Hydrothermal Synthesis of Nitrogen-Doped and Excitation-Dependent Carbon Quantum Dots for Selective Detection of Fe3+ in Blood Plasma
Abstract
:1. Introduction
2. Experimental Section
2.1. Production and Characterization of CQDs through Hydrothermal Method
2.2. pH Stability of the CQDs
2.3. Sensing of Metal Ions
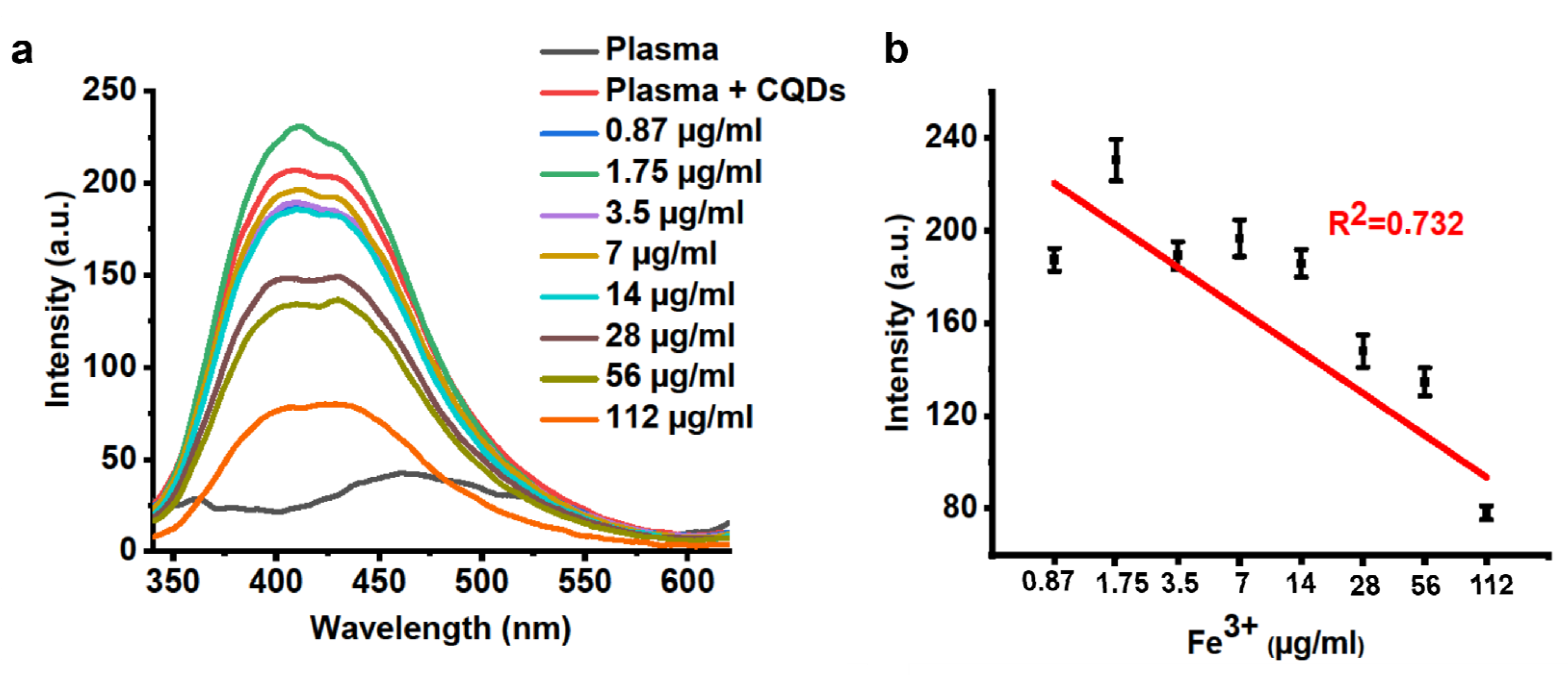
2.4. Sensing of Fe3+ Ions in Blood Plasma
2.5. Cytotoxic Activity of CQDs
2.6. Antimicrobial Activity of CQDs
3. Results and Discussion
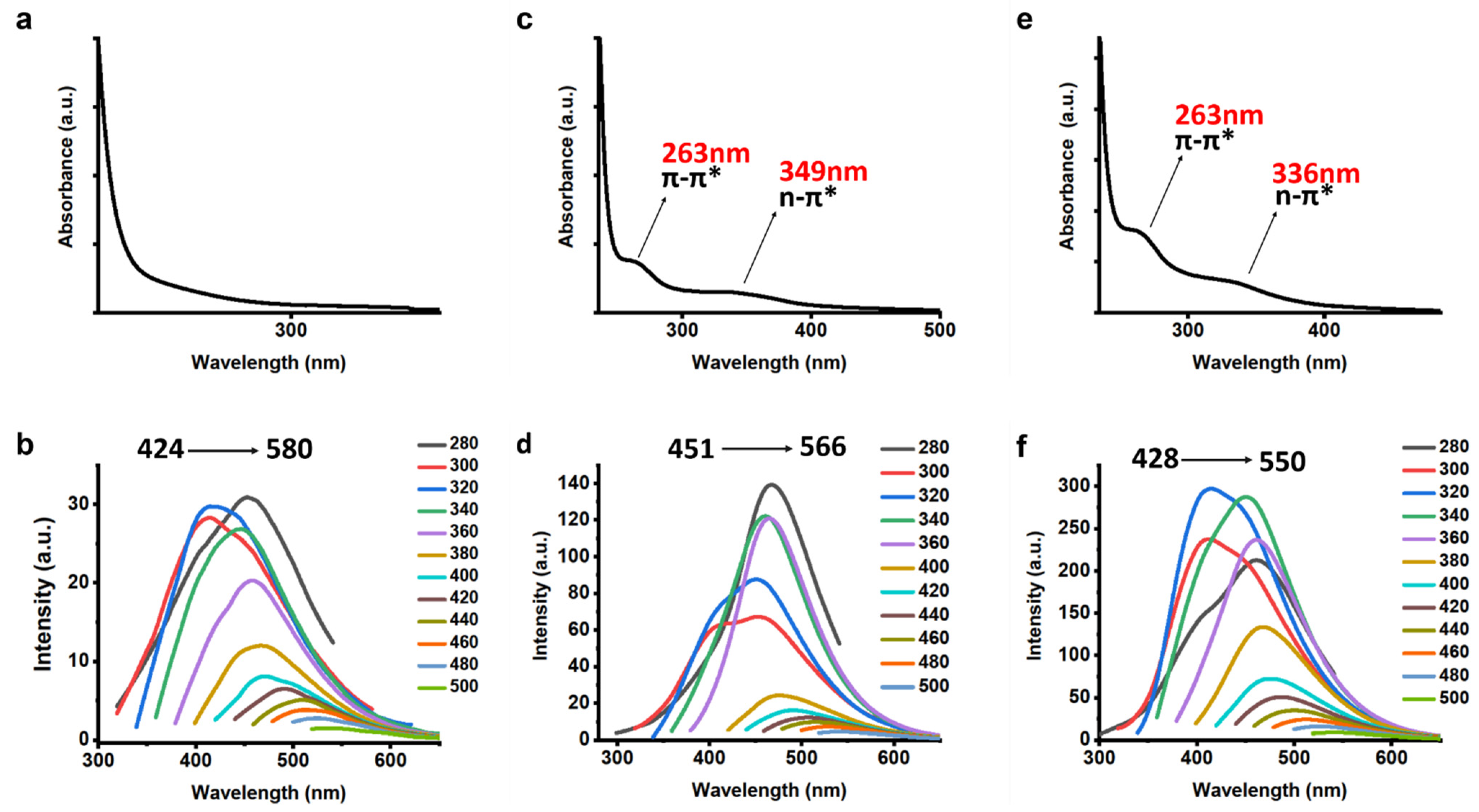
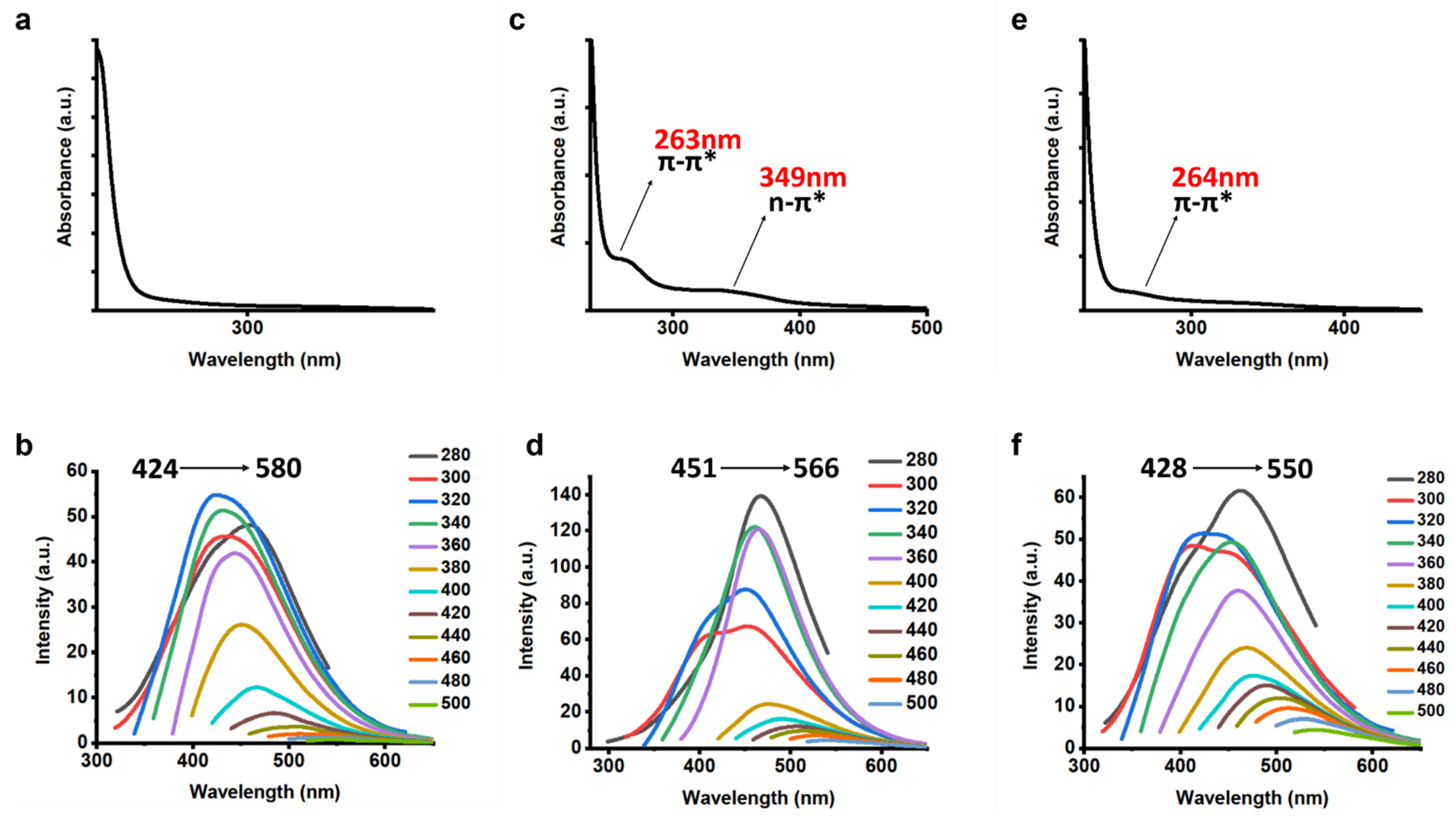
3.1. Characterization of CQDs
3.2. Effect of Experimental Parameters
3.3. Further Characterization of CQDs
3.4. Sensing of Fe3+ Ions
3.5. Cytotoxicity and Antibacterial Activity of CQDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, M.H.; Liu, R.S. Carbon Nitride Quantum Dots and Their Applications. In Phosphors, Up Conversion Nano Particles, Quantum Dots and Their Applications; Springer: Singapore, 2016; Volume 2, pp. 485–502. [Google Scholar] [CrossRef]
- Chan, K.K.; Yap, S.H.K.; Yong, K.T. Biogreen Synthesis of Carbon Dots for Biotechnology and Nanomedicine Applications. Nano-Micro Lett. 2018, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Mintz, K.J.; Zhou, Y.; Leblanc, R.M. Recent Development of Carbon Quantum Dots Regarding Their Optical Properties, Photoluminescence Mechanism, and Core Structure. Nanoscale 2019, 11, 4634–4652. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Semeniuk, M.; Yi, Z.; Poursorkhabi, V.; Tjong, J.; Jaffer, S.; Lu, Z.H.; Sain, M. Future Perspectives and Review on Organic Carbon Dots in Electronic Applications. ACS Nano 2019, 13, 6224–6255. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and Their Applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, J.; Li, X.; Zhou, W.; Wang, Z.; He, P.; Ding, G.; Xie, X.; Kang, Z.; Jiang, M. Large-Scale Fabrication of Heavy Doped Carbon Quantum Dots with Tunable-Photoluminescence and Sensitive Fluorescence Detection. J. Mater. Chem. A 2014, 2, 8660–8667. [Google Scholar] [CrossRef]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining Carbon Dots: Synthesis and Biomedical and Optoelectronic Applications. Nano Today 2016, 11, 565–586. [Google Scholar] [CrossRef]
- Sun, X.; Lei, Y. Fluorescent Carbon Dots and Their Sensing Applications. TrAC-Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Mao, C. Fluorescent Carbon Nanoparticles Derived from Candle Soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef]
- Qiao, Z.A.; Wang, Y.; Gao, Y.; Li, H.; Dai, T.; Liu, Y.; Huo, Q. Commercially Activated Carbon as the Source for Producing Multicolor Photoluminescent Carbon Dots by Chemical Oxidation. Chem. Commun. 2010, 46, 8812–8814. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In Vivo NIR Fluorescence Imaging, Biodistribution, and Toxicology of Photoluminescent Carbon Dots Produced from Carbon Nanotubes and Graphite. Small 2012, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon Dots for Multiphoton Bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, H.; Jorge, P.A.S.; Fernandes, J.R.A.; Esteves da Silva, J.C.G. Hg(II) Sensing Based on Functionalized Carbon Dots Obtained by Direct Laser Ablation. Sens. Actuators B Chem. 2010, 145, 702–707. [Google Scholar] [CrossRef]
- Hu, S.; Liu, J.; Yang, J.; Wang, Y.; Cao, S. Laser Synthesis and Size Tailor of Carbon Quantum Dots. J. Nanopart. Res. 2011, 13, 7247–7252. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Lu, Y.; Liu, C.; Yang, W. Carbon Quantum Dots Displaying Dual-Wavelength Photoluminescence and Electrochemiluminescence Prepared by High-Energy Ball Milling. Carbon 2015, 94, 472–478. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The Photoluminescence Mechanism in Carbon Dots (Graphene Quantum Dots, Carbon Nanodots, and Polymer Dots): Current State and Future Perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Gao, M.X.; Liu, C.F.; Wu, Z.L.; Zeng, Q.L.; Yang, X.X.; Wu, W.B.; Li, Y.F.; Huang, C.Z. A Surfactant-Assisted Redox Hydrothermal Route to Prepare Highly Photoluminescent Carbon Quantum Dots with Aggregation-Induced Emission Enhancement Properties. Chem. Commun. 2013, 49, 8015–8017. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Cao, F.; Wang, L.; Wang, Z.; Leng, Y. Hydrothermal Synthesis of Nitrogen and Boron Doped Carbon Quantum Dots with Yellow-Green Emission for Sensing Cr(VI), Anti-Counterfeiting and Cell Imaging. RSC Adv. 2017, 7, 48386–48393. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Yang, Y.; Ma, C.Q.; Liu, X.; Wang, H.; Xu, B. Synthesis of Carbon Quantum Dots by Chemical Vapor Deposition Approach for Use in Polymer Solar Cell as the Electrode Buffer Layer. Carbon 2016, 109, 598–607. [Google Scholar] [CrossRef]
- Kuo, T.R.; Sung, S.Y.; Hsu, C.W.; Chang, C.J.; Chiu, T.C.; Hu, C.C. One-Pot Green Hydrothermal Synthesis of Fluorescent Nitrogen-Doped Carbon Nanodots for in Vivo Bioimaging. Anal. Bioanal. Chem. 2016, 408, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, C.; Hu, C.; Yang, W.; Zhao, C.; Wang, S.; Zhang, M.; Zhao, J.; Wang, X.; Qiu, J. Solvothermal Conversion of Coal into Nitrogen-Doped Carbon Dots with Singlet Oxygen Generation and High Quantum Yield. Chem. Eng. J. 2017, 320, 570–575. [Google Scholar] [CrossRef]
- Mitra, S.; Chandra, S.; Pathan, S.H.; Sikdar, N.; Pramanik, P.; Goswami, A. Room Temperature and Solvothermal Green Synthesis of Self Passivated Carbon Quantum Dots. RSC Adv. 2013, 3, 3189–3193. [Google Scholar] [CrossRef]
- Choi, Y.; Kang, B.; Lee, J.; Kim, S.; Kim, G.T.; Kang, H.; Lee, B.R.; Kim, H.; Shim, S.H.; Lee, G.; et al. Integrative Approach toward Uncovering the Origin of Photoluminescence in Dual Heteroatom-Doped Carbon Nanodots. Chem. Mater. 2016, 28, 6840–6847. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Q.; Ni, Y. Facile Microwave-Assisted Solid-Phase Synthesis of Highly Fluorescent Nitrogen-Sulfur-Codoped Carbon Quantum Dots for Cellular Imaging Applications. Chem. A Eur. J. 2015, 21, 13004–13011. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.T.; Kang, Z. One-Step Ultrasonic Synthesis of Water-Soluble Carbon Nanoparticles with Excellent Photoluminescent Properties. Carbon 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Liu, Y.; Yu, H.; Kang, Z.; Lee, S.T. Synthesis of Fluorescent Carbon Nanoparticles Directly from Active Carbon via a One-Step Ultrasonic Treatment. Mater. Res. Bull. 2011, 46, 147–151. [Google Scholar] [CrossRef]
- Ma, Z.; Ming, H.; Huang, H.; Liu, Y.; Kang, Z. One-Step Ultrasonic Synthesis of Fluorescent N-Doped Carbon Dots from Glucose and Their Visible-Light Sensitive Photocatalytic Ability. New J. Chem. 2012, 36, 861–864. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Zong, J.; Zhang, J.; Li, C. One-Pot Hydrothermal Synthesis of Graphene Quantum Dots Surface-Passivated by Polyethylene Glycol and Their Photoelectric Conversion under near-Infrared Light. New J. Chem. 2012, 36, 97–101. [Google Scholar] [CrossRef]
- Liu, J.J.; Li, D.; Zhang, K.; Yang, M.; Sun, H.; Yang, B. One-Step Hydrothermal Synthesis of Nitrogen-Doped Conjugated Carbonized Polymer Dots with 31% Efficient Red Emission for In Vivo Imaging. Small 2018, 14, 1703919. [Google Scholar] [CrossRef]
- Karadag, S.N.; Ustun, O.; Yilmaz, A.; Yilmaz, M. The fabrication of excitation-dependent fluorescence boron/nitrogen co-doped carbon quantum dots and their employment in bioimaging. Chem. Phys. 2022, 562, 111678. [Google Scholar] [CrossRef]
- Hwang, E.; Lee, B. Synthesis of a Fluorescence Sensor Based on Carbon Quantum Dots for Detection of Bisphenol A in Aqueous Solution. Korean J. Chem. Eng. 2022, 39, 1324–1332. [Google Scholar] [CrossRef]
- Kim, S.; Yoo, B.K.; Choi, Y.; Kim, B.S.; Kwon, O.H. Time-Resolved Spectroscopy of the Ensembled Photoluminescence of Nitrogen- and Boron/Nitrogen-Doped Carbon Dots. Phys. Chem. 2018, 20, 11673–11681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Ma, D.K.; Zhuang, Y.; Zhang, X.; Chen, W.; Hong, L.L.; Yan, Q.X.; Yu, K.; Huang, S.M. One-Pot Synthesis of N-Doped Carbon Dots with Tunable Luminescence Properties. J. Mater. Chem. 2012, 22, 16714–16718. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-Based Dots Co-Doped with Nitrogen and Sulfur for High Quantum Yield and Excitation-Independent Emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Zheng, M.; Zhang, L.; Zhao, H.; Xie, Z.; Jing, X.; Haddad, R.E.; Fan, H.; Sun, Z. Formation Mechanism and Optimization of Highly Luminescent N-Doped Graphene Quantum Dots. Sci. Rep. 2014, 4, 5294. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Sun, Y.P.; Wang, X.; Lu, F.; Cao, L.; Meziani, M.J.; Luo, P.G.; Gu, L.; Monica Veca, L. Doped Carbon Nanoparticles as a New Platform for Highly Photoluminescent Dots. J. Phys. Chem. C 2008, 112, 18295–18298. [Google Scholar] [CrossRef]
- Xu, J.; Cui, K.; Gong, T.; Zhang, J.; Zhai, Z.; Hou, L.; Zaman, F.U.; Yuan, C. Ultrasonic-Assisted Synthesis of N-Doped, Multicolor Carbon Dots toward Fluorescent Inks, Fluorescence Sensors, and Logic Gate Operations. Nanomaterials 2022, 12, 312. [Google Scholar] [CrossRef]
- Wang, H.; Sun, P.; Cong, S.; Wu, J.; Gao, L.; Wang, Y.; Dai, X.; Yi, Q.; Zou, G. Nitrogen-Doped Carbon Dots for “Green” Quantum Dot Solar Cells. Nanoscale Res. Lett. 2016, 11, 27. [Google Scholar] [CrossRef]
- Li, H.; Su, D.; Gao, H.; Yan, X.; Kong, D.; Jin, R.; Liu, X.; Wang, C.; Lu, G. Design of Red Emissive Carbon Dots: Robust Performance for Analytical Applications in Pesticide Monitoring. Anal. Chem. 2020, 92, 3198–3205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, X.; Li, W.; Huang, X.; Li, Z.; Zhang, W.; Zhang, X.; Zou, X.; Shi, J. Preparation of Boron Nitrogen Co-Doped Carbon Quantum Dots for Rapid Detection of Cr(VI). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 243, 118807. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, T.; Zhang, X.; Li, H.; Jia, X.; Zhao, S.; Yang, Z.; Liu, X. Nitrogen–Doped Carbon Quantum Dots as Fluorescent Nanosensor for Selective Determination and Cellular Imaging of ClO−. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 271, 120941. [Google Scholar] [CrossRef] [PubMed]
- Coates, T.D. Physiology and Pathophysiology of Iron in Hemoglobin-Associated Diseases. Free Radic. Biol. Med. 2014, 72, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.; Payne, S.M. Regulation of Iron Transport Systems in Enterobacteriaceae in Response to Oxygen and Iron Availability. J. Inorg. Biochem. 2014, 133, 110–117. [Google Scholar] [CrossRef]
- Proos Vedin, N.; Lundberg, M. Protein Effects in Non-Heme Iron Enzyme Catalysis: Insights from Multiscale Models. J. Biol. Inorg. Chem. 2016, 21, 645–657. [Google Scholar] [CrossRef]
- Baker, R.D.; Greer, F.R. Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0–3 Years of Age). Pediatrics 2021, 126, 1040–1050. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and Cancer: More Ore to Be Mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Shangguan, J.; Huang, J.; He, D.; He, X.; Wang, K.; Ye, R.; Yang, X.; Qing, T.; Tang, J. Highly Fe3+-Selective Fluorescent Nanoprobe Based on Ultrabright N/P Codoped Carbon Dots and Its Application in Biological Samples. Anal. Chem. 2017, 89, 7477–7484. [Google Scholar] [CrossRef]
- Gong, X.; Lu, W.; Paau, M.C.; Hu, Q.; Wu, X.; Shuang, S.; Dong, C.; Choi, M.M.F. Facile Synthesis of Nitrogen-Doped Carbon Dots for Fe3+ Sensing and Cellular Imaging. Anal. Chim. Acta 2015, 861, 74–84. [Google Scholar] [CrossRef]
- Liu, W.; Diao, H.; Chang, H.; Wang, H.; Li, T.; Wei, W. Green Synthesis of Carbon Dots from Rose-Heart Radish and Application for Fe3+ Detection and Cell Imaging. Sens. Actuators B Chem. 2017, 241, 190–198. [Google Scholar] [CrossRef]
- Aslandaş, A.M.; Balci, N.; Arik, M.; Şakiroğlu, H.; Onganer, Y.; Meral, K. Liquid Nitrogen-Assisted Synthesis of Fluorescent Carbon Dots from Blueberry and Their Performance in Fe3+ Detection. Appl. Surf. Sci. 2015, 356, 747–752. [Google Scholar] [CrossRef]
- Ge, L.; Yu, H.; Ren, H.; Shi, B.; Guo, Q.; Gao, W.; Li, Z.; Li, J. Photoluminescence of Carbon Dots and Their Applications in Hela Cell Imaging and Fe3+ Ion Detection. J. Mater. Sci. 2017, 52, 9979–9989. [Google Scholar] [CrossRef]
- Yang, R.; Guo, X.; Jia, L.; Zhang, Y.; Zhao, Z.; Lonshakov, F. Green Preparation of Carbon Dots with Mangosteen Pulp for the Selective Detection of Fe3+ Ions and Cell Imaging. Appl. Surf. Sci. 2017, 423, 426–432. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, C.; Song, J.; Li, H.; Du, D.; Lin, Y. Drug-Derived Bright and Color-Tunable N-Doped Carbon Dots for Cell Imaging and Sensitive Detection of Fe3+ in Living Cells. ACS Appl. Mater. Interfaces 2017, 9, 7399–7405. [Google Scholar] [CrossRef]
- Lu, W.; Gong, X.; Nan, M.; Liu, Y.; Shuang, S.; Dong, C. Comparative Study for N and S Doped Carbon Dots: Synthesis, Characterization and Applications for Fe3+ Probe and Cellular Imaging. Anal. Chim. Acta 2015, 898, 116–127. [Google Scholar] [CrossRef]
- Chandra, S.; Laha, D.; Pramanik, A.; Ray Chowdhuri, A.; Karmakar, P.; Sahu, S.K. Synthesis of Highly Fluorescent Nitrogen and Phosphorus Doped Carbon Dots for the Detection of Fe3+ Ions in Cancer Cells. Luminescence 2016, 31, 81–87. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, W.; Song, W.; Liu, J.; Ren, C.; Wu, J.; Liu, D.; Chen, H. Red Emission B, N, S-Co-Doped Carbon Dots for Colorimetric and Fluorescent Dual Mode Detection of Fe3+ Ions in Complex Biological Fluids and Living Cells. ACS Appl. Mater. Interfaces 2017, 9, 12663–12672. [Google Scholar] [CrossRef]
- Rong, M.; Feng, Y.; Wang, Y.; Chen, X. One-Pot Solid Phase Pyrolysis Synthesis of Nitrogen-Doped Carbon Dots for Fe3+ Sensing and Bioimaging. Sensors Actuators, B Chem. 2017, 245, 868–874. [Google Scholar] [CrossRef]
- Molkenova, A.; Amangeldinova, Y.; Aben, D.; Sayatova, S.; Atabaev, T.S. Quick Synthesis of Fluorescent Nitrogen-Doped Carbon Nanoparticles for Selective and Sensitive Fe(III) Detection in Water. Sens. Bio-Sens. Res. 2019, 23, 100271. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Shang, X.; Chao, D.; Zhou, L.; Zhang, H. Highly Sensitive and Selective Detection of Fe3+ by Utilizing Carbon Quantum Dots as Fluorescent Probes. Chem. Phys. Lett. 2018, 705, 1–6. [Google Scholar] [CrossRef]
- Maity, M.; Bera, K.; Pal, U.; Khamaru, K.; Maiti, N.C. Sensing of Iron(III) Ion via Modulation of Redox Potential on Biliverdin Protected Silver Nanosurface. ACS Appl. Nano Mater. 2018, 1, 6099–6111. [Google Scholar] [CrossRef]
- Jain, A.; Wadhawan, S.; Kumar, V.; Mehta, S.K. Colorimetric Sensing of Fe3+ Ions in Aqueous Solution Using Magnesium Oxide Nanoparticles Synthesized Using Green Approach. Chem. Phys. Lett. 2018, 706, 53–61. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Guo, Z.; Ma, H.; Zhang, Y.; Wang, B.; Bin, D.; Wei, Q. Highly Selective Fluorescent Chemosensor for Detection of Fe3+ Based on Fe3O4@ZnO. Sci. Rep. 2016, 6, 23558. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Zhang, W.L.; Lv, F.; Guo, S. Recent Progress in Two-Dimensional Inorganic Quantum Dots. Chem. Soc. Rev. 2018, 47, 586–625. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Z.; Xue, Z.; Huang, C.; Shan, Y.; Liu, C.; Qin, X.; Yang, W.; Chen, X.; Wang, T. Understanding the Selective Detection of Fe3+ Based on Graphene Quantum Dots as Fluorescent Probes: The Ksp of a Metal Hydroxide-Assisted Mechanism. Anal. Chem. 2017, 89, 12054–12058. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Woo, J.Y.; Han, C.S. Colorimetric Detection of Fe(III) Ions Using Label-Free Gold Nanoparticles and Acidic Thiourea Mixture. Sens. Actuators, B Chem. 2013, 181, 114–118. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Xu, L.; Chen, W. Undefined Nanoparticle-Based Environmental Sensors. Elsevier 2010, 70, 265–274. [Google Scholar]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Wu, P.; Li, W.; Wu, Q.; Liu, Y.; Liu, S. Hydrothermal Synthesis of Nitrogen-Doped Carbon Quantum Dots from Microcrystalline Cellulose for the Detection of Fe3+ Ions in an Acidic Environment. RSC Adv. 2017, 7, 44144–44153. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Han, Q.; Jin, C.; Zhang, J.; Wang, B. A Fluorescence Turn-off Chemosensor Based on N-Doped Carbon Quantum Dots for Detection of Fe3+ in Aqueous Solution. Mater. Lett. 2015, 141, 366–368. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Kong, W.; Liu, J.; Liu, Y.; Tong, C.; Zhang, X.; Kang, Z. Ultra-Sensitive and Selective Hg2+ Detection Based on Fluorescent Carbon Dots. Mater. Res. Bull. 2013, 48, 2529–2534. [Google Scholar] [CrossRef]
- Zhuo, Y.; Miao, H.; Zhong, D.; Zhu, S.; Yang, X. One-Step Synthesis of High Quantum-Yield and Excitation-Independent Emission Carbon Dots for Cell Imaging. Mater. Lett. 2015, 139, 197–200. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Wu, P.; Li, J.; Liu, S.; Jin, C.; Zhan, X. Effect of Reaction Temperature on Properties of Carbon Nanodots and Their Visible-Light Photocatalytic Degradation of Tetracyline. RSC Adv. 2015, 5, 75711–75721. [Google Scholar] [CrossRef]
- Shaikh, A.F.; Tamboli, M.S.; Patil, R.H.; Bhan, A.; Ambekar, J.D.; Kale, B.B. Bioinspired Carbon Quantum Dots: An Antibiofilm Agents. J. Nanosci. Nanotechnol. 2018, 19, 2339–2345. [Google Scholar] [CrossRef]
- Asghar, K.; Qasim, M.; Das, D. One-Pot Green Synthesis of Carbon Quantum Dot for Biological Application. AIP Conf. Proc. 2017, 1832, 50117. [Google Scholar] [CrossRef]
- P, K.; Cherian, A.R.; Sirimahachai, U.; Thadathil, D.A.; Varghese, A.; Hegde, G. Detection of Picric Acid in Industrial Effluents Using Multifunctional Green Fluorescent B/N-Carbon Quantum Dots. J. Environ. Chem. Eng. 2022, 10, 107209. [Google Scholar] [CrossRef]
- Pan, L.; Sun, S.; Zhang, L.; Jiang, K.; Lin, H. Near-Infrared Emissive Carbon Dots for Two-Photon Fluorescence Bioimaging. Nanoscale 2016, 8, 17350–17356. [Google Scholar] [CrossRef]
- Serhan, M.; Sprowls, M.; Jackemeyer, D.; Long, M.; Perez, I.D.; Maret, W.; Tao, N.; Forzani, E. Total Iron Measurement in Human Serum with a Smartphone. In Proceedings of the 2019 AIChE Annual Meeting, Orlando, FL, USA, 10–15 November 2019; pp. 1–3. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.S.; Zhong, N.; Gao, Q.Y.; Xiong, H.M. Highly Efficient Red-Emitting Carbon Dots with Gram-Scale Yield for Bioimaging. Langmuir 2017, 33, 12635–12642. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Zhang, C.Y. A Low-Temperature Solid-Phase Method to Synthesize Highly Fluorescent Carbon Nitride Dots with Tunable Emission. Chem. Commun. 2013, 49, 8605–8607. [Google Scholar] [CrossRef]
- Chen, B.B.; Li, R.S.; Liu, M.L.; Zhang, H.Z.; Huang, C.Z. Self-Exothermic Reaction Prompted Synthesis of Single-Layered Graphene Quantum Dots at Room Temperature. Chem. Commun. 2017, 53, 4958–4961. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, Y.; Xu, Y.; Xiang, Q.; Sun, L.; Zhang, W.; Li, W.; Si, Y.; Luo, Z. A Highly Nanoporous Nitrogen-Doped Carbon Microfiber Derived from Bioresource as a New Kind of ORR Electrocatalyst. Nanoscale Res. Lett. 2019, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Al Awak, M.M.; Wang, P.; Wang, S.; Tang, Y.; Sun, Y.P.; Yang, L. Correlation of Carbon Dots’ Light-Activated Antimicrobial Activities and Fluorescence Quantum Yield. RSC Adv. 2017, 7, 30177–30184. [Google Scholar] [CrossRef]
- Meziani, M.J.; Dong, X.; Zhu, L.; Jones, L.P.; Lecroy, G.E.; Yang, F.; Wang, S.; Wang, P.; Zhao, Y.; Yang, L.; et al. Visible-Light-Activated Bactericidal Functions of Carbon “Quantum” Dots. ACS Appl. Mater. Interfaces 2016, 8, 10761–10766. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Bao, Y.-W.; Wu, F.-G. Carbon Dots for Sensing and Killing Microorganisms. C J. Carbon Res. 2019, 5, 33. [Google Scholar] [CrossRef]
- Anand, A.; Unnikrishnan, B.; Wei, S.C.; Chou, C.P.; Zhang, L.Z.; Huang, C.C. Graphene Oxide and Carbon Dots as Broad-Spectrum Antimicrobial Agents-a Minireview. Nanoscale Horiz. 2019, 4, 117–137. [Google Scholar] [CrossRef]
- Khan, Z.M.S.H.; Rahman, R.S.; Shumaila; Islam, S.; Zulfequar, M. Hydrothermal treatment of red lentils for the synthesis of fluorescent carbon quantum dots and its application for sensing Fe3+. Opt. Mater. (Amst). 2019, 91, 386–395. [Google Scholar] [CrossRef]
- Li, G.; Lv, N.; Bi, W.; Zhang, J.; Ni, J. Nitrogen-doped carbon dots as a fluorescence probe suitable for sensing Fe3+ under acidic conditions. New J. Chem. 2016, 40, 10213–10218. [Google Scholar] [CrossRef]
- Hong, D.; Deng, X.; Liang, J.; Li, J.; Tao, Y.; Tan, K. One-step hydrothermal synthesis of down/up-conversion luminescence F-doped carbon quantum dots for label-free detection of Fe3+. Microchem. J. 2019, 151, 104217. [Google Scholar] [CrossRef]
- Lu, M.; Duan, Y.; Song, Y.; Tan, J.; Zhou, L. Green preparation of versatile nitrogen-doped carbon quantum dots from watermelon juice for cell imaging, detection of Fe3+ ions and cysteine, and optical thermometry. J. Mol. Liq. 2018, 269, 766–774. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. Facile synthesis of novel carbon quantum dots from biomass waste for highly sensitive detection of iron ions. Mater. Res. Bull. 2020, 124, 110730. [Google Scholar] [CrossRef]
- Zhao, S.; Song, X.; Chai, X.; Zhao, P.; He, H.; Liu, Z. Green production of fluorescent carbon quantum dots based on pine wood and its application in the detection of Fe3+. J. Clean. Prod. 2020, 263, 121561. [Google Scholar] [CrossRef]
- Wu, F.; Yang, M.; Zhang, H.; Zhu, S.; Zhu, X.; Wang, K. Facile synthesis of sulfur-doped carbon quantum dots from vitamin B1 for highly selective detection of Fe3+ ion. Opt. Mater. (Amst). 2018, 77, 258–263. [Google Scholar] [CrossRef]
- Deng, X.; Feng, Y.; Li, H.; Du, Z.; Teng, Q.; Wang, H. N-doped carbon quantum dots as fluorescent probes for highly selective and sensitive detection of Fe3+ ions. Particuology 2018, 41, 94–100. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, X.; Ma, Y.; Qian, T.; Wang, C.; Chu, F. Facile and cost-effective preparation of carbon quantum dots for Fe3+ ion and ascorbic acid detection in living cells based on the “on-off-on” fluorescence principle. Appl. Surf. Sci. 2019, 469, 911–916. [Google Scholar] [CrossRef]
- Malavika, J.P.; Shobana, C.; Ragupathi, M.; Kumar, P.; Lee, Y.S.; Govarthanan, M.; Selvan, R.K. A sustainable green synthesis of functionalized biocompatible carbon quantum dots from Aloe barbadensis Miller and its multifunctional applications. Environ. Res. 2021, 200, 111414. [Google Scholar] [CrossRef]
- Tan, A.; Yang, G.; Wan, X. Ultra-high quantum yield nitrogen-doped carbon quantum dots and their versatile application in fluorescence sensing, bioimaging and anti-counterfeiting. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2021, 253, 119583. [Google Scholar] [CrossRef]
- Mahani, M.; Karimi-Mazidi, P.; Khakbaz, F.; Torkzadeh-Mahani, M. Carbon quantum dots—Annexin V probe: photoinduced electron transfer mechanism, phosphatidylserine detection, and apoptotic cell imaging. Microchim. Acta 2022, 189, 69. [Google Scholar] [CrossRef]
- Huo, X.; Liu, L.; Bai, Y.; Qin, J.; Yuan, L.; Feng, F. Facile synthesis of yellowish-green emitting carbon quantum dots and their applications for phoxim sensing and cellular imaging. Anal. Chim. Acta 2022, 1206, 338685. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yao, Z.; Zhang, B.; Liu, E.; Hu, X.; Xue, W.; Fan, J. Biocompatible sulfur nitrogen co-doped carbon quantum dots for highly sensitive and selective detection of dopamine. Colloids Surf. B Biointerfaces 2021, 205, 111874. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, L.; Zhao, S.; Liu, X.; Yang, Y.; Du, J.; Li, Q.; Yu, S. Green-emissive carbon quantum dots with high fluorescence quantum yield: Preparation and cell imaging. Front. Mater. Sci. 2021, 15, 253–265. [Google Scholar] [CrossRef]
- Yu, C.; Qin, D.; Jiang, X.; Zheng, X.; Deng, B. N-doped carbon quantum dots from osmanthus fragrans as a novel off-on fluorescent nanosensor for highly sensitive detection of quercetin and aluminium ion, and cell imaging. J. Pharm. Biomed. Anal. 2021, 192, 113673. [Google Scholar] [CrossRef] [PubMed]
- Xu, O.; Wan, S.; Zhang, Y.; Li, J.; Zhu, X. A unique dual-excitation carbon quantum dots: Facile synthesis and application as a dual-“on-off-on” fluorescent probe. Sens. Actuators B Chem. 2021, 340, 129904. [Google Scholar] [CrossRef]
- Wang, C.; Yang, M.; Shi, H.; Yao, Z.; Liu, E.; Hu, X.; Guo, P.; Xue, W.; Fan, J. Carbon quantum dots prepared by pyrolysis: Investigation of the luminescence mechanism and application as fluorescent probes. Dye Pigment. 2022, 204, 110431. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, S.; Ustun, O.; Ghosigharehaghaji, A.; Tavaci, T.; Yilmaz, A.; Yilmaz, M. Hydrothermal Synthesis of Nitrogen-Doped and Excitation-Dependent Carbon Quantum Dots for Selective Detection of Fe3+ in Blood Plasma. Coatings 2022, 12, 1311. https://doi.org/10.3390/coatings12091311
Aydin S, Ustun O, Ghosigharehaghaji A, Tavaci T, Yilmaz A, Yilmaz M. Hydrothermal Synthesis of Nitrogen-Doped and Excitation-Dependent Carbon Quantum Dots for Selective Detection of Fe3+ in Blood Plasma. Coatings. 2022; 12(9):1311. https://doi.org/10.3390/coatings12091311
Chicago/Turabian StyleAydin, Selin, Oguzhan Ustun, Atena Ghosigharehaghaji, Taha Tavaci, Asli Yilmaz, and Mehmet Yilmaz. 2022. "Hydrothermal Synthesis of Nitrogen-Doped and Excitation-Dependent Carbon Quantum Dots for Selective Detection of Fe3+ in Blood Plasma" Coatings 12, no. 9: 1311. https://doi.org/10.3390/coatings12091311






