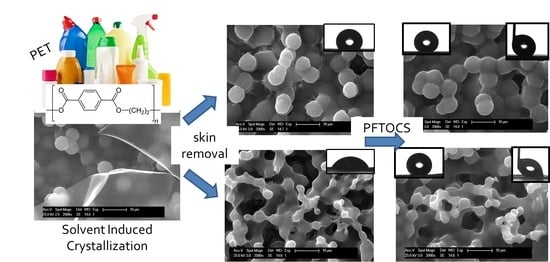
Facile Preparation of Hydrophobic PET Surfaces by Solvent Induced Crystallization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
3. Results and Discussion
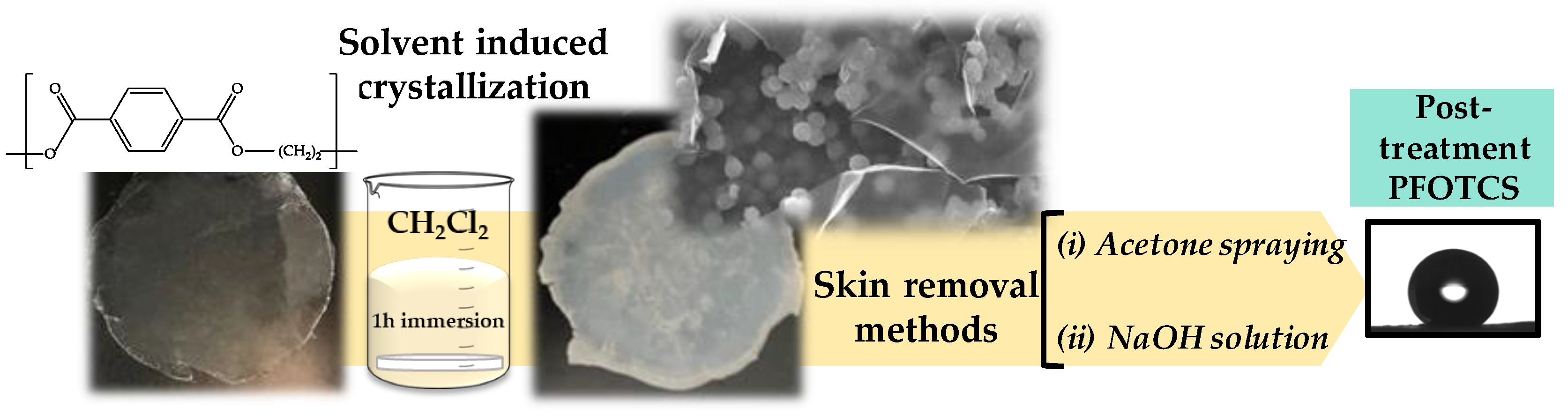
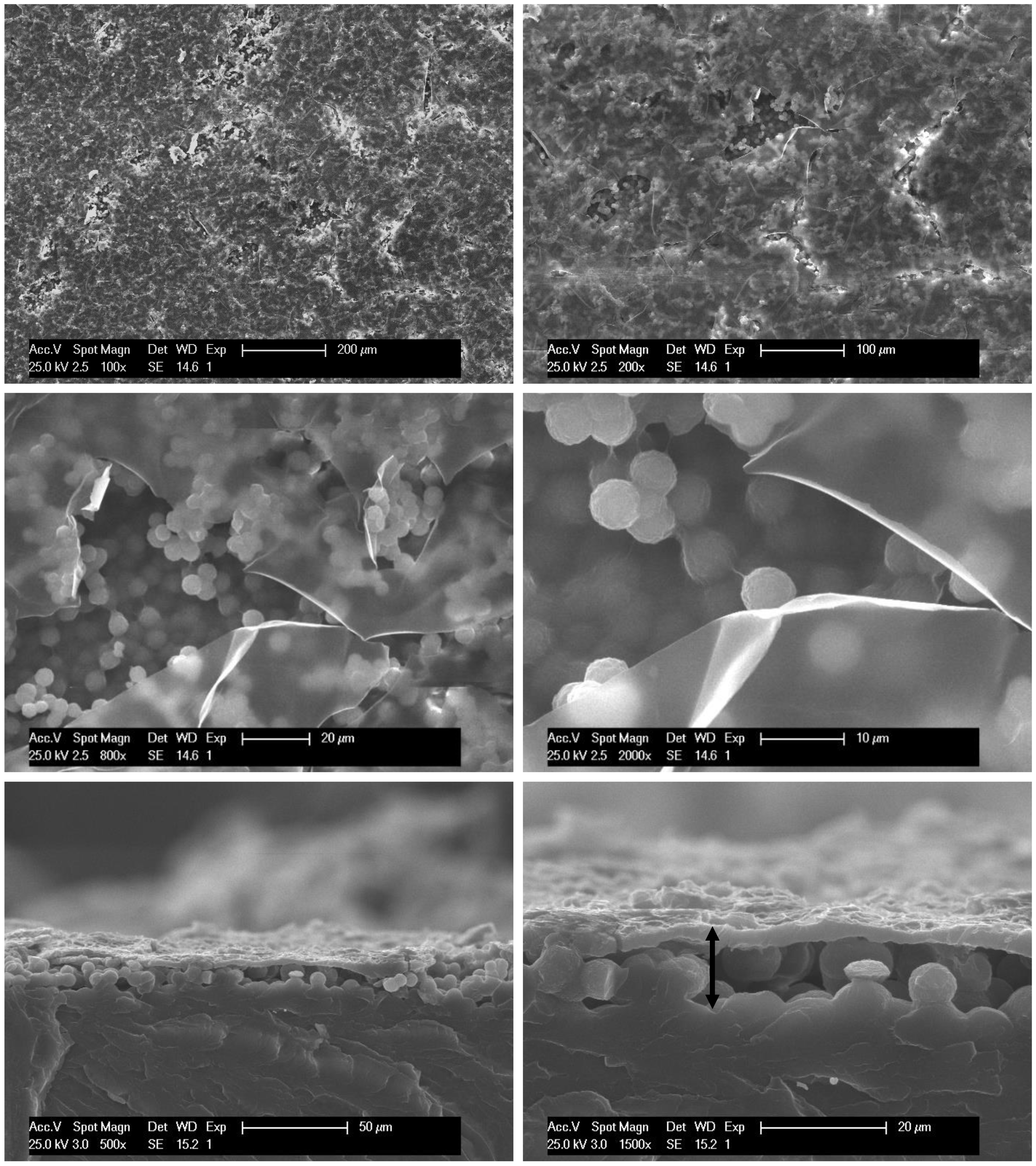
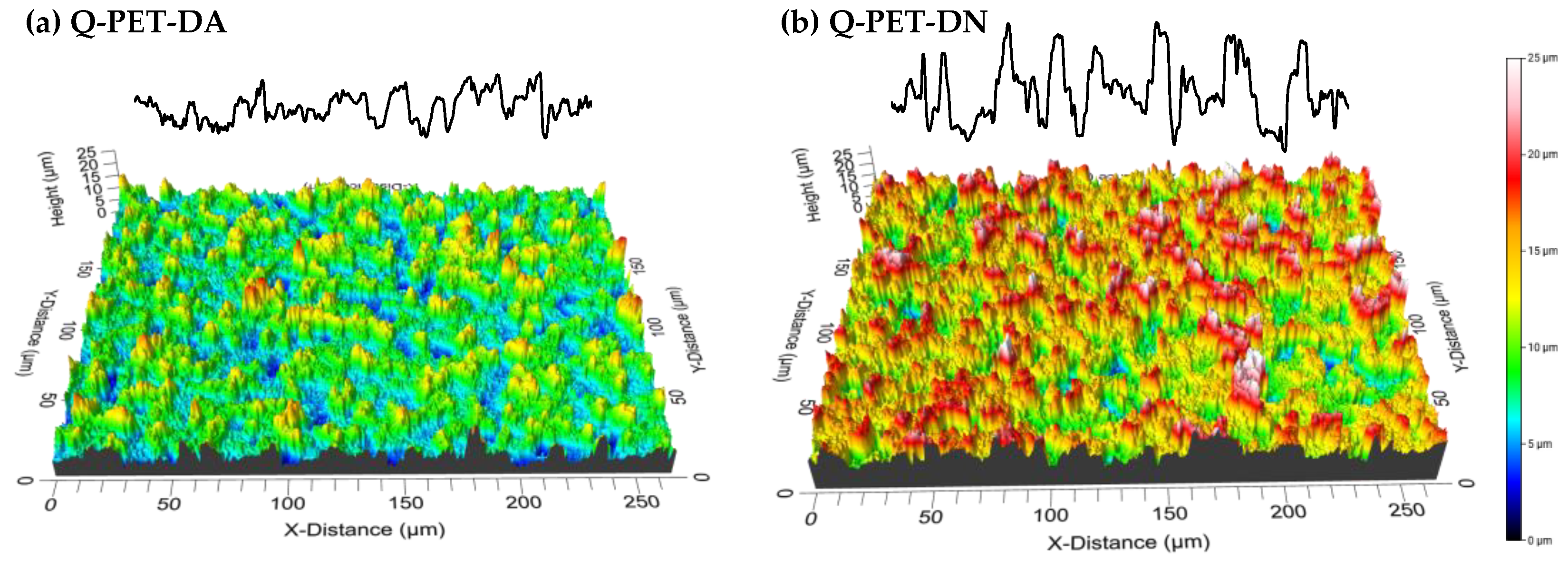
3.1. Roughness Generation by Solvent-Induced PET Crystallization
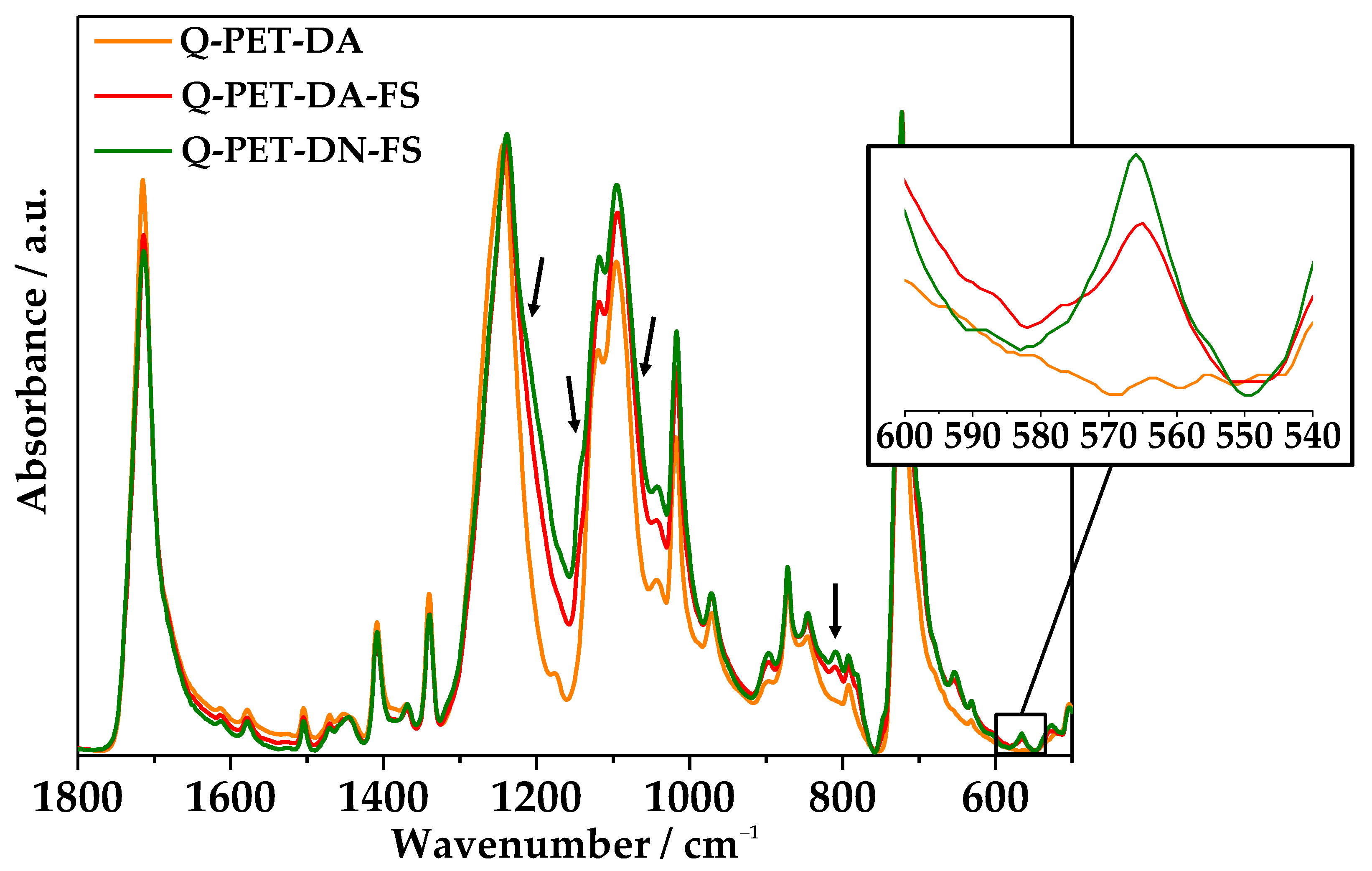
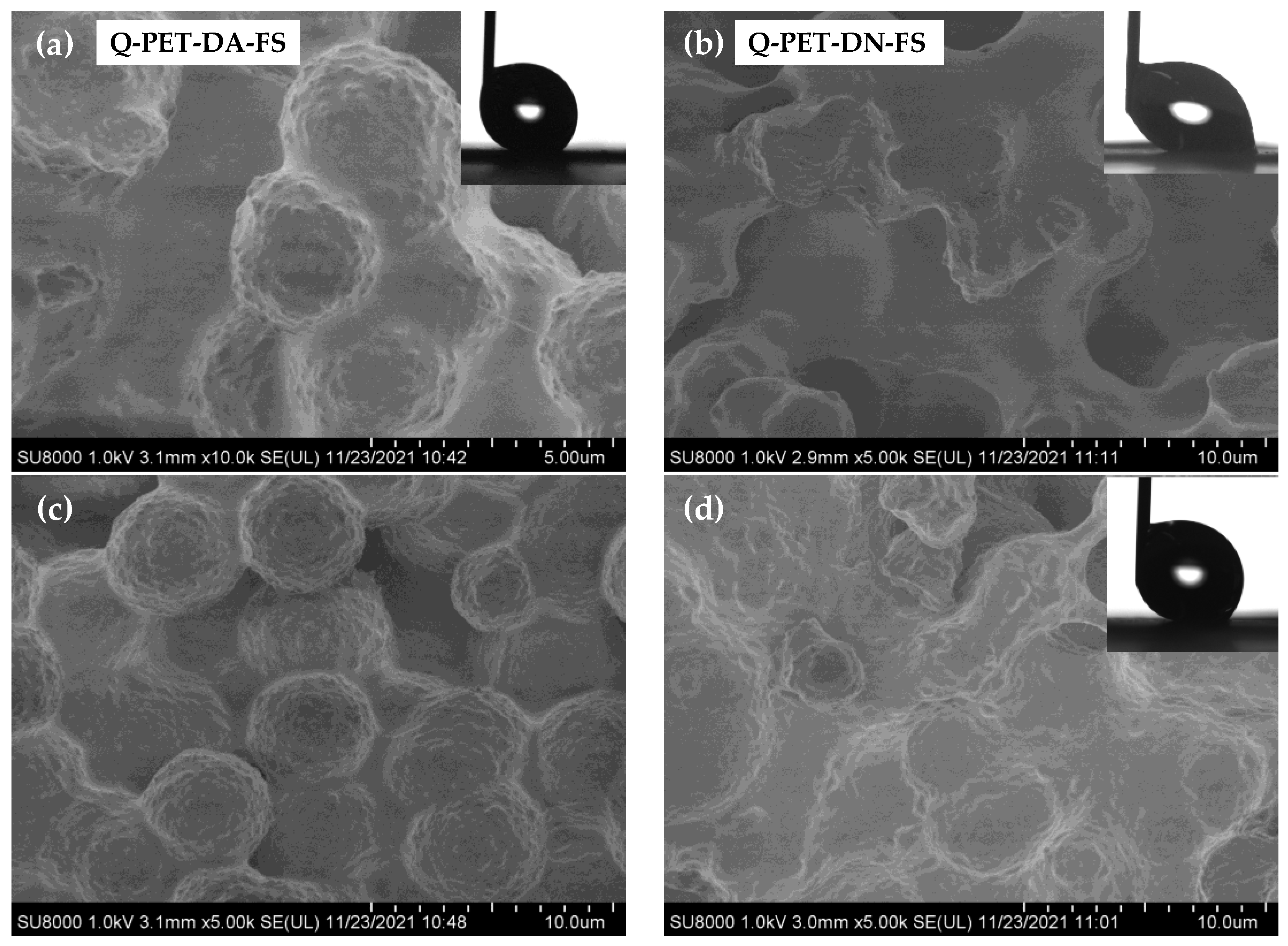
3.2. Chemical Modification by PFOTCS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sethi, S.K.; Manik, G. Recent Progress in Super Hydrophobic/Hydrophilic Self-Cleaning Surfaces for Various Industrial Applications: A Review. Polym.—Plast. Technol. Eng. 2018, 57, 1932–1952. [Google Scholar] [CrossRef]
- Weng, C.; Wang, F.; Zhou, M.; Yang, D.; Jiang, B. Fabrication of hierarchical polymer surfaces with superhydrophobicity by injection molding from nature and function-oriented design. Appl. Surf. Sci. 2018, 436, 224–233. [Google Scholar] [CrossRef]
- Ai, J.; Guo, Z. Biomimetic polymeric superamphiphobic surfaces: Their fabrication and applications. Chem. Commun. 2019, 55, 10820–10843. [Google Scholar] [CrossRef] [PubMed]
- Naz, A.; Sattar, R.; Siddiq, M. Polymer membranes for biofouling mitigation: A review. Polym. Technol. Mater. 2019, 58, 1829–1854. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Encinas, N.; Yang, C.Y.; Geyer, F.; Kaltbeitzel, A.; Baumli, P.; Reinholz, J.; Mailänder, V.; Butt, H.J.; Vollmer, D. Submicrometer-Sized Roughness Suppresses Bacteria Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 21192–21200. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coat. 2020, 142, 105557. [Google Scholar] [CrossRef]
- GMR Plastics Europe; Conversion Market & Strategy GmbH. Plastics—The Facts 2019; Volume 14, p. 35. Available online: https://www.readkong.com/page/plastics-the-facts-2019-an-analysis-of-european-6681489 (accessed on 20 December 2021).
- Das, S.; Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. A Review on Superhydrophobic Polymer Nanocoatings: Recent Development and Applications. Ind. Eng. Chem. Res. 2018, 57, 2727–2745. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.; Han, S.; Kim, K.J. Improvement of hydrophobic properties of polymer surfaces by plasma source ion implantation. Surf. Coat. Technol. 2006, 200, 4763–4769. [Google Scholar] [CrossRef]
- Gotoh, K.; Shohbuke, E.; Kobayashi, Y.; Yamada, H. Wettability control of PET surface by plasma-induced polymer film deposition and plasma/UV oxidation in ambient air. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 1–10. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Narmukhamedova, A.R.; Melnikova, G.B.; Muslimova, I.B.; Yeszhanov, A.B.; Zhatkanbayeva, Z.K.; Chizhik, S.A.; Zdorovets, M.V. Preparation of hydrophobic PET track-etched membranes for separation of oil–water emulsion. Membranes 2021, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Gärtner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, F.; Soldera, M.; Baumann, R.; Lasagni, A.F. Hierarchical microtextures embossed on PET from laser-patterned stamps. Materials 2021, 14, 1756. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Soldera, M.; Wang, W.; Milles, S.; Deng, K.; Voisiat, B.; Nielsch, K.; Lasagni, A.F. Wettability control of polymeric microstructures replicated from laser-patterned stamps. Sci. Rep. 2020, 10, 22428. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, C.R.; Guittard, F.; Darmanin, T. Recent advances in the study and design of parahydrophobic surfaces: From natural examples to synthetic approaches. Adv. Colloid Interface Sci. 2017, 241, 37–61. [Google Scholar] [CrossRef]
- Oh, J.H.; Park, C.H. Robust Fluorine-Free Superhydrophobic PET Fabric Using Alkaline Hydrolysis and Thermal Hydrophobic Aging Process. Macromol. Mater. Eng. 2018, 303, 1700673. [Google Scholar] [CrossRef]
- Oh, J.H.; Moon, M.W.; Park, C.H. Effect of crystallinity on the recovery rate of superhydrophobicity in plasma-nanostructured polymers. RSC Adv. 2020, 10, 10939–10948. [Google Scholar] [CrossRef]
- Afonso, E.; Martínez-Gómez, A.; Tiemblo, P.; García, N. Industrially viable method for producing all-polymer hydrophobic surfaces apt for slippery liquid-infused substrates. Appl. Surf. Sci. 2021, 535, 147728. [Google Scholar] [CrossRef]
- Desai, A.B.; Wilkes, G.L. Solvent-Induced Crystallization of Polyethylene Terephthalate. J. Polym. Sci. Part C Polym. Symp. 1974, 319, 291–319. [Google Scholar] [CrossRef]
- Ouyang, H.; Lee, W.H.; Ouyang, W.; Shiue, S.T.; Wu, T.M. Solvent-induced crystallization in poly(ethylene terephthalate) during mass transport: Mechanism and boundary condition. Macromolecules 2004, 37, 7719–7723. [Google Scholar] [CrossRef]
- Zhao, N.; Weng, L.; Zhang, X.; Xie, Q.; Zhang, X.; Xu, J. A lotus-leaf-like superhydrophobic surface prepared by solvent-induced crystallization. ChemPhysChem 2006, 7, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Gupper, A.; Kazarian, S.G. Study of solvent diffusion and solvent-induced crystallization in syndiotactic polystyrene using FT-IR spectroscopy and imaging. Macromolecules 2005, 38, 2327–2332. [Google Scholar] [CrossRef]
- Takashi, N.; Masashi, M.; Katsuhiko, N.; Motonori, M.; Yasukiyo, U. The Lowest Surface Free Energy Based on -CF3 Alignment. Langmuir 1999, 15, 4321–4323. [Google Scholar]
- Everson, I.; Bendall, B.; Murray, A. Roughness parameters. CCAMLR Sci. 1999, 6, 117–123. [Google Scholar]
- Lawton, E.L.; Cates, D.M. Liquid-induced crystallization of poly(ethylene terephthalate). J. Appl. Polym. Sci. 1969, 13, 899–909. [Google Scholar] [CrossRef]
- Jameel, H.; Noether, H.D.; Rebenfeld, L. The effects of orientation and crystallinity on the solvent-induced crystallization of poly(ethylene terephthalate). II. Physical structure and morphology. J. Appl. Polym. Sci. 1982, 27, 773–793. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The role of crystalline, mobile amorphous and rigid amorphous fractions in the performance of recycled poly (ethylene terephthalate) (PET). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Yang, S.L.; Jiang, J.M.; Wu, C.X. Crystallization characteristics of weakly branched poly(ethylene terephthalate). Polymer 2005, 46, 11142–11148. [Google Scholar] [CrossRef]
- Kulshreshtha, A.K.; Khan, A.H.; Madan, G.L. X-ray diffraction study of solvent-induced crystallization in polyester filaments. Polymer 1978, 19, 819–823. [Google Scholar] [CrossRef]
- Lin, S.B.; Koenig, J.L. Spectroscopic Characterization of Solvent-Induced Crystallization of Pet. J. Polym. Sci. Part A-2 Polym. Phys. 1983, 21, 1539–1558. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. FTIR spectroscopic analysis of poly(ethylene terephthalate) on crystallization. Eur. Polym. J. 2012, 48, 1586–1610. [Google Scholar] [CrossRef]
- Prorokova, N.P.; Khorev, A.V.; Vavilova, S.Y. Chemical method of surface activation of poly(ethylene terephthalate) fibre materials: Part 1. Study of the modifying effect of sodium hydroxide solutions and products made from quaternary ammonium salts. Fibre Chem. 2009, 41, 158–163. [Google Scholar] [CrossRef]
- Anjum, A.S.; Ali, M.; Sun, K.C.; Riaz, R.; Jeong, S.H. Self-assembled nanomanipulation of silica nanoparticles enable mechanochemically robust super hydrophobic and oleophilic textile. J. Colloid Interface Sci. 2020, 563, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Škvarla, J.; Luxbacher, T.; Nagy, M.; Sisol, M. Relationship of surface hydrophilicity, charge, and roughness of PET foils stimulated by incipient alkaline hydrolysis. ACS Appl. Mater. Interfaces 2010, 2, 2116–2127. [Google Scholar] [CrossRef]

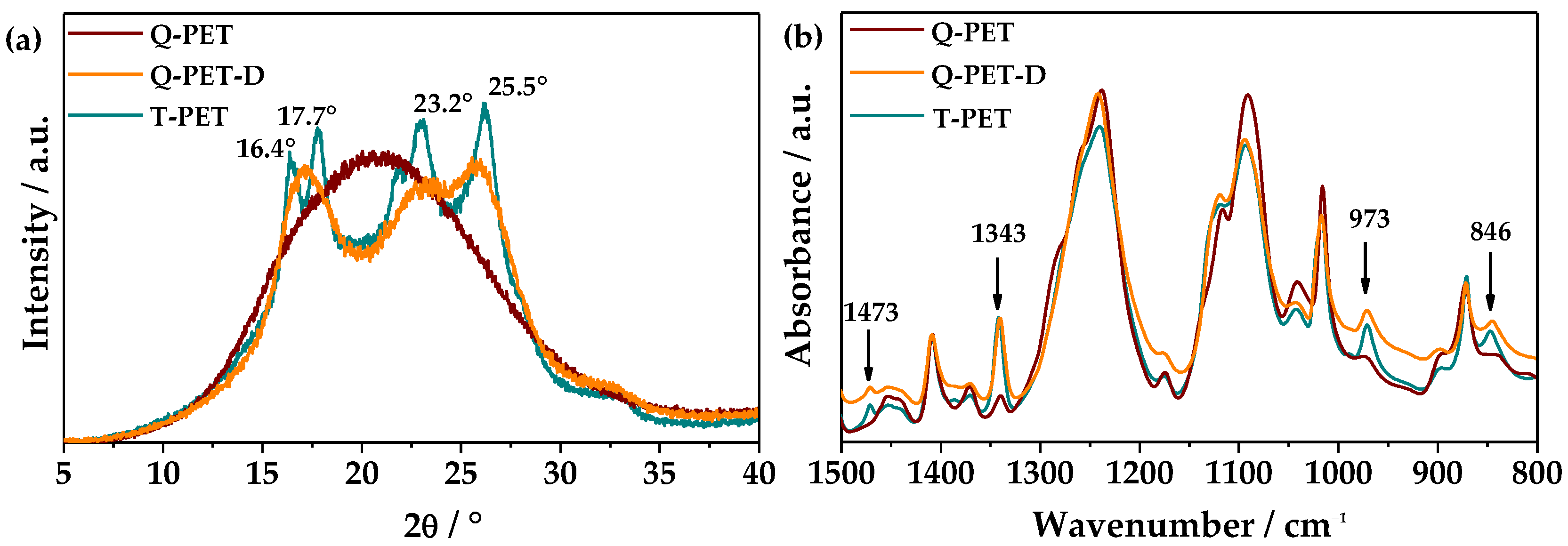

| Sample Name | ΔHcc/J·g−1 | ΔHm/J·g−1 | Xc/% |
|---|---|---|---|
| Q-PET | 33.7 | 34.8 | 0.8 |
| Q-PET-D | 0 | 42.3 | 30.2 |
| T-PET | 0 | 38.3 | 27.3 |
| Sample Name | θw/° | Sa | Sq | Spv | Sz | Ssk | Sku |
|---|---|---|---|---|---|---|---|
| Q-PET | 72 ± 2 | 0.08 ± 0.02 | 0.11± 0.02 | 2.2 ± 0.5 | 1.2 ± 0.3 | 0.4 ± 0.2 | 5.2 ± 0.9 |
| Q-PET-DA | 125 ± 3 | 2.00 ± 0.02 | 2.52 ± 0.03 | 20.0 ± 0.4 | 16.7 ± 0.2 | 0.49 ± 0.04 | 3.3 ± 0.1 |
| Q-PET-DA-FS | 156 ± 2 | 2.2 ± 0.2 | 2.8 ± 0.2 | 23 ± 2 | 16 ± 1 | 0.10 ± 0.05 | 3.0 ± 0.2 |
| Q-PET-DN | 66 ± 2 | 3.3 ± 0.7 | 4.1 ± 0.8 | 28 ± 3 | 24 ± 3 | 0.2 ± 0.1 | 2.9 ± 0.3 |
| Q-PET-DN-FS | 154 ± 2 | 4.2 ± 0.4 | 5.2 ± 0.5 | 32 ± 3 | 27 ± 2 | 0.3 ± 0.2 | 2.6 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, E.; Martínez-Gómez, A.; Huerta, A.; Tiemblo, P.; García, N. Facile Preparation of Hydrophobic PET Surfaces by Solvent Induced Crystallization. Coatings 2022, 12, 137. https://doi.org/10.3390/coatings12020137
Afonso E, Martínez-Gómez A, Huerta A, Tiemblo P, García N. Facile Preparation of Hydrophobic PET Surfaces by Solvent Induced Crystallization. Coatings. 2022; 12(2):137. https://doi.org/10.3390/coatings12020137
Chicago/Turabian StyleAfonso, Elisabet, Aránzazu Martínez-Gómez, Andrea Huerta, Pilar Tiemblo, and Nuria García. 2022. "Facile Preparation of Hydrophobic PET Surfaces by Solvent Induced Crystallization" Coatings 12, no. 2: 137. https://doi.org/10.3390/coatings12020137