S, Wong LS, Rajamani R, Tanislaus Antony AC, Subbaih SK, Janakiraman AK, Aminuzzaman M, Subramaniyan V, Sekar M, et al. An In Vitro Study of the Antifungal Efficacy of Zinc Oxide Nanoparticles against Saccharomyces cerevisiae. Coatings. 2022; 12(12):1988. https://doi.org/10.3390/coatings12121988
Chicago/Turabian StyleTan, Eng Pei, Sinouvassane Djearamane, Ling Shing Wong, Ranjithkumar Rajamani, Anto Cordelia Tanislaus Antony, Suresh Kumar Subbaih, Ashok Kumar Janakiraman, Mohammod Aminuzzaman, Vetriselvan Subramaniyan, Mahendran Sekar, and et al. 2022. "An In Vitro Study of the Antifungal Efficacy of Zinc Oxide Nanoparticles against Saccharomyces cerevisiae" Coatings 12, no. 12: 1988. https://doi.org/10.3390/coatings12121988
APA StyleTan, E. P., Djearamane, S., Wong, L. S., Rajamani, R., Tanislaus Antony, A. C., Subbaih, S. K., Janakiraman, A. K., Aminuzzaman, M., Subramaniyan, V., Sekar, M., & Selvaraj, S. (2022). An In Vitro Study of the Antifungal Efficacy of Zinc Oxide Nanoparticles against Saccharomyces cerevisiae. Coatings, 12(12), 1988. https://doi.org/10.3390/coatings12121988










