An Effect of Co–W Barrier Sublayer on the Functional Characteristics of Au–Ru Contact Coatings
Abstract
:1. Introduction
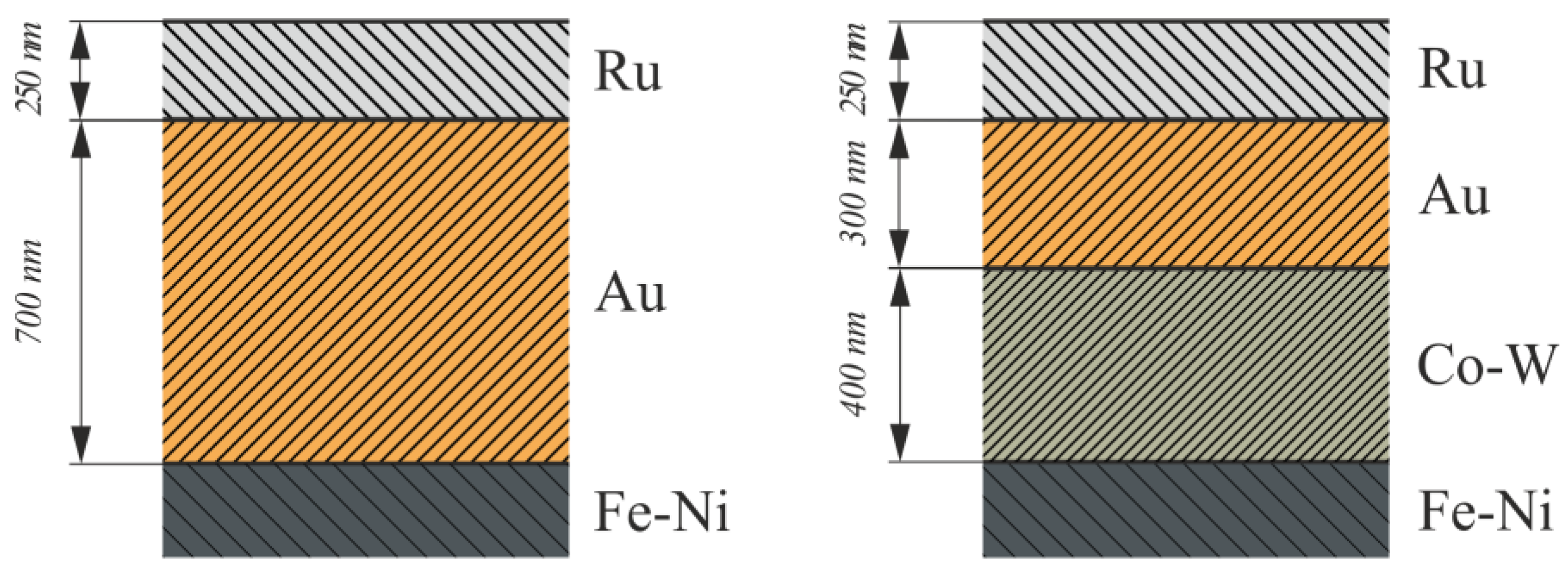
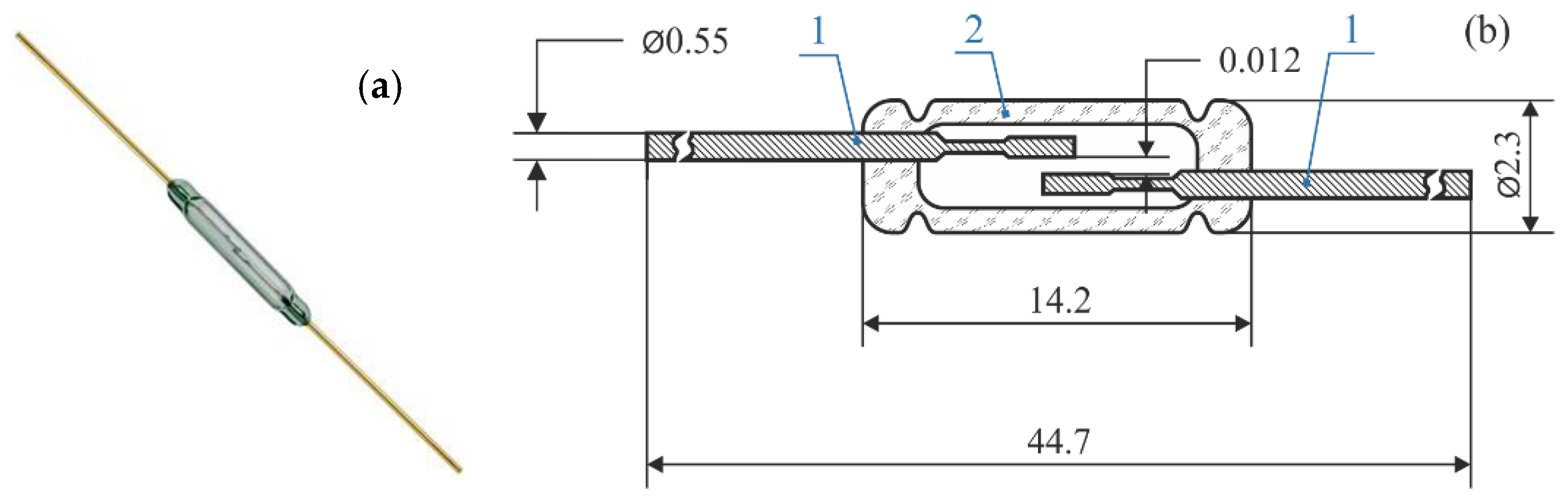
2. Materials and Methods
3. Results and Discussion
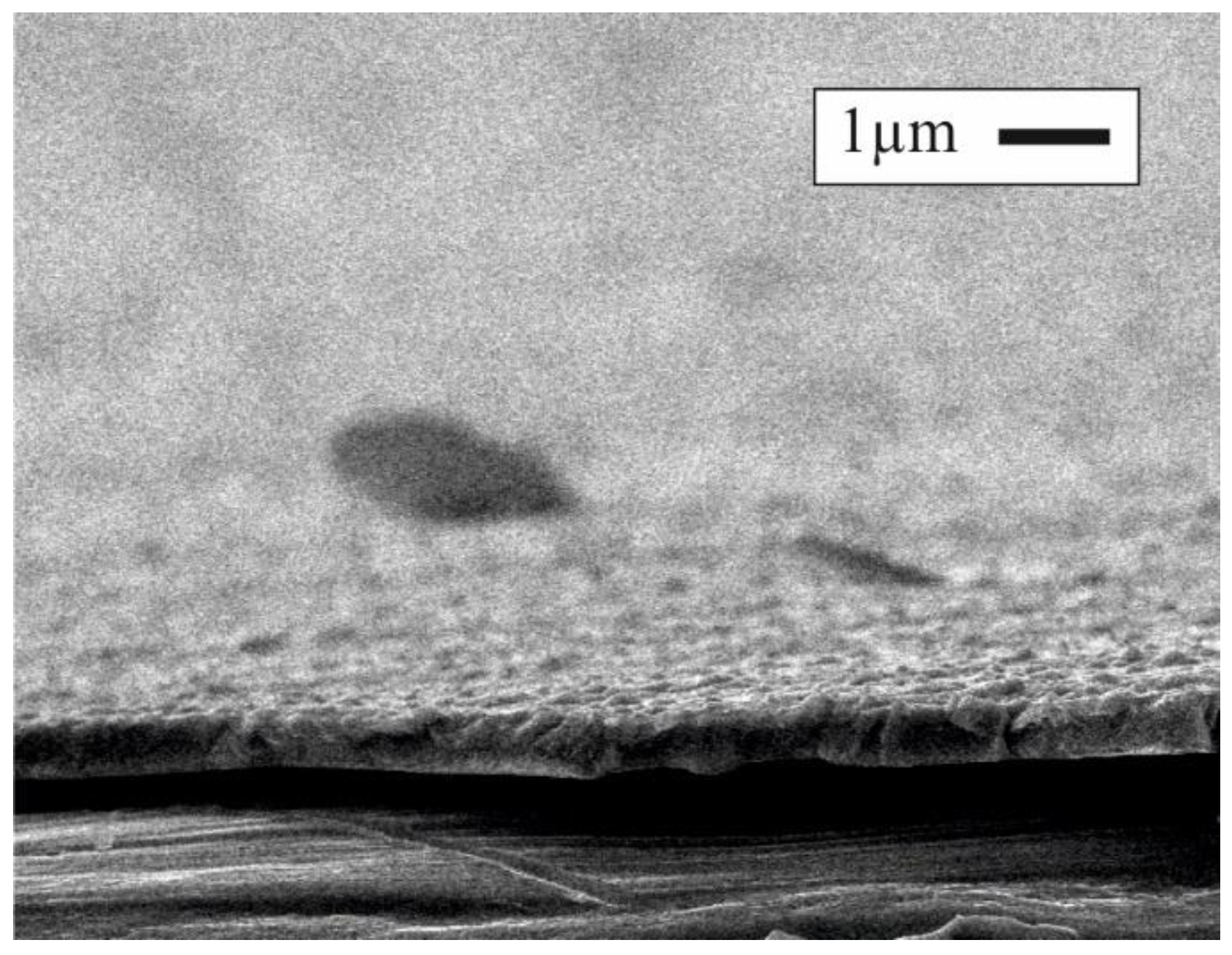
3.1. SEM Images
3.2. Physical–Morphology Characteristics
3.3. Transient Resistance
3.4. Commutation Tests (Temporal Evolution of Transient Resistance)
4. Conclusions
- The Co–W barrier sublayer reduced the average roughness of Au–Ru coatings from 35 to 32 nm and increased their microhardness from 395 to 413 HV. At the same time, the porosity of such coatings did not change significantly, namely, from 4.1% to 3.9%, but average pore sizes decreased.
- The blades with Au–Ru coatings without a Co–W sublayer exhibited a greater variation in the transient resistance during an increase in the pressing force within the range of 0.25–1 N with a lower Rc-value equal to 12 mΩ.
- The Co–W barrier sublayer increased the service time of the tested reed switches: in the low-power mode by more than 4 × 106 switching cycles, and in mean-power mode by more than 1.8 × 106 switching cycles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shishkina, L.; Lokshtanova, O.G.; Karabanov, S.M. Electroplated Coatings for Magnetically Operated Contacts (Reed Switches). Coatings 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Murugan, V.K.; Jia, Z.; Syaranamual, G.J.; Gan, C.L.; Huang, Y.; Chen, Z. An investigation into different nickel and nickel–phosphorus stacked thin coatings for the corrosion protection of electrical contacts. Surf. Coat. Technol. 2016, 300, 95–103. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Frank, C.; Zangari, W.G.; Köçkar, H.; Alper, M.; Rizal, C.; Magagnin, L.; Protsenko, V.; Arunachalam, R.; Rezvanian, A.; et al. Development of electrodeposited multilayer coatings: A review of fabrication, microstructure, properties and applications. Appl. Surf. Sci. Adv. 2021, 6, 100141. [Google Scholar] [CrossRef]
- Braunovich, M.; Konchits, V.V.; Myshkin, N.K. Electrical Contacts; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Gamburg, Y.D. Electrochemical Crystallization of Metals and Alloys; Yanus-K: Moscow, Russia, 1997; 384p. (In Russian) [Google Scholar]
- Karabanov, S.М.; Suvorov, D.V. Ruthenium Based Nanoscale Contact Coatings for Magnetically Controlled MEMS Switches. Int. J. Mechan. Mater. Eng. 2019, 3, 170–173. [Google Scholar]
- Ralbag, N.; Felner, I.; Avnir, D. New reed switch design based on magnetic silver. Mater. Res. Express 2019, 6, 126329. [Google Scholar] [CrossRef]
- Karabanov, S.М.; Lokshtanova, О.G. Complex composition of the sulphamate electrolytes used for ruthenium coating. Zh. Prikl. Khimii 2008, 18, 961–964. (In Russian) [Google Scholar]
- Bernasconi, R.; Lucotti, A.; Nobili, L.; Magagnin, L. Ruthenium Electrodeposition from Deep Eutectic Solvents. J. Electrochem. Soc. 2018, 165, D620. [Google Scholar] [CrossRef]
- William, D.S.; Qiang, H. Ruthenium Electrodeposition from Water-in-Salt Electrolytes and the Influence of Tetrabutylammonium. J. Electrochem. Soc. 2020, 167, 062509. [Google Scholar]
- Shishkina, L.; Karabanov, S.M.; Lokshtanova, O.G. Experience of Development and Application of the Electroplated Coatings Used in the Industrial Production of Magnetically Operated Contacts (Reed Switches). Electropl. Surf. Treat. 2011, 2, 20–26. (In Russian) [Google Scholar]
- Kondati-Natarajan, S.; Nies, C.-L.; Nolan, M. The role of Ru passivation and doping on the barrier and seed layer properties of Ru-modified TaN for copper interconnects. J. Chem. Phys. 2020, 152, 144701. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, Y.; Teng, C.; Cao, H. Recent Advances in Barrier Layer of Cu Interconnects. Materials 2020, 13, 5049. [Google Scholar] [CrossRef] [PubMed]
- Popczyk, M.; Łosiewicz, B. The Influence of Current Density of Electrodeposition on the Electrochemical Properties of Ni-Mo Alloy Coatings. Solid State Phenom. 2015, 228, 269–272. [Google Scholar] [CrossRef]
- Ma, L.; Xi, X.; Nie, Z.; Dong, T.; Mao, Y. Electrodeposition and Characterization of Co-W Alloy from Regenerated Tungsten Salt. Int. J. Electrochem. Sci. 2017, 12, 1034–1051. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Sabour Rouhaghdam, A.R. Ni–W electrodeposited coatings: Characterization, properties and applications. Surf. Coat. Technol. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Lee, H.B. Synergy between corrosion and wear of electrodeposited Ni–W coating. Tribol. Lett. 2013, 50, 407–419. [Google Scholar] [CrossRef]
- Udompanit, N.; Wangyao, P.; Henpraserttae, S.; Boonyongmaneerat, Y. Wear response of composition-modulated multilayer Ni–W coatings. Adv. Mater. Res. 2014, 1025–1026, 302–309. [Google Scholar] [CrossRef]
- Suvorov, D.V.; Gololobov, G.P.; Tarabrin, D.Y.; Slivkin, E.V.; Karabanov, S.M.; Tolstoguzov, A.B. Electrochemical Deposition of Ni–W Crack-Free Coatings. Coatings 2018, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Kublanovsky, V.S.; Yapontseva, Y.S. Electrocatalytic Properties of Co-Mo Alloys Electrodeposited from a Citrate-Pyrophosphate Electrolyte. Electrocatalysis 2014, 5, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Tsyntsarua, N.; Cesiulis, H.; Pellicer, E.; Celis, J.-P.; Sort, J. Structural, magnetic, and mechanical properties of electrodeposited cobalt-tungsten alloys: Intrinsic and extrinsic interdependencies. Electrochim. Acta 2013, 104, 94–103. [Google Scholar] [CrossRef]
- Shishkina, L.; Lokshtanova, O.G. Electroplated Coatings for Reed Switches. Bull. SPbSTI 2011, 11, 122–125. (In Russian) [Google Scholar]
- Karabanov, S.M.; Verlov, N.A.; Suvorov, D.V.; Gololobov, G.P.; Slivkin, E.V. Properties of contact coatings based on ruthenium nanofilms for MEMS switches. Tech. Phys. Lett. 2015, 14, 56–63. [Google Scholar] [CrossRef]
- Gololobov, G.P.; Arefiev, A.S.; Tregulov, V.R.; Utochkin, I.G.; Kireeva, O.V. The study of the surfaces of magnetically controlled contacts by atomic force microscopy. Vestn. RGRTU 2003, 13, 66–69. (In Russian) [Google Scholar]
- Gololobov, G.P.; Suvorov, D.V.; Serpova, M.A.; Arefiev, A.S. The study of contact electrical resistance of coatings on the basis of refractory metal alloys Co-W, Co-Mo, Ni-W, Ni-Mo. J. Phys. Conf. Ser. 2020, 889, 012017. [Google Scholar] [CrossRef]
- Pyatin, Y.M. Materials in Instrumentation and Automation; Mashinostroenie: Moscow, Russia, 1982; 528p. (In Russian) [Google Scholar]
- Jones, T. Electroplating of ruthenium. Met. Finish. 2001, 6, 121–128. [Google Scholar] [CrossRef]






| Deposition Characteristic | Layers | ||
|---|---|---|---|
| Co–W | Au | Ru | |
| Composition and concentration of electrolytes, g·dm−3 | CoSO4–56 Na2WO4–99 C6H8O7–80 H3BO4–40 C12H25SO4Na–1 | K[Au(CN)2]–10 KH2PO4–45 K2HPO4–150 K3C6H5O7–65 ТlН2РO4–0.8 | (NH4)3[Ru2(µ-N)(H2O)2Cl8]–15 (NH4)2 SO4–50 |
| Current density, mA·cm−2 | 5–15 | 1–2.5 | 10–30 |
| pH | 7 | 5.5–6.5 | 1.5–2.0 |
| Temperature, °C | 60 | 60 | 60 |
| Characteristic | Au–Ru Coatings | Au–Ru Coatings with Co–W Sublayer | |||||
|---|---|---|---|---|---|---|---|
| Fe–Ni | Au | Ru | Fe–Ni | Co–W | Au | Ru | |
| Area of pores ρ, % | - | 5.8 | 4.1 | - | 6.2 | 4.4 | 3.9 |
| Roughness h, nm | 20 | 32 | 35 | 20 | 25 | 28 | 32 |
| Microhardness, HV | 345 | 354 | 395 | 345 | 420 | 371 | 413 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gololobov, G.P.; Suvorov, D.V.; Karabanov, S.M.; Slivkin, E.V.; Tolstoguzov, A. An Effect of Co–W Barrier Sublayer on the Functional Characteristics of Au–Ru Contact Coatings. Coatings 2022, 12, 161. https://doi.org/10.3390/coatings12020161
Gololobov GP, Suvorov DV, Karabanov SM, Slivkin EV, Tolstoguzov A. An Effect of Co–W Barrier Sublayer on the Functional Characteristics of Au–Ru Contact Coatings. Coatings. 2022; 12(2):161. https://doi.org/10.3390/coatings12020161
Chicago/Turabian StyleGololobov, Gennady P., Dmitriy V. Suvorov, Sergey M. Karabanov, Evgeniy V. Slivkin, and Alexander Tolstoguzov. 2022. "An Effect of Co–W Barrier Sublayer on the Functional Characteristics of Au–Ru Contact Coatings" Coatings 12, no. 2: 161. https://doi.org/10.3390/coatings12020161






